Abstract
The complexities that underlie the cognitive impairment and neurodegeneration characteristic of Alzheimer's disease have yet to be completely understood, although many factors in disease pathogenesis have been identified. Particularly important in disease development seem to be mitochondrial disturbances. As pivotal role players in cellular metabolism, mitochondria are pertinent to cell survival and thus any deviation from their operation is certainly fatal. In this review, we describe how the dynamic balance of mitochondrial fission and fusion in particular is a necessary aspect of cell proliferation and that, as the cell ages, such balance is inevitably compromised to yield a destructive environment in which the cell cannot exist. Evidence for such disturbance is abundant in Alzheimer disease. That is, the dynamic balance of fission and fusion in AD is greatly shifted toward fission, and, as a result, affected neurons contain abnormal mitochondria that are unable to meet the metabolic demands of the cell. Moreover, mitochondrial distribution in AD cells is perinuclear, with few metabolic organelles in the distal processes where they are normally distributed in healthey cells and where they are needed for exocytosis, ion channel pumps, and synaptic function, among other things. AD neurons are thus characterized by increases in reactive oxidative species and decreases in metabolic capability, and notably, these changes are evident very early in AD progression. We therefore believe that oxidative stress and altered mitochondrial dynamics contribute to the precipitation of AD pathology and thus cognitive decline. These implications provide a window for therapeutic intervention (i.e., mitochondrial protection) that has the potential to significantly deter AD progression if adequately developed. Current treatment strategies under investigation are herein described.
The uncertainty surrounding the causes of the vastly debilitating neurodegenerative disorder Alzheimer's disease (AD) has fostered much debate. That it is the leading cause of senile dementia in the US, affecting 15% of people aged >65 years and almost 50% of those aged >85 years,[1] generates further controversy and warrants increased investigation, as the aging population demands an effective therapeutic measure to facilitate disease prevention/control. Fortunately, studies have begun to connect the pathological ‘dots’ that characterize the disease, such that the immeasurably complex interactions that yield AD are now becoming clear.
The disorder is characterized by progressive neuronal loss and an accompanying cognitive deterioration that eventually proves fatal. Extracellular aggregates of amyloid-β (Aβ)-containing senile plaques have long been implicated in disease onset and progression, as mutations in the amyloid-β protein precursor (AβPP) are known to initiate early onset, familial AD,[2,3] and concentrations of anti-Aβ antibody have been shown to be an effective marker for the disease and its progression.[4] Similarly, the microtubule associated protein tau has been confirmed as a role player in AD, as its hyperphosphorylated, aggregated fibrils occupy neuronal space in vulnerable regions of AD brains (i.e., hippocampus/cortices) in the form of neurofibrillary tangles (NFTs).[5,6] Notably, these hallmarks of AD, while once thought to be primary mediators of the sporadic form of the disease (responsible for 90–95% of all AD cases[7]), are now understood to be secondary role players in AD pathogenesis.[8] Although much is unclear about the origins of AD, evidence supports the role of mitochondrial dynamics as a potential suspect.
Indeed, malfunctions in mitochondria have been documented in AD brains.[9,10] Given that mitochondria are immensely pertinent to cellular proliferation as they are the metabolic and energy centres of the cell, and that abnormalities in mitochondrial dynamics widely precede many of the hallmark pathologies of AD,[9,10] it is not unreasonable to investigate them as progenitors of the disease. Moreover, the mitochondria provide a potential access point for therapeutic intervention that, if appropriately harnessed, could yield remarkable treatment strategies for patients and as yet unaffected individuals. As we shall see, although there are still some points that must be clarified, the prognosis for mitochondria as instigators of AD is strong, and thus an effective preventative measure in this regard will certainly be beneficial.
1. Mitochondrial Dynamics: Fission, Fusion and Function
Mitochondria are not static organelles, but are dynamic bodies that constantly divide and fuse within the cell as the environment demands.[11] They are composed of an inner membrane (almost entirely impermeable) and an outer membrane (permeable to ions and small molecules), such that establishment of an proton gradient during oxidative phosphorylation is possible. Maintenance of membrane integrity is vital to mitochondrial and cellular functioning: 95% of the cell's energy supply is generated in mitochondria via the citric acid (tricarboxylic acid [TCA]) cycle and oxidative phosphorylation.
As such, the number and morphology of the mitochondria in a cell are controlled by delicate balance of organelle fission and fusion mechanisms.[9,11] In particular, genetic inactivation of fission results in elongation of mitochondria, whereas inactivation of fusion yields fragmentation.[12,13] It is through this balance that the cell ultimately maintains mitochondrial integrity and homogeneity: fusion allows the exchange of lipid membranes and mitochondrial contents, such as mitochondrial DNA (mtDNA), and fission (coupled with fusion and autophagy) allows sequestration and elimination of irreversibly damaged mitochondria and mitochondrial content.[14-16] Both processes effectively lower the percentage of defective mitochondria in the cell and ensure stability in cellular proliferation; indeed, metabolism, energy production, calcium signaling, reactive oxidative species (ROS) production, apoptosis and senescence all depend on the balance of fission and fusion.[17-22] Conversely, dynamic distortion (i.e., excessive fragmentation/elongation) results in inefficiencies in cell functioning, if not cell death. Studies have specifically demonstrated a direct reduction in metabolism and loss of mtDNA after dynamic misbalance.[9,19,23-25]
In fission, at least two proteins are involved: a large guanosine triphosphatase (GTPase), dynamin-like protein 1 (DLP-1) and a small molecule Fis1.[26-28] DLP-1 is a member of the conserved dynamin large GTPase superfamily that controls membrane tabulation and fission; it is primarily a cytosolic protein that is recruited to punctuate spots on the mitochondrial surface.[28] DLP-1 is believed to oligomerize to form large ring-like complexes that, once bound to the mitochondrial surface, hydrolyze guanosine triphosphate (GTP) to constrict and twist tubules to initiate fission.[29] Fis1 then acts as a receptor to DLP-1, recruiting the complex-forming protein to the appropriate mitochondria. As such, Fis1 is evenly distributed along the mitochondrial surface.[30] Fission produces two daughter organelles with cristae and other inner structural remodeling, the mechanisms of which are not entirely clear.[26,27]
Fusion, on the other hand, is regulated by three large GTPases: mitofusin 1 (Mfn1), mitofusin 2 (Mfn2) and optic atrophy protein 1 (OPA-1).[26-28] Mfn1/2 are transmembrane proteins localized to the outer mitochondrial membrane that appear to play similar roles in fusion, although they function independently of one another and have different rates of GTP hydrolysis.[19,28,31] Mechanistically, the two proteins form homo- or hetero-oligomeric complexes (through interactions of their coiled-coil extracellular domains) and tether two mitochondria together for fusion of the outer membranes.[31,32] Inner membrane fusion, while not as completely understood, occurs primarily through the functioning of OPA-1, which faces the intermembrane space, in correspondence with Mfn1.[19,33]
Within the cellular environment, mitochondrial dynamics are manipulated by regulations involving the above listed proteins, among others. While much of this dynamic interplay is yet unknown, studies have revealed the interactions and regulations that utilize DLP-1 to instigate or prevent mitochondrial fission.[28] Specifically, post-translational modifications of the fission protein have been examined and include phosphorylation, SUMOylation (i.e. the covalent attachment of a small ubiquitin-like modifier [SUMO]), ubiquitylation and nitrosylation.[34-39] Phosphorylation of DLP-1 at two serine residues (Ser616 and Ser637) has been demonstrated, although only that of Ser616 has been confirmed as actually facilitating fission. That is, while phosphorylation of Ser616 by mitosis promoting factor has been shown to directly induce mitochondrial fission,[34,35,39] there is still debate over the effect of Ser637 phosphorylation: two groups indicate Ser637 phosphorylation by protein kinase A inhibits fission[34,35] (while its dephosphorylation by calcineurin promotes fission[40]) and another group indicates that Ser637 phosphorylation by calcium/calmodulin dependent protein kinase-1α induces DLP-1 translocation to mitochondria, thereby enhancing fission.[41] Further investigation is thus required to elaborate on the specificities of DLP-1 dynamics.
Similarly, SUMOylation of DLP-1 by SUMO-1 protects the protein from degradation, effectively enlarging the DLP-1 pool, and facilitates its translocation to the mitochondria from the cytosol.[28,36] S-nitrosylation of DLP-1 activates its GTPase activity and thus instigates fission.[42]
In addition to fission/fusion processes, mitochondrial activity depends on the physical location of the organelles within the cell. The distribution of mitochondria is orchestrated by the cytoskeleton and associated proteins such that regions with high metabolic requirements receive the highest concentrations of mitochondria.[9,43] Notably, mitochondrial distribution is affected by mitochondrial dynamics: both fission mutants with elongated mitochondria (i.e. DLP-1 mutants) and fusion mutants with short, rounded mitochondria (i.e. OPA-1 mutants) cause distribution changes within the cell.[44-46] Because a cell's functioning strongly relies on its ability to generate energy in the appropriate location at the appropriate time, the position of mitochondria within the cell greatly affect its performance, and thus the balance of fission and fusion is pivotal. Even more crucially, because neurons are particularly dependent on mitochondrial integrity as they have much greater energy demands than any other cell type (including the extremely energy taxing functioning of ion channels and pumps, synaptic transmission, axonal/dendritic transport of signal molecules and vesicles, etc.[28,47]), this balance is pertinent to brain functioning. Any perturbations in mitochondrial fission/fusion and function would therefore expose the neuron to disastrous consequences which, as we shall see, include the neurodegeneration typical of AD.
2. Mitochondrial Mechanistic Malfunction in Alzheimer's Disease (AD): Altered Metabolism Due to Dysfunctional Dynamics
Metabolic defects in AD are one of the best documented abnormalities in the disease.[10] In fact, neuropsychological testing and neuroimaging studies have demonstrated that such abnormalities precede evidence for functional impairment or brain atrophy attributed to traditional pathologies of neurodegeneration (i.e. senile plaques and tangles).[9,10] Damage to the components and structure of mitochondria are also well documented in AD,[48,49] and oxidative stress has recently been strongly implicated in disease pathogenesis.[8] As the process of oxidative phosphorylation inevitