Abstract
Arabidopsis dnd1 and dnd2 mutants lack cyclic nucleotide-gated ion channel proteins and carry out avr/R-mediated defense with a greatly reduced hypersensitive response (HR). They also exhibit elevated broad-spectrum disease resistance and constitutively elevated salicylic acid (SA) levels. We examined the contributions of NPR1, SID2 (EDS16), NDR1 and EIN2 to dnd phenotypes. Mutations that affect SA accumulation or signaling (sid2, npr1 and ndr1) abolished the enhanced resistance of dnd mutants against Pseudomonas syringae pv. tomato and Hyaloperonospora parasitica but not Botrytis cinerea. When SA-associated pathways were disrupted, the constitutive activation of NPR1-dependent and NPR1-independent/SA-dependent pathways was redirected toward PDF1.2-associated pathways. This PDF1.2 over-expression was down-regulated after infection by P. syringae. Disruption of ethylene signaling abolished the enhanced resistance to B. cinerea but not P. syringae or H. parasitica. However, loss of NPR1, SID2, NDR1 or EIN2 did not detectably alter the reduced HR in dnd mutants. The susceptibility of dnd ein2 plants to B. cinerea despite their reduced-HR phenotype suggests that cell death repression is not the primary cause of dnd resistance to necrotrophic pathogens. The partial restoration of resistance to B. cinerea in dnd1 npr1 ein2 triple mutants indicated that this resistance is not entirely EIN2-dependent. The above findings indicate that the broad spectrum resistance of dnd mutants occurs due to activation and/or sensitization of multiple defense pathways, yet none of the investigated pathways are required for the reduced-HR phenotype.
Additional Keywords: RPS2, triple mutant, HLM1, AtCNGC2, AtCNGC4, Pseudomonas syringae pv. tomato DC3000, Arabidopsis thaliana
INTRODUCTION
Plants have numerous defenses against pathogen attack, some of which are constitutive while others are induced by contact with the pathogen. Specific recognition of pathogens can occur via direct or indirect interaction of the products of host resistance (R) genes with corresponding pathogen avirulence (avr) gene products (reviewed in (Jones and Dangl 2006; Nimchuk et al. 2003). This “gene-for-gene” recognition rapidly induces an array of host defense responses, through signaling pathways that include cellular ion fluxes, production of reactive oxygen intermediates (ROI), MAP kinase cascades and accumulation of salicylic acid (SA), with contributions from many signaling proteins (Glazebrook 2005; Hammond-Kosack and Parker 2003; Jones and Dangl 2006; Nimchuk et al. 2003). Compatible interactions in which host or pathogen lack the cognate R or avr gene exhibit similar, albeit slower and weaker, defense-associated changes in gene expression (Lucas 1998; Tao et al. 2003). It is of interest to understand the signaling mechanisms that activate inducible plant defense responses.
A characteristic feature of avr/R–mediated resistance is the hypersensitive response (HR) – the programmed cell death of a small number of host cells at the site of pathogen attack (Greenberg and Yao 2004; Heath 2000). While the HR has been hypothesized to limit access of biotrophs to host resources, several studies have indicated that the HR can be separated from other aspects of avr/R–mediated resistance (Bendahmane et al. 1999; del Pozo and Lam 1998; Jakobek and Lindgren 1993; Kohm et al. 1993; Yu et al. 2000; Yu et al. 1998). The HR apparently can contribute to defense through death of the host cell, and/or by contributing to the activation of defense in adjacent cells and to the activation of systemic acquired resistance (SAR) thoughout the plant (reviewed in (Heath 2000)).
We previously isolated Arabidopsis dnd1 and dnd2 mutants that exhibit a “defense, no death” phenotype (Yu et al. 2000; Yu et al. 1998). These plants carry out avr/R-mediated defense responses despite substantial absence of the HR, but also exhibit constitutively elevated SA levels, reduced plant size and elevated broad-spectrum disease resistance (Yu et al. 2000; Yu et al. 1998). The dnd1 and dnd2/hlm1 mutations carry stop codons that disrupt the cyclic nucleotide-gated ion channel proteins AtCNGC2 and AtCNGC4 respectively (Balague et al. 2003; Clough et al. 2000; Jurkowski et al. 2004). A separate Arabidopsis cpr22 mutation caused fusion of two other cyclic nucleotide-gated ion channel proteins, AtCNGC11 and AtCNGC12 (Yoshioka et al. 2006). cpr22 plants exhibit constitutive defense signaling and Ca2+-dependent programmed cell death, but unlike the dnd/hlm mutants they still develop a normal HR and single gene knockouts of AtCNGC11 or AtCNGC12 do not confer dnd-like phenotypes (Balague et al. 2003; Clough et al. 2000; Jurkowski et al. 2004; Urquhart et al. 2007; Yoshioka et al. 2001; Yoshioka et al. 2006). Impacts of these ion channel mutations on defense are not surprising given the importance of ion fluxes in plant defense signaling (Nurnberger and Scheel 2001), but the means by which the dnd and other CNGC mutants alter defense remain unclear.
AtCNGC2 and AtCNGC4 are more closely related to each other than to other Arabidopsis CNGCs (Maser et al. 2001), but the two genes are functionally non-redundant in that loss of either can cause dnd phenotypes. They may, however, form a heterotetramer ion channel, as is known to occur with animal CNGC channels (Zhong et al. 2003). Study of AtCNGC2 and AtCNGC4 has demonstrated conductance of Ca2+ and K+, but not Na+, by AtCNGC2 (Ali et al. 2007; Hua et al. 2003; Leng et al. 2002; Leng et al. 1999; Tornero and Dangl 2001), and conductance of K+ and Na+ by AtCNGC4 (Balague et al. 2003). AtCNGC2 and AtCNGC4 have different binding affinities for calmodulin isoforms, suggesting differential regulation of channel activity (Kohler and Neuhaus 2000). Additionally, expression of these genes is differentially regulated, as AtCNGC2 is constitutively expressed regardless of treatment, while AtCNGC4 is induced by treatment with avirulent Xanthomonas or with methyl-jasmonate (Balague et al. 2003). Studies using transgenic dnd1 and dnd2 plants expressing bacterial salicylate hydroxylase (nahG+), which catabolizes SA, suggested that SA is required for the elevated resistance of dnd mutants but not for the loss of HR (Clough et al. 2000; Jurkowski et al. 2004). PAD4 is also required for elevated resistance in dnd1 and dnd2/hlm1, but not for other phenotypes of these mutants (Jirage et al. 2001).
A number of components of plant defense pathways have been revealed by analysis of Arabidopsis mutants with increased disease susceptibility. NDR1, for example, is required for the function of many R proteins that possess coiled-coil, nucleotide binding site and leucine-rich repeat domains (CC-NB-LRR), while many R proteins with a N-terminal domain homologous to Toll and the interleukin-1 receptor (TIR-NB-LRR) require EDS1 and PAD4 (Aarts et al. 1998; Feys et al. 2001), defining at least two separate pathways for defense signaling. The existence of a third pathway is indicated by the finding that the RPP7 and RPP8 genes for resistance to Hyaloperonospora parasitica activate defenses independently of EDS1 and NDR1 (McDowell et al. 2000). Two very important classes of mutants with enhanced disease susceptibility include eds5/sid1 and eds16/sid2, which are impaired in SA accumulation (Nawrath and Metraux 1999; Rogers and Ausubel 1997; Volko et al. 1998), and npr1/nim1, which fail to respond to exogenously applied SA (Cao et al. 1994; Delaney et al. 1995; Shah et al. 1997). SA is involved in avr/R-mediated defenses and it is required for establishment of SAR and for basal resistance to some virulent pathogens (Cao et al. 1994; Nawrath and Metraux 1999). Mutant ndr1 plants exhibit a partial reduction in SA accumulation after infection (Shapiro and Zhang 2001), while EDS16/SID2 encodes isochorismate synthase, a central protein in SA biosynthesis whose absence largely eliminates SA production (Wildermuth et al. 2001). NPR1 acts downstream of SA to mediate activation of defense genes ((Cao et al. 1994; Delaney et al. 1995); reviewed in Pieterse and Van Loon, 2004) and also influences SA levels, which are often elevated in npr1 plants (Ryals et al. 1996; Shah et al. 1997). However, some SA-dependent defense responses are independent of NPR1 (e.g., (Bowling et al. 1997; Glazebrook et al. 1996; Rate et al. 1999)).
Analysis of Arabidopsis mutants impaired in jasmonic acid (JA) or ethylene biosynthesis and/or perception has revealed that these two signaling molecules act in concert to induce plant defenses against necrotrophic pathogens (Balbi and Devoto 2007; Knoester et al. 1999; Lorenzo et al. 2003; Penninckx et al. 1998; Staswick et al. 1998; Thomma et al. 1999). For example, the resistance of Arabidopsis against the pathogens P. syringae and H. parasitica is known to be mediated primarily through SA-mediated signaling pathways rather than JA/ethylene pathways while in contrast, defense against B. cinerea is mediated primarily through JA/ethylene pathways (Balbi and Devoto 2007; Feys and Parker 2000; Pieterse and Van Loon 2004; Spoel et al. 2003; Thomma et al. 1999); there is also a small contribution to defense against B. cinerea from basal SA accumulation not involving SID2-mediated SA biosynthesis (Ferrari et al. 2003; Govrin and Levine 2002)). There is evidence of crosstalk between ethylene and JA responses. For example, expression of some JA-responsive genes is antagonized by ethylene (Ellis and Turner 2001; Rojo et al. 1999), and promotion of ozone-induced cell death by ethylene is antagonized by JA (Overmyer et al. 2003; Overmyer et al. 2000; Tuominen et al. 2004). There is also complex and biologically significant crosstalk between SA-dependent and JA/ethylene-dependent defense pathways that, for example, can lead to NPR1-mediated suppression of JA signaling and defenses (Balbi and Devoto 2007; Feys and Parker 2000; Pieterse and Van Loon 2004; Spoel et al. 2003). Crosstalk between pathways leading to defense and stress responses likely serves to fine-tune plant responses to multiple biotic and abiotic stresses.
