Abstract
Introduction
Spinal α2-adrenoceptor stimulation produces analgesia in neuropathic pain states, and this effect in animals is blocked by inhibitors of brain derived neurotrophic factor (BDNF) function. In rats, α2-adrenoceptor stimulation normally inhibits acetylcholine release, but excites release after nerve injury. We examined the roles of BDNF and excitatory Gs protein in this change.
Methods
Male rats underwent L5-L6 spinal nerve ligation (SNL) and their lumbar spinal dorsal horns with or without spinal BDNF infusion were used for either synaptosome preparation for acetylcholine release or immunostaining for choline acetyltransferase.
Results
SNL did not alter spontaneous release from synaptosomes or choline acetyltransferase-immunoreactivity in the spinal dorsal horn, but reduced KCl-evoked acetylcholine release. Dexmedetomidine inhibited KCl-evoked acetylcholine release in synaptosomes from normal rats, but excited KCl-evoked release in synaptosomes from SNL rats, and both effects were blocked by the α2-adrenoceptor antagonist, idazoxan. Spinal infusion of an antibody to BDNF reduced choline acetyltransferase-immunoreactivity in the spinal dorsal horn in both normal and SNL rats, and abolished facilitation of KCl-evoked acetylcholine release by dexmedetomidine in SNL rats. Dexmedetomidine’s facilitation of acetylcholine release was also blocked by inhibitors of Gs function.
Discussion
The increased reliance of spinal α2-adrenoceptors on cholinergic stimulation to cause analgesia after nerve injury reflects in part a shift from direct inhibition to direct excitation of spinal cholinergic neurons. Our results suggest this shift relies on an interaction with Gs proteins and BDNF.
Introduction
Better treatment for chronic neuropathic pain has been sought for decades. However, only a few drugs, including oral gabapentin and monoamine re-uptake inhibitors, and epidural clonidine have been approved to treat chronic neuropathic pain. These drugs all share a common mechanism which involves stimulation of spinal α2-adorenoceptors which in turn results in spinal release of acetylcholine to relieve neuropathic pain.1-7 We have previously demonstrated that inhibitory M2 subtype muscarinic receptors are up-regulated in primary sensory afferents after nerve injury.8 Thus, activation of a spinal noradrenergic-cholinergic interaction is a key strategy to treat neuropathic pain.
Activation of α2-adrenoceptors directly reduces pain transmission by reducing pronociceptive transmitter release including substance P and glutamate from primary afferent terminals,9 and by hyperpolarizing spinal interneurons via G-protein mediated activation of potassium channels.10 This occurs in both normal and neuropathic animals. In contrast, the α2-adrenergic – cholinergic circuit predominates as a mechanism for analgesia after peripheral nerve injury. We previously demonstrated that clonidine inhibits acetylcholine release in spinal cord slices and synaptosomes in normal rats, consistent with the classical inhibitory effect of the G-protein coupled α2-adrenoceptors.3 Surprisingly, after peripheral nerve injury clonidine enhances rather than inhibits acetylcholine release from these preparations in rats with a peripheral nerve injury model of neuropathic pain,3 consistent with behavioral observations that intrathecal atropine abolishes the analgesic effect of intrathecal clonidine in nerve injured rats, but not in normal rats.5,6
α2-Adrenoceptors normally activate inhibitory, pertussis toxin-sensitive Gi/o proteins to reduce cAMP production and reduce neurotransmitter release.11 Under some conditions, α2-adrenoceptors have been shown to couple with stimulatory Gs-proteins,12,13 which activate voltage-gated Ca2+ channels and could enhance Ca2+-dependent neurotransmitter release. We have previously shown, using quantitative ligand binding, that the total number of α2-adrenoceptors in the spinal cord is unchanged after nerve injury, but that the efficacy of G-protein coupling from spinal α2-adrenoceptors increases.14 One goal of the current study was to test whether α2-adrenoceptor-mediated facilitation of acetylcholine release after nerve injury requires activation of Gs proteins.
Brain-derived neurotrophic factor (BDNF) may provide the stimulus for the shift in action of α2-adrenoceptor agonists on cholinergic neurons. We recently demonstrated that peripheral nerve injury increases descending noradrenergic axon density via BDNF-dependent mechanisms in rats.15 Interestingly, spinal infusion of BDNF antibody not only blocked the increase in noradrenergic axon density but also reduced the analgesic efficacy of the spinally administered clonidine after nerve injury.15 A final goal of the current study was to test whether BDNF is involved in the shift in α2-adrenoceptor agonist effect on acetylcholine release after nerve injury and/or in the expression of the synthetic enzyme for acetylcholine, choline acetyltransferase (ChAT) in the spinal dorsal horn.
Materials and Methods
Animals
Male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN), weighing 180-280 g, were used. All experiments were approved by the Animal Care and Use Committee at Wake Forest University (Winston Salem, North Carolina). Animals were housed under a 12-h light-dark cycle, with free access to food and water.
Surgical preparations
Spinal nerve ligation (SNL)
As previously described, 16 animals were anesthetized with 2% isoflurane in oxygen, the right L6 transverse process was removed and the right L5 and L6 spinal nerves were tightly ligated using 5–0 silk suture. Animals were allowed to recover for 3-14 days.
Anti-BDNF treatment
Animals were anesthetized with 2% isoflurane and intrathecal catheterization was performed as previously described. 17 A small puncture was made in the atlanto-occipital membrane of the cisterna magnum and a polyethylene catheter (ReCathCO LLC, Allison Park, PA), 7.5 cm, was inserted so that the caudal tip reached the lumbar enlargement of the spinal cord. Animals were allowed at least 5 days to recover from the surgery. As previously described, 15 a sheep polyclonal antibody to BDNF (AB1513p, Chemicon, Temecula, CA; 5 μg/day) or control sheep IgG (Sigma Chemical CO., St. Louis, MO; 5 μg/day) was delivered in sterile saline via osmotic minipump (0.5 μl/h, model 2002, Alzet, Cupertino, CA). One day before SNL surgery, animals were anesthetized and the osmotic minipump was implanted subcutaneously on the back of the rat and connected to the intrathecal catheter. After 11 days of spinal infusion, animals were used for synaptosome studies or immunohistochemistry.
Acetylcholine release from synaptosomes
Crude synaptosomes from the lumbar spinal dorsal horn were prepared as previously described 3 with minor modifications. Under deep anesthesia with 5% isoflurane, animals were killed by decapitation, and a 1 cm length of the spinal cord containing the lumbar enlargement, measured by the ruler, was quickly removed and placed in oxygenated (with 95% O2–5% CO2) ice-cold Krebs buffer containing 124 mM NaCl, 3 mM KCl, 2 mM MgSO4, 2 mM CaCl2, 1.25 mM KH2PO4, 25 mM NaHCO3, and 10 mM glucose, pH 7.35. The dorsal quadrants of the spinal cord ipsi-and contralateral to SNL were removed and separately homogenized in ice-cold sucrose (0.32 M)-HEPES (10 mM) buffer, pH 7.4. Each synaptosome preparation contained 4 dorsal quadrants of spinal cord, either unilateral from 4 SNL rats or bilateral from 2 normal rats. The initial homogenate was centrifuged at 1,000xG for 5 min and the resulting supernatant was centrifuged again at 10,000xG for 12 min. The supernatant was discarded and the pellet was resuspended in Krebs buffer. Acetylcholine release from synaptosomes was determined after loading with [3H]-choline which results in rapid uptake of choline, acetylation to acetylcholine, and release of [3H]-acetylcholine with depolarization. 18,19 We did not, however, confirm in the current experiments that released radioactivity was completely in the form of [3H]-acetylcholine. After incubation with 1 μM choline chloride (combination of both triturated and unlabeled choline) for 20 min at 37°C, the synaptosome-containing solution was centrifuged at 10,000XG for 5 min and the pellet was resuspended in Krebs buffer. Each preparation was divided into six equal aliquots and placed on Whatman filters in temperature controlled perfusion chambers (SF-12, Brandel, Gaithersburg, MD). Synaptosomes were perfused with Krebs buffer (0.67 ml/min) for 20 min to remove free radioactivity, and then fractions were collected every 5 min for 20 min. Antagonists for α2-adrenoceptors (idazoxan, 1 μM) or Gs function (suramin, 10 μM or NF449, 10 μM) were present during the perfusion including the wash-out period. After a 10 min baseline collection, synaptosomes were perfused with dexmedetomidine alone for 2 min, then stimulated with 12 mM KCl-Krebs buffer (in mM: 115 NaCl, 12 KCl, 2 MgSO4, 2 CaCl2, 1.25 KH2PO4, 25 NaHCO3, and 10 glucose, pH 7.35) containing dexmedetomidine for 3 min. An inhibitor of acetylcholine transporter hemicholinium-3 (10 μM) was present during perfusion to inhibit reuptake of acetylcholine. [3H]-acetylcholine release from synaptosomes in each fraction was measured by a liquid scintillation counter (LS6500, Beckman Coulter Inc., Fullerton, CA). In some experiments, total radioactivity in each preparation was calculated from radioactivity present in the collected fractions and filters from six chambers. All chemicals were purchased from Sigma Chemical CO. except [3H]-choline (Perkin Elmer, Boston, MA) and NF449 (Tocris Bioscience, Ellisville, MO). Dexmedetomidine, idazoxan, suramin, and NF449 were dissolved in distilled water and then diluted with Krebs buffer.
Immunohistochemistry
Immunostaining for ChAT was performed in the sheep IgG or BDNF antibody-infused spinal cord of normal and SNL rats, as previously described 20 with minor modifications. Under deep anesthesia with 100 mg/kg pentobarbital, animals were perfused intracardially with cold phosphate buffered saline containing 1 % sodium nitrite and subsequently with 4 % paraformaldehyde in 0.1 M phosphate buffer (ph 7.4). The L4-L6 spinal cord was removed, postfixed in the same fixative for 3 h, and cryoprotected with 30 % sucrose in 0.1 M phosphate buffer for 48 h at 4 °C. Tissues were then sectioned on a cryostat at a 40 μm thickness. After being pretreated with 0.3 % hydrogen peroxide and 1.5 % normal donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), the sections were incubated for 24 h at 4 °C in a goat anti-ChAT antibody (1:500, AB144P, Millipore, Billerica, MA) in 1.5 % normal donkey serum. Subsequently, the sections were incubated in biotinylated donkey anti-goat IgG (1:200, Vector Laboratories, Burlingame, CA), processed using Elite Vectastain ABC kit (Vector) according to the manufacturer instructions, and then developed by the standard glucose oxidase-nickel method.
For quantification of ChAT immunoreactivity, four to five L4-L6 spinal cord sections were randomly selected from each rat. Images of both ipsilateral and contralateral dorsal horns of SNL or normal rats were captured using a digital charge coupled device camera. Using image analysis software (SigmaScan, Jandel Scientific Inc., San Rafael, CA), pixels of ChAT-immunoreactive objects within the area of the dorsal horn containing lamina I to IV were quantified based on a constant threshold of optical density. Data are expressed as percentage of ChAT-immunoreactive pixels in total pixels of the quantified area. The person performing image analysis was blinded to treatment.
Statistical analyses
Data were normally distributed and are presented as mean ± SE. Differences among groups were determined using one or two-way analysis of variance (ANOVA) as appropriate. P< 0.05 was considered significant. SigmaStat, version 3.0 (Systat Software, Chicago, IL) was used for data analysis.
Results
Synaptosome study
Total [3H]-choline uptake (sum of released radioactivity and that retained in filters) in the synaptosome preparation did not differ between normal rats (78000 ± 1500 cpm, n=14) and rats 14 days after SNL surgery (SNL-ipsilateral, 79000 ± 2700 cpm; SNL-contralateral, 76000 ± 2900 cpm, n=16). SNL also did not alter spontaneous [3H]-acetylcholine release from synaptosomes compared to normal (% total radioactivity: normal, 1.69 ± 0.17%; SNL-ipsilateral, 1.70 ± 0.15 %; SNL-contralateral, 1.74 ± 0.21 %).
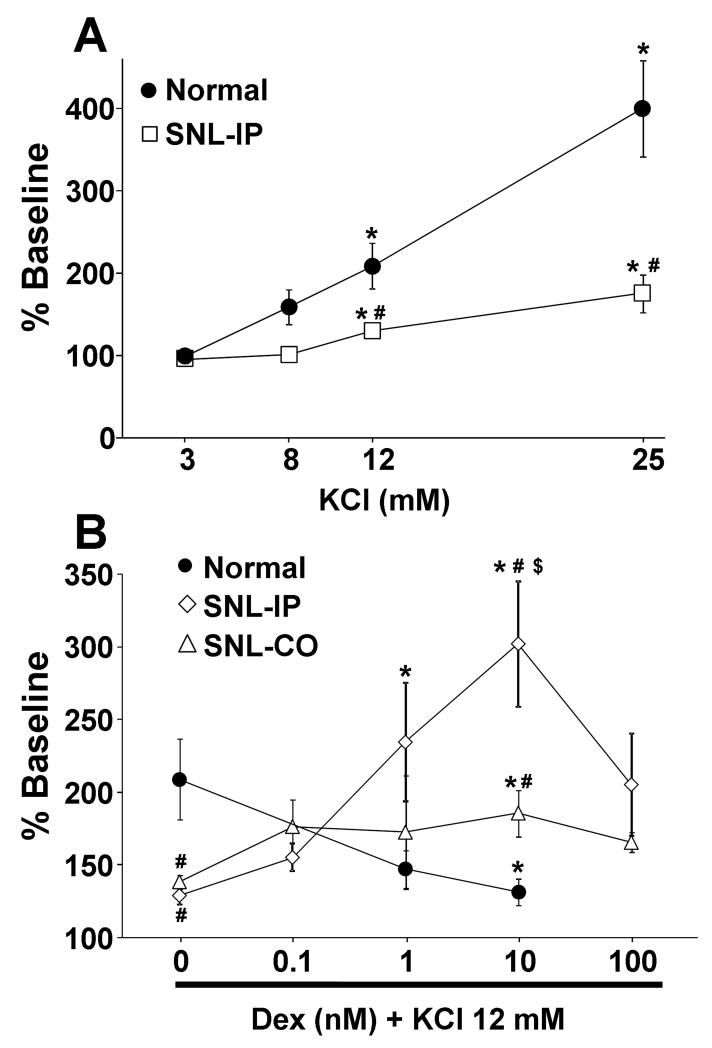
In synaptosomes from normal animals, KCl increased [3H]-acetylcholine release in a concentration dependent manner (Fig. 1 A). At 14 days after SNL in synaptosomes ipsilateral to surgery, the concentration response of KCl on [3H]-acetylcholine release significantly differed from that in synaptosomes from normal rats (Fig. 1 A; p<0.001). Dexmedetomidine inhibited KCl-evoked [3H]-acetylcholine release in a concentration dependent manner in synaptosomes from normal rats, but enhanced evoked release in synaptosomes ipsilateral to SNL surgery (Fig. 1 B). KCl-evoked [3H]-acetylcholine release was also decreased in synaptosomes contralateral to SNL surgery (Fig. 1 B, p<0.05), and dexmedetomidine also increased KCl-evoked [3H]-acetylcholine release in synaptosomes contralateral to surgery, but to a lesser extent than in those ipsilateral to SNL surgery (Fig. 1B; p<0.05). Dexmedetomidine-induced enhancement of evoked [3H]-acetylcholine release peaked at 10 nM and decreased at 100 nM. Base on these results, we selected 12 mM KCl and 10 nM dexmedetomidine for the subsequent studies.
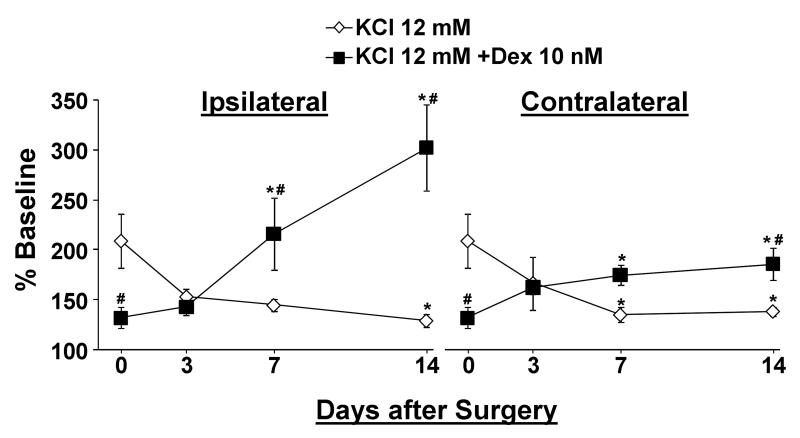
We next examined the time course of changes in KCl-evoked [3H]-acetylcholine release and the effect of dexmedetomidine on this release after SNL surgery. KCl-evoked [3H]-acetylcholine release decreased in a time dependent manner in synaptosomes from both sides of the spinal cord after SNL surgery (Fig. 2). The effect of dexmedetomidine on KCl-evoked [3H]-acetylcholine release shifted from inhibition in normal animals (Day 0) to no effect at 3 days after SNL surgery to enhancement by 7 days after surgery in synaptosomes from dorsal spinal cord ipsilateral to injury (Fig. 2; p<0.05). A similar pattern, although of lesser magnitude, was observed in spinal cord tissue contralateral to injury (Fig.2; p<0.05).
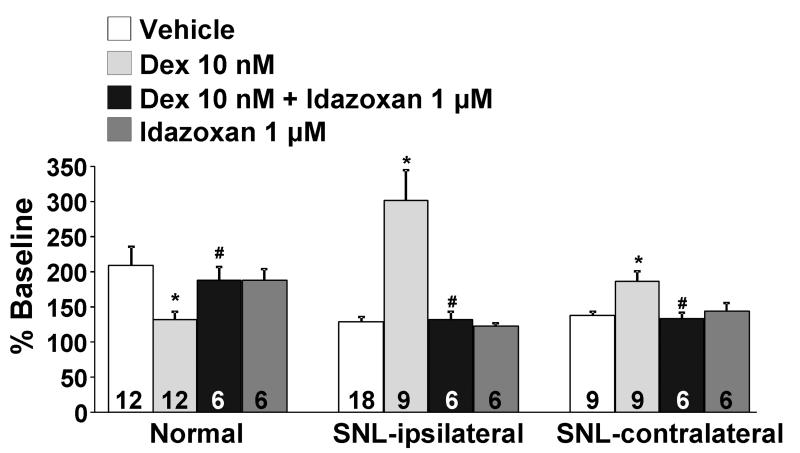
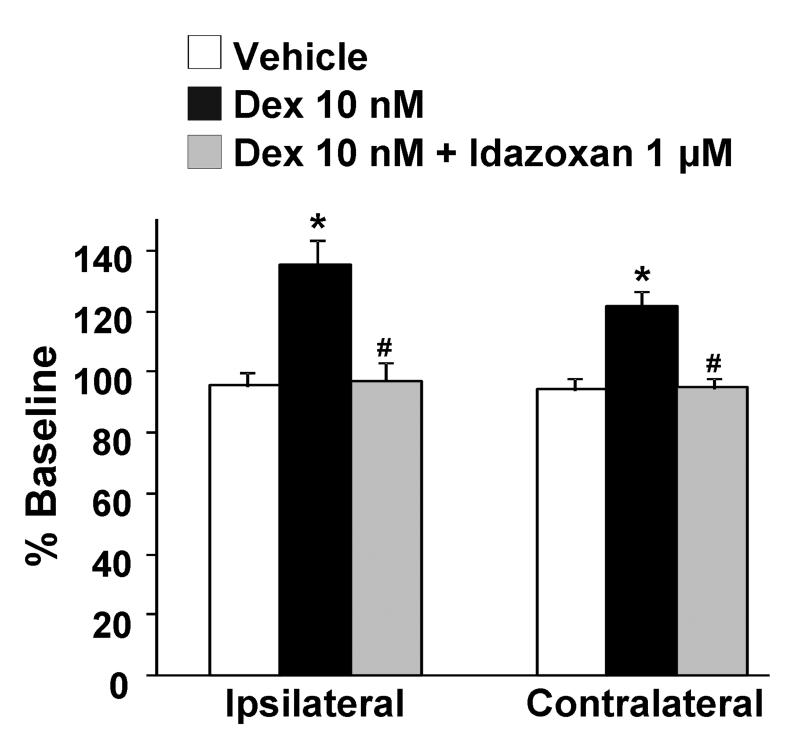
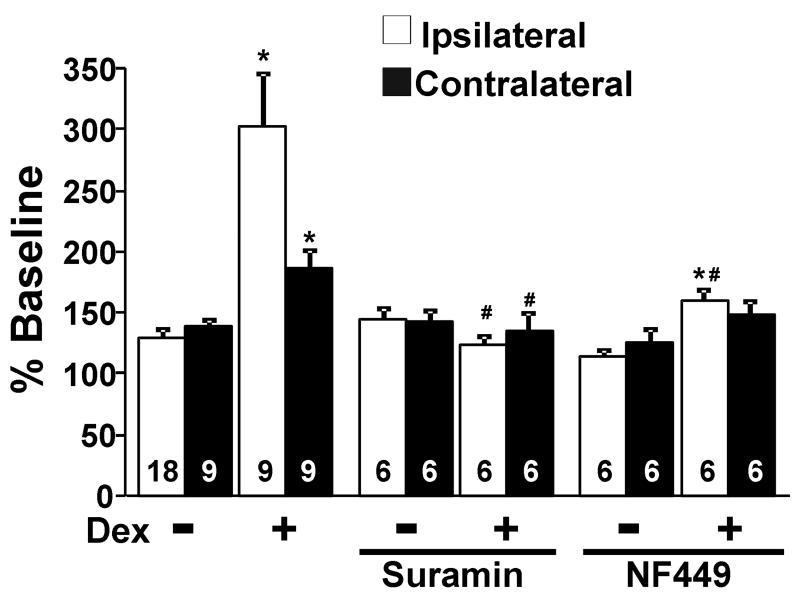
The selective α2-adrenoceptor antagonist idazoxan (1 μM), which did not affect KCl-evoked [3H]-acetylcholine release alone, abolished the inhibitory effect of dexmedetomidine on KCl-evoked [3H]-acetylcholine release in normal rats and its facilitation after SNL in both ipsi-and contralateral to surgery (Fig. 3). Dexmedetomidine alone, in the absence of KCl, slightly but significantly increased [3H]-acetylcholine release in synaptosomes ipsi-and contralateral to SNL (Fig. 4, p<0.05). This direct excitatory effect of dexmedetomidine on basal [3H]-acetylcholine release was also blocked by idazoxan (Fig. 4). Perfusion of synaptosomes with the selective Gs inhibitors, suramin (10 μM) and NF449 (10 μM), neither of which affected KCl-evoked [3H]-acetylcholine release alone, blocked the facilitatory effect of dexmedetomidine on KCl-evoked [3H]-acetylcholine release from synaptosomes ipsilateral to surgery (Fig. 5). In synaptosomes contralateral to surgery, suramin-treatment significantly decreased dexmedetomidine effect (p<0.05).
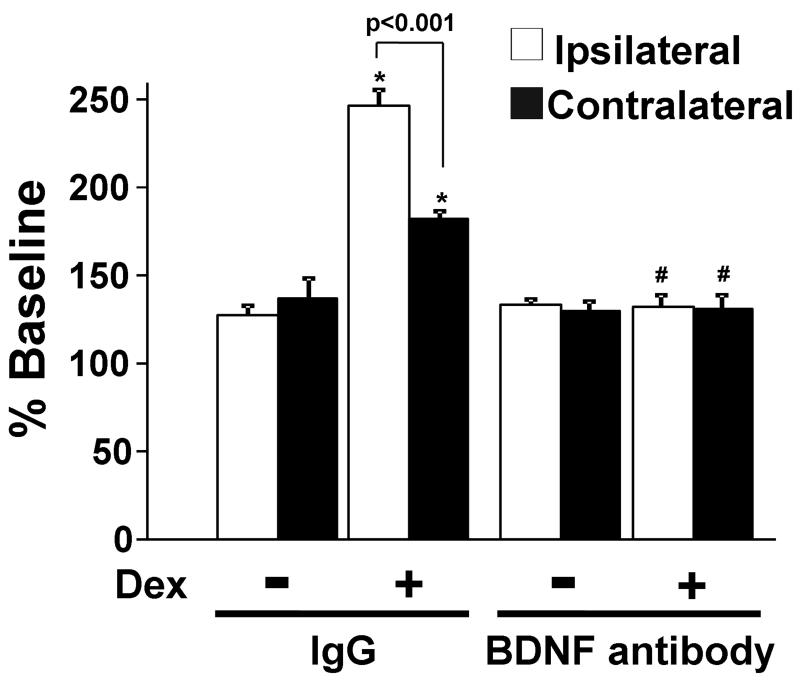
To examine whether blockade of BDNF signaling affects the facilitatory effect of dexmedetomidine on KCl-evoked [3H]-acetylcholine release in synaptosomes after nerve injury, animals received continuous intrathecal infusion of BDNF antibody (5 μg/day, n=8) or control IgG (5 μg/day, n=8) starting from 1 day prior to SNL and synaptosome experiments were performed at 10 days after the surgery. Since we only made two synaptosome preparations in each group, we did not compare total [3H]-choline uptake and basal [3H]-acetylcholine release between treatments. In IgG-treated animals, dexmedetomidine significantly enhanced KCl-evoked [3H]-acetylcholine release bilaterally compared to KCl alone (Fig. 6). In BDNF antibody-treated animals, KCl-evoked [3H]-acetylcholine release did not differ from IgG-treated animals, but dexmedetomidine’s enhancement of release was absent.
Immunohistochemistry for ChAT
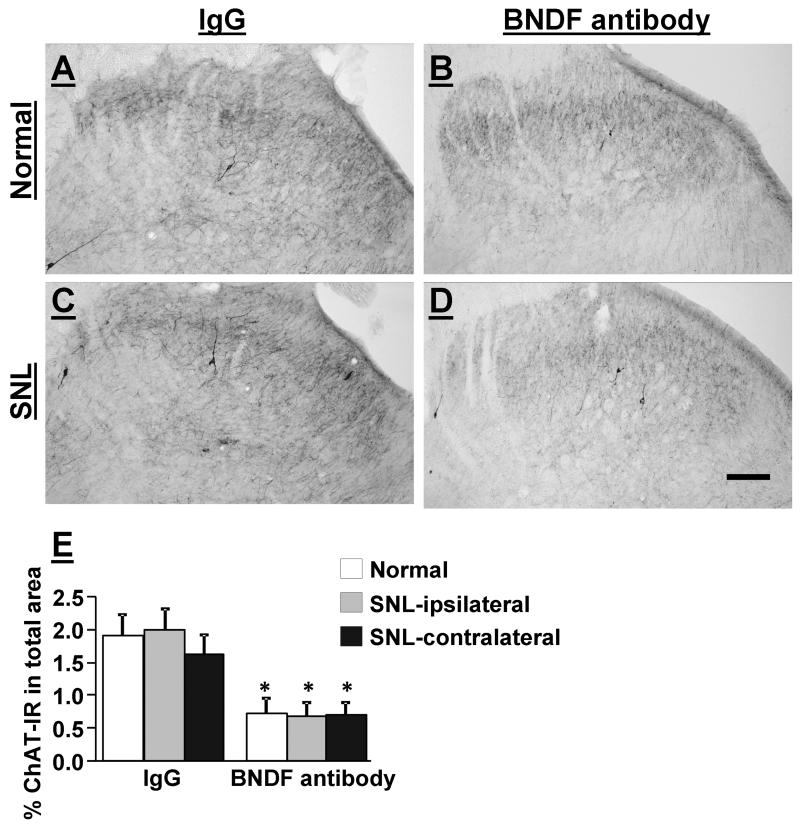
Figure 7 A-D depict ChAT-immunoreactivity in the L5 spinal dorsal horn in normal and SNL rats treated with a spinal infusion of IgG (5 μg/day) or BDNF (5 μg/day) for 11 days. ChAT-immunoreactivity in the L5 spinal dorsal horn was found mainly in axons but a few ChAT-positive cells were also found in both normal and SNL animals. In IgG-treated groups (n=4), there was no difference between tissues from normal and SNL rats in ChAT-immunoreactivity in the spinal dorsal horn (Fig. 7 E). In BDNF antibody-treated groups (n=4), ChAT-immunoreactivity in the spinal dorsal horn significantly decreased similarly in both normal and SNL rats compared to the IgG-treated group (p<0.05).
Discussion
Several lines of evidence support a key role for spinal α2-adrenoceptors and spinal cholinergic activation in analgesia from various pharmacologic interventions in animals with hypersensitivity from nerve injury and in humans with neuropathic pain.21 Although drugs may target multiple mechanisms for analgesia, gabapentin, antidepressants, and clonidine clearly reduce hypersensitivity in animals with peripheral nerve injury by direct or indirect activation of α2-adrenoceptors and subsequent acetylcholine release in the spinal cord.1-7 The current study confirms our previous observation3 of a shift from direct inhibition to direct enhancement of evoked acetylcholine release from a preparation that includes spinal cholinergic terminals when exposed to α2-adrenoceptor agonists. We extend these observations by determining the time course of this effect, its bilateral nature, and probing its mechanisms, which likely involve a shift in G-protein species interacting with these receptors and in a direct or indirect action of BDNF.
In the current study, [3H]-choline uptake in synaptosomes and ChAT-immunoreactivity in the spinal dorsal horn did not differ between normal and SNL animals, suggesting a lack of anatomic plasticity of cholinergic neurons and fibers in this model of neuropathic pain. However, SNL resulted in a bilateral decrease in KCl-evoked acetylcholine release in synaptosomes, consistent with other studies that peripheral nerve injury reduced KCl-evoked acetylcholine release in the spinal cord slices and in vivo microdialysis in the spinal cord.22,23 These data suggest that cholinergic terminals may be less excitable after SNL, and that the tonic inhibitory cholinergic tone on sensory processing shown in normal animals24 may be reduced in this state of hypersensitivity. The current study does not determine the source of cholinergic terminals affected by SNL, whether from descending neuronal terminals, interneurons, or the central terminals of primary afferents.9,22 We did previously observe, however, that unilateral peripheral nerve injury results in a bilaterally decreased KCl-induced Ca2+ response in dorsal root ganglion neurons, 8 consistent with a reduction in evoked acetylcholine release from afferent terminals in the spinal cord. Further studies would be required to determine the relative contribution of each of the cholinergic terminal sources to this change after peripheral nerve injury.
Activation of α2-adrenoceptors classically inhibits voltage-gated Ca2+ channels to reduce neurotransmitter release via pertussis toxin -sensitive Gi/o protein-dependent mechanisms, 11 and this mechanism was likely responsible for the decreased in KCl-evoked acetylcholine release by dexmedetomidine in synaptosomes from normal animals. This direct inhibition of acetylcholine release may partially underlie behavioral studies which show that antinociception in normal rats from intrathecal clonidine is not reversed by intrathecal injection of the muscarinic antagonist, atropine, or by a selective toxin to cholinergic neurons. 5,6 Thus, acetylcholine probably plays a small or no role in antinociception from spinal α2-adrenoceptor agonists in normal states.
This state of affairs is altered after nerve injury. We show here that dexmedetomidine enhancement of evoked acetylcholine release in synaptosomes occurs bilaterally in the spinal cord after nerve injury and that this depends on Gs proteins. We previously showed that peripheral nerve injury increases the efficacy of G-protein coupling from exposure to α2-adrenoceptor agonists, 14 perhaps reflecting this novel interaction with Gs proteins. In vitro, α2-adrenoceptors in transfected cells couple not only with Gi-proteins but also with Gs-proteins. 12 Although one can argue that this merely reflects over-expression of G-protein species in these artificial cell lines, in vivo data also support an interaction between α2-adrenoceptors and Gs proteins in some circumstances. For example, in the pregnant rat cervix, α2-adrenoceptors change their balance of Gi to Gs-coupling depending on the day of pregnancy. 13 Whether peripheral nerve injury increases Gs-coupling of α2-adrenoceptors in the spinal dorsal horn cholinergic terminals by an alteration in expression of Gs or Gi proteins or by post-translational modification of either these proteins or the α2-adrenoceptors is under current study.
BDNF regulates survival and differentiation of neurons during development and in adulthood, and can be released to induce plasticity in pathologic states. The most likely sources of BDNF in the spinal cord after peripheral nerve injury include the terminals of primary afferents and resident glia. 15,25-27 We and others have recently shown that peripheral nerve injury bilaterally increases BDNF content in the spinal dorsal horn. 15,28 BDNF plays important roles in synaptogenesis and plasticity in cholinergic motor neurons, as demonstrated by increased survival and axonal growth of cholinergic motor neurons in rats after spinal cord injury from spinal infusion of BDNF or over-expression of BDNF by gene-transfer, 29,30 and prevention of cholinergic neuron loss in the rat brainstem after hypoglossal nerve transection by intracerebroventricular infusion of BNDF. 31 The current study demonstrates that tonic BDNF signaling may be important to regulate the density of cholinergic fibers in the normal spinal cord dorsal horn, since spinal infusion of BDNF antibody reduced ChAT-immunoreactivity in the spinal dorsal horn. Interestingly, increased BDNF in the spinal cord after peripheral nerve injury does not further increase the density of cholinergic fibers. Additionally, the increase in BDNF content and signaling in the spinal cord after peripheral nerve injury indirectly or directly affects the action of α2-adrenoceptors on cholinergic terminals since injury-induced transformation of dexmedetomidine’s effect on acetylcholine release from inhibition to enhancement was abolished by spinal infusion of a BDNF antibody. These results coincide with a reduction in the anti-hypersensitivity effect of intrathecal clonidine after SNL injury from spinal infusion of a BDNF antibody. 15 These results suggest that BDNF is essential for maintenance of ChAT expression in the spinal dorsal horn and also for enhanced spinal acetylcholine release by α2-adrenoceptor stimulation after nerve injury. On the other hand, spinal BDNF antibody infusion itself did not alter KCl-evoked acetylcholine release, suggesting that regulation of α2-adrenoceptor function and depolarization-induced acetylcholine in the spinal dorsal horn cholinergic terminals reflects different mechanisms. However, the current study does not address whether BDNF affects expression and/or Gs-coupling of α2-adrenoceptors on the cholinergic terminals. Further study will be required to clarify these points.
In summary, peripheral nerve injury alters α2-adrenoceptor function on spinal cholinergic terminals from inhibition to facilitation via Gs-coupling. This shift requires BDNF which plays a pivotal role in expression of ChAT in the spinal dorsal horn and α2-adrenoceptor-mediated facilitation of spinal acetylcholine release after nerve injury. These results suggest that functional alterations of α2-adrenoceptors and actions of BDNF on cholinergic terminals after peripheral nerve injury are important for the analgesia by drugs commonly used to treat neuropathic pain and which rely in part on engagement of the spinal noradrenergic-cholinergic pathway.
Summary Statement.
Brain derived nerve growth factor in the spinal cord is needed for normal synthesis of acetylcholine by cholinergic neurons and excitation of these neurons by α2-adrenoceptor agonists after nerve injury.
Acknowledgements
This work was supported by grants NS57594 to JE and DA24826 to KH from the National Institute of Health, Bethesda, Maryland.
Footnotes
From: the Department of Anesthesiology, WFUSM, Winston Salem, NC
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–84. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashida K, Parker R, Eisenach JC. Oral gabapentin activates spinal cholinergic circuits to reduce hypersensitivity after peripheral nerve injury and interacts synergistically with oral donepezil. Anesthesiology. 2007;106:1213–9. doi: 10.1097/01.anes.0000267605.40258.98. [DOI] [PubMed] [Google Scholar]
- 3.Obata H, Li X, Eisenach JC. alpha2-Adrenoceptor activation by clonidine enhances stimulation-evoked acetylcholine release from spinal cord tissue after nerve ligation in rats. Anesthesiology. 2005;102:657–62. doi: 10.1097/00000542-200503000-00027. [DOI] [PubMed] [Google Scholar]
- 4.Obata H, Saito S, Koizuka S, Nishikawa K, Goto F. The monoamine-mediated antiallodynic effects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg. 2005;100:1406–10. doi: 10.1213/01.ANE.0000149546.97299.A2. table of contents. [DOI] [PubMed] [Google Scholar]
- 5.Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90:509–14. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Paqueron X, Conklin D, Eisenach JC. Plasticity in action of intrathecal clonidine to mechanical but not thermal nociception after peripheral nerve injury. Anesthesiology. 2003;99:199–204. doi: 10.1097/00000542-200307000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Takasu K, Honda M, Ono H, Tanabe M. Spinal alpha(2)-adrenergic and muscarinic receptors and the NO release cascade mediate supraspinally produced effectiveness of gabapentin at decreasing mechanical hypersensitivity in mice after partial nerve injury. Br J Pharmacol. 2006;148:233–44. doi: 10.1038/sj.bjp.0706731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashida KI, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–68. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 10.Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555:515–26. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–61. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eason MG, Kurose H, Holt BD, Raymond JR, Liggett SB. Simultaneous coupling of alpha 2-adrenergic receptors to two G-proteins with opposing effects. Subtype-selective coupling of alpha 2C10, alpha 2C4, and alpha 2C2 adrenergic receptors to Gi and Gs. J Biol Chem. 1992;267:15795–801. [PubMed] [Google Scholar]
- 13.Gal A, Ducza E, Minorics R, Klukovits A, Galik M, Falkay G, Gaspar R. The roles of alpha2-adrenoceptor subtypes in the control of cervical resistance in the late-pregnant rat. Eur J Pharmacol. 2009;615:193–200. doi: 10.1016/j.ejphar.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 14.Bantel C, Eisenach JC, Duflo F, Tobin JR, Childers SR. Spinal nerve ligation increases alpha2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005;1038:76–82. doi: 10.1016/j.brainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida KI, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–55. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 17.Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–6. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- 18.Jope RS. Acetylcholine turnover and compartmentation in rat brain synaptosomes. J Neurochem. 1981;36:1712–21. doi: 10.1111/j.1471-4159.1981.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 19.Pittel Z, Cohen S, Fisher A, Heldman E. Differential long-term effect of AF64A on [3H]ACh synthesis and release in rat hippocampal synaptosomes. Brain Res. 1992;586:148–51. doi: 10.1016/0006-8993(92)91386-s. [DOI] [PubMed] [Google Scholar]
- 20.Paqueron X, Li X, Bantel C, Tobin JR, Voytko ML, Eisenach JC. An obligatory role for spinal cholinergic neurons in the antiallodynic effects of clonidine after peripheral nerve injury. Anesthesiology. 2001;94:1074–81. doi: 10.1097/00000542-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Eisenach JC. Muscarinic-mediated analgesia. Life Sci. 1999;64:549–54. doi: 10.1016/s0024-3205(98)00600-6. [DOI] [PubMed] [Google Scholar]
- 22.Dussor GO, Jones DJ, Hulsebosch CE, Edell TA, Flores CM. The effects of chemical or surgical deafferentation on [3H]-acetylcholine release from rat spinal cord. Neuroscience. 2005;135:1269–76. doi: 10.1016/j.neuroscience.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136–45. doi: 10.1016/j.pain.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 24.Zhuo M, Gebhart GF. Tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1991;46:211–22. doi: 10.1016/0304-3959(91)90078-C. [DOI] [PubMed] [Google Scholar]
- 25.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 26.Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen H, Chung JM, Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Brain Res Mol Brain Res. 1999;64:186–92. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- 28.Miletic G, Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci Lett. 2002;319:137–40. doi: 10.1016/s0304-3940(01)02576-9. [DOI] [PubMed] [Google Scholar]
- 29.Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–84. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- 30.Xu K, Uchida K, Nakajima H, Kobayashi S, Baba H. Targeted retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene prevents loss of mouse (twy/twy) anterior horn neurons in vivo sustaining mechanical compression. Spine (Phila Pa 1976) 2006;31:1867–74. doi: 10.1097/01.brs.0000228772.53598.cc. [DOI] [PubMed] [Google Scholar]
- 31.Tuszynski MH, Mafong E, Meyer S. Central infusions of brain-derived neurotrophic factor and neurotrophin-4/5, but not nerve growth factor and neurotrophin-3, prevent loss of the cholinergic phenotype in injured adult motor neurons. Neuroscience. 1996;71:761–71. doi: 10.1016/0306-4522(95)00440-8. [DOI] [PubMed] [Google Scholar]