Abstract
HIV-1-infected opiate abusers often exhibit an accelerated form of HIV-1 associated dementia and enhanced neurological dysfunction. Productive HIV-1 infection of microglia and perivascular macrophages and the resultant secretion of neurotoxic molecules by these cells contribute to this phenomenon. In order to understand the role of morphine in this process, we performed a genome-wide association study at the microRNA (miRNA) and protein levels in human monocyte-derived macrophages (h-mdms). A total of 26 differentially expressed miRNA were identified (p < 0.01), of which hsa-miR-15b and hsa-miR-181b had the greatest increase and decrease in expression levels, respectively. Computational analysis predicted fibroblast growth factor-2 (FGF-2) as the strongest target gene for hsa-miR15b. Of note, we observed a decrease in FGF-2 protein expression in response to morphine. Both hsa-miR-15b and hsa-miR-181b have several predicted gene targets involved in inflammation and T-cell activation pathways. In this context, we observed induction of MCP-2 and IL-6 by morphine. Moreover, proteomic analysis revealed the induction of mitochondrial superoxide dismutase in response to morphine treatment. HIV-1 infection did not induce mitochondrial superoxide dismutase. Collectively, these observations demonstrate that morphine induces inflammation and oxidative stress in h-mdms thereby contributing to expansion of HIV-1 CNS reservoir expansion and disease progression. Of note, differentially expressed miRNAs (hsa-miR-15b and 181-b) may have a potential role in regulating these processes.
Keywords: CNS, HIV-1, miRNA, morphine, proteomics, macrophages
Introduction
Opiate abuse has the potential to destabilize neuronal functions and in Human Immunodeficiency virus-1 (HIV-1)-infected individuals might lead to an accelerated form of HIV-1 associated dementia (HAD). Not surprisingly, HIV-1 infected individuals that abuse opiates exhibit more severe neuropathology at autopsy [Bell et al., 2006]. Importantly however, the molecular biology underlying these clinical observations needs elucidation. Our current understanding of HIV-1 neuropathogenesis suggests that key features include infiltration of macrophages into the CNS, formation of microglial nodules and multinucleated giant cells, astrocyte activation and neuronal loss, particularly in the hippocampus and basal ganglia. In addition, evidence also suggests dysregulation of synaptodendritic communication among neurons in the absence of cell death [Ellis et al., 2007]. Since productive viral replication occurs in perivascular macrophages and in microglia, the ultimate effects on the functioning of neurons is mediated by viral proteins and neurotoxins secreted from these infected cells [Gonzalez-Scarano and Martin-Garcia, 2005; Hauser et al., 2007].
A strong relationship seems to exist between opiate usage and HIV-1 neuropathogenesis, as the former correlates with the severity of the CNS disease in heroin-abusing cohorts [Bokhari et al., 2009]. Heroin is a semi-synthetic ester of morphine and exerts its effect by converting to morphine in the brain [Rook et al., 2006]. Hence, morphine is the drug of choice to study HIV-1 neuropathogenesis in opiate-abusing cohorts. Opiates promote HIV-1 propagation in immune cells while suppressing immune functions. Immunomodulatory activities of opiates also include inhibition of antibody responses, induction of chemotaxis in mononuclear cells, alteration of macrophage functions and modulation of cytokine production [Bussiere et al., 1993; Ho et al., 2003; Rojavin et al., 1993]. Of note, chronic opioid drug exposure often leads to altered patterns of gene activation and increased oxidative stress which might eventually lead to synaptodendritic miscommunications and neuronal injury [Ellis et al., 2007; Turchan-Cholewo et al., 2006]. Generation of reactive oxygen species (ROS) can lead to oxidation of proteins and DNA, peroxidation of lipids, and eventually cell death [Hsiao et al., 2009]. A consequence of the production of ROS involves induction of pro-inflammatory cytokine biosynthesis suggesting complex interplay between CNS inflammation and oxidative stress [Turchan-Cholewo et al., 2009].
Genome-wide association studies enable identification of unknown biochemical pathways involved in clinical observations. Such studies also aid in generating molecular tools by which these pathways might be elucidated. Additionally, simultaneous analysis at the micro RNA (miRNA) and protein levels provides unique insights towards developing potential small interfering RNA or miRNA based therapeutic moieties that could abrogate the associated pathologies. Few studies have performed proteomic analysis associated with morphine or HIV-1 infection [Li et al., 2009; Ricardo-Dukelow et al., 2007; Yang et al., 2007]. A recent study performed miRNA-based analysis to identify role of hsa-mir-23b in regulation of the μ-opioid receptor [Wu et al., 2009]. None of the studies thus far have simultaneously performed genome-wide miRNA and protein level analysis. In this study, we focused our attention on human monocyte-derived macrophages (h-mdms) and their response during exposure to morphine and infection with HIV-1. We utilized miRNA microarray and 2D-gel electrophoresis based analyses to determine differential expression and deduce potential correlations and unique features associated with morphine exposure or HIV-1 associated insults.
Materials and Methods
Isolation & differentiation of human monocyte derived macrophages
h-mdms were generated by differentiation of peripheral blood mononuclear cells (PBMCs) obtained from healthy, HIV-1 seronegative donors as previously described with several modifications [Dave and Pomerantz, 2004]. Dynabeads® CD8 (Invitrogen, Carlsbad, CA) were utilized to deplete CD8+ T cells from PBMCs according to the manufacturer's instructions. CD8-PBMCs (5 × 106 cells in 10-cm petri dishes) were allowed to adhere and differentiate into h-mdms for 7-10 days prior to any treatment. CD8-PBMCs were differentiated in DMEM containing 10% heat-inactivated FBS, 10% heat-inactivated horse serum, 2mM L-glutamine, 50U penicillin G/mL, 50μg streptomycin/mL, 0.5ng granulocyte-macrophage colony stimulating factor/mL and 0.5ng macrophage colony stimulating factor/mL. Additional fresh media was added at 2-3 day intervals. At the end of differentiation, adherent h-mdms were washed 3 times in the differentiation media and cultured in Optimem I serum-free medium (Invitrogen, Carlsbad, CA). h-mdms were infected with HIV-1 isolates, YU-2 or JR-FL (MOI = 0.1 pg p24/cell) for 2h [National Institute of Health AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. pYU-2 (Catalog #: 1350; Contributor: Dr. B. Hahn & Dr. G.M. Shaw) JR-FL (Catalog #: 395; Contributor: Dr. I.S.Y. Chen)]. After 2h, cells were washed extensively with PBS to remove input virus. h-mdms were cultured in DMEM containing 10% heat inactivated FBS for 5 days post infection (dpi) [Dave and Pomerantz, 2004]. h-mdms were treated with morphine (Sigma-Aldrich, St. Louis, MO) in Optimem I reduced serum medium (Invitrogen, Carlsbad, CA).
miRNA analysis
Total RNA was extracted from h-mdms at the end of the treatment with mirVana™ miRNA isolation kit according to manufacturer's instructions (Ambion, Austin, TX). In order to obtain a representative profile of miRNA expression, h-mdms were isolated from three individual donors and were treated with 0.1μM morphine for 1h and 24h. Total RNA was isolated from these h-mdms at the end of 1h and 24h treatments along with those from control, to ensure detection of early and late expressing miRNAs. Equivalent amounts of total RNA from both 1h and 24h samples from the three donors was pooled (4 × 3 = 12μg) to eliminate differences between the donors. miRNA extraction and miRNA hybridization were performed at LC Sciences (Houston, TX). miRNA from control and morphine-treated h-mdms was labeled either with Cy3 or Cy5, respectively. Labeled miRNA was hybridized to an array with probe content from Release 12.0 of the miRBase sequence database that included 856 mature human miRNAs. Cy3/Cy5 signal ratios were determined to identify differentially expressed miRNAs. A four probe repeat system was utilized to perform statistical analysis. Each miRNA probe was spotted four times on the array and as a result four columns were generated on the heat map.
miRNA gene target prediction
Web-based tools at the miRDB website (http://www.mirdb.org) in conjunction with the Panther database (http://www.pantherdb.org) of compiled pathways were utilized to identify potential pathways and target genes through which miRNAs (differentially expressed in response to morphine) might intersect. The MirTarget2 prediction tool assigned a score to each of the gene targets in the range from 50-100 (with a score of 100 being the most likely gene target for the miRNA). A predicted target with score greater than 80 is most likely to be bonafide, while targets with scores below 60 require additional evidence to be considered valid [Thomas et al., 2003; Wang, 2008; Wang and El Naqa, 2008].
