Abstract
Galectin-3, a ß-galactoside-binding lectin, is found in cellular and extracellular location of the cell and has pleiotropic biological functions such as cell growth, cell adhesion and cell-cell interaction. It may exhibit anti or pro-apoptotic activity depending on its localization and post-translational modifications. Two important post-translational modifications of galectin-3 have been reported: its cleavage and phosphorylation. Cleavage of galectin-3 was reported to be involved with angiogenic potential and apoptotic resistance. Phosphorylation of galectin-3 regulates its sugar-binding ability. In this report we have identified novel tyrosine phosphorylation sites in galectin-3 as well as the kinase responsible for its phosphorylation. Our results demonstrate that tyrosines at position 79, 107 and 118 can be phosphorylated in vitro and in vivo by c-Abl kinase. Tyrosine 107 is the main target of c-Abl. Expression of galectin-3 Y107F mutant in galectin-3 null SK-Br-3 cells leads to morphological changes and increased motility compared to wild type galectin-3. Further investigation is needed to better understand the functional significance of the novel tyrosine phosphorylated sites of galectin-3.
Keywords: phosphorylation, kinase, galectin-3
1. Introduction
Galectin-3 is a member of animal lectins that bind β -galactosides through evolutionarily conserved sequence elements of the carbohydrate recognition domain (CRD) [1]. Galectin-3 plays an important role in tumorigenesis and progression through regulating cell proliferation, apoptosis, cell adhesion, invasion, angiogenesis and metastasis by binding to the carbohydrate moiety of cell surface glycoproteins or glycolipids [2-4]. The coding sequence of galectin-3 gene produces a protein with calculated molecular weight equal to 26.1 kDa molecular mass that comprises 250 amino acid residues. However, its apparent molecular weight as determined by electrophoretic mobility is around 30 kDa [1]. Galectin-3 has a unique chimera type structure consisting of three different domains: NH2-terminal domain, a repeated collagen-like sequence rich in proline, glycine, tyrosine and glutamine residues, and a COOH-terminal domain [2]. The NH2-terminal domain consists of 12 amino acids containing two serine phosphorylation sites at positions 6 and 12, which act as substrates for casein kinase 1 [5]. The serine phosphorylations appear to be related to fibroblast quiescence/replicative competence in vitro, lectin export from nuclei, e.g. in response to apoptotic stress, alterations in signaling/gene expression with cell-type specificity and binding ability to its ligands. Serine phosphorylation of galectin-3 reduces its binding to the glycoprotein ligands laminin and asialomucin, whereas the dephosphorylation fully restores the sugar binding activity [6-9].
The collagen-like domain contains two matrix metalloproteinases (MMPs) recognition sites and is made up of repetitive sequence of nine amino acid residues rich in proline, glycine, tyrosine and glutamine and lacks charged or large side-chain hydrophobic residues. The COOH-terminal domain contains a single carbohydrate recognition domain (CRD) consisting of 140 amino acid residues, which defines the molecular characteristics of galectin family, i.e. their affinity to carbohydrates [2, 10, 11]. Galectin-3 predominantly localizes in the cytoplasm, can import into the nucleus through at least two pathways; via passive diffusion and/or active transport [12, 13]. It also gets secreted from the cytoplasm via the non-classical secretion pathway, since galectin-3 contains no consensus signal sequence for either secretion or nuclear translocation [14, 15]. Serine phosphorylation plays an important role in translocation of galectin-3 between nucleus and cytoplasm. Galectin-3 also contains the GSK-3ß phosphorylation consensus sequence (S92XXXS96). Shimura et al demonstrated that phosphorylation of galectin-3 by GSK-3ß is mediated by Axin [16]. Although the role of this phosphorylation is not clear yet, it indicates importance of galectin-3 in Wnt pathway.
In 2001 Yamazaki et al demonstrated that galectin-3 can also be phosphorylated on tyrosine residues. Their findings indicated that galectin-3 is a novel member of signaling proteins downstream of tyrosine kinase, and suggested that it plays roles in supporting repair or survival of the injured hepatocytes rather than their proliferation [17].
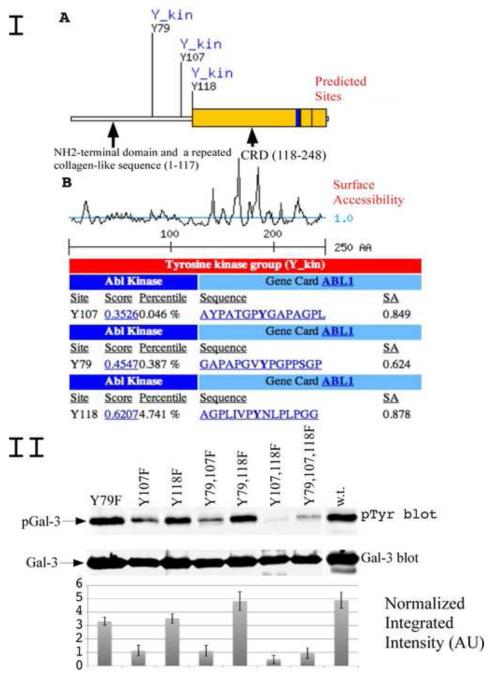
In order to identify the tyrosine phosphorylation sites in galectin-3, we performed bioinformatic scan and found novel possible sites within repeated collagen-like sequence rich in glycine, proline tyrosine and glutamine residues, which can be phosphorylated by c-Abl and Arg (c-Abl related gene) kinase (Fig 1 I).
Figure1.
I. Bioinformatic search predicting possible tyrosine phosphorylation sities of galectin-3 and the cognate protein kinase. (A) A diagram depicting newly identified galectin-3 phosphorylation sites and potential kinase. Indicated are NH2-terminal domain, a repeated collagen-like sequence and carbohydrate binding domain (CRD).
(B) A diagram showing surface accessibility, scores and amino acids around predicted phosphorylation sites.
II. c-Abl phosphorylation of galectin-3 in vitro. Recombinant GST-Galectin-3 mutants of predicted tyrosine sites and galectin-3 wild type were used as substrate for active c-Abl in in vitro assay. The reaction was stopped by adding sample buffer, resolved on 10% polyacrilomide gel, and immunoblotted using anti-pTyr antibody (top) or anti-galectin-3 antibody (bottom). The normalized integrated intensity was calculated as Band integrated intensity/Line normalization factor, anti-galectin-3 blot was used as reference channel.
c-Abl is a nonreceptor tyrosine kinase ubiquitously expressed and highly conserved in metazoan evolution. c-Abl and the product of its paralog gene, Arg (ABL2), resemble Src family kinases and consist of a catalytic domain that is preceded by a variable N-terminal region of 60 or 80 residues, a SH3, and a SH2 domain [18]. Activity of c-Abl protein is negatively regulated by its SH3 domain, and deletion of the SH3 domain turns c-Abl into an oncogene [19]. The presence of c-Abl in multiple cellular compartments suggests that the protein might move from one place to another within the cell, transducing signals in response to physiological stimuli. Wild-type c-Abl protein does not transform fibroblasts or hematopoietic cells, even when overexpressed, suggesting that Abl kinase activity is regulated tightly in cells [20]. Deletions and point mutations in the Abl SH3 domain that prevent binding of proline-rich SH3 ligands, activate c-Abl kinase activity in vivo, resulting in elevated tyrosine phosphorylation of c-Abl and other proteins and cellular transformation [18].
In the present study we confirm the presence of phosphorylation at Tyr 79, Tyr 107 and Tyr 118 residues of galectin-3 and demonstrate that these sites are targeted by c-Abl kinase in vitro as well as in vivo.
2. Materials and methods
2.1. Cell lines and antibodies
The human breast cancer cell line SK-Br-3 and MDA-MB-435 were maintained in McCoy medium (Invitrogen Corporation) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals). Customized polyclonal rabbit anti-galectin-3 antibody against the recombinant whole molecule was created by Zymed Laboratories (South San Francisco, CA). Monoclonal rat anti-galectin-3 antibody was isolated from the supernatant of hybridoma TIB-166 (American Type Culture Collection, Rockville, MD). pTyr blot was performed with anti-pTyr antibody coupled with IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA). Mouse anti-ß-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO)
2.2. Plasmid constructs
Galectin-3 was PCR amplified from a pcDNA-Gal-3 wild type vector and subcloned into pECFP, pEGFP (BD Bioresearch, Palo Alto, CA), pGex-6p (Amersham, Piscataway, NJ), pCMV-Tag1 (Stratagene, La Jolla, CA) vectors as EcoRI-BamHI, BamHI-EcoRI, BamHI-EcoRI and BglII-XhoI fragment respectively. pSGT-c-Abl PP and KM was a kind gift from Dr. Giulio Superti-Furga (Center for Molecular Medicine of the Austrian Academy of Sciences). c-Abl was PCR amplified from pSGT-c-Abl P242/249E (c-Abl PP) and K290M (c-Abl KM) and subcloned into pEYFP vector (BD Bioresearch, Palo Alto, CA) as BamHI-HindIII fragment. In experiments using EGFP, ECFP and YCFP-tagged proteins, images were acquired with an Olympus (Melville, NY) IX71 microscope supporting a Hamamatsu ORCA-ER video camera.
2.3. Western Blot Analysis
Cells were grown up to 80% confluence, and whole-cell lysates were prepared in lysis buffer [20 mM Tris-HCl (pH 7.4), 0.1% SDS (Fisher Scientific, Pittsburgh, PA), 1.0% Triton X-100, 0.25% sodium deoxycholate, 1 mM EGTA, 1 mM EDTA, 5 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin (Sigma, St. Louis, MO). Equal amount of protein was loaded on the gel and resolved by 10% SDS-PAGE and electroblotted onto polyvinylidene difluoride membrane (Immobilon PSQ, Millipore, MA). Membranes were quenched in a solution of TBS, containing 0.1% Casein and 0.1% Tween-20 for 60 min or in 2% Gelatin from cold water fish skin (Teleostean gelatin) in case of phosphoblots (Sigma, St. Louis, MO) in TBST on a rotary shaker. Blots were incubated with appropriate primary antibodies according to manufactures instructions, washed and then incubated with appropriate secondary antibodies conjugated with IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA) or Alexa Fluor 680 (Invitrogen Corporation) for 30 minutes at room temperature. After incubation with both primary and the secondary antibodies, membranes were washed four times with TBST (TBS, containing 0.1% Tween-20) at 5-minute intervals. Immunoblots were visualized and density of each band was quantitated using the Odyssey infrared imaging system and Odyssey application software (LI-COR Biosciences, Lincoln, NE).
2.4. Co-immunoprecipitation Assays
Galectin-3 was purified using rat monoclonal TIB-166 immunoprecipitation, β-catenin and TCF-4 were purified using β-catenin and TCF-4 antibody from BD Bio-Sciences (San Diego, CA). Monoclonal rat anti-galectin-3 antibody was isolated from the supernatant of hybridoma (catalogue no. TIB-166; American Type Culture Collection, Rockville, MD). After extensive washes, galectin-3 presence in the immunoprecipitates was determined using polyclonal antibody HL31. Customized polyclonal rabbit anti-galectin-3 antibody against the recombinant whole molecule was created by Zymed Laboratories (South San Francisco, CA). 5 mg of cell extracts from exponentially growing Sk-Br-3 cells were incubated with 10 μg of appropriative antibody, or control rat IgG precoupled to 50 μl of protein G agarose-beads (Pharmacia, Uppsala, Sweden) for 2 h at 4°C. The beads were washed twice with 10 ml of lysis buffer, twice with 10 ml of lysis buffer containing 0.5 M LiCl, and twice with 10 ml of phosphate-buffered saline. Beads were boiled with x1 sample buffer and loaded on the gel.