In the present study we used epistasis analysis to examine the contributions of NPR1-, SID2-, NDR1-, and EIN2-associated pathways to expression of the distinct defense phenotypes that arise in dnd1 and dnd2 mutants. Introduction of npr1, ndr1, sid2, or ein2 impacted some but not all of the dnd phenotypes, and unanticipated redirection of defense signaling was observed.
RESULTS
Morphology of double mutants carrying dnd1 or dnd2
Morphologically, dnd1 and dnd2 plants exhibit dwarf rosettes in comparison to wild-type Columbia plants (Yu et al. 2000; Yu et al. 1998). We introduced mutations that perturb R gene-mediated signaling (ndr1), SA-mediated defense signaling (sid2 and npr1) or ethylene-mediated defense signaling (ein2) into the dnd1 and dnd2 backgrounds. The resulting plant lines were grown in numerous independent experiments and Figure 1 provides a representative example of the reproducibly altered rosette morphology that was observed for some genotypes. Homozygous npr1, sid2, ndr1, and ein2 single mutant plants were similar to wild-type Columbia in size and appearance. None of these mutations, when introduced into the dnd1 or dnd2 backgrounds, completely reversed the dwarf phenotype. However, the ndr1 and the sid2 mutations slightly but consistently relieved the dwarf rosette size of dnd1 and dnd2 plants (Fig. 1A, 1B). In contrast, dnd1 npr1 lines exhibited an exacerbation of the dwarf rosette phenotype (Fig. 1A). The dnd1 npr1 plants also displayed macroscopic spontaneous lesions in the absence of pathogen, and a wrinkled leaf phenotype (Fig. 1C). Unlike the effects seen in the dnd1 background, introduction of npr1 into the dnd2 genetic background partially relieved the dwarf rosette phenotype, and dnd2 npr1 plants did not exhibit spontaneous lesions or wrinkled leaves (Fig. 1D). Introduction of the ein2 mutation into the dnd1 and dnd2 backgrounds did not alter the dwarf phenotype (Fig 1A, 1D). Triple-mutant dnd1 npr1 ein2 and dnd2 npr1 ein2 plants were similar in size to dnd1 and dnd2 single mutants respectively, although like npr1 ein2 plants, their color was a more pale green than wildtype or dnd plants (Fig. 1D). These triple mutants exhibited ruffled leaf edges.
Figure 1.
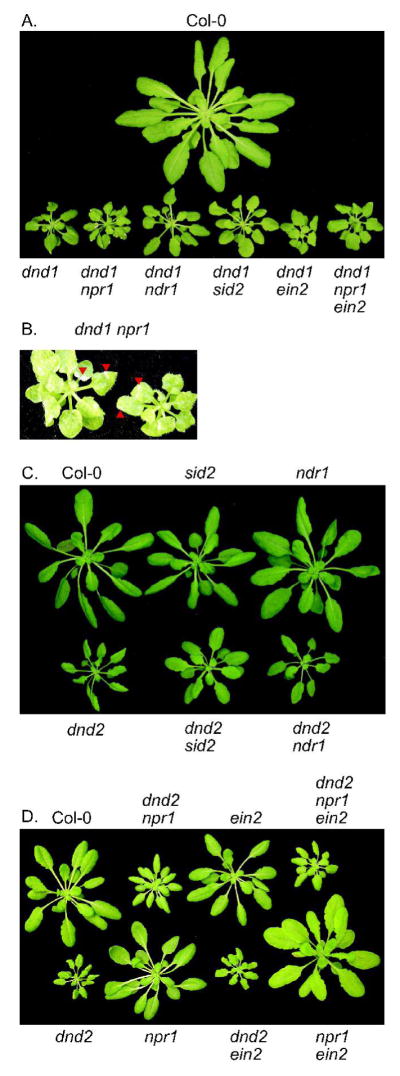
Effect of various mutations on the rosette morphology of dnd1 and dnd2 plants. (A) Two-month-old dnd1 and double mutant plants. (B) Spontaneous lesions on two month old dnd1 npr1 plants. Some lesions are indicated with red arrows. (C) Five week old dnd2, dnd2 sid2 and dnd2 ndr1 plants. (D) Five-week-old dnd2 plant with double mutants dnd2 npr1 and dnd2 ein2, and triple mutant dnd2 npr1 ein2.
NPR1-independent expression of β-glucanase-2
The npr1-1 genetic background used to construct double mutants in this study contains a β-glucanase transcriptional reporter fusion (BGL2::GUS); npr1-1 plants fail to induce BGL2::GUS expression in response to exogeneous application of SA (Cao et al. 1994). We noted strong GUS staining in dnd1 npr1 and dnd2 npr1 plants in the absence of pathogens and without SA application, showing that mutations in dnd1 and dnd2 activate BGL2 (PR-2) via an NPR1-independent pathway (Fig. 2A, 2B).
Figure 2.
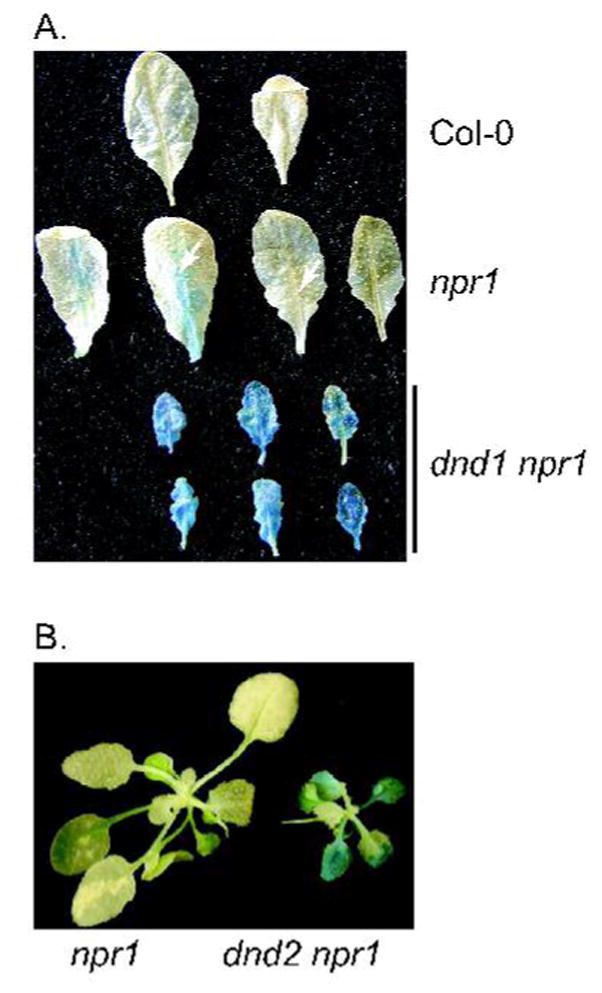
β-glucanase expression phenotypes of single and double mutants. Mutants npr1, dnd1 npr1 and dnd2 npr1 carry the BGL2 promoter region fused to the β-glucuronidase reporter gene. (A) Constitutive expression of BGL2::GUS in leaves of non-inoculated dnd1 and dnd1 npr1 plants. (B) Constitutive expression of BGL2::GUS in non-inoculated dnd2 and dnd2 npr1 plants.
Salicylic acid production in dnd npr1 and dnd sid2 double mutants
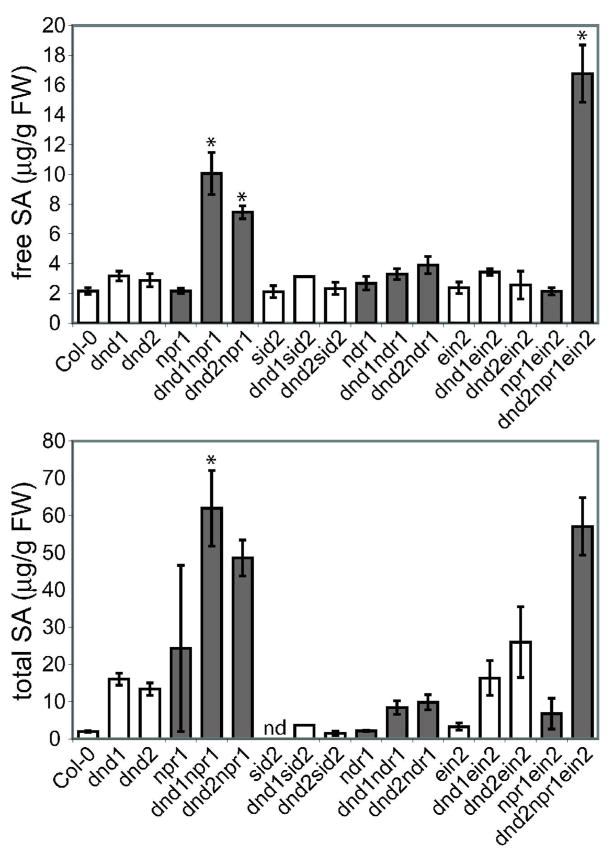
Leaves of dnd1 and dnd2 mutants accumulate high levels of SA (Jurkowski et al. 2004; Yu et al. 1998). We measured SA for dnd1 and dnd2 mutants carrying mutations in npr1, sid2, ndr1or ein2. As expected, levels of total SA in the dnd1 or dnd2 background were markedly reduced when the sid2 mutation, which significantly impairs SA biosynthesis, was present (Fig. 3). A much smaller effect was seen for free SA (Fig. 3), and we noted that the dnd mutants exhibited SID2-independent production of SA. In both the dnd1 and dnd2 backgrounds, the presence of the npr1 mutation correlated with a large increase in SA to levels higher than those seen for npr1, suggesting impacts on SA feedback regulation (see Discussion). The effect of the ndr1 mutation was less clear. In one of two experiments, levels of both conjugated and free SA were lower in dnd1 ndr1 than in dnd1, but plants in the other experiment showed little to no effect of the ndr1 mutation on total or free SA in either the dnd1 or dnd2 background. As expected, the ein2 mutation had little to no effect on SA levels in either dnd1 or dnd2 (Fig. 3).