Western blot analysis
Cell-free culture supernatants were obtained from control h-mdms, h-mdms treated for 24h with 0.1μM morphine and 0.1μM morphine plus 1μM naloxone (Sigma-Aldrich, St. Louis, MO). In addition, cell-free culture supernatants were obtained from HIV-1 YU-2 infected h-mdms (5 dpi) along with those treated with 0.1μM morphine and 0.1μM morphine plus 1.0μM naloxone (Sigma-Aldrich, St. Louis, MO). Supernatants were concentrated 25× on Amicon Ultra-15 Centrifugal filter device (with 3000 Molecular Weight Cut-off) according to manufacturer's instructions (Millipore, Billerica, MA). Concentrated supernatants were subjected to western blot analysis and probed with a monoclonal anti-fibroblast growth factor-2 (FGF-2) antibody (Calbiochem, San Diego, CA) at a concentration of 0.1μg/mL in 5% skimmed milk for 1h. Blots were washed extensively with PBS containing 0.1% Tween-20 (Sigma-Aldrich, St. Louis, MO) and subsequently probed with goat-anti mouse horse radish peroxidase conjugated secondary antibody at a concentration of 4 ng/mL (Thermo Scientific, Rockford, IL). Enhanced chemiluminescent plus detection kit (GE healthcare, Piscataway, NJ) was utilized to detect the secondary antibody. Band intensities were determined with the aid of Quantity One 1D analysis software (Biorad, Hercules, CA) and were normalized with protein concentration in the concentrated supernatants.
Cytokine array
Uninfected and HIV-1-infected h-mdms (5dpi) were incubated in Optimem-I reduced serum medium (Invitrogen, Carlsbad, CA) for 48h. Subsequently, they were treated with 0.1μM morphine for 24h and cell-free culture supernatants were obtained from morphine-treated and control h-mdms for cytokine array analysis. Culture supernatants (1mL) were utilized to probe a human cytokine antibody array (AAH-CYT3) according to manufacturer's instructions (RayBiotech, Norcross, GA).
Two-dimensional gel electrophoresis and MALDI-TOF MS analysis
Control h-mdms, HIV-1 YU-2 infected and morphine-treated h-mdms were washed 3 times with cold PBS. Cells were harvested by scraping in cold PBS and the resultant cell-suspension was centrifuged at 1200 rpm at 4°C to pellet cells. Protein extraction, gel electrophoresis and MALDI-TOF MS analysis was performed at the Proteomics Core (Temple University). Briefly, protein extracts were prepared by lysing cells in 2D buffer containing 8M urea, 2% CHAPS, 40mM Tris, 60mM DTT, and 1× protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN). For the first dimension, proteins were resolved in an immobilized pH gradient ranging from pI 4-7 or 6-10. For the second dimension, proteins were resolved on 10-14% SDS-poly-acrylamide gels. Resolved proteins in the 2D Gels were stained with Sypro-Ruby fluorescent stain. Fluorescence images were captured with FLA-5000 Fluor Imager and the images were analyzed by PDQuest Software Version 8.0. Spots were detected by the software and the images were manually edited to remove artifacts and correct mismatches. Individual spot volumes were calculated by density/area integration. Gels were run in duplicate and to eliminate gel-to-gel variation, individual spot volumes for each gel were normalized relative to total valid spot volume of that gel. Normalized spot volume was utilized to determine differential expression. Select differentially expressed spots were excised using an Xcise automated robotic system followed by identification of in-gel tryptic digested peptides by MALDI-TOF MS analysis [Duan et al., 2008].
Statistical Analysis
Statistically significant differences between control and test groups were determined by Student's t tests. p values of ≤ 0.05 were considered statistically significant.
Results
A genome-wide analysis of miRNA expression associated with morphine treatment of h-mdms was performed to identify gene targets and biochemical pathways with the objective of understanding HIV-1 neuropathogenesis in opioid-abusing cohorts. These investigations were supplemented by analysis of select proteins including growth factors (FGF-2) and pro-inflammatory cytokines that may be regulated as a result of miRNAs that were differentially expressed in response to morphine or Guanine Nucleotide Binding-protein couple receptor (GPCR) signalling pathways. In order to compare the response of h-mdms to morphine or HIV-1 infection, cytokine and FGF-2 expression and proteomics studies were performed.
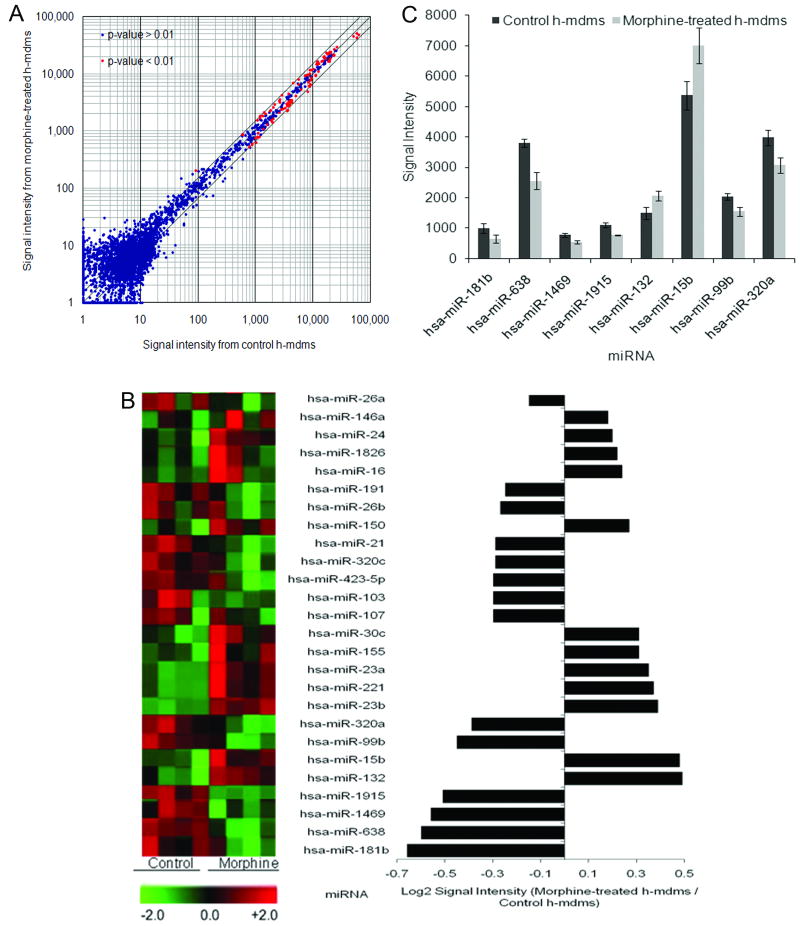
Fluorescence intensity of normalized signal from Cy5 or Cy3 labeled probes hybridized to the array was calculated and a heat-map was generated to determine differential miRNA expression (Fig. 1A). Approximately, 26 miRNAs exhibited differential expression with log2 values ± 0.6 range (p < 0.01) (Fig. 1B). Of these 27 miRNAs, 12 miRNAs were up-regulated. Signal strengths of these differentially expressed miRNAs were compared with log2 ratios to identify miRNA that had both maximal change in expression level and high abundance. Based on this analysis, we identified hsa-miR-15b and hsa-miR-638 with a log2 ratio of 0.48 and -0.6, respectively. In addition, we identified hsa-miR181b which showed the strongest down-regulation in morphine-treated h-mdms with log2 ratio of -0.66 (Fig. 1C).