2.5. c-Abl Kinase assay
c-Abl Kinase assay was preformed using the HTScan Abl1 kinase assay kit (Cell Signaling Technology, MA) according to the manufacturer’s instructions.
2.6. Galectin-3 phosphopeptide mapping
Two-dimensional phosphopeptide mapping was performed according to previously described protocols [21, 22]. Briefly, 32P-labeled recombinant galectin-3 wild type and mutants were resolved using 10% SDS-PAGE, transferred to a PVDF membrane, excised, and 32P incorporation in galectin-3 was determined by Cherenkov counting. Following incubation with 0.5% PVP in 100 mM acetic acid for 30 min at 37°C and extensive washes, the protein samples were digested with 10 mg sequencing grade modified trypsin (Promega, Madison, WI) in 50 mM ammonium bicarbonate buffer for 2 h at 37°C and with additional 10 mg of trypsin overnight (this method routinely allowed recovery of 90-95% of the initial radioactivity in galectin-3). The eluted peptides were washed twice with 50 mM ammonium bicarbonate buffer and once with pH 1.9 TLC-electrophoresis buffer (2.2% Formic acid and 7.8% acetic acid in water). Samples were spotted on cellulose TLC plates (Merck KGaA, Darmstadt, Germany) and separated using the Hunter thin-layer chromatography system (CBS Scientific, Del Mar, CA) in pH 1.9 buffer for 30 min at 1250 volts. The plates were dried overnight and subjected to second dimension chromatographic separation in a phospho-chromatography buffer (37.5% n-Butanol, 25% Pyridine and 7.5% acetic acid). The plates were dried and phosphopeptide spots were visualized by autoradiography and phosphor imaging.
2.7. Wound Healing Assay
A wound healing assay was performed according to published protocol [23].
3. Results
3.1 Possible tyrosine phosphorylation sites of galectin-3 by bioinformatic search
Four possible serine phosphorylations sites within N-terminal and collagen-like domain were found. Yamazaki et al demonstrated that galectin-3 carries tyrosine phosphorylation, however the possible phosphorylation sites were not revealed in this study [17]. Bioinformatic search using SCANSITE 2.0 (http://scansite.mit.edu) with low stringency indicates that galectin-3 can be phosphorylated by c-Abl and Arg kinases on Tyr 79, 107 and 118 (Fig 1 IA). High stringency scan left only Tyr 107 with a score of 0.3526 and a percentile of 0.046 (Fig. 1 IB).
3.2 Confirmation of tyrosine phosphorylation at predicted sites by site-directed mutagenesis
To investigate if galectin-3 can be phosphorylated on tyrosine residues, we substituted the tyrosine residues 79, 107 and 118 with phenylalanine and created single, double and triple mutants. All muteins were expressed in bacteria as GST fusion proteins and purified as described [24]. Pure muteins as well as wild type galectin-3 were used as substrate for active c-Abl kinase in an in vitro assay (Fig. 1 II). The results confirm that c-Abl can phosphorylate galectin-3 in vitro. When mutations were performed at the three predicted sites, phosphorylation of galectin-3 decreased. Tyr 107 is the major residue phosphorylated by c-Abl kinase as its mutation alone or in combination with other sites showed minimum phosphorylation of the protein. These results also indicated the presence of some other unknown site/s with low stoichiometry. Level of phosphotyrosine fraction of galectin-3 was measured and normalized to the level of total galectin-3 in each line using Odyssey 3.0 software.
3.3 Confirmation of tyrosine phosphorylation at predicted sites by 2-D phosphopeptide mapping
To confirm the identity of the phosphorylation sites by an independent method, we performed 2-D phosphopeptide mapping of in vitro phosphorylated recombinant muteins and compared its 2-D tryptic phosphopeptide maps with that of wild type galectin-3 and CRD domain only (consisting of amino acids 120 to 250) (Fig. 2 I). According to predicted tryptic map all three residues should be located on the same peptide. The actual tryptic 2-D phosphopeptide map of wild type galectin-3 shows one major spot with smear around it and its position on the 2-D map correlates with the predicted position of a phosphorylated galectin-3 tryptic peptide, while CRD domain does not show this spot. 2-D phosphopeptide maps of mutated proteins demonstrate that the mutation in Tyr 107 results in complete disappearance of the major fraction of phosphorylation, whereas in other muteins some of the some phosphorylation could be seen indicating that Tyr 107 is a major phosphorylated site on galectin-3. Collectively, the phosphopeptide mapping results confirmed the data from Western blot analysis and demonstrate that c-Abl is responsible for tyrosine phosphorylation of galectin-3 and Tyr 107 is a major phosphorylated site.
Figure 2.
I. Direct phosphorylation of galectin-3 by c-Abl in vitro. Approximately 1 μg of recombinant wild-type or mutated galectin-3 proteins used as substract for active c-Abl in in vitro assay. 100 μl kinase assay buffer containing 100 μM ATP and 25 μCi of [g-32P] ATP with recombinant galectin-3 and active c-Abl was incubated for 20 min at 30°C. After reaction was stopped with sample buffer, boiled samples were subjected to phosphopeptide map analysis as described in Materials and Methods.
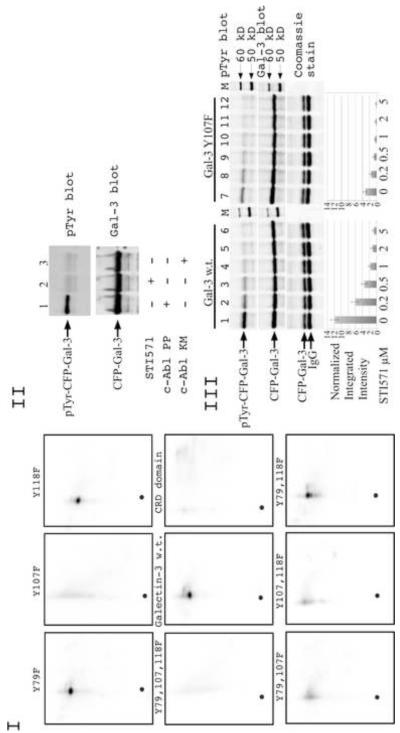
II. Phosphorylation of galectin-3 by c-Abl in vivo. Wild type galectin-3 protein was expressed in SK-Br-3 cell with constitutively active c-Abl PP (lane 1,2) and kinase dead c-Abl KM (lane3). Cells in lane 2 were treated with 10 μM of c-Abl inhibitor STI571. Galectin-3 was immunoprecipitated using anti-galectin-3 (TIB166) antibody and loaded on gel. The samples were resolved using 10% SDS-PAGE and immunoblotted with anti-Gal-3 (HL31) (top) and anti-phosphotyrosine antibodies (bottom).
III. Dose-dependent inhibition of c-Abl activity by STI571. SK-Br-3 cells were co-transfected with c-Abl PP and wild type (lane 1-6) or Y107F (lane 7-12) galectin-3. STI571 was added to cell medium overnight. Cells were lysed and galectin-3 was immunoprecipitated using anti-Gal-3 (TIB166) antibody and loaded on gel. The samples were resolved using 10% SDS-PAGE gel and immunoblotted with anti-Gal-3 (HL31) (top) and anti-phosphotyrosine antibody (middle). To confirm equal loading of immunoprecipitated proteins 15% of IP mixture was run on 10% SDS-PAGE and visualized with Coomassie blue stain. The normalized integrated intensity was calculated as Band integrated intensity/Lane normalization factor, anti-galectin-3 blot was used as reference channel. The band having the highest integrated intensity is assigned a normalization factor of 1.0. This reference band is then used to calculate the normalization factor for all other selected lanes by dividing the band integrated intensity by the integrated intensity of the reference band. Then the normalization factor was used to calculate the normalized integrated intensity.
3.4. In vivo phosphorylation of tyrosine residues
To demonstrate the ability of c-Abl to phosphorylate galectin-3 in vivo we constructed plasmids expressing constitutively active c-Abl PP and kinase dead mutant c-Abl KM fused with EYFP protein as well as wild type and Y107F mutant galectin-3 fused with ECFP protein under the control of the CMV promoter.
SK-Br-3 cells were co-transfected with plasmids expressing EYFP-c-Abl PP and ECFP-Gal-3. One of the culture dishes was treated with c-Abl inhibitor STI571. Western blot analysis performed with galectin-3 immunoprecipitated from these cells clearly demonstrates that STI571 treatment resulted in the reduction of tyrosine phosphorylation of galectin-3 confirming our suggestion that c-Abl is the kinase responsible for most tyrosine phosphorylations of galectin-3 in the cells under these conditions (Fig. 2 II lane 2). To address the possibility that STI571 may block other tyrosine kinases and thus prevent tyrosine phosphorylation of galectin-3, we co-transfected SK-Br-3 cells with plasmids expressing EYFP-c-Abl KM and ECFP-Gal-3 (Fig. 2 II lane 3). A reduction in tyrosine phosphorylation, which was comparable to STI571 treatment, was observed. That indicates that other tyrosine kinases in Sk-Br-3 cells phosphorylate galectin-3 with low stoichiometry under these conditions.
Dose-dependent inhibition of tyrosine phosphorylation in wild type and Y107F galectin-3 is shown in Figure 2 III. The results show a decrease in phosphorylation with increasing concentration of the inhibitor. However, a weak band could still be seen at higher concentrations confirming our earlier presumption that some other unknown low stoichiometry site may be present. To ensure that STI571 acts on a timescale that is consistent with its use in our experiments, time course analysis of STI571 inhibition was performed (data not shown). Both the experiments did not reveal any significant differences between wild type and mutated protein except difference in the level of total phosphorylation.
3.5 Interaction of galectin-3 with c-Abl in vivo
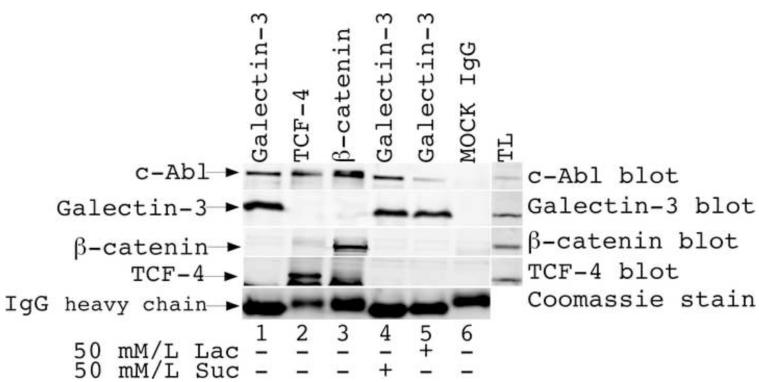
To analyze if galectin-3 and c-Abl exhibit interactions in vivo, we performed co-immunoprecipitation studies (Fig. 3). MDA-MB-435 cells expressing both galectin-3 and c-Abl were utilized for immunoprecipitation of galectin-3 with TIB166 antibody in the presence of sucrose and lactose. Immunoblotting with c-Abl antibody revealed the presence of c-Abl protein in immunoprecipitated mixture as well as in immunoprecipitates with ß-catenin and TCF-4 antibody. ß-catenin and TCF-4 were used as positive control because their interactions with c-Abl have been established earlier [25, 26]. c-Abl was also found in immunoprecipitates from cells treated with lactose indicating that this interaction is not dependent on galectin’s carbohydrate-binding properties.
Figure 3.
Coimmunoprecipitation of c-Abl with Gal-3. MDA-MB-435 cell lysates were immunoprecipitated using anti-galectin-3 (lanes 1,4,5), anti-TCF-4 (lane 2) and anti-β-catenin (lane 3) antibody and immunoblotted with anti-c-Abl, anti-TCF-4, anti-β-catenin and anti-galectin-3 polyclonal antibodies. Some cells were treated with sucrose and lactose (line 4,5 respectively). To confirm equal loading of immunoprecipitated proteins 15% of IP mixture was run on 10% SDS-PAGE and visualized with Coomassie blue stain.
3.6. Effect of tyrosine phosphorylation on cell morphology
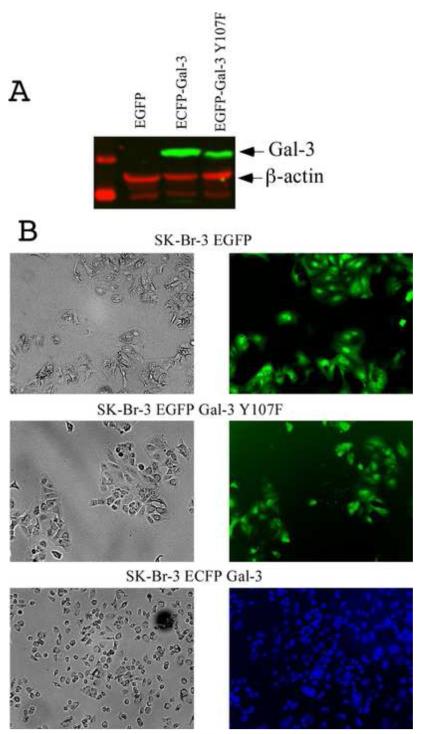
To analyze if tyrosine phosphorylation affects cell morphology, SK-Br-3 cell lines stably expressing EGFP, ECFP-Gal-3 and EGFP-Gal-3 Y107F were established. We decided to use CFP fused galectin-3 wild type to prevent possible overlap between wild type and mutant protein labeled with GFP. Selected clones were screened to confirm the expression of transfected proteins. Cellular phenotypes were visualized on the second day after splitting (Fig. 4). It is interesting to notice that cells expressing wild type galectin-3 are different compared to cells expressing mutated galectin-3 as well as cells expressing only EGFP protein.
Figure 4.
Photomicrograph depicting the morphology of Sk-Br-3 stable cell lines expressing wild type and Y107F galectin-3. (A) Western blot analysis of Sk-Br-3 clones: transfected with EGFP (lane 1), ECFP-Gal-3 (lane 2) and EGFP-Gal-3 Y107F (lane 3).
(B) Cellular shapes were visualized 2 days after splitting. Cells were grown under identical conditions. Left (phase contrast) and right (fluorescent light) panels represent same area on plate, x20.
3.7 Effect of tyrosine phosphorylation on cell motility
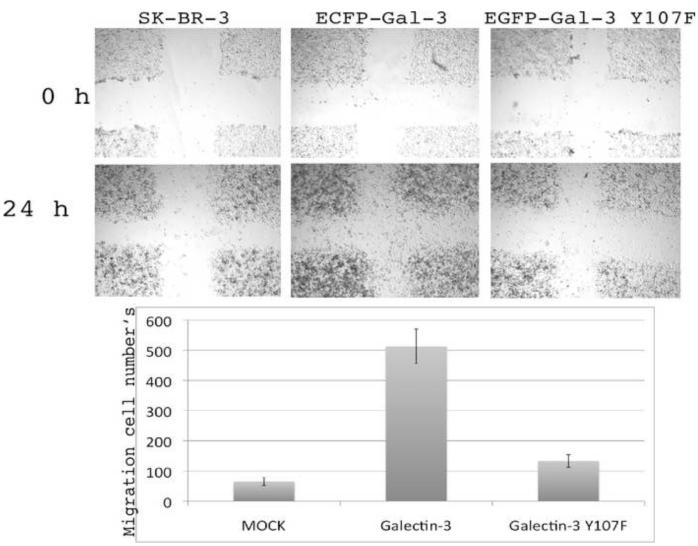
We used SK-Br-3 stable cell lines expressing galectin-3 and Y107F mutant to examine whether absence of tyrosine phosphorylation at that position affects cancer cell motility. As shown in Figure 5, over-expression of galectin-3 induced cell motility, whereas galectin-3 Y107F did not show a significantly increased motility compared to the mock transfected cells. These results suggest that galectin-3 promotes breast cancer cell motility partially through tyrosine phosphorylation.
Figure 5.
Wound healing assay of SK-Br-3 cells were performed with stable cell lines constituvely expressing galectin-3 wild type and galectin-3 Y107F mutant. Cells were grown under identical conditions.
