Abstract
Herein, we report that N-alkyl-octahydroisoquinolin-1-one-8-carboxamides are a novel class of readily synthesized, selective κ-opioid receptor (KOR) ligands. A striking feature of this class of compounds is the absence of any basic nitrogen atoms. Many of these compounds have demonstrated exclusive affinity for the KOR over not only the δ-opioid receptor and the μ-opioid receptor but also 38 other G protein-coupled receptor targets. The general binding affinity of this class of compounds for the KOR combined with a streamlined route for analogue synthesis provide strong motivation for pursuing this interesting new scaffold as a basis toward new probes targeting the KOR.
Keywords: κ-Opioid receptor, isoquinolones
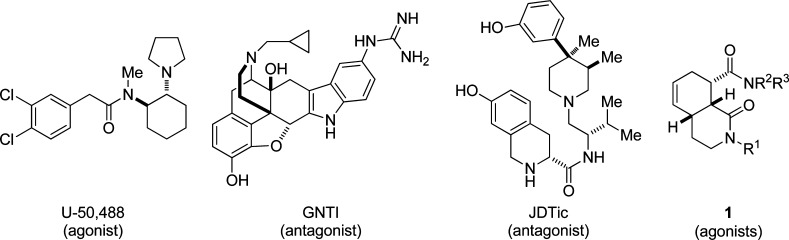
The κ-οpioid receptor (KOR) plays a significant role in a broad range of physiological functions1−3 that include, inter alia, addiction,4,5 depression,6−8 and pain relief.9 Additionally, the naturally occurring hallucinogen salvinorin A is a potent and selective KOR agonist implicating the KOR in diseases of human perception.10 Accordingly, there is great interest in discovering agents that are able to positively or negatively modulate KOR function. In addition to a robust and still-evolving collection of peptide-derived and natural product ligands that interact with the KOR, synthetic small molecules are of particular interest as potential therapeutic compounds.11−13 Three notable recent examples of the latter are the aryl acetamide class of KOR agonists, exemplified by U-50,488,14 first introduced by VonVoigtlander and Szmuszkovicz, the guandine derivative GNTI15 developed by Portoghese and co-workers, and the tetrahydroisoquinoline JDTic16,17 developed by Carroll and co-workers (Figure 1). Herein, we report that comparatively simple and synthetically accessible octahydroisoquinolone carboxamides of the general structure 1 represent a new class of selective KOR ligands. This new chemotype is distinct from most known small molecule KOR ligands in that the nitrogen atoms present are neutral by their involvement in amide bond resonance. Eight of the compounds reported here bind to the KOR at concentrations <1 μM with no measurable affinity for any other tested neurotransmitter receptor. Functional studies reveal individual compounds of this chemotype to be full agonists of varying efficacy.
Figure 1.
Examples of known synthetic KOR ligands and the general isoquinolone amide structure.
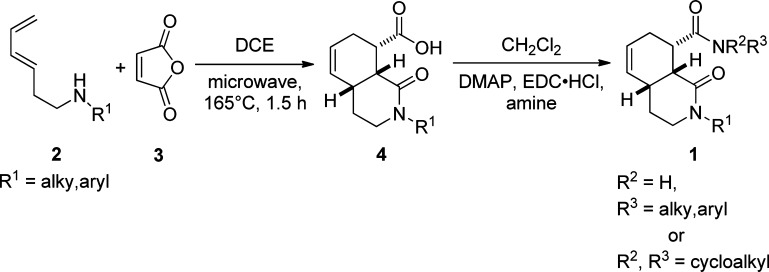
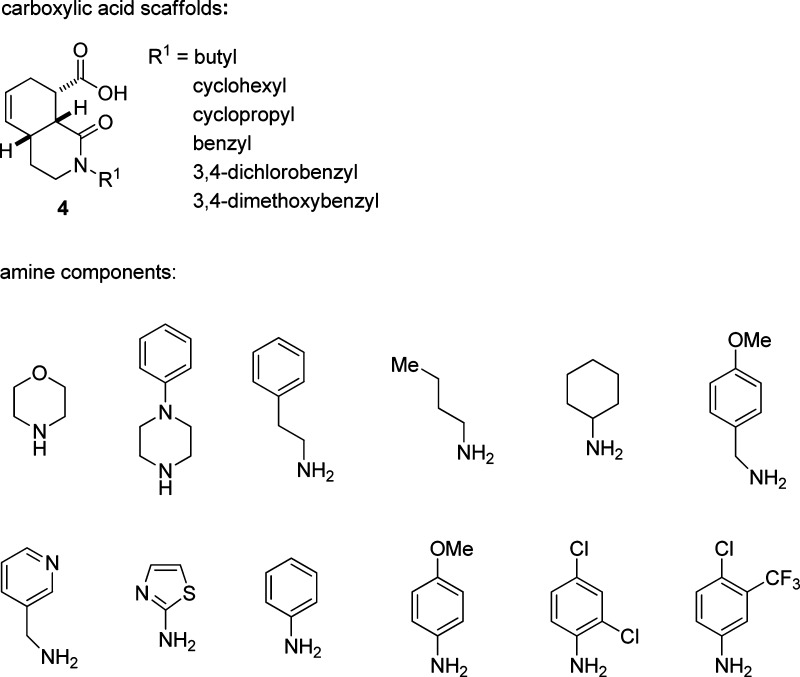
Recently, we reported an efficient synthesis of octahydroisoquinolone carboxylic acids utilizing a tandem Diels−Alder/acylation sequence (Scheme 1).18 Subsequent elaboration of the carboxylic acids via carbodiimide coupling with a selection of amines afforded an initial set of 72 octahydroisoquinolone carboxamides. The requisite amine-containing dienes 2 are readily obtained18,19 bearing diverse R1 groups. A tandem Diels−Alder/acylation reaction sequence between these dienes and maleic anhydride 3 then affords the carboxylic acid scaffolds 4 in good yields (68−80%). The exclusive cis, cis relative configuration of the bicyclic framework and pendant carboxylic acid group in the product 4 is consistent with either a Diels−Alder reaction followed by an intramolecular acylation or the inverse sequence.20 The acid scaffolds 4 were further diversified by carbodiimide-mediated coupling with a selection of 12 commercially available amines to afford the octahydroisoquinolone carboxamides 1. The nature of the carboxylic acid and amine components utilized in the construction of this initial library of octahydroisoquinolone carboxamides is detailed in Figure 2. This sequence provides an efficient and high-yielding route to a potentially vast collection of octahydroisoquinolone carboxamides, as demonstrated by the synthesis of this initial 72 member compound set.
Scheme 1. Synthesis of Octahydroisoquinolone Carboxamides.
Figure 2.
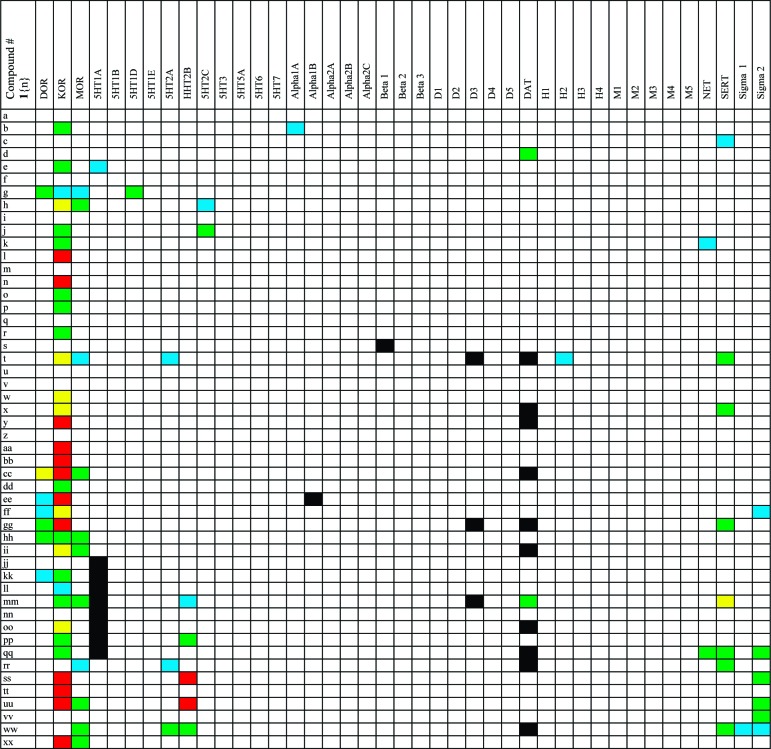
Components for the synthesis of the octahydroisoquinolone amide products 1.
Our interest in the development of methods to enable the synthesis of compound collections is partially motivated by a curiosity in the biological profile of the final products of these efforts. To this end, 50 library compounds were selected to represent the diverse range of functional groups in the collection and subsequently screened against 41 individual G protein-coupled receptor (GPCR) assays using the resources of the NIMH Psychoactive Drug Screening Program. Compounds were first screened at each GPCR target at a constant concentration (10 μM) to identify active compounds.21 Active compounds from initial binding screens were selected for Ki determinations using radioligand binding assays. A summary of the full results of the secondary binding screen is depicted in heatmap format in Figure 3. Most striking is a general selectivity trend of the compounds for the KOR over not only the δ-opioid receptor (DOR) and the μ-opioid receptor (MOR) but also against the other GPCR targets screened. Several of the compounds screened were found to be both highly selective for the KOR and remarkably potent, most notably compounds 1l, 1n, 1y, 1aa, 1bb, and 1tt. Furthermore, the majority of the compounds screened (35 out of 50) possesses experimentally significant KOR binding (Ki < 10 μM), a remarkable hit ratio from a set of compounds not synthesized with the target assay in mind.
Figure 3.
Activity of the isoquinonlone carboxamide compounds in secondary binding assays. Key: white, Ki > 10 μM/primary screen missed; blue, Ki = 5−10 μM; green, Ki = 1−5 μM; yellow, Ki = 0.5−1 μM; red, Ki < 0.5 μM; and black, no data available. For the complete binding data (Ki values) on these compounds, see the Supporting Information.
Isolated members of this compound set display binding affinity for other GPCR targets. For example, compounds 1ss and 1uu show potent binding affinity for the 5HT2B receptor in addition to the KOR, the activation of which has been linked to pulmonary hypertension.22 Compound 1mm displayed selective, submicromolar affinity for the serotonin transporter, and compound 1g was marginally selective for the DOR over the KOR, providing promising entry points for future optimization studies. At this time, however, we were most interested in the KOR binding activity that appears to be generally characteristic for this structural class of compounds.
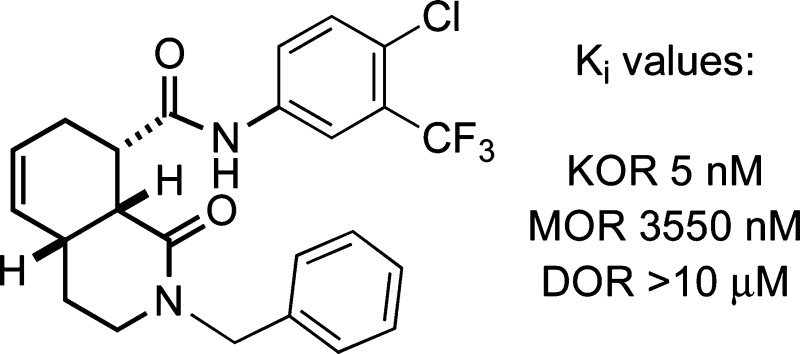
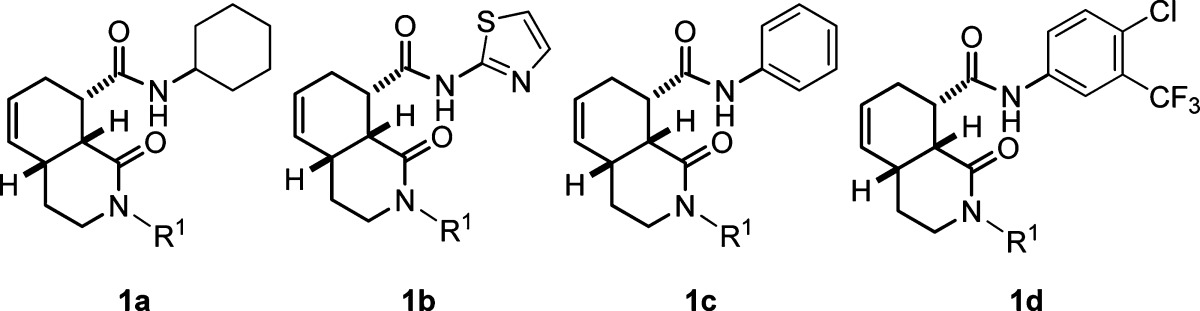
The substituents on the 12 most potent compounds and the numerical secondary binding data (Ki values) for the DOR, KOR, and MOR receptor assays are shown in Table 1. The data confirm high selectivity for the KOR over the DOR and to a lesser extent the MOR for most of these examples, except for one case where R2 = phenyl (entry 6). Compounds where R2 = 4-chloro-3-trifluoromethylphenyl were shown to be >10-fold selective for the KOR over the MOR/DOR and to have the highest potency among the four sets of amide derivatives examined (entries 9−12). While compound 1xx containing a benzyl group in the R1 position was highly potent, not all compounds containing benzyl at R1 had similarly high affinity (cf. entries 1, 3, 6, and 12 in Table 1).