Fig. 1.
Morphine treatment of h-mdms induces differential miRNA expression. miRNA isolated from morphine-treated and control h-mdms were labeled with Cy5 or Cy3, respectively. A: Labeled miRNA were hybridized simultaneously to a miRNA array (Sanger Version 12.0) to detect morphine-induced differential miRNA expression. The majority of the differentially expressed miRNAs had <0.5 fold change in expression level as compared to control. The outer lines along the median indicate a 0.5 fold change in expression levels. B: A total of 26 different miRNAs with P-values ≤ 0.01 were identified to exhibit differential expression. The heat-map of these miRNAs is depicted on the right-side. Green signal on the heat-map indicates a decrease in miRNA expression and red signal indicates an increase in miRNA expression level as compared to control (range: -2.0 to +2.0). C: Eight of these 26 miRNAs had a log2 ratio ≥ 0.05 and P-values 0.01.
A bioinformatics approach was utilized to identify gene targets and associated biochemical pathways that might be regulated by hsa-miR-15b, hsa-miR-181b and hsa-miR-638 that may explain accelerated disease progression in opioid abusing cohorts. The miRDB database predicted 600 gene targets for hsa-miR15b of which 105 genes targets had a score greater than 80. The highest score (100) was associated with FGF-2. hsa-miR-15b was predicted to bind at four locations in the 3′-UTR (1113, 5066, 5599 & 5640). The core sequence (5′-AGCAGCAC-3′) was conserved at all the four identified locations in the 3′-UTR. We identified 936 gene targets for hsa-miR-181b of which 185 gene targets had a score greater than 80. Of note, tumor necrosis factor alpha (TNF-α) is a gene target for hsa-miR181b as well as related miRNAs hsa-miR181a-d. As compared with hsa-miR-15b, hsa-miR-638 had only 6 predicted gene targets. Gene targets identified for hsa-miR-638, cyclin G2 (score 72) and transcription elongation regulator 1-like factor (score 65) were predicted to have an involvement in p53 and platelet-derived growth factor (PDGF) signaling pathways (Table 1).
Table 1.
| Predicted Target Genes | ||||
|---|---|---|---|---|
| Symbol | Name | Biochemical pathway | miRNA | Score |
| FGF-2 | Fibroblast grow th factor 2 | FGF Signalling pathw ay | hsa-miR-15b | 100 |
| CCNE1 | Cyclin E1 | p53 pathw ay feedback loops 2 | hsa-miR-15b | 97 |
| BTRC | Beta-transducin repeat containing | Wnt Signalling pathw ay | hsa-miR-15b | 96 |
| CD80 | CD80 molecule | T-cell activation pathw ay | hsa-miR-15b | 92 |
| DCLK1 | Doublecortin-like kinase 1 | Inflammation mediated by chemokine and cytokine signalling pathways | hsa-miR-15b | 90 |
| SMURF1 | SMAD specific E3 ubiquitin protein ligase 1 | Ubiquitin proteasome pathway | hsa-miR-15b | 90 |
| TBL1XR1 | Transducin (beta)-like 1 X-linked receptor 1 | Wnt Signalling pathw ay | hsa-miR-15b | 85 |
| ARHGAP5 | Rho GTPase activating protein 5 | PDGF signalling pathw ay | hsa-miR-15b | 84 |
| SMAD7 | SMAD family member 7 | TGF-β signalling pathway | hsa-miR-15b | 84 |
| CDC25A | Cell division cycle 25 homolog A | p53 pathw ay | hsa-miR-15b | 82 |
| PCDH9 | Protocadherin 9 | Cadherin Signalling Pathw ays | hsa-miR-15b | 82 |
| XDH | Xanthine dehydrogenase | Adenine and hypoxanthine salvage pathw ay | hsa-miR-15b | 82 |
| NFAT5 | Nuclear factor of activated T-cells 5, tonicity-responsive | Inflammation mediated by chemokine and cytokine signalling pathways | hsa-miR-181b | 97 |
| ARHGEF3 | Rho guanine nucleotide exchange factor (GEF) 3 | Heterotrimeric G-protein signaling pathw ay-Gq alpha and Go alpha mediated pathw ay | hsa-miR-181b | 97 |
| CPOX | Coproporphyrinogen oxidase | Heme biosynthesis | hsa-miR-181b | 97 |
| ACVR2B | Activin A receptor, type IIB | TGF-beta signaling pathw ay | hsa-miR-181b | 96 |
| RAP1B | RAP1B, member of RAS oncogene family | Heterotrimeric G-protein signaling pathw ay-Gi alpha and Gs alpha mediated pathw ay | hsa-miR-181b | 94 |
| TCERG1 | Transcription elongation regulator 1 | PDGF signaling pathway | hsa-miR-181b | 94 |
| TBL1X | Transducin (beta)-like 1×-linked | Wnt signaling pathw ay | hsa-miR-181b | 92 |
| ATM | Ataxia telangiectasia mutated | p53 pathw ay | hsa-miR-181b | 91 |
| ACVR2A | Activin A receptor, type IIA | TGF-beta signaling pathw ay | hsa-miR-181b | 91 |
| SIPA1L2 | Signal-induced proliferation-associated 1 like 2 | Heterotrimeric G-protein signaling pathw ay-Gq alpha and Go alpha mediated pathw ay | hsa-miR-181b | 89 |
| BAI3 | Brain-specific angiogenesis inhibitor 3 | p53 pathw ay | hsa-miR-181b | 89 |
| TBL1XR1 | Transducin (beta)-like 1 X-linked receptor 1 | Wnt signaling pathw ay | hsa-miR-181b | 89 |
| KRAS | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | p53 pathw ay feedback loops 2 | hsa-miR-181b | 88 |
| PCDHAC2 | Protocadherin alpha subfamily C, 2 | Cadherin signaling pathw ay | hsa-miR-181b | 88 |
| GNB4 | Guanine nucleotide binding protein (G protein), beta polypeptide 4 | Cortocotropin releasing factor receptor signaling pathw ay | hsa-miR-181b | 88 |
| SIRT1 | Sirtuin (silent mating type information regulation 2 homolog) 1 | p53 pathw ay | hsa-miR-181b | 86 |
| PER3 | Period homolog 3 (Drosophila) | Circadian clock system | hsa-miR-181b | 86 |
| FKBP1A | FK506 binding protein 1A, 12kDa | TGF-beta signaling pathw ay | hsa-miR-181b | 85 |
| TBPL1 | (TATA-binding protein) TBP-like 1 | General transcription by RNA polymerase I | hsa-miR-181b | 83 |
| ITGA2 | Integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) | Inflammation mediated by chemokine and cytokine signalling pathways | hsa-miR-181b | 83 |
| CALM1 | Calmodulin 1 (phosphorylase kinase, delta) | T cell activation | hsa-miR-181b | 82 |
| PCDHAC1 | Protocadherin alpha subfamily C, 1 | Cadherin signaling pathw ay | hsa-miR-181b | 82 |
| PCDHA1-8, 10, 11, 13 | Protocadherin alpha 8 | Cadherin signaling pathw ay | hsa-miR-181b | 82 |
| CAMK2D | Calcium/calmodulin-dependent protein kinase II delta | Inflammation mediated by chemokine and cytokine signalling pathways | hsa-miR-181b | 81 |
| UBE2D1 | Ubiquitin-conjugating enzyme E2D 1 (UBC4/5 homolog, yeast) | Ubiquitin proteasome pathway | hsa-miR-181b | 80 |
| TNF | Tumor necrosis factor (TNF superfamily, member 2) | Apoptosis signaling pathw ay | hsa-miR-181b | 80 |
| CCNG2 | Cyclin G2 | p53 pathw ays | hsa-miR-638 | 72 |
| TCERG1L | Transcription elongation regulator 1-like | PDGF signaling pathway | hsa-miR-638 | 65 |
To identify metabolic pathways and biological processes that might be regulated by hsa-miR-15b and hsa-miR-181b through intersection with target genes (score > 80), we performed an alternate search utilizing the miRDB and Panther databases. Pathways with the greatest potential for regulation by hsa-miR-15b include the p53 and Wnt signaling pathways, T-cell activation pathway and most importantly chemokine and cytokine mediated inflammation pathways. These pathways were also identified for hsa-miR-181b; however, the target genes were different. For example in the p53 pathway, CDC25A (Cell division cycle homolog A) is a target for hsa-miR-15b, while ATM (Ataxia telangiectasia mutated) is targeted by hsa-miR181b. In addition, G-protein, TGF-β & Cadherin signaling pathways were also identified as potentially regulated by hsa-miR-181b. Of note, several members of the protocadherin-α family that participate in the Cadherin signalling pathway were predicted as target genes for hsa-miR-181b (Table 1).