Figure 3.
Salicylic acid (SA) levels in single, double and triple mutants. Free (unconjugated) and total SA in leaves of four-week old plants. Four replicates were measured for each genotype within each experiment; error bars represent standard error of the mean. Similar genotypes are grouped by shading; asterisks denote plant lines for which SA levels were significantly different from the parental line that is left-most within the similarly-shaded group of bars (ANOVA P < 0.05; Tukey test). Similar results were obtained in a second, independent experiment. nd: not detectable.
HR- phenotype of dnd plants is not relieved by npr1, ndr1, sid2, or ein2
The dnd mutants were isolated in a mutant screen for plants that failed to exhibit the HR in response to high titer of P. syringae pv. glycinea Race 4 expressing avrRpt2 (Yu et al. 2000; Yu et al. 1998). We conducted a similar assay by inoculating dnd1 and dnd2 double mutants and single mutant parents with 1 × 108 cfu/mL of Psg Race 4 expressing avrRpt2, and assessing HR at 24 hours. As expected, wild-type Columbia and the mutants npr1, sid2, ein2 and npr1 ein2 exhibited a strong HR, while a weak/intermediate HR was observed for ndr1 mutants. Mutant plants of dnd1, dnd2, and their double mutants with npr1, sid2, ein2 and ndr1, as well as the triple mutants dnd1 npr1 ein2 and dnd2 npr1 ein2, did not show an HR (Table 1). Thus, mutation of NPR1, SID2, NDR1 or EIN2 did not relieve the loss of HR phenotype of the dnd mutants.
Table 1.
Hypersensitive response of dnd1 and dnd2 mutants in combination with mutations in npr1, ndr1, sid2, and ein2.
| Genotype (number of experiments) | HR (Psg avrRpt2+) | HR (Psg, no avr) |
|---|---|---|
| Col-0 (3) | + | − |
| dnd1 (3) | − | − |
| dnd2 (2) | − | − |
| npr1 (3) | + | − |
| dnd1 npr1 (1) | − | − |
| dnd2 npr1 (3) | − | − |
| ndr1 (3) | +/− | − |
| dnd1 ndr1 (2) | − | − |
| dnd2 ndr1 (2) | − | − |
| sid2 (3) | + | − |
| dnd1 sid2 (2) | − | − |
| dnd2 sid2 (2) | − | − |
| ein2 (2) | + | − |
| dnd1 ein2 (1) | − | − |
| dnd2 ein2 (2) | − | − |
| npr1 ein2 (3) | +/− | − |
| dnd1 npr1 ein2 (1) | − | − |
| dnd2 npr1 ein2 (2) | − | − |
One-month-old plants were vacuum infiltrated with 108 cfu/ml P. syringae pv. glycinea Race 4 carrying an empty plasmid vector (no avr) or expressing avrRpt2.
Distinct impacts of npr1, sid2 and ndr1 on growth of P. syringae
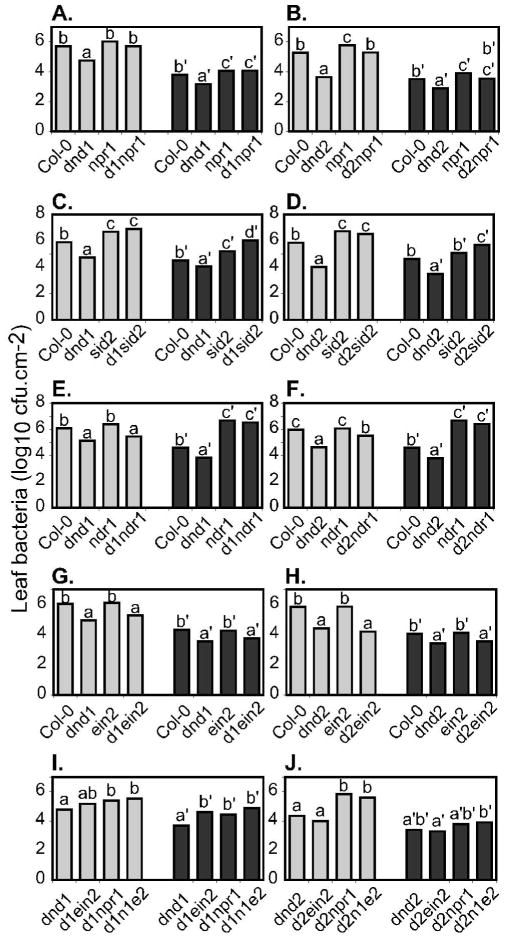
Impacts of the npr1, sid2, ein2 and ndr1 mutations on the defense responses of dnd mutants were further examined by measuring the growth of virulent Pseudomonas syringae pv. tomato strain DC3000 (Pst DC3000) or avirulent Pst DC3000 expressing avrRpt2 (Fig. 4). The sections of Fig. 4 identify instances in which there were significant differences between host genotypes in the amount of bacterial growth observed, as determined by ANOVA for the combined data from three or more independent experiments. As previously reported, populations of both virulent and avirulent Pst DC3000 were restricted in leaves of dnd1 and dnd2 mutants relative to wild-type Columbia (Yu et al. 2000; Yu et al. 1998).
Figure 4.
Bacterial populations in leaves of single, double and triple mutant plants. Leaf bacterial populations were determined three days after plants (4–5 weeks old) were inoculated by vacuum infiltration with 5 × 104 cfu/ml Pst DC3000 (light grey bars) or DC3000 + avrRpt2 (dark bars). Least squares means from the indicated number of independent experiments are presented. Within each set of four genotypes treated with the same bacterial strain, bars marked with the same letter were not significantly different (ANOVA P<0.05). Data are from a total of 17 experiments, and wildtype Columbia was included in 16 of these experiments. Genotypes tested in comparison to wild-type and single-mutant controls were (A) dnd1 npr1 (3 experiments), (B) dnd2 npr1 (4 experiments), (C) dnd1 sid2 (3 experiments) (D) dnd2sid2 (4 experiments), (E) dnd1 ndr1 (3 experiments), (F) dnd2 ndr1 (3 experiments), (G) dnd1 ein2 (5 experiments), (H) dnd2 ein2 (7 experiments), (J) dnd1 npr1 ein2 (4 experiments), and (K) dnd2 npr1 ein2 (3 experiments). In figure labels, genotypes are abbreviated as follows: d1 = dnd1; d2 = dnd2; d1n1e2 = dnd1npr1ein2; d2n1e2 = dnd2npr1ein2.
The ability of dnd1 and dnd2 plants to restrict bacterial growth was compromised by npr1; levels of both virulent and avirulent Pst DC3000 were significantly higher in leaves of dnd1 npr1 and dnd2 npr1 than in dnd1 and dnd2 respectively (Fig. 4A, 4B). Double mutants carried bacterial populations similar to those found in the npr1 single mutant except in the case of dnd2 npr1 plants inoculated with virulent bacteria (Fig. 4A, 4B), suggesting that NPR1-independent defense pathways partially contribute to the enhanced resistance of dnd2 plants to virulent Pst DC3000.
Restriction of growth of virulent and avirulent Pst DC3000 was entirely dependent on SID2 for both dnd1 and dnd2. Interestingly, growth of Pst DC3000 expressing avrRpt2 was higher in leaves of dnd1 sid2 and dnd2 sid2 than in leaves of sid2 alone (Fig. 4C and 4D).
The ndr1 mutation, which impairs resistance mediated by RPS2, RPM1 and RPS5 (Century et al. 1995), disrupted the ability of both dnd1 and dnd2 to restrict growth of Pst DC3000 expressing avrRpt2 (Fig. 4E and 4F). Intriguingly, the ndr1 mutation also impacted resistance against virulent Pst DC3000 in dnd2 plants. Pst DC3000 growth was similar in dnd1 and dnd1 ndr1 plants (Fig. 4E). However, in dnd2 ndr1 plants, Pst DC3000 population sizes were reproducibly intermediate between those found in dnd2 leaves and those in ndr1 leaves (Fig. 4F). The elevated restriction of virulent Pst DC3000 caused by dnd2 exhibits a partial dependence on NDR1 that is not seen for dnd1. Impairment of ethylene responses by the ein2 mutation did not detectably alter the ability of dnd1 or dnd2 to restrict bacterial growth. In dnd1 ein2 and dnd2 ein2 leaves, virulent and avirulent Pst DC3000 numbers were restricted to levels similar to those in dnd1 and dnd2 leaves, respectively (Fig. 4G, 4H). In a separate set of experiments that focused on triple mutants, although dnd1 plants did restrict growth of avirulent Pst DC3000 to a greater extent than dnd1 ein2, loss of EIN2 again did not observably alter bacterial growth for the other host and bacterial genotypes (Fig. 4I and 4J). As one way to address if the NPR1-independent defenses of dnd plants are activated through JA/ethylene pathways we constructed dnd npr1 ein2 triple mutants, but saw no further effect. With virulent and avirulent Pst DC3000 bacteria, leaf population levels were similar in dnd npr1 ein2 triple mutants and dnd npr1 double mutants (Fig. 4I and 4J).