Table 1. Selected Binding Data for Four Sets of Potent Isoquinolone Carboxamide Compounds.
|
Ki (μM) |
||||||
|---|---|---|---|---|---|---|
| entry | compound | scaffold | R1 | DOR | KOR | MOR |
| 1 | 1l | 1a | benzyl | >10 | 0.30 | >10 |
| 2 | 1n | 1a | cylcohexyl | >10 | 0.17 | >10 |
| 3 | 1y | 1b | benzyl | >10 | 0.20 | >10 |
| 4 | 1aa | 1b | cylcohexyl | >10 | 0.16 | >10 |
| 5 | 1bb | 1b | n-butyl | >10 | 0.36 | >10 |
| 6 | 1cc | 1c | benzyl | 0.85 | 0.15 | 2.57 |
| 7 | 1ee | 1c | cylcohexyl | >10 | 0.07 | 8.94 |
| 8 | 1gg | 1c | 3,4-dichloro-benzyl | 1.11 | 0.26 | 2.70 |
| 9 | 1ss | 1d | cylcopropyl | >10 | 0.49 | >10 |
| 10 | 1tt | 1d | cylcohexyl | >10 | 0.11 | >10 |
| 11 | 1uu | 1d | n-butyl | >10 | 0.19 | 2.22 |
| 12 | 1xx | 1d | benzyl | >10 | 0.005 | 3.55 |
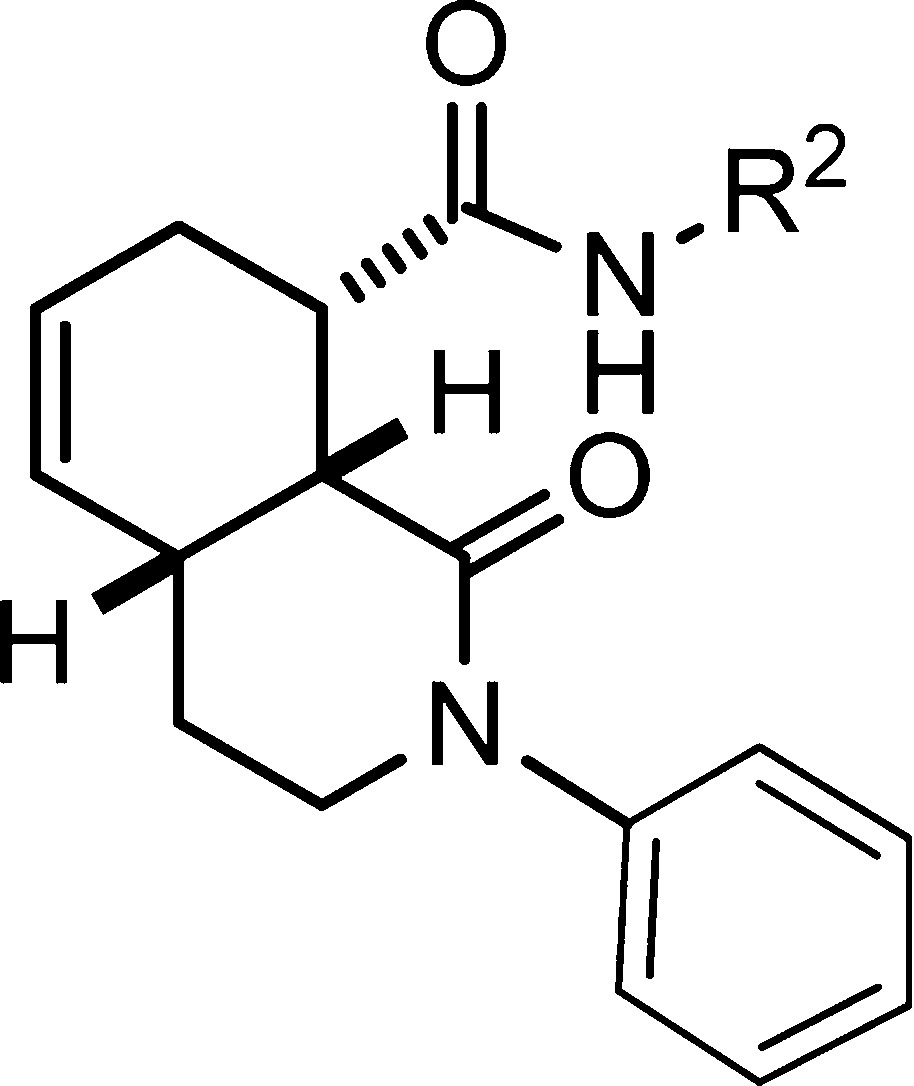
To further investigate the effect of the substituents on potency, four additional compounds were synthesized according to the protocol in Scheme 1 and screened for DOR, KOR, and MOR binding in the primary and secondary assays described above. These compounds, while not more potent than compound 1{50} add a further three examples of selective and potent KOR ligands in this structural class of compounds (Table 2).