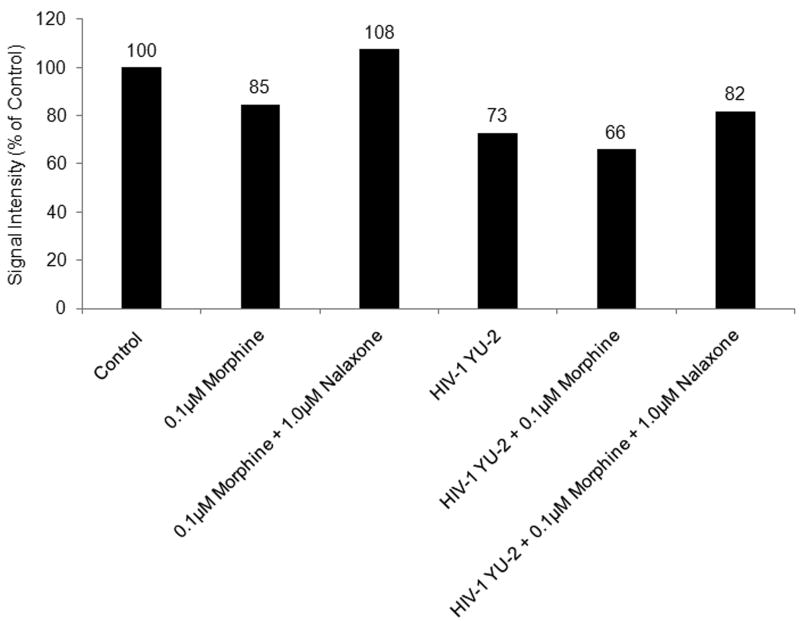
As FGF-2 was predicted to be a strongest gene target for hsa-miR-15b, we performed western blot analysis of cell-free h-mdm culture supernatants to correlate their expression in response to morphine treatment. In addition, FGF-2 secretion was quantified during HIV-1 infection alone or in combination with morphine treatment. For this analysis, cell-free h-mdm culture supernatants that had been subjected to equivalent concentration (25 ×) were utilized. FGF-2 levels were decreased to 85% and 66% of control in cell-free culture supernatants from morphine-treated h-mdms and morphine-treated h-mdms infected with HIV-1, respectively. These effects seem to be mediated through the μ-opioid receptor as, naloxone was able to reverse the morphine-induced decrease only in the absence of HIV-1 infection. In HIV-1-infected h-mdms treated with morphine, naloxone increased the secretion of FGF-2 from 66% to 82% of control suggesting additional mechanisms might affect hsa-miR-15b expression and FGF-2 secretion during HIV-1 infection (Fig. 2).
Fig. 2.
Morphine treatment of h-mdms decreases secretion of FGF-2. Cell-free culture supernatants were concentrated to equivalent volumes (25×) on ultra-filtration membranes (cut-off: 3,000 kDa). Concentrated cell-free culture supernatants from uninfected and HIV-1 YU-2-infected h-mdms at 5dpi were either treated with 0.1μM morphine or 0.1μM morphine and 1.0μM naloxone for a duration of 24h. These concentrated cell-free supernatants along with the same from untreated controls were subjected to Western blot analysis. Image-quantitation of the blot suggests FGF-2 secretion was decreased to 85% and 66% in response to morphine treatment and morphine treatment during HIV-1 infection, respectively. Signal intensities depicted were normalized with protein concentration and compared as % of control.
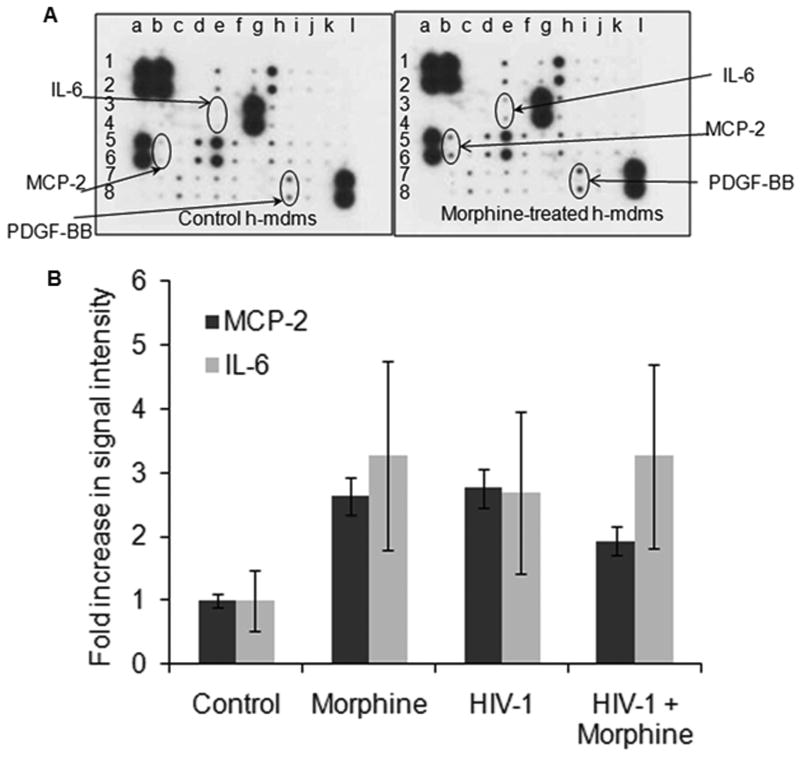
Several gene targets identified from the miRNA analysis suggested potential miRNA mediated regulation of the pro-inflammatory cytokines and chemokines. In order to quantitate secretion of pro-inflammatory cytokines and chemokines from h-mdms upon treatment with morphine, we utilized a human cytokine antibody array. We also analyzed changes in secretion of these molecules from HIV-1 JR-FL infected h-mdms alone or in combination with morphine treatment as inflammation is an important aspect of HIV-1 neuropathogenesis. Both, morphine-treatment and HIV-1 JR-FL infection of h-mdms induced secretion of monocyte chemoattractant protein 2 (MCP-2) and interleukin 6 (IL-6) (Fig. 3). MCP-2 secretion was increased 2.6 (p value = 0.003) and 2.8 (p value = 0.0001) fold over control in morphine treated and HIV-1 JR-FL infected h-mdms, respectively. The differences in MCP-2 secretion from morphine treated or HIV-1 infected h-mdms were statistically insignificant (p value = 0.322). When h-mdms were treated with morphine during HIV-1 JR-FL infection, MCP-2 secretion was increased 1.9 fold over control and was significantly less than the increase triggered by individual treatment conditions (p value = 0.002). IL-6 secretion was increased 3.3 (p value = 0.0005) and 2.7 (p value = 0.011) fold over control in morphine treated and HIV-1 JR-FL infected h-mdms, respectively. The differences in IL-6 secretion from morphine treated or HIV-1 infected h-mdms were statistically insignificant (p value ≥ 0.05). When h-mdms were treated with morphine during HIV-1 JR-FL infection, IL-6 secretion was increased 3.3 fold over control and was similar to the increases triggered by individual treatment conditions (p value ≥ 0.05). TNF-α secretion was not detected in culture supernatants from control, morphine-treated or HIV-1-infected h-mdms. While TNF-α was not detected in this analysis, it should be noted that 50 pg/mL of TNF-α was detected during HIV-1 YU-2 infection of h-mdms by ELISA.
Fig. 3.
Morphine-induced secretion of inflammatory cytokines and chemokines. A: Cell-free culture supernatants from morphine-treated, HIV-1 JR-FL-infected, HIV-1 JR-FL-infected plus morphine-treated or control h-mdms were subjected to an inflammatory chemokine and cytokine antibody array analysis. B: Quantitation of signal intensities revealed that morphine treatment and HIV-1 infection of h-mdms increased secretion of MCP-2 and IL-6. A cumulative effect was not observed when HIV-1-infected h-mdms were treated with morphine. Increase in PDGF-BB secretion was not statistically significant. No induction of TNF-α was observed in this array analysis.
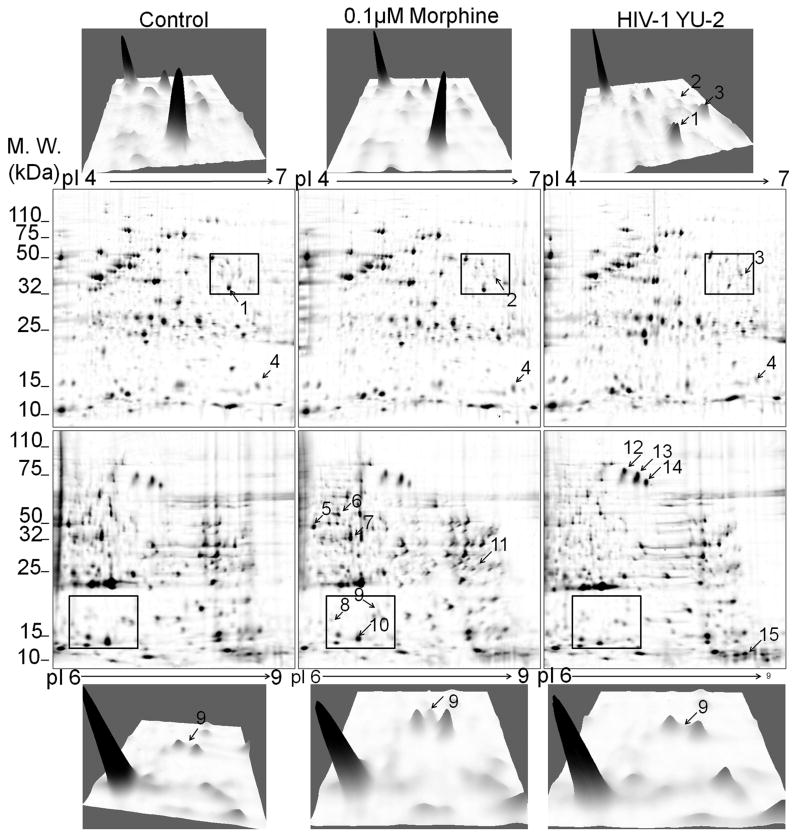
Both morphine treatment and HIV-1 infection altered expression at select proteins. Hence, in order to identify changes in overall protein expression associated with morphine treatment or HIV-1 infection, we performed 2D-gel analysis of proteins isolated from control, morphine-treated and HIV-1 YU-2 infected h-mdms (Fig. 4). Densitometric analysis of proteins resolved on 2D gels was performed to determine qualitative and quantitative changes in protein expression. Both morphine treatment and HIV-1 infection decreased protein expression with the latter having a greater effect. As compared to control h-mdms, 48% and 57% of different protein spots had decreased signal intensity in morphine-treated and HIV-1 YU-2 infected h-mdms, respectively. A stronger decrease in overall protein expression as a result of HIV-1 YU-2 infection was reflected in the number of protein spots that had a greater than 0.5 fold decrease. The number of proteins with greater than 0.5 fold decrease as compared to control, was 2 and 9 (p value ≤ 0.05) for morphine and HIV-1 YU-2 infected h-mdms, respectively. The number of proteins with greater than 0.5 fold increase as compared to control, was 3 and 9 (p value ≤ 0.05) for morphine treated and HIV-1 YU-2 infected h-mdms, respectively (Supplementary Fig. 1, Supplementary Fig. 2).
Fig. 4.
Morphine treatment of h-mdms induces differential protein expression. Protein extracts from control h-mdms, morphine-treated and HIV-1 YU-2-infected h-mdms were subjected to 2D gel electrophoresis. Proteins were resolved in first dimension by isoelectric focusing at pI 4-7 and 6-9. Signal intensities of resolved protein spots from morphine-treated and HIV-1 YU-2-infected h-mdms was compared to the same from control h-mdms. The 3D plot on the top row shows change in protein expression from within the square in the pI 4-7 gel electrophoresis. Both morphine and HIV-1 infection decrease intensity of spot 1. In addition, spot 2 was induced by morphine treatment and spot 3 was induced in response to HIV-1 YU-2 infection. The 3D plot in the bottom row shows changes in protein expression from within the square in the pI 6-9 gel electrophoresis. Spot 9 was induced only in response to morphine treatment.