4. Discussion
Galectin-3 is ubiquitously expressed in normal tissues as well as in a variety of tumors, and the level of expression varies by origin of the tissue. The intensity of its expression and localization depends on tumorigenicity, invasiveness and metastatic potential of a cell and has resulted in the use of galectin-3 as a biomarker for variety of tumors. Galectin-3 can shuttle between the nucleus and the cytoplasm [27-29] and is involved in the fundamental processes such as pre-mRNA splicing [30], cell-cycle progression [31, 32], proliferation [33], and apoptosis [34-37] mainly through intracellular protein-protein interactions and lectin-carbohydrate interactions. However, the functions of galectin-3 are regulated not only by its expression level and cellular localization, but also by post-translational modifications like phosphorylation. It was established that human galectin-3 is phosphorylated at different serine residues with serine 6 being the main site phosphorylated by casein kinase I [5]. Protein phosphorylated at serine 6 showed reduced binding to laminin and asialomucin. Dephosphorylation of galectin-3 restored the sugar binding capacity. Interestingly, mutations at serine 6 resulted in a diminished ability of galectin-3 to protect cells from cisplatin-induced apoptosis [5]. GSK-3ß is responsible for phosphorylation of serine 92 and 96 and these phosphorylations are mediated by Axin [16]. The biological significance of these phosphorylation sites has not been reported so far.
As early as in 2001, phosphorylation of tyrosine residue/s of endogenous galectin-3 was demonstrated for the first time. However the position of the phosphorylated tyrosine residue/s and tyrosine kinase that phosphorylates galectin-3 was not determined [17]. The authors demonstrated that the hepatic galectin-3 has the potential to function as a novel member of signaling proteins downstream of unknown tyrosine kinase. Although the nature of the interaction was unclear, Menon and Hughes hypothesized that galectin-3 can interact with proteins carrying SH3 domains through PXXP motifs found in primary structure of galectin-3 [38].
c-Abl kinase is a tyrosine kinase containing a SH3 domain, and has been implicated in the processes of cell differentiation, cell division, cell adhesion, and stress response. Truncated c-Abl can fuse with other genes and turn oncogenic: for example in the case of Bcr (breakpoint cluster region)-abl [39]. Our results on the in vitro phosphorylation of wild type, single, double and triple mutants of galectin-3 as substrate confirmed that c-Abl is the kinase responsible for phosphorylation of galectin-3 (Fig. 1 II). Immunoblotting studies with anti-phosphotyrosine antibody and 2-D phosphopeptide mapping point out that the main phosphorylation site on galectin-3 is tyrosine 107. Although bioinformatic search suggested that all three tyrosines at amino acid position 79, 107 and 118 can be phosphorylated, our results (Fig. 1 and 2) clearly demonstrate that c-Abl can phosphorylate some other yet unknown tyrosine residue/s since some of the phosphorylation could still be seen even in triple mutant. In vivo experiment (Fig. 2 II) also indicates the possibility of other yet unknown tyrosine kinase/s to phosphorylate galectin-3 as weak bands were observed in cells treated with c-Abl inhibitor STI571 and cell co-transfected with kinase dead c-Abl. The phosphorylation may play a role in the interactions of galectin-3 with other proteins that are targeted by c-Abl kinase. It was reported that galectin-3 binds to β-catenin/TCF-4 complex, co-localizes with β-catenin in the nucleus, and induces the transcriptional activity of TCF-4 leading to up-regulation cyclin D1 and c-myc expression [33]. Bcr-Abl and active c-Abl physically interact with β-catenin, and its oncogenic tyrosine kinase activity is required to phosphorylate β-catenin at tyrosine 86 and tyrosine 654 residues. β-catenin with these post-translational modifications binds to the TCF-4 transcription factor, thus representing a transcriptionally active pool that is responsible for increased expression of cyclinD1 and c-myc [26]. Our results suggest the possibility that the nuclear localization and activation of β-catenin may be dependent on its interaction with galectin-3 as well as its tyrosine phosphorylation triggered by active c-Abl.
Although our results demonstrate that tyrosine 107 is the main target of c-Abl kinase, however, we cannot exclude the possibility that in other environments the main phosphorylation site can be different or all three tyrosine residues can be equally phosphorylated. Since the in vitro studies indicate a clear interaction between c-Abl and galectin-3, we analyzed this interaction in vivo utilizing immunoprecipitation techniques. Immunoprecipitated galectin-3 pulled-down endogenous c-Abl together with other proteins even in presence of lactose indicating that binding of galectin-3 to c-Abl is independent of carbohydrate binding properties of galectin-3. This finding strengthens the hypothesis of Menon and Hughes that c-Abl and galectin-3 interaction involve SH3 domain of c-Abl.
Next, in vivo experiments were performed in breast cancer cell cells SK-Br-3; that do not express galectin-3 and c-Abl, the results show that transfected c-Abl is responsible for a significant part of tyrosine phosphorylation of galectin-3 in SK-Br-3 cells (Fig. 2 II). However this finding does not exclude the possibility that in other cells galectin-3 can be targeted by various tyrosine kinases.
Next we co-transfected wild type or Y107F galectin-3 with constitutively active c-Abl PP in SK-Br-3 cells and studied the effect of the c-Abl inhibitor STI571 in a dose dependent manner. Although the total level of tyrosine phosphorylation was lower in case of mutated galectin-3, kinetic of phosphorylation was similar pointed out that this mutation does not change the affinity of the two proteins.
We also measured the time course of STI571 inhibition for c-Abl. Wild type and mutant galectin-3 were immunoprecipitated after incubation with STI571 for various amounts of time. The phosphorylation of galectin-3 drop significantly upon STI571 addition, with maximum inhibition being achieved in approximately 6 hours (data not shown). These experiments show that the majority of c-Abl inhibition occurs within first hour and confirm our previous data that Y107F mutation does not change the affinity between c-Abl and galectin-3.
To study the effect of galectin-3 tyrosine phosphorylation cell morphology we created SK-Br-3 cell clones stably expressing GFP protein only, GFP-Gal-3 and CFP-Gal-3 Y107F. Although, both wild type and Y107F mutant protein clones expressed galectin-3 at equal levels, SK-Br-3 cells expressing wild type galectin-3 were much smaller and rounded compared to cells expressing GFP protein only and mutated galectin-3. As the changes in cell shape are associated with cytoskeleton reorganization and focal adhesion, in addition to a plethora of other biological responses and altered by specific external stimuli, we presume that the observed differences in cell shape could be either due to interaction of phosphorylated galectin-3 with cytoskeleton proteins directly or interactions with receptor proteins and thus vary the signaling output. As a result, the cells harboring wild type protein are smaller in size and more motile compared to the ones carrying mutated protein. Trans-membrane crosstalk between the galectin-3 lattice and caveaolin-1 phosphorylated by c-Abl on tyrosine 14 promotes focal adhesion (FA) turnover by stabilizing FA kinase within FAs defining interdependent roles for galectin-3 and pY14Cav1 in tumor cell migration [40, 41]. Our experiments did not reveal any significant difference in subcellular localization of galectin-3 between wild type and Y107F mutant, therefore we presume that the differences in cell morphology could be due to altered interactions with the binding partners in the two variants.
Recent data demonstrate that galectin-3 is a centrosome-associated protein that is required for epithelial morphogenesis [42]. One of the functions of centrosome is to control key aspects of cell morphology necessary for normal development. We can suggest that the galectin-3 and centrosome interaction dependent from tyrosine phosphorylation and can affect cell morphology through this mechanism. Further investigation is needed to better understand the functional significance of novel tyrosine phosphorylated sites of galectin-3.
Galectin-3 regulates intrinsic cell motility by regulating the activity of transcriptional factors and its over-expression results in enhanced adhesion to extracellular matrix components, cell motility, and in vitro invasiveness [43, 44]. Our results demonstrate that cells expressing galectin-3 Y107F variant showed reduced migration in wound healing assay (Figure 5). This result confirms the role of galectin-3 tyrosine phosphorylation in cell motility. Since c-Abl kinase is either not expressed in SK-Br-3 cells or its expression level is very low, we assumed that the observed effects are the result of galectin-3 phosphorylation by other as yet unknown kinase/s.
It will be of interest to determine the effects of phosphorylation of tyrosine 107 by c-Abl kinase on other phosphorylation events of galectin-3 and vice versa, to explore the potential regulatory role of each phosphorylation event on the other. In summary, this novel finding shows that c-Abl is responsible for tyrosine phosphorylation of galectin-3 at residues Tyr 79, 107 and 118 and Tyr 107 is a major phosphorylation site.
Off note: when this work was in progress Li et al published the paper where confirmed by indirect methods phosphorylations of two sites from three sites [45].
Acknowledgements
This work was supported by NIH R37CA46120 (A. Raz). The authors would like to thank Victor Hogan for editing the manuscript.
References
- [1].Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- [2].Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- [3].Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer. Int. J. Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- [4].Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim. Biophys. Acta. 2006;1760:616–635. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- [5].Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J. Biol. Chem. 1993;268:26712–26718. [PubMed] [Google Scholar]
- [6].Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J. Biol. Chem. 2002;277:6852–6857. doi: 10.1074/jbc.M107668200. [DOI] [PubMed] [Google Scholar]
- [7].Takenaka Y, Fukumori T, Yoshii T, Oka N, Inohara H, Kim HR, Bresalier RS, Raz A. Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol. Cell. Biol. 2004;24:4395–4406. doi: 10.1128/MCB.24.10.4395-4406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mazurek N, Conklin J, Byrd JC, Raz A, Bresalier RS. Phosphorylation of the beta-galactoside-binding protein galectin-3 modulates binding to its ligands. J. Biol. Chem. 2000;275:36311–36315. doi: 10.1074/jbc.M003831200. [DOI] [PubMed] [Google Scholar]
- [9].Mazurek N, Sun YJ, Price JE, Ramdas L, Schober W, Nangia-Makker P, Byrd JC, Raz A, Bresalier RS. Phosphorylation of galectin-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Res. 2005;65:10767–10775. doi: 10.1158/0008-5472.CAN-04-3333. [DOI] [PubMed] [Google Scholar]
- [10].Gong HC, Honjo Y, Nangia-Makker P, Hogan V, Mazurak N, Bresalier RS, Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- [11].Herrmann J, Turck CW, Atchison RE, Huflejt ME, Poulter L, Gitt MA, Burlingame AL, Barondes SH, Leffler H. Primary structure of the soluble lactose binding lectin L-29 from rat and dog and interaction of its non-collagenous proline-, glycine-, tyrosine-rich sequence with bacterial and tissue collagenase. J. Biol. Chem. 1993;268:26704–26711. [PubMed] [Google Scholar]
- [12].Nakahara S, Hogan V, Inohara H, Raz A. Importin-mediated nuclear translocation of galectin-3. J. Biol. Chem. 2006;281:39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- [13].Nakahara S, Oka N, Wang Y, Hogan V, Inohara H, Raz A. Characterization of the nuclear import pathways of galectin-3. Cancer Res. 2006;66:9995–10006. doi: 10.1158/0008-5472.CAN-06-1772. [DOI] [PubMed] [Google Scholar]
- [14].Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- [15].Moutsatsos IK, Wade M, Schindler M, Wang JL. Endogenous lectins from cultured cells: nuclear localization of carbohydrate-binding protein 35 in proliferating 3T3 fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1987;84:6452–6456. doi: 10.1073/pnas.84.18.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, Kikuchi A, Kuwano H, Raz A. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–3537. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- [17].Yamazaki K, Kawai A, Kawaguchi M, Hibino Y, Li F, Sasahara M, Tsukada K, Hiraga K. Simultaneous induction of galectin-3 phosphorylated on tyrosine residue, p21(WAF1/Cip1/Sdi1), and the proliferating cell nuclear antigen at a distinctive period of repair of hepatocytes injured by CCl4. Biochem. Biophys. Res. Com. 2001;280:1077–1084. doi: 10.1006/bbrc.2000.4193. [DOI] [PubMed] [Google Scholar]
- [18].Superti-Furga G, Courtneidge SA. Structure-function relationships in Src family and related protein tyrosine kinases. Bioessays. 1995;17:321–330. doi: 10.1002/bies.950170408. [DOI] [PubMed] [Google Scholar]
- [19].Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- [20].Van Etten RA. Cycling, stressed-out and nervous: cellular functions of c-Abl. Trends Cell Biol. 1999;9:179–186. doi: 10.1016/s0962-8924(99)01549-4. [DOI] [PubMed] [Google Scholar]
- [21].Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods in Enzymology. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- [22].Luo KX, Hurley TR, Sefton BM. Cyanogen bromide cleavage and proteolytic peptide mapping of proteins immobilized to membranes. Methods in Enzymology. 1991;201:149–152. doi: 10.1016/0076-6879(91)01014-s. [DOI] [PubMed] [Google Scholar]
- [23].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- [24].Nangia-Makker P, Raz T, Tait L, Hogan V, Fridman R, Raz A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007;67:11760–11768. doi: 10.1158/0008-5472.CAN-07-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rao AS, Kremenevskaja N, von Wasielewski R, Jakubcakova V, Kant S, Resch J, Brabant G. Wnt/beta-catenin signaling mediates antineoplastic effects of imatinib mesylate (gleevec) in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. 2006;91:159–168. doi: 10.1210/jc.2005-1381. [DOI] [PubMed] [Google Scholar]
- [26].Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li SY, Davidson PJ, Lin NY, Patterson RJ, Wang JL, Arnoys EJ. Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology. 2006;16:612–622. doi: 10.1093/glycob/cwj089. [DOI] [PubMed] [Google Scholar]
- [28].Davidson PJ, Li SY, Lohse AG, Vandergaast R, Verde E, Pearson A, Patterson RJ, Wang JL, Arnoys EJ. Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import. Glycobiology. 2006;16:602–611. doi: 10.1093/glycob/cwj088. [DOI] [PubMed] [Google Scholar]
- [29].Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12:329–337. doi: 10.1093/glycob/12.5.329. [DOI] [PubMed] [Google Scholar]
- [30].Lannoo N, Van Damme EJ. Nucleocytoplasmic plant lectins. Biochim. Biophys. Acta. 2009 doi: 10.1016/j.bbagen.2009.07.021. [DOI] [PubMed] [Google Scholar]
- [31].Kim HR, Lin HM, Biliran H, Raz A. Cell cycle arrest and inhibition of anoikis by galectin-3 in human breast epithelial cells. Cancer Res. 1999;59:4148–4154. [PubMed] [Google Scholar]
- [32].Lin HM, Pestell RG, Raz A, Kim HR. Galectin-3 enhances cyclin D(1) promoter activity through SP1 and a cAMP-responsive element in human breast epithelial cells. Oncogene. 2002;21:8001–8010. doi: 10.1038/sj.onc.1205820. [DOI] [PubMed] [Google Scholar]
- [33].Shimura T, Takenaka Y, Tsutsumi S, Hogan V, Kikuchi A, Raz A. Galectin-3, a novel binding partner of beta-catenin. Cancer Res. 2004;64:6363–6367. doi: 10.1158/0008-5472.CAN-04-1816. [DOI] [PubMed] [Google Scholar]
- [34].Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin. Cancer Res. 2001;7:661–668. [PubMed] [Google Scholar]
- [35].Fukumori T, Oka N, Takenaka Y, Nangia-Makker P, Elsamman E, Kasai T, Shono M, Kanayama HO, Ellerhorst J, Lotan R, Raz A. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66:3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- [36].Fukumori T, Takenaka Y, Oka N, Yoshii T, Hogan V, Inohara H, Kanayama HO, Kim HR, Raz A. Endogenous galectin-3 determines the routing of CD95 apoptotic signaling pathways. Cancer Res. 2004;64:3376–3379. doi: 10.1158/0008-5472.CAN-04-0336. [DOI] [PubMed] [Google Scholar]
- [37].Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
- [38].Menon RP, Hughes RC. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 1999;264:569–576. doi: 10.1046/j.1432-1327.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- [39].Shaul Y. c-Abl: activation and nuclear targets. Cell Death. Differ. 2000;7:10–16. doi: 10.1038/sj.cdd.4400626. [DOI] [PubMed] [Google Scholar]
- [40].Sanguinetti AR, Mastick CC. c-Abl is required for oxidative stress-induced phosphorylation of caveolin-1 on tyrosine 14. Cell Signal. 2003;15:289–298. doi: 10.1016/s0898-6568(02)00090-6. [DOI] [PubMed] [Google Scholar]
- [41].Goetz JG, Joshi B, Lajoie P, Strugnell SS, Scudamore T, Kojic LD, Nabi IR. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J. Cell Biol. 2008;180:1261–1275. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Koch A, Poirier F, Jacob R, Delacour D. Galectin-3, a Novel Centrosome-associated Protein, Required for Epithelial Morphogenesis. Mol. Biol. Cell. 2009 doi: 10.1091/mbc.E09-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, Chun KH. Galectin-3 increases gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–1045. e1031–1032. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- [44].O’Driscoll L, Linehan R, Liang YH, Joyce H, Oglesby I, Clynes M. Galectin-3 expression alters adhesion, motility and invasion in a lung cell line (DLKP), in vitro. Anticancer Res. 2002;22:3117–3125. [PubMed] [Google Scholar]
- [45].Li X, Ma Q, Wang J, Liu X, Yang Y, Zhao H, Wang Y, Jin Y, Zeng J, Li J, Song L, Li P, Qian X, Cao C. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death. Differ. 2010 doi: 10.1038/cdd.2010.8. [DOI] [PubMed] [Google Scholar]