Table 2. Additional Isoquinolone Carboxamide Analogues and Opioid Receptor Binding Profiles.
|
Ki (μM) |
||||
|---|---|---|---|---|
| compound | R2 | DOR | KOR | MOR |
| 1yy | 4-chloro-3- trifluoromethylphenyl | >10 | 0.10 | >10 |
| 1zz | 4-chloro-3-methylphenyl | >10 | 0.03 | >10 |
| 1aaa | 2,6-difluorophenyl | >10 | 5.00 | >10 |
| 1bbb | 2,4-dichlorophenyl | >10 | 0.29 | >10 |
Compounds 1xx, 1yy, and 1zz were further evaluated in KOR functional binding assays to determine whether they behave as agonists or antagonists (Table 3). Thus, the concentration-dependent (ranging from 0.01 to 10000 nM) inhibition of isoproterenol-stimulated cAMP accumulation in hKOR-expressing HEK293T cells was measured for both the test compounds and U-69,593 (a known KOR full agonist). The three test compounds 1xx, 1yy, and 1zz were all found to be full agonists (Emax = 100%) as compared to U-69,593. The EC50 for compound 1zz is over 2 orders of magnitude greater than the EC50 for the similar analogue 1yy; thus, given the two analogues' similar binding affinities, the functional data suggest that the efficacy of this new chemotype can be modulated by subtle structural modifications such as exchanging a triflouoromethyl substituent for a methyl group.
Table 3. KOR cAMP Reporter Assay Secondary Functional Binding Results.
| compound | KOR agonist EC50 values (μM) |
|---|---|
| 1xx | 0.063 |
| 1yy | 0.073 |
| 1zz | >10 |
| U-69,593 (reference) | 0.002 |
In summary, we have discovered an attractive new scaffold for KOR modulator discovery, with a number of representative analogues showing high potency and selectivity toward the KOR. The synthetic methodology required to procure these compounds is robust and provides an efficient and straightforward route to analogues. The synthesis, screening, and evaluation of additional analogues are ongoing and will be reported in due course.
Acknowledgments
We are grateful to Benjamin Neuenswander for HR-MS and compound purification.
Abbreviations
KOR, κ-opioid receptor; DOR, δ-opioid receptor; MOR, μ-opioid receptor; GPCR, G protein-coupled receptor.
Supporting Information Available
Experimental details and characterization data for all new compounds, structures, and Ki values for each compound in all active assays shown in Figure 3, purity assessment for all compounds, and assay protocols. This material is available free of charge via the Internet at http://pubs.acs.org.