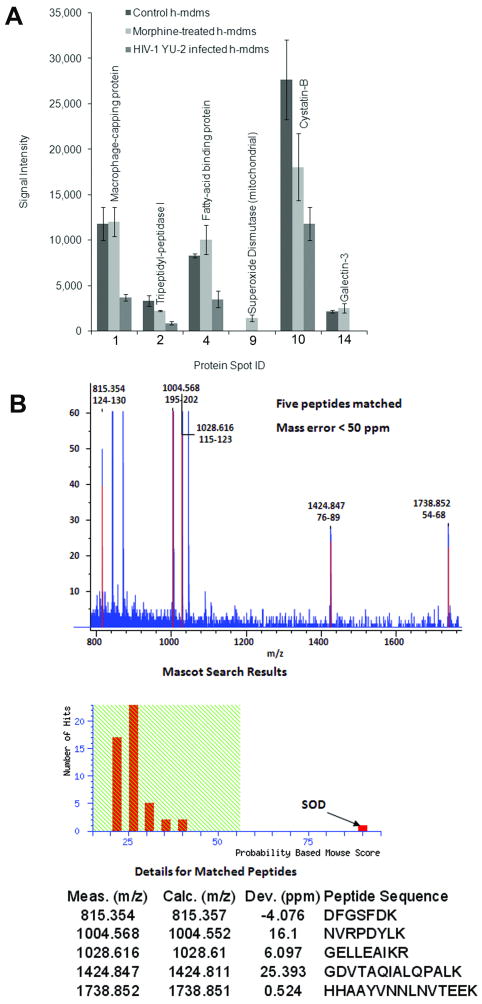
A select group of proteins that exhibited differential expression between morphine-treated and HIV-1 YU-2 infected h-mdms were identified by MALDI-TOF MS analysis (Fig. 5A & B). Mitochondrial superoxide dismutase (spot 9; Fig. 4) was not detected in control or HIV-1 YU-2 infected h-mdms. It was specifically induced during morphine treatment. Similarly, Galectin-3 (Gal-3) was not detected during HIV-1 infection. Gal-3 expression was not increased significantly in response to morphine treatment (1.15 fold over control; p value = 0.47). Macrophage capping protein (spot 1; Fig. 4) expression was decreased by 0.3 fold over control (p value = 0.02) in HIV-1 infected h-mdms. Morphine did not alter expression of macrophage capping protein (p value = 0.93). Both morphine treatment and HIV-1 infection decreased expression of Tripeptidyl-peptidase I (spot 2; Fig. 4) by 0.68 and 0.27 fold over control, respectively. However, the decrease was statistically significant only in the case of HIV-1 infection (p value = 0.03). Similar decrease in expression levels were observed for Cystatin B (spot 10; Fig. 4). Both morphine treatment and HIV-1 infection decreased expression of Cystatin B (spot 10; Fig. 4) by 0.65 and 0.43 fold over control, respectively. However, the decrease was statistically significant only in the case of HIV-1 infection (p value = 0.04). Expression of fatty acid binding protein (spot 4; Fig. 4) was increased 1.21 fold over control by morphine and decreased by 0.43 fold over control during HIV-1 infection. However, the change in expression levels was significant only during HIV-1 infection (p value = 0.02).
Fig. 5.
Mitochondrial superoxide dismutase was induced by morphine treatment of h-mdms. Differentially-expressed protein spots were identified by MALDI-TOF MS analysis. A: Majority of these protein spots were suppressed as a result of HIV-1 YU-2 infection. Identity of 6 of the 15 spots was successfully confirmed by MALDI-TOF MS analysis. B. Only 1 of these 6 spots was specifically induced by morphine and was identified as mitochondrial superoxide dismutase.
Discussion
These data demonstrate the potential mechanisms through which HIV-1 disease progression may accelerate in opioid abusing individuals. In this study, morphine treatment of h-mdms lead to differential miRNA and protein expression that have potential impacts key inflammation and oxidative stress processes that lead to expansion of the HIV-1 viral reservoir in the CNS. Differentially expressed miRNAs, hsa-miR-15b and hsa-miR-181b have several targets in the pro-inflammatory pathways. Of note, FGF-2 is likely to be targeted by hsa-miR-15b (score = 100). FGF-2 secretion was down-regulated by morphine and the hsa-miR-15b was up-regulated under similar treatment conditions. Pro-inflammatory response including the induction of MCP-2 and IL-6 secretion similar to that observed during HIV-1 infection, was also observed upon morphine treatment of h-mdms revealing commonality of some pathways, while induction of mitochondrial Superoxide Dismutase reflects on unique aspects of morphine induced metabolic changes in CNS.
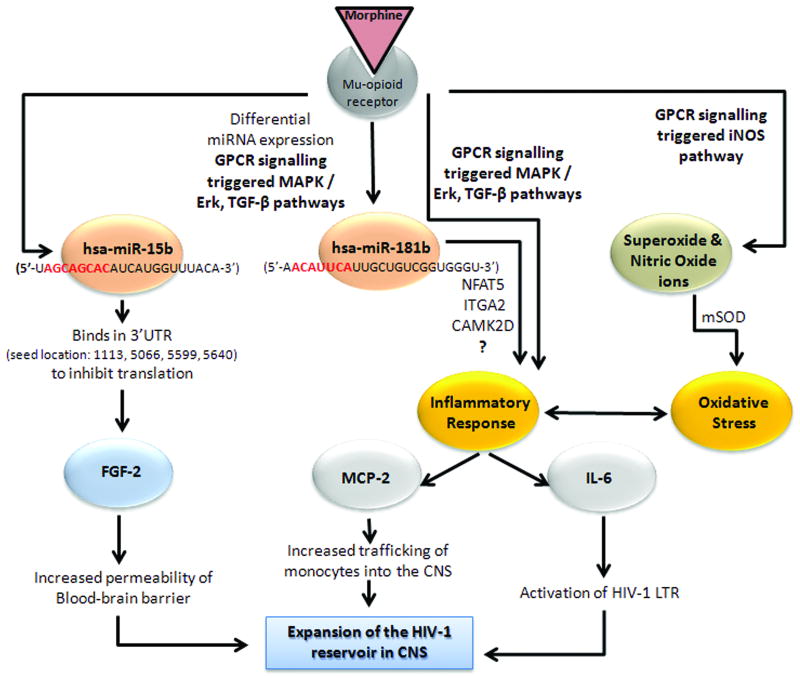
The molecular mechanisms that lead to differential miRNA expression in response to morphine in h-mdms are not known. However, recent studies elucidating regulation of miRNA pathways provide some insights. Morphine induced differential miRNA expression may be the result of activation of biochemical pathways that sense environmental stress cues. For example, mitogen-activated protein kinase / extracellular signal-regulated kinase (MAPK / ERK) pathway phosphorylates the HIV TAR RNA-binding protein (TRBP). TRBP and DICER are essential components of the miRNA generating machinery. Phosphorylation of TRBP stabilizes these complexes and enhances miRNA production [Paroo et al., 2009]. MAPK may also regulate activity of Argonaute-2 (Ago2) protein by phosphorylation. Together these molecules can transduce environmental cues to affect stability and abundance of miRNAs. Morphine induced differential miRNA expression may also be the result of specific regulation of individual miRNAs. Such specificity may be imparted when RNA Polymerase II or III are utilized for transcription of primary miRNAs (pri-miRNAs). Another pathway that regulates expression of specific miRNAs is Transforming Growth Factor β (TGF-β). TGF-β facilitates interaction of SMAD proteins with one of the RNA helicases (p68) in the large Drosha complex [Winter et al., 2009]. It is highly probable that both the MAPK / ERK and TGF-β pathways are involved in regulation differential miRNA expression in response to morphine treatment of h-mdms as both these biochemical pathways are known to be triggered by GPCR signalling resulting from interaction of morphine with the μ-opioid receptor [Chen and Sommer, 2009; Happel et al., 2008] (Fig. 6).
Fig. 6.
Mechanisms through which morphine contributes to expansion of HIV-1 reservoir in CNS. Interaction of morphine with the μ-opioid receptor is known to trigger GPCR signaling pathways that can regulate miRNA expression, pro-inflammatory response and oxidative stress. Each of these biochemical responses induced by morphine may lead to expansion of the HIV-1 viral reservoir in the CNS and explain the accelerated disease progression in opioid abusing cohorts.
Morphine induced pro-inflammatory response in h-mdms might be regulated by MAPK and TGF-β as has been demonstrated in human monocytes [Happel et al., 2008; Mahajan et al., 2005]. The role of chemokines and cytokines in the context of inflammation of CNS during HIV-1 infection has been extensively studied [Avison et al., 2004; Kaul, 2008]. Both, MCP-2 and IL-6 have been reported to be secreted in response to HIV-1 infection of microglia in the CNS [Schwartz et al., 2000; Wang and Gabuzda, 2006]. However, the role of these two proteins is yet to be elucidated in context of potential pro-inflammatory responses resulting from morphine treatment of h-mdms or microglia. Cross-talk between MCP-2, IL-6 and other proinflammatory cytokines and chemokines can potentially lead to expansion of HIV-1 reservoir in CNS [Williams et al., 2009]. Absence of TNF-α induction appears as an unusual anomaly, as it is known to play a crucial role in HIV-1 neuropathogenesis [Huang et al., 2005; Yu et al., 2007; Zheng et al., 2008]. Studies in U937, a human monocytic cell line and in activated mouse peritoneal macrophages suggested secretion of TNF-α [Peng et al., 2000; Sawaya et al., 2008]. Even so, TNFα was not detected in cell-free culture supernatants of morphine-treated h-mdms, while it was detected in cell-free supernatants of HIV-1 infected and phorbol 12-myristate 13-acetate (PMA)-treated h-mdms. In addition, we did not observe activation of nuclear factor kappa B (NFkB).
Morphine could potentially compromise the integrity of the blood brain barrier with chronic usage by decreasing secretion of FGF-2. Direct evidence of the effect of morphine on the integrity of blood barrier is lacking. Existing data suggests that in mice chronic administration of heroin might impede tumor growth. In androgen-responsive SC115 carcinoma cells, opioid peptides inhibited cell growth, while FGF-2 induced proliferation of these cells [Jiang et al., 1993]. In another study with human umbilical vein endothelial cells, FGF-2 was shown to mediate angioprotection against HIV-1 gp120 toxicity [Langford et al., 2005]. Hence, decrease in FGF-2 levels might have potential implications for HIV-1 neuropathogenesis in HIV-1-infected opiate abusers by permitting increased viral traffic into CNS. In addition, FGF-2 is an important component of neuron-microglia cross-talk, allowing the latter to exert protective influences [Figueiredo et al., 2008]. It is possible that chronic usage of morphine or heroin deteriorates the ability of microglia to protect neurons and might provide a potential explanation of the often observed neuronal loss in HIV-1 infected drug abusers [Langford et al., 2004].
Mitochondrial oxidative stress and free radical production is often observed in microglial cells treated with morphine [Raut and Ratka, 2009; Turchan-Cholewo et al., 2009]. Generation of nitric oxide is an important feature of this oxidative stress [Stefano et al., 2001]. Additionally, production of superoxide ions has also been observed [Sharp et al., 1985; Stoll-Keller et al., 1997]. Morphine has been reported to inhibit PMA-induced monocyte macrophage conversion with superoxide ions playing a crucial role. In this study, superoxide dismutase both inhibited the PMA induced change in monocyte phenotype as well as attenuated the effect of morphine [Hatsukari et al., 2006]. Apart from the current study, induction of mitochondrial superoxide dismutase has thus far not been observed in h-mdms, human macrophages or microglia. However, induction of the mitochondrial superoxide dismutase was observed in a study in mice and was associated with blocking development of opiate-induced antinociceptive tolerance [Muscoli et al., 2007]. Additionally, potential for interplay between oxidative stress and cytokine regulation exists, suggesting complex underlying mechanisms associated with how h-mdms and microglia might respond to morphine [Turchan-Cholewo et al., 2009]. Exclusive induction of the mitochondrial superoxide dismutase is not surprising. In fact, HIV-1 Tat has been shown to suppress expression of the same isoform in HeLa cells [Flores et al., 1993].
In this study, we observed a down-regulation of Gal-3 expression in response to only HIV-1 YU-2 infection of h-mdms. Gal-3 is a β-galactosidase binding protein, which has been previously shown to function as an anti-apoptotic protein in anaplastic carcinoma cells. In this study, expression of Gal-3 was inversely correlated with p53 expression [Lavra et al., 2009]. Morphine did not alter Gal-3 expression and we never observed apoptosis in morphine treated h-mdms. Perhaps Gal-3 protects morphine treated h-mdms from apoptosis. Gal-3 has also been shown to play a role in the morphine induced impairment of innate immune response in a Pneumococcal pneumonia mice model. Gal-3 secretion was greatly diminished in cell-free fractions of bronchoalveolar lavage fluid at 4h after infection in morphine-treated mice. Levels were similar to placebo at later time-points. Significantly, no change in Gal-3 expression was observed in the cell fraction of bronchoalveolar lavage fluid [Wang et al., 2005]. Hence it is possible that Gal-3 has a role in protecting morphine-treated h-mdms from apoptosis and at the same time impairs innate immune response. In the context of HIV-1 infection, several cell-types transfected with a tat expression vector had an increase in Gal-3 expression [Fogel et al., 1999]. In addition, microarray analysis of lymphatic tissue from HIV-1-infected individuals suggested increased expression of Gal-3 correlates with transition from acute to asymptomatic phase. We did not observe an increase in Gal-3 expression in HIV-1-infected h-mdms. Perhaps alteration in Gal-3 expression in response to HIV-1 infection is dependent on cell-type and kinetics.
Genome-wide association studies provide insights into unknown aspects of a biological phenomenon and aid in validating previously described observations. In the context of HIV-1 neuropathogenesis and morphine this study presents a unique perspective. Novel association was observed at miRNA and protein level for hsa-miR-15b and FGF-2. Several potential means to intersect in inflammation pathways through miRNAs hsa-miR-15b and hsa-miR-181b were identified. Our analysis also indicates commonality of processes as reflected in induction of MCP-2 and IL-6 and divergence as in induction of mitochondrial superoxide dismutase. As well, at the whole genome proteomics level, the overall changes induced via morphine were subtle compared with HIV-1 YU-2 infection. Further studies would aid in determining the actual target and quantify the biological consequence of the observed differential expression. Identification of miRNA with potential ability to regulate these biochemical pathways presents a unique opportunity to dissect underlying molecular mechanisms and unravel potential clinical applications.
Supplementary Material
Supplementary Figure 1: Differential protein expression in response to morphine treatment of h-mdms. Signal intensity of all the resolved protein spots was compared between morphine-treated and control h-mdms. Gels were run in duplicate and each spot represents average of the signal intensity. The outer lines along the median indicate a 0.5 fold change in expression levels.
Supplementary Figure 2: Differential protein expression in response to HIV-1 YU-2-infection of h-mdms. Signal intensity of all the resolved protein spots was compared between HIV-1-infected and control h-mdms. Gels were run in duplicate and each spot represents average of the signal intensity. The outer lines along the median indicate a 0.5 fold change in expression levels.
Acknowledgments
We would like to thank Drs. T. Dianne Langford, Bassel E. Sawaya & Martyn K. White for critical reading of the manuscript and Dr. Salim Merali for proteomics analysis in his core facility. In addition, acknowledgements are due to the members of the Center for Neurovirology for their cooperation and advice and Cynthia Schriver for editorial assistance.