Altered chlorotic responses to Pst in dnd1 double mutants
Pathogen population size and disease damage to the host (symptoms such as chlorosis and cell death) do not always correlate. We monitored the development of disease symptoms in dnd1 double mutants inoculated by vacuum infiltration with either virulent Pst DC3000 or Pst DC3000 expressing avrRpt2. As previously observed in many laboratories, wild-type Columbia plants inoculated with virulent Pst DC3000 first exhibited chlorosis approximately three days after inoculation (not shown), and more successfully limited disease damage relative to immunocompromised genotypes such as sid2 or npr1 (Fig. 5). Note that in two other experiments, sid2 plants inoculated with Pst DC3000 expressing avrRpt2 exhibited more evident chlorosis on their leaves than is shown in the experiment of Fig. 5. As expected from previous studies, dnd1 plants inoculated with either virulent or avirulent Pst DC3000 remained asymptomatic up to and beyond seven days after inoculation, indicating significant resistance even in the absence of an avr/R interaction (Fig. 5).
Figure 5.
Disease symptoms in double and triple mutants. Two month old dnd1 double or triple mutant plants seven days after inoculation with Pst DC3000 +/− avrRpt2 (2 × 105 cfu/ml) by vacuum infiltration. Chlorosis was evident three days post-inoculation in susceptible lines. This experiment was repeated three times with similar results.
Although bacterial growth in double mutant dnd1 npr1 plants was high as in npr1 mutants (Fig. 4), disease symptoms of dnd1 npr1 plants were more like dnd1 plants, with only minimal chlorosis or other disease symptoms after inoculation with either virulent or avirulent Pst DC3000 (Fig. 5). In contrast, dnd1 sid2 plants, like sid2 plants, developed chlorosis after inoculation with virulent or avirulent Pst DC3000 (Fig. 5). These results suggest that dnd1 mutants suppress symptom development in a NPR1-independent manner, but this suppression may require elevated SA levels. ndr1 plants exhibited chlorosis (similar to wild-type plants) when inoculated with virulent Pst DC3000, but severity of chlorosis was significantly greater when inoculated with Pst DC3000 expressing avrRpt2 (Fig. 5 – also reported in (Century et al. 1995)). The pattern of chlorosis seen for dnd1 ndr1 plants in response to virulent and avirulent Pst DC3000 was comparable to that seen for ndr1 (Fig. 5).
The dnd1 mutants and the dnd1 ein2 double mutants were quite similar in overall symptom development in response to either virulent or avirulent Pst DC3000 (Fig. 5). Likewise, dnd1 npr1 and dnd1 npr1 ein2 plants showed similar symptom development. This is consistent with our overall observations that ein2 has minimal impact on dnd phenotypes. Double mutant npr1 ein2 plants developed much less chlorosis than npr1 single mutants, providing a particularly pronounced example of the previous observation that ethylene insensitivity can enhance the disease tolerance of Arabidopsis, tomato, soybean and Nicotiana to P. syringae and other bacteria (Bent et al. 1992; Hoffman et al. 1999; Knoester et al. 1998; Lund et al. 1998).
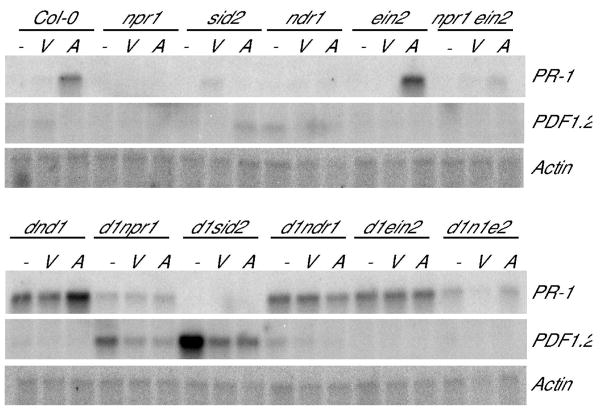
SA-dependent, NPR1-independent PR-1 expression
Replicated RNA blot analyses were conducted to assess how npr1, sid2, ndr1, and ein2 mutations alter dnd-associated expression of PR-1 and PDF1.2, standard marker genes for SA-dependent and JA/ethylene-dependent defense responses respectively. As previously reported, both dnd1 and dnd2 exhibited constitutive PR-1 expression in the absence of pathogens, and greater PR-1 expression in response to avirulent Pst (Fig. 6 and (Jurkowski et al. 2004; Yu et al. 1998)). Also as expected, single mutants npr1, sid2, and ndr1 failed to show substantial levels of PR-1 gene expression 24 hours after inoculation whereas ein2 plants resembled wild-type Columbia (Fig. 6). In double mutants the constitutive PR-1 gene expression of dnd1 plants was reduced but not eliminated by npr1, and no further induction was seen in response to avirulent Pst DC3000 (Fig. 6). Expression of PR-1 was not detected for dnd1 sid2 plants even when inoculated with virulent or avirulent Pst DC3000 (Fig. 6). Taken together, these data suggest that dnd1 mutation results in the activation of a SA-dependent, NPR1-independent pathway leading to PR-1 expression.
Figure 6.
PR-1 and PDF1.2 expression in dnd1 double and triple mutants. Month-old plants were inoculated with Pst DC3000 +/− avrRpt2 (2×106 cfu/ml) by vacuum infiltration, and leaf tissue for RNA extraction was collected 24 hours later. Control plants were not inoculated. Actin served as a loading control. Similar results were obtained in independent experiments.
The dnd1 ndr1 plants retained constitutive PR-1 gene expression similar to the dnd1 single mutant (Fig. 6). No additional PR-1 induction was seen in infected dnd1 ndr1 plants (Fig. 6). In replicated experiments involving ein2, PR-1 gene expression was essentially unchanged between dnd1 and dnd1 ein2 plants, or between dnd1 npr1 and dnd1 npr1 ein2 plants, providing another instance where ein2 had little or no effect on dnd phenotypes (Fig. 6).
PDF1.2 expression in dnd1 is promoted if SA pathways are blocked, and is suppressed by Pst DC3000 infection
In light of the crosstalk that can occur between SA and JA/ethylene defense signaling, we also investigated how dnd and the other mutations altered expression of PDF1.2. As expected from previous reports, RNA blot analyses did not reveal notable PDF1.2 expression in Columbia or in npr1, sid2, ndr1, or ein2 mutants before or after inoculation with virulent or avirulent Pst DC3000 (Fig. 6). We found that PDF1.2 expression was also minimal in dnd1, dnd1 ndr1 and dnd1 ein2 plants (Fig. 6). However, although dnd1 plants did not exhibit constitutive PDF1.2 expression, substantial levels of PDF1.2 RNA were reproducibly observed in non-inoculated dnd1 npr1 and dnd1 sid2 plants (Fig. 6). The observed PDF1.2 expression was EIN2-dependent, as it was reduced in dnd1 npr1 ein2 plants. The constitutive defense signaling of dnd mutants is apparently directed to JA/ethylene pathways when SA/NPR1-mediated pathways are blocked. Of equal interest, in multiple replicates, the strong PDF1.2 expression in non-inoculated dnd1 npr1 and dnd1 sid2 plants was significantly reduced when plants were inoculated with either virulent Pst or avirulent Pst expressing avrRpt2 (Fig. 6).
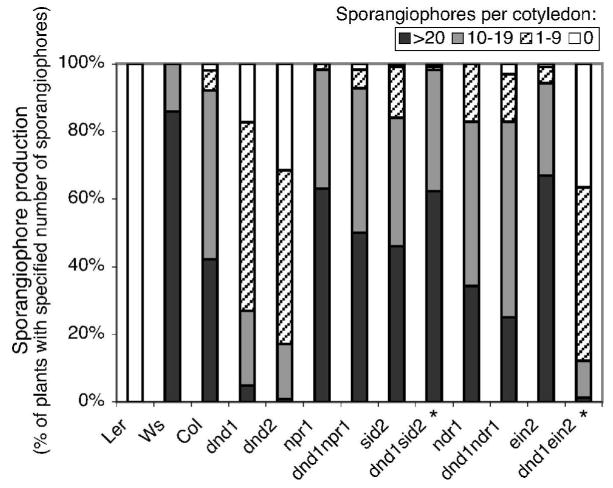
Requirement for NPR1, SID2 and NDR1 for dnd1 resistance to virulent H. parasitica
To further evaluate the disease resistance phenotypes of dnd1 double mutants, we inoculated seedlings with an isolate of the oomycete downy mildew pathogen H. parasitica (Emco5). The Landsberg erecta allele of RPP8 confers resistance to Emco5, while Columbia plants are susceptible to Emco5 and carry a non-functional allele of RPP8 (McDowell et al. 1998). Columbia dnd1 plants exhibited strong resistance to this virulent isolate of H. parasitica, as expected (Yu et al. 1998), although this resistance was not as effective as that conferred by RPP8 (Fig. 7; c.f. Ler). This strong level of “compatible interaction resistance” to Emco5 by dnd1 plants was significantly compromised by introduction of npr1, sid2, or ndr1 into the dnd1 genotype (Fig. 7). Notably, dnd1 sid2 plants supported significantly higher sporangiophore production than did sid2 plants (Fig. 7). In contrast, the dnd1 ein2 mutant retained the disease resistance phenotype of the dnd1 parental line. These data indicate that NPR1, SID2, and NDR1, but not EIN2, are necessary for dnd1-mediated resistance to virulent H. parasitica.
Figure 7.
Production of H. parasitica sporangiophores on cotyledons of dnd1 double mutants seven days after inoculation with Emco5 isolate. Data from two separate experiments are combined (total number of plants tested for each genotype ranged between 44 and 61). Asterisks designate double mutants that were significantly different from the corresponding non-dnd1 single mutant (e.g., dnd1 sid2 compared to sid2), determined by ANOVA (p<0.05; Tukey test). Sporangiophore production on dnd1 was significantly different from all other genotypes except dnd1 ein2.
Loss of resistance to B. cinerea in dnd ein2 is reversed by npr1
To evaluate JA/ethylene-mediated defense capacity in dnd1 and dnd2 mutants, we inoculated plants with the necrotrophic fungal pathogen B. cinerea. Govrin and Levine previously reported that the loss-of-HR dnd1 plants do not support B. cinerea growth (Govrin and Levine 2000); we also observed less B. cinerea growth on dnd mutants relative to wild-type plants (Fig. 8). We noted a striking susceptibility to B. cinerea in dnd1 ein2 and dnd2 ein2 plants compared to dnd1 and dnd2 respectively (Fig. 8), indicating a dependence on ethylene signaling for dnd1- and dnd2-mediated resistance to B. cinerea. Note that dnd ein2 double mutants retain the defective HR of dnd mutants (Table 1).
Figure 8.
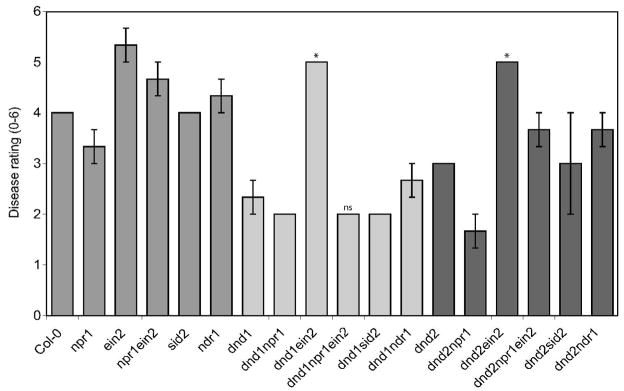
Disease ratings of dnd1 double and triple mutants inoculated with B. cinerea. Two-month-old plants were inoculated with 2 × 105 B. cinerea spores/ml and rated for disease symptoms 3 dpi. Mean ± SE of disease ratings from three independent experiments are shown for all lines except dnd1 npr1 ein2, which lacks SE data because only two replicates were completed. Asterisks identify severity scores that were significantly different from the left-most line with same shading (Col-0, dnd1 or dnd2), determined by ANOVA (P < 0.05; Tukey test). Disease rating scale: 0 = no disease, 6 = extensive disease (see Methods).
Independently replicated RNA blot analyses showed strong PDF1.2 expression in dnd1 and dnd2 plants challenged with B. cinerea (Fig. 9). No induction of PDF1.2 expression over that for mock-inoculated materials was seen for dnd1 ein2 or dnd2 ein2 plants infected with B. cinerea, but elevated expression was seen in the dnd1 and dnd2 backgrounds in the presence of the npr1, sid2, and ndr1 mutations (Fig. 9). Interestingly, although inoculated dnd1 ein2 and dnd2 ein2 exhibited minimal PDF1.2 expression, expression was elevated in dnd1 npr1 ein2 and dnd2 npr1 ein2 mutants (Fig. 9). These triple mutants carrying a defective EIN2 were substantially more resistant to B. cinerea than ein2 singles or dnd ein2 double mutants (Fig. 8).
Figure 9.
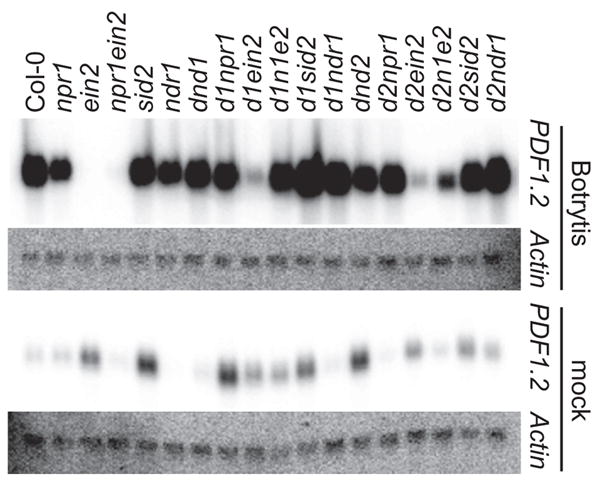
PDF1.2 expression in dnd1 double mutants inoculated with B. cinerea. Two-month-old plants were inoculated with 2 × 105 B. cinerea spores/ml. Sample order is the same for both blots. Tissue samples were collected from mock-inoculated (−) or inoculated (+) plants three days post-inoculation. Actin served as a loading control. Experiment was performed twice with similar results.
DISCUSSION
Plants carrying mutations in DND1 (AtCNGC2) or DND2 (AtCNGC4, HLM1) show multiple phenotypes, including reduced or absent HR, dwarfing, enhanced resistance to virulent and avirulent pathogens, elevated salicylic acid levels, and constitutive expression of defense marker genes (Balague et al. 2003; Clough et al. 2000; Govrin and Levine 2000; Jirage et al. 2001; Jurkowski et al. 2004; Yu et al. 2000; Yu et al. 1998). It has been unclear how these cyclic nucleotide-gated ion channels, and mutation of these channels, are tied in to normal defense pathways. Here we identify some of the well-characterized defense pathways that mediate the enhanced resistance of dnd1 and dnd2 mutants, and show that plant defense pathways are activated in interesting ways in dnd double and triple mutants. Our findings reinforce the concept that plant defense is controlled by regulatory networks rather than linear pathways, and that specific elements of the plant response (e.g., pathogen growth restriction, SA production, expression of defense-associated genes, disease lesions, HR and dwarfing) are regulated in overlapping but partially separable ways.
The enhanced resistance of dnd1 and dnd2 plants to virulent and avirulent P. syringae pv. tomato (Pst), and of dnd1 plants to virulent H. parasitica, was dependent on NPR1 and required salicylic acid (SA) synthesized through the SID2-encoded isochorismate synthase, indicating that salicylic acid signaling mediated through NPR1 is an important contributor to the enhanced resistance of these mutants. SID2-deficient dnd1 and dnd2 plants still carried higher levels of SA than were found in sid2 mutants, presumably due to SA production via a second isochorismate synthase or the phenylalanine ammonium lyase (PAL) pathway (Wildermuth et al. 2001). Notably, the constitutive PR-1 expression of dnd1 plants was reduced in dnd1 npr1 plants, but was undetectable in dnd1 sid2. Together with the observation that dnd1 npr1 and dnd2 npr1 plants showed activation of the β-glucanase/PR-2 promoter, this suggests that NPR1-independent, SA-dependent pathways leading to PR gene expression are activated in dnd1 and dnd2. Activation of NPR1-independent SA-dependent pathways has been previously observed (Clarke et al. 2000; Greenberg 2000; Nandi et al. 2003; Shah et al. 1999; Shah et al. 2001). We found that resistance to Pst and to H. parasitica, and constitutive expression of PR-1, appear to be dependent specifically on SA produced via SID2, suggesting that SID2 activity may be required to produce SA in appropriate cellular locations, or to sufficient levels, for defense activation.
Although NPR1-independent pathways were activated in dnd1 and dnd2 plants, they were not effective in defense against virulent Pst and H. parasitica or avirulent Pst, since these pathogens were no less successful on dnd1 or dnd2 plants mutated at NPR1 or SID2 than on npr1 and sid2 single mutants. Interestingly, dnd1 sid2 and dnd2 sid2 plants supported higher populations of avirulent Pst than did sid2 plants, and dnd1 sid2 plants were more susceptible to H. parasitica than sid2 plants (this paper; H.W.J. and J.T.G. unpublished results).
dnd1 mutants show reduced symptom development when inoculated with P. syringae. This phenotype was maintained in dnd1 npr1 plants, but lost in dnd1 sid2 and dnd1 ndr1 plants, suggesting that SA is required for dnd1-mediated suppression of symptom development via an NPR1-independent pathway. As ndr1 plants are impaired in SA accumulation after inoculation (Shapiro and Zhang 2001), the observation that dnd1 ndr1 plants show chlorotic symptoms similar to ndr1 plants is consistent with a requirement for SA for dnd1-mediated disease symptom suppression.
dnd1 npr1 and dnd2 npr1 mutants showed increased SA levels compared to dnd1 and dnd2, presumably due to the loss of feedback regulation of SA accumulation by NPR1 (Delaney et al. 1995; Shah et al. 1997; Wildermuth et al. 2001). Similar increases in SA levels due to npr1 have been reported for other constitutive defense mutants including ssi2 (Shah et al. 2001) and the cpr mutants (Clarke et al. 2000). Interestingly, dnd1 npr1 and dnd2 npr1 plants responded differently to this increase in SA. While dnd2 npr1 plants showed a slight size increase compared to dnd2 single mutants, dnd1 npr1 plants showed exacerbated dwarfing compared to dnd1, as well as spontaneous lesion formation. NPR1 may repress or promote cell death depending on the cellular context: for example, NPR1 represses the HR but promotes spontaneous cell death in the lesion mimic mutant agd2 (Rate and Greenberg 2001) and promotes lesion development in the hrl1 lesion mimic mutant (Devadas et al. 2002). Evidently NPR1 suppresses lesion formation in dnd1 but not dnd2.
Small differences between dnd1 and dnd2 mutants were also observed in experiments with virulent Pst DC3000, where the npr1 mutation entirely disrupted the elevated resistance of dnd1 but only partially disrupted dnd2 resistance, and the ndr1 mutation partially disrupted the elevated resistance of dnd2 but not dnd1. Although the phenotypic impacts on the plant caused by loss of the DND1/CNGC2 and DND2/CNGC4 ion channels is overall quite similar, these results point to subtle differences in pathways activated in dnd1 as opposed to dnd2 mutants.
Returning to the discussion of plant morphologies, triple-mutant studies with dnd1 showed that introduction of ein2 into the dnd1 npr1 mutants prevented the development of spontaneous lesions, and restored them to a rosette size similar to dnd1, suggesting that ethylene signaling is involved in lesion formation in these plants. Ethylene has previously been implicated as a regulator of ozone-induced lesion formation in Arabidopsis (Rao et al. 2002; Tuominen et al. 2004).
Since NDR1 is required for resistance mediated by the genes RPM1, RPS2, and RPS5 (Century et al. 1995), it was not surprising to see an NDR1 requirement for the enhanced resistance of dnd1 and dnd2 to Pst DC3000 expressing avrRpt2, recognized by RPS2-expressing plants. However, dnd2 also showed a partial requirement for NDR1 for resistance to virulent Pst DC3000. Although dnd1 plants did not show a statistically significant NDR1 requirement for resistance to virulent Pst, mutation of ndr1 eliminated dnd1 resistance to virulent H. parasitica. Evidently, the enhanced resistance of both dnd1 and dnd2 to certain virulent pathogens requires NDR1. However, other phenotypes of the dnd1 and dnd2 mutants, including SA accumulation, dwarfing, and constitutive PR-1 expression showed only a partial NDR1 requirement, or no requirement for NDR1. This mirrors previous findings for the role of PAD4 in the dnd phenotypes: both dnd1 and dnd2 have previously been shown to require PAD4 for resistance to virulent P. syringae, although dnd1 and dnd2 SA levels, constitutive PR-1 expression and rosette size were unaffected by the pad4 mutation (Jirage et al. 2001). The requirement for both PAD4 and NDR1, considered to define separate signaling pathways downstream of distinct groups of R genes (Aarts et al. 1998; Feys et al. 2001), in the enhanced resistance of dnd1 and dnd2 to P. syringae and H. parasitica suggests that these mutants activate multiple defense pathways. However, it is important to note that measurements of SA levels in dnd1 and dnd2 plants impaired in either PAD4 (Jirage et al. 2001) or NDR1 (this paper) were performed on non-inoculated plants. Since both PAD4 and NDR1 are involved in accumulation of SA post-inoculation (Jirage et al. 1999; Shapiro and Zhang 2001; Zhou et al. 1998), it is also possible that impaired disease resistance in dnd pad4 or dnd ndr1 double mutants is simply due to impaired SA accumulation upon infection. If so, this would explain the apparent uncoupling of SA accumulation and PR-1 expression from enhanced resistance seen in dnd1 ndr1 plants.
In other lesion mimic mutants, similar uncoupling of resistance from phenotypes such as PR gene expression and SA accumulation has been seen (Clarke et al. 1998; Greenberg and Yao 2004; Yoshioka et al. 2006). The cpr22 mutant, which results from a fusion of two cyclic nucleotide-gated ion channel genes (Yoshioka et al. 2006), provides a particularly relevant example. Epistasis analyses indicated that the enhanced resistance of cpr22 to virulent H. parasitica and P. syringae pathogens required functional NDR1, PAD4 and EDS1 genes, while other phenotypes such as stunting, constitutive PR-1 expression, spontaneous lesions, and SA accumulation were independent of NDR1, PAD4 and EDS1 (Yoshioka et al. 2006).
None of the defense mutations introduced into dnd1 or dnd2 led to restoration of the HR in response to challenge with avirulent Pst DC3000. In other mutants that lack the HR, alteration of SA signaling or SAR induction has been shown to restore the HR: the HR was restored in agd2 mutants that lacked a functional NPR1 gene (Rate and Greenberg 2001), and introduction of the npr1 mutation, depletion of SA by nahG, or induction of SAR restored the HR in the hrl1 mutant (Devadas and Raina 2002). As previously mentioned, dnd1 npr1 plants showed spontaneous lesions not seen for dnd1, and these were suppressed by introduction of ein2. However, an HR in response to inoculation with avirulent pathogen was still absent. Like the HR, normal rosette size was not restored by introduction of any of the defense mutations introduced into dnd1 or dnd2. Slight size increases were seen when sid2 or ndr1 were introduced into dnd1 or dnd2, or npr1 into dnd2. It has previously been shown that expression of the bacterial salicylate hydroxylase gene nahG+ in dnd1 and dnd2 only partially relieves the dwarf phenotype, suggesting, as do the results reported here, that other factors beside the level of SA affect rosette size in these mutants (Clough et al. 2000; Jurkowski et al. 2004). These two aspects of the dnd phenotypes, rosette size and lack of HR, are clearly affected by mechanisms/pathways beyond those that were explicitly examined in the present study.
EIN2 is important for defense against B. cinerea but is relatively uninvolved in resistance to Pst DC3000 and H. parasitica, SA accumulation, and PR-1 expression (Balbi and Devoto 2007; Pieterse and Van Loon 2004; Thomma et al. 1999). This was also true in ein2 double mutants with dnd1 or dnd2. Previous work has suggested that the enhanced resistance of dnd1 to B. cinerea is due to its deficient programmed cell death response (Govrin and Levine 2000). While altered programmed cell death may be a contributing factor to the elevated resistance of dnd mutants to B. cinerea, the ethylene pathway is more significant. dnd1 ein2 and dnd2 ein2 plants still had a deficient HR in response to Pst, yet were highly susceptible to B. cinerea.
The JA/ethylene defense pathways that are induced by wounding, herbivory and necrotrophic pathogens are often monitored by tracking PDF1.2 expression, and PDF1.2 expression shows EIN2-dependence (Balbi and Devoto 2007; Penninckx et al. 1998; Pieterse and Van Loon 2004; Thomma et al. 1998). In the present study, PDF1.2 was expressed after challenge with B. cinerea except in ein2, dnd1 ein2 and dnd2 ein2 lines, as might be predicted. Interestingly, we observed constitutive expression of PDF1.2 in dnd1 npr1 and dnd1 sid2 plants that is absent in dnd1, npr1 or sid2 single mutants. Apparently, the constitutively elevated defense activation in dnd mutants is channeled preferentially toward NPR1- and SA-dependent pathways, but is channeled toward PDF1.2-associated pathways when SA-associated pathways are not available. This PDF1.2 over-expression in dnd1 npr1 and dnd1 sid2 plants was down-regulated after inoculation with virulent or avirulent Pst DC3000. Although cross-talk between JA and SA pathways is partially understood (Balbi and Devoto 2007; Beckers and Spoel 2006; De Vos et al. 2006; Dong 2004; Pieterse and Van Loon 2004; Spoel et al. 2003), the mechanism that directs this defense signaling toward and away from PDF1.2 pathways in plants carrying dnd mutations is not known, and could be examined through future study both of host factors and of pathogen effectors.
Possibly related to the preceding matter, RPS2-mediated defense was operational in dnd sid2 double mutants (DC3000 grew less if it expressed avrRpt2; Fig. 4C and 4D), but growth of Pst DC3000 expressing avrRpt2 was substantially higher in leaves of dnd1 sid2 and dnd2 sid2 than in leaves dnd1, dnd2 or sid2 alone. The virulence contribution of AvrRpt2 may be greater when dnd mutations are present in the sid2 background, and/or the redirection of dnd-activated defense signaling toward PDF1.2-associated pathways may prevent effective activation of R gene-mediated defenses that can otherwise operate in a sid2 mutant.
Of further interest, dnd1 npr1 ein2 and dnd2 npr1 ein2 triple mutants inoculated with B. cinerea showed restoration of resistance and of PDF1.2 expression that was absent in dnd1 ein2 and dnd2 ein2. Mutation of NPR1 presumably allows activation of EIN2-independent JA/ethylene defense pathways and reduces damage from B. cinerea by releasing the suppression of JA/ethylene responses mediated by NPR1 (Spoel et al. 2003). Together with the observation that mutation of genes from several different defense pathways impairs the enhanced resistance of dnd mutants, the above findings suggest that the loss of cyclic nucleotide-gated ion channels in dnd1 and dnd2 plants, rather than activating a particular defense pathway, produces a generalized defense activation signal. This is consistent with recent findings (Ali et al. 2007) suggesting that NO may be the, or one of the, relevant signals. The signal derived from loss of DND/CNGC ion channels is preferentially transduced through SA-mediated pathways, is directed to JA/ethylene pathways if the SA pathways are disrupted, and can be further redirected if both SA and JA/ethylene pathways are disrupted, or upon pathogen infection.
MATERIALS AND METHODS
Growth conditions
Unless noted otherwise, all plants were grown in 9 hour photoperiods at 22°C. Light intensity was in the range of 100–180 μE. Plants were grown on Sunshine Mix #1 and irrigated from below with distilled water.
Generation of double and triple mutants
Unless specifically noted, plant lines referenced by a lower-case gene symbol were homozygous for the mutant allele. All double and triple mutants described here were created using dnd1 or dnd2 as the pollen-recipient plant. The mutant alleles used were dnd1-1 (Yu et al. 1998), dnd2-1 (Jurkowski et al., 2004), npr1-1 (Cao et al. 1994), ein2-1 (Guzman and Ecker 1990), ndr1-1 (Century et al. 1995), sid2-2 (eds16-1) (Dewdney et al. 2000), and npr1-1 ein2-1 (Clarke et al. 2001). All mutants were generated in the Arabidopsis thaliana Columbia genetic background. The dnd1-1 mutation was confirmed with a dCAPS marker (MboI restriction site) using the primer pair 5′-TGCAGGCAGTGTTTTGGTTA and 5′-ATGAGATTAAGAGCAAAACCCGA. The dnd2-1 mutation was confirmed with a dCAPS marker (NlaIII restriction site) using the primer pair 5′-TCCAAATGGGTTCGAGCAT and 5′-GCAATCTTGAACTGAATCC. Mutants carrying the npr1-1 mutation were identified by screening respective F2 populations with a previously described CAPS marker (Cao et al. 1997). Mutant lines containing the ein2-1 allele were identified by plating F2 seeds on half strength MS plates containing 10 μM 1-amino-cyclopropane-1-carboxylic acid (ACC) and allowing the seedlings to germinate in the dark for 3–4 days. Seedlings that displayed ethylene insensitivity were transplanted into soil. All dwarf plants exhibiting ethylene insensitivity were subsequently sequenced at the ein2-1 allele. Triple mutants of dnd1 npr1 ein2 and dnd2 npr1 ein2 were first selected on half strength MS plates containing 10 μM ACC, sequenced at ein2-1, and then checked for homozygosity at npr1-1 by PCR. The ndr1-1 mutation was detected using the primer pair 5′-AATCTACTACGACGATGTCCAC and 5′-GTAACCGATGGCAACTTTCAC. The sid2-2 mutation was detected using the primer pair 5′-TTACGGTAATCGCGGAAGAG and 5′-AAGCTTGCAAGAGTGCAACA.
Plant growth and histochemical GUS assay
Plant growth characteristics such as rosette size relative to control genotypes were noted for multiple plants in each of numerous experiments across multiple years; single representative plants are shown in Fig. 1. The histochemical GUS assay was performed as described (Cao et al. 1994).
Pathogen assays
To determine bacterial growth in leaves, one-month-old plants were inoculated with Pst DC3000 carrying either avrRpt2 or the empty pVSP61 vector at 5 × 104 cfu/ml by vacuum infiltration. Three days post-inoculation (dpi), homogenized leaf tissue was dilution-plated on selective media as previously described (Yu et al. 1998). For each experiment, four leaf samples were taken per genotype. Each leaf sample comprised a total of four leaf discs taken from two plants.
For observation of disease symptoms, two month old plants were inoculated with Pst DC3000 carrying either avrRpt2 or the empty pVSP61 vector at 2 × 105 cfu/ml by vacuum infiltration; symptoms were observed 3 dpi. HR assays were performed by vacuum-infiltrating two month old plants with P. syringae pv. glycinea Race 4 carrying either avrRpt2 or the empty pVSP61 vector at 108 cfu/ml; tissue collapse was scored 24 hours post inoculation using a 0–5 scale in which 0 = no collapse; 1 = minor damage to less than 5 % of leaves; 2 = some watersoaked and/or collapsed tissue present on 5–35 % of leaves; 3 = 35–75 % of leaves watersoaked and/or collapsed; 4 = widespread coalescing areas of collapsed leaf tissue; 5 = total collapse of all leaves. Combined average of scores from multiple experiments were summarized for Table 1 as: 0–1.9 = “ −”, 2–2.9 = “ +/−”, 3–3.9 = “ +”, 4–5 = “ ++.” Additional experiments with Pst DC3000 and the plant lines from this study used spray inoculation of two to three week old seedlings as per (Tornero and Dangl 2001), but the results were variable (poorly reproducible within our lab, and too often failing to reproduce published results from other labs), so data for those experiments are not reported. H. parasitica Emco5 assays were performed as previously described (McDowell et al. 2000). Sporangiophore counts per seedling were grouped into four categories prior to ANOVA tests (see Figure 7). For B. cinerea assays, due to leaf size differences between genotypes, whole plant phenotype tests (spray inoculation) were chosen over lesion size measurement on detached leaves (droplet inoculation; whole plant disease phenotypes correlate with detached leaf phenotypes; (Denby et al. 2004; Govrin and Levine 2000; Mengiste et al. 2003). B. cinerea cultures were grown on potato dextrose agar at room temperature for 7–10 days. Spores were scraped from the agar surface and resuspended in potato dextrose broth at 2 × 105 spores/mL. Two and a half month old plants were lightly sprayed with the spore suspension; domes were placed over the plants to maintain high humidity, and disease assessments were made seven dpi. The pot label identifying the genotype was obscured until plants had been rated for disease symptoms. Disease rating scale used: 0 = no detectable lesions; 1 = small rare lesions, no fungal growth visible; 2 = lesions on up to 10% of leaves, little to no fungal growth visible; 3 = significant necrosis of leaves (10–30% of leaves) and visible fungal growth; 4 = extensive fungal growth with death of 30–60% of leaves; 5 = extensive fungal growth with death of 60–80% of leaves; 6 = fungus overgrew plants; less than 10% of green leaves remain. Inoculation conditions were optimized to provide a wide spread of scores between the most and least susceptible genotypes.
Northern blot analysis
RNA isolation was conducted using either mini-to-midi RNA isolation kits (Invitrogen) or RNeasy kits (Qiagen) following manufacturer’s instructions. Northern blots were probed as previously described (Jurkowski et al. 2004). All RNA blot findings are based on independent biological replicates, and in most cases were performed three or more times.
Quantification of salicylic acid
Both free and total (including conjugated) salicylic acid were quantified from non-inoculated leaf tissue of 4-week-old plants as described (Vanacker et al. 2001). Between 0.2 and 0.5 g of leaf tissue per sample was utilized. The experiment was performed twice, using entirely independent materials and in two separate years.
Acknowledgments
The authors would like to thank N. Keuler and P. Esker for their substantial contributions to the statistical analyses, I-c. Yu for initial construction of some plant lines, J. Clarke and X. Dong for providing npr1 ein2 seeds, M. Wildermuth for providing information on sid2-2 primers, T. Mengiste for providing the B. cinerea culture and advice in conducting assays, and J. Bergelson for the use of her HPLC for SA measurements. This work was primarily supported by USDA-NRI grant 2001-35319-09888 to A.B. Experiments in JTG’s laboratory were done with support from NIH grant R01 GM54292 and NSF grant IOB-0450207.
LITERATURE CITED
- Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:10306–10311. doi: 10.1073/pnas.95.17.10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balague C, Lin B, Alcon C, Flottes G, Malmstrom S, Kohler C, Neuhaus G, Pelletier G, Gaymard F, Roby D. HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell. 2003;15:365–379. doi: 10.1105/tpc.006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi V, Devoto A. Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 2007 doi: 10.1111/j.1469-8137.2007.02292.x. [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Spoel SH. Fine-Tuning Plant Defence Signalling: Salicylate versus Jasmonate. Plant biology (Stuttgart, Germany) 2006;8:1–10. doi: 10.1055/s-2005-872705. [DOI] [PubMed] [Google Scholar]
- Bendahmane A, Kanyuka K, Baulcombe DC. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell. 1999;11:781–791. doi: 10.1105/tpc.11.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent A, Innes R, Ecker J, Staskawicz B. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Bowling SA, Gordon AS, Dong X. Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarker JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Century KS, Holub EB, Staskawicz BJ. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc Natl Acad Sci USA. 1995;92:6597–6601. doi: 10.1073/pnas.92.14.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Aarts N, Feys BJ, Dong X, Parker JE. Constitutive disease resistance requires EDS1 in the Arabidopsis mutants cpr1 and cpr6 and is partially EDS1-dependent in cpr5. Plant J. 2001;26:409–420. doi: 10.1046/j.1365-313x.2001.2641041.x. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Liu Y, Klessig DF, Dong X. Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell. 1998;10:557–569. doi: 10.1105/tpc.10.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu I-c, Lippok B, Smith RK, Bent AF. The Arabidopsis dnd1 “defense, no death’ gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci (USA) 2000;97:9323–9328. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Van Zaanen W, Koornneef A, Korzelius JP, Dicke M, Van Loon LC, Pieterse CM. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006;142:352–363. doi: 10.1104/pp.106.083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo O, Lam E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr Biol. 1998;8:R896. doi: 10.1016/s0960-9822(07)00555-6. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denby KJ, Kumar P, Kliebenstein DJ. Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. Plant J. 2004;38:473–486. doi: 10.1111/j.0960-7412.2004.02059.x. [DOI] [PubMed] [Google Scholar]
- Devadas SK, Enyedi A, Raina R. The Arabidopsis hrl1 mutation reveals novel overlapping roles for salicylic acid, jasmonic acid and ethylene signalling in cell death and defence against pathogens. Plant J. 2002;30:467–480. doi: 10.1046/j.1365-313x.2002.01300.x. [DOI] [PubMed] [Google Scholar]
- Devadas SK, Raina R. Preexisting systemic acquired resistance suppresses hypersensitive response-associated cell death in Arabidopsis hrl1 mutant. Plant Physiol. 2002;128:1234–1244. doi: 10.1104/pp.010941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewdney J, Reuber TL, Wildermuth MC, Devoto A, Cui J, Stutius LM, Drummond EP, Ausubel FM. Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J. 2000;24:205–218. doi: 10.1046/j.1365-313x.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- Dong X. NPR1, all things considered. Current opinion in plant biology. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ellis C, Turner JG. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell. 2001;13:1025–1033. doi: 10.1105/tpc.13.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD4, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313x.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Feys BJ, Moisan LJ, Newman MA, Parker JE. Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. Embo J. 2001;20:5400–5411. doi: 10.1093/emboj/20.19.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys BJ, Parker JE. Interplay of signaling pathways in plant disease resistance. Trends Genet. 2000;16:449–455. doi: 10.1016/s0168-9525(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Rogers EE, Ausubel FM. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics. 1996;143:973–982. doi: 10.1093/genetics/143.2.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- Govrin EM, Levine A. Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR) Plant Molec Biol. 2002;48:267–276. doi: 10.1023/a:1013323222095. [DOI] [PubMed] [Google Scholar]
- Greenberg JT. Positive and negative regulation of salicylic acid-dependent cell death and pathogen resistance in Arabidopsis lsd6 and ssi1 mutants. Mol Plant Microbe Interact. 2000;13:877–881. doi: 10.1094/MPMI.2000.13.8.877. [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Yao N. The role and regulation of programmed cell death in plant-pathogen interactions. Cellular microbiology. 2004;6:201–211. doi: 10.1111/j.1462-5822.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Parker JE. Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol. 2003;14:177–193. doi: 10.1016/s0958-1669(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant molecular biology. 2000;44:321–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- Hoffman T, Schmidt JS, Zheng X, Bent AF. Isolation of ethylene insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol. 1999;119:935–949. doi: 10.1104/pp.119.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Leng Q, Berkowitz GA. Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiol. 2003;132:1353–1361. doi: 10.1104/pp.103.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobek JL, Lindgren PB. Generalized Induction of Defense Responses in Bean Is Not Correlated with the Induction of the Hypersensitive Reaction. Plant Cell. 1993;5:49–56. doi: 10.1105/tpc.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Zhou N, Cooper B, Clarke JD, Dong X, Glazebrook J. Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J. 2001;26:395–407. doi: 10.1046/j.1365-313x.2001.2641040.x. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jurkowski GI, Smith RK, Jr, Yu IC, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF. Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol Plant Microbe Interact. 2004;17:511–520. doi: 10.1094/MPMI.2004.17.5.511. [DOI] [PubMed] [Google Scholar]
- Knoester M, Pieterse CM, Bol JF, Van Loon LC. Systemic resistance in Arabidopsis induced by rhizobacteria requires ethylene-dependent signaling at the site of application. Mol Plant Microbe Interact. 1999;12:720–727. doi: 10.1094/MPMI.1999.12.8.720. [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM. Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C, Neuhaus G. Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett. 2000;471:133–136. doi: 10.1016/s0014-5793(00)01383-1. [DOI] [PubMed] [Google Scholar]
- Kohm BA, Goulden MG, Gilbert JE, Kavanagh TA, Baulcombe DC. A potato virus × resistance gene mediates an induced, nonspecific resistance in protoplasts. Plant Cell. 1993;5:913–920. doi: 10.1105/tpc.5.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Hua BG, Fromm H, Berkowitz GA. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002;128:400–410. doi: 10.1104/pp.010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Yao W, Berkowitz GA. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA. Plant Pathology and Plant Pathogens. Blackwell Science; Oxford, United Kingdom (Malden, MA): 1998. [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Cuzick A, Can C, Beynon J, Dangl JL, Holub EB. Downy mildew (Peronospora parasitica) resistance genes in Arabidopsis vary in functional requirements for NDR1, EDS1, NPR1 and salicylic acid accumulation. Plant J. 2000;22:523–529. doi: 10.1046/j.1365-313x.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- McDowell JM, Dhandaydham M, Long TA, Aarts MG, Goff S, Holub EB, Dangl JL. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell. 1998;10:1861–1874. doi: 10.1105/tpc.10.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste T, Chen X, Salmeron J, Dietrich R. The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell. 2003;15:2551–2565. doi: 10.1105/tpc.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Kachroo P, Fukushige H, Hildebrand DF, Klessig DF, Shah J. Ethylene and jasmonic acid signaling affect the NPR1-independent expression of defense genes without impacting resistance to Pseudomonas syringae and Peronospora parasitica in the Arabidopsis ssi1 mutant. Mol Plant Microbe Interact. 2003;16:588–599. doi: 10.1094/MPMI.2003.16.7.588. [DOI] [PubMed] [Google Scholar]
- Nawrath C, Metraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchuk Z, Eulgem T, Holt IIIBF, Dangl JL. Recognition and response in the plant immune system. Annu Rev Genet. 2003;37:579–609. doi: 10.1146/annurev.genet.37.110801.142628. [DOI] [PubMed] [Google Scholar]
- Nurnberger T, Scheel D. Signal transmission in the plant immune response. Trends Plant Sci. 2001;6:372–379. doi: 10.1016/s1360-1385(01)02019-2. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjarvi J. Ozone-sensitive arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Van Loon LC. NPR1: the spider in the web of induced resistance signaling pathways. Current opinion in plant biology. 2004;7:456–464. doi: 10.1016/j.pbi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee HI, Davis KR. Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J. 2002;32:447–456. doi: 10.1046/j.1365-313x.2002.01434.x. [DOI] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT. The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell. 1999;11:1695–1708. doi: 10.1105/tpc.11.9.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rate DN, Greenberg JT. The Arabidopsis aberrant growth and death2 mutant shows resistance to Pseudomonas syringae and reveals a role for NPR1 in suppressing hypersensitive cell death. Plant J. 2001;27:203–211. doi: 10.1046/j.0960-7412.2001.1075umedoc.x. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM. Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell. 1997;9:305–316. doi: 10.1105/tpc.9.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo E, Leon J, Sanchez-Serrano JJ. Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 1999;20:135–142. doi: 10.1046/j.1365-313x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hu MD. Systemic Acquired Resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Klessig DF. The Arabidopsis ssi1 mutation restores pathogenesis-related gene expression in npr1 plants and renders defensin gene expression salicylic acid dependent. Plant Cell. 1999;11:191–206. doi: 10.1105/tpc.11.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Nandi A, Klessig DF. A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J. 2001;25:563–574. doi: 10.1046/j.1365-313x.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- Shah J, Tsui F, Klessig DF. Characterization of a salicylic acid-insensitive mutant (sai1) of Arabidopsis thaliana, identified in a selective screen utilizing the SA-inducible expression of the tms2 gene. Mol Plant Microbe Interact. 1997;10:69–78. doi: 10.1094/MPMI.1997.10.1.69. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Zhang C. The Role of NDR1 in Avirulence Gene-Directed Signaling and Control of Programmed Cell Death in Arabidopsis. Plant Physiol. 2001;127:1089–1101. [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux JP, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CM. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell. 2003;15:760–770. [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Tao Y, Xie Z, Chen W, Glazebrook J, Chang HS, Han B, Zhu T, Zou G, Katagiri F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell. 2003;15:317–330. doi: 10.1105/tpc.007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch-Mani B, Vogelsang R, Cammue BP, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Tierens KF, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1102. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Dangl JL. A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. Plant J. 2001;28:475–481. doi: 10.1046/j.1365-313x.2001.01136.x. [DOI] [PubMed] [Google Scholar]
- Tuominen H, Overmyer K, Keinanen M, Kollist H, Kangasjarvi J. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J. 2004;39:59–69. doi: 10.1111/j.1365-313X.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- Urquhart W, Gunawardena AH, Moeder W, Ali R, Berkowitz GA, Yoshioka K. The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant molecular biology. 2007;65:747–761. doi: 10.1007/s11103-007-9239-7. [DOI] [PubMed] [Google Scholar]
- Vanacker H, Lu H, Rate DN, Greenberg JT. A role for salicylic acid and NPR1 in regulating cell growth in Arabidopsis. Plant J. 2001;28:209–216. doi: 10.1046/j.1365-313x.2001.01158.x. [DOI] [PubMed] [Google Scholar]
- Volko SM, Boller T, Ausubel FM. Isolation of new Arabidopsis mutants with enhanced disease susceptibility to Pseudomonas syringae by direct screening. Genetics. 1998;149:537–548. doi: 10.1093/genetics/149.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Kachroo P, Tsui F, Sharma SB, Shah J, Klessig DF. Environmentally sensitive, SA-dependent defense responses in the cpr22 mutant of Arabidopsis. Plant J. 2001;26:447–459. doi: 10.1046/j.1365-313x.2001.2641039.x. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell. 2006;18:747–763. doi: 10.1105/tpc.105.038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I-c, Fengler KA, Clough SJ, Bent AF. Identification of Arabidopsis mutants exhibiting an altered hypersensitive response in gene-for-gene disease resistance. Mol Plant-Microbe Interact. 2000;13:277–286. doi: 10.1094/MPMI.2000.13.3.277. [DOI] [PubMed] [Google Scholar]
- Yu I-c, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lai J, Yau KW. Selective heteromeric assembly of cyclic nucleotide-gated channels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5509–5513. doi: 10.1073/pnas.0931279100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell. 1998;10:1021–1030. doi: 10.1105/tpc.10.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]