We thank the National Institute of General Medical Sciences (GM-49093 and PO50-GM069663), the National Institute of Mental Health's Psychoactive Drug Screening Program [Contract #HHSN-271-2008-000025-C (NIMH-PDSP)], and RO1DA017204 for financial support.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- McCurdy C. R.; Prisinzano T. E.. Opioid receptor ligands. In Burger's Medicinal Chemistry, Drug Discovery and Development, 7th ed.; Abraham D. J., Rotella D. P., Eds.; John Wiley & Sons: New York, NY, 2010. [Google Scholar]
- Aldrich J. V.; McLaughlin J. P. Peptide kappa opioid receptor ligands: Potential for drug development. AAPS J. 2009, 11, 312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf M. D.; Coop A. Kappa opioid antagonists: Past successes and future prospects. AAPS J. 2005, 7, E704–E722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano T. E.; Tidgewell K.; Harding W. W. k Opioids as potential treatments for stimulant dependance. AAPS J. 2005, 7, E592–E599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick S. D.; Maisonneuve I. M.; Raucci J.; Archer S. Kappa-opioid inhibition of morphine and cocaine self-administration. Brain Res. 1995, 681, 147–152. [DOI] [PubMed] [Google Scholar]
- Reindl J. D.; Rowan K.; Carey A. N.; Peng X.; Neumeyer J. L.; McLaughlin J. P. Antidepressant-like effects of the novel kappa opioid antagonist MCL-144B in the forced-swim test. Pharamcology 2008, 81, 229–235. [DOI] [PubMed] [Google Scholar]
- Knoll A. T.; Meloni E. G.; Thomas J. B.; Carroll F. I.; Carlezon W. A. Jr. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J. Pharmacol. Exp. Ther. 2007, 323, 838–845. [DOI] [PubMed] [Google Scholar]
- Mague S. D.; Pliakas A. M.; Todtenkopf M. S.; Thomasiewicz H. C.; Zhang Y.; Stevens W. C. J.; Jones R. M.; Portoghese P. S.; Carlezon W. A. Jr. Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 2003, 305, 323–330. [DOI] [PubMed] [Google Scholar]
- Millan M. J. Kappa-opioid receptor-mediated antinociception in the rat. 1. Comparative actions of mu-opioids and kappa-opioids against noxious thermal, pressure and electrical stimuli. J. Pharmacol. Exp. Ther. 1989, 251, 334–341. [PubMed] [Google Scholar]
- Roth B. L.; Baner K.; Westkaemper R.; Siebert D.; Rice K. C.; Steinberg S.; Ernsberger P.; Rothman R. B. Salvinorin A: A potent naturally occurring nonnitrogenous kappa opioid selective sgonist. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor A.; Matosiuk D. Non-peptide opioid receptor ligands—Recent advances. Part I. Agonists. Curr. Med. Chem. 2002, 9, 1567–1589. [DOI] [PubMed] [Google Scholar]
- Kaczor A.; Matosiuk D. Non-peptide opioid receptor ligands—Recent advances. Part II. Antagonists. Curr. Med. Chem. 2002, 9, 1591–1603. [DOI] [PubMed] [Google Scholar]
- For a recently reported KOR agonist, see Kracht D.; Rack E.; Schepmann D.; Fröhlich R.; Wünsch B. Stereoselective synthesis and structure−affinity relationships of bicyclic receptor agonists. Org. Biomol. Chem. 2010, 8, 212–225. [DOI] [PubMed] [Google Scholar]
- VonVoigtlander P. F.; Szmuszkovicz J. Benzeneacetamide amines: Structurally novel non-μ opioids. J. Med. Chem. 1982, 25, 1125–1126. [DOI] [PubMed] [Google Scholar]
- Jones R. M.; Portoghese P. S. 5′-Guanidinonaltrindole, a highly selective and potent κ-opioid receptor antagonist. Eur. J. Pharmacol. 2000, 396, 49–52. [DOI] [PubMed] [Google Scholar]
- Cueva J. P.; Cai T. B.; Mascarella S. W.; Thomas J. B.; Navarro H. A.; Carroll F. I. Synthesis and in vitro opioid receptor functional antagonism of methyl-substituted analogues of (3R)-7-hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). J. Med. Chem. 2009, 52, 7463–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T. B.; Zou Z.; Thomas J. B.; Brieaddy L.; Navarro H. A.; Carroll F. I. Synthesis and in vitro opioid receptor functional antagonism of analogues of the selective kappa receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). J. Med. Chem. 2008, 51, 1849–1860. [DOI] [PubMed] [Google Scholar]
- Frankowski K. J.; Hirt E. E.; Zeng Y.; Neuenswander B.; Fowler D.; Schoenen F.; Aubé J. Synthesis of N-alkyl-octahydroisoquinolin-1-one-8-carboxamide libraries using a tandem Diels−Alder/acylation sequence. J. Comb. Chem. 2007, 9, 1188–1192. [DOI] [PubMed] [Google Scholar]
- Plietker B.; Seng D.; Fröhlich R.; Metz P. High pressure intramolecular Diels−Alder reactions of vinylsulfonates and vinylsulfonamides. Tetrahedron 2000, 56, 873–879. [Google Scholar]
- For a more detailed discussion of these possible pathways, see ref (18).
- Data were measured as the mean % inhibition (N = 4 determinations) for compounds tested at receptor subtypes. Significant inhibition is considered >50%. For full experimental details, see the Supporting Information.
- Launay J.-M.; Hervé P.; Peoc'h K.; Tournois C; Callebert J; Neigil C. G.; Etienne N.; Drouet L.; Humbert M.; Simonneau G.; Maroteaux. L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nature Med. 2002, 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.