Contract grant sponsor: National Institute of Drug Abuse
Contract grant number: 5P01DA023860-020003
References
- Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, Greenberg RN, Berger JR. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–32. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- Bokhari SM, Yao H, Bethel-Brown C, Fuwang P, Williams R, Dhillon NK, Hegde R, Kumar A, Buch SJ. Morphine enhances Tat-induced activation in murine microglia. J Neurovirol. 2009:1–10. doi: 10.1080/13550280902913628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Cytokine reversal of morphine-induced suppression of the antibody response. J Pharmacol Exp Ther. 1993;264:591–7. [PubMed] [Google Scholar]
- Chen Y, Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–7. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- Dave RS, Pomerantz RJ. Antiviral effects of human immunodeficiency virus type 1-specific small interfering RNAs against targets conserved in select neurotropic viral strains. J Virol. 2004;78:13687–96. doi: 10.1128/JVI.78.24.13687-13696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Kelsen SG, Merali S. Proteomic analysis of oxidative stress-responsive proteins in human pneumocytes: insight into the regulation of DJ-1 expression. J Proteome Res. 2008;7:4955–61. doi: 10.1021/pr800295j. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Pais TF, Gomes JR, Chatterjee S. Neuron-microglia crosstalk up-regulates neuronal FGF-2 expression which mediates neuroprotection against excitotoxicity via JNK1/2. J Neurochem. 2008;107:73–85. doi: 10.1111/j.1471-4159.2008.05577.x. [DOI] [PubMed] [Google Scholar]
- Flores SC, Marecki JC, Harper KP, Bose SK, Nelson SK, McCord JM. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc Natl Acad Sci U S A. 1993;90:7632–6. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S, Guittaut M, Legrand A, Monsigny M, Hebert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383–7. doi: 10.1093/glycob/9.4.383. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83:956–63. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Hatsukari I, Hitosugi N, Dinda A, Singhal PC. Morphine modulates monocyte-macrophage conversion phase. Cell Immunol. 2006;239:41–8. doi: 10.1016/j.cellimm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–86. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WZ, Guo CJ, Yuan CS, Douglas SD, Moss J. Methylnaltrexone antagonizes opioid-mediated enhancement of HIV infection of human blood mononuclear phagocytes. J Pharmacol Exp Ther. 2003;307:1158–62. doi: 10.1124/jpet.103.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Huang Y, Erdmann N, Peng H, Zhao Y, Zheng J. The role of TNF related apoptosis-inducing ligand in neurodegenerative diseases. Cell Mol Immunol. 2005;2:113–22. [PubMed] [Google Scholar]
- Jiang Y, Weinberg J, Wilkinson DA, Emerman JT. Effects of steroid hormones and opioid peptides on the growth of androgen-responsive Shionogi carcinoma (SC115) cells in primary culture. Cancer Res. 1993;53:4224–9. [PubMed] [Google Scholar]
- Kaul M. HIV's double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–94. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Crews L, Masliah E. The role of mitochondrial alterations in the combined toxic effects of human immunodeficiency virus Tat protein and methamphetamine on calbindin positive-neurons. J Neurovirol. 2004;10:327–37. doi: 10.1080/13550280490520961. [DOI] [PubMed] [Google Scholar]
- Langford D, Hurford R, Hashimoto M, Digicaylioglu M, Masliah E. Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120. BMC Neurosci. 2005;6:8. doi: 10.1186/1471-2202-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavra L, Ulivieri A, Rinaldo C, Dominici R, Volante M, Luciani E, Bartolazzi A, Frasca F, Soddu S, Sciacchitano S. Gal-3 is stimulated by gain-of-function p53 mutations and modulates chemoresistance in anaplastic thyroid carcinomas. J Pathol. 2009;218:66–75. doi: 10.1002/path.2510. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao X, Zhong LJ, Yang HY, Wang Q, Pu XP. Effects of chronic morphine treatment on protein expression in rat dorsal root ganglia. Eur J Pharmacol. 2009;612:21–8. doi: 10.1016/j.ejphar.2009.03.049. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Aalinkeel R, Chawda RP, Sykes DE, Nair MP. Morphine modulates chemokine gene regulation in normal human astrocytes. Clin Immunol. 2005;115:323–32. doi: 10.1016/j.clim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–9. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–22. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ, Jr, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol. 2000;68:723–8. [PubMed] [Google Scholar]
- Raut A, Ratka A. Oxidative damage and sensitivity to nociceptive stimulus and opioids in aging rats. Neurobiol Aging. 2009;30:910–9. doi: 10.1016/j.neurobiolaging.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Dukelow M, Kadiu I, Rozek W, Schlautman J, Persidsky Y, Ciborowski P, Kanmogne GD, Gendelman HE. HIV-1 infected monocyte-derived macrophages affect the human brain microvascular endothelial cell proteome: new insights into blood-brain barrier dysfunction for HIV-1-associated dementia. J Neuroimmunol. 2007;185:37–46. doi: 10.1016/j.jneuroim.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53:997–1006. doi: 10.1016/0024-3205(93)90122-j. [DOI] [PubMed] [Google Scholar]
- Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. Pharmacokinetics and pharmacokinetic variability of heroin and its metabolites: review of the literature. Curr Clin Pharmacol. 2006;1:109–18. doi: 10.2174/157488406775268219. [DOI] [PubMed] [Google Scholar]
- Sawaya BE, Deshmane SL, Mukerjee R, Fan S, Khalili K. TNF Alpha Production in Morphine-Treated Human Neural Cells Is NF-kappaB-Dependent. J Neuroimmune Pharmacol. 2008 doi: 10.1007/s11481-008-9137-z. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Catez P, Rohr O, Lecestre D, Aunis D, Schaeffer E. Functional interactions between C/EBP, Sp1, and COUP-TF regulate human immunodeficiency virus type 1 gene transcription in human brain cells. J Virol. 2000;74:65–73. doi: 10.1128/jvi.74.1.65-73.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–5. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- Stefano GB, Cadet P, Fimiani C, Magazine HI. Morphine stimulates iNOS expression via a rebound from inhibition in human macrophages: nitric oxide involvement. Int J Immunopathol Pharmacol. 2001;14:129–138. [PubMed] [Google Scholar]
- Stoll-Keller F, Schmitt C, Thumann C, Schmitt MP, Caussin C, Kirn A. Effects of morphine on purified human blood monocytes. Modifications of properties involved in antiviral defenses. Int J Immunopharmacol. 1997;19:95–100. doi: 10.1016/s0192-0561(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–15. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006;23:109–19. doi: 10.1016/j.nbd.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–34. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- Wang J, Gabuzda D. Reconstitution of human immunodeficiency virus-induced neurodegeneration using isolated populations of human neurons, astrocytes, and microglia and neuroprotection mediated by insulin-like growth factors. J Neurovirol. 2006;12:472–91. doi: 10.1080/13550280601039659. [DOI] [PubMed] [Google Scholar]
- Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14:1012–7. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics. 2008;24:325–32. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–43. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang L, Law PY, Wei LN, Loh HH. Long-term morphine treatment decreases the association of mu-opioid receptor (MOR1) mRNA with polysomes through miRNA23b. Mol Pharmacol. 2009;75:744–50. doi: 10.1124/mol.108.053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Sun ZS, Zhu YP. Proteomic analysis of rat prefrontal cortex in three phases of morphine-induced conditioned place preference. J Proteome Res. 2007;6:2239–47. doi: 10.1021/pr060649o. [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Tu H, Waters S, Pan W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–8. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]
- Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, Morgello S, Chen S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. J Neuroimmunol. 2008;200:100–10. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Differential protein expression in response to morphine treatment of h-mdms. Signal intensity of all the resolved protein spots was compared between morphine-treated and control h-mdms. Gels were run in duplicate and each spot represents average of the signal intensity. The outer lines along the median indicate a 0.5 fold change in expression levels.
Supplementary Figure 2: Differential protein expression in response to HIV-1 YU-2-infection of h-mdms. Signal intensity of all the resolved protein spots was compared between HIV-1-infected and control h-mdms. Gels were run in duplicate and each spot represents average of the signal intensity. The outer lines along the median indicate a 0.5 fold change in expression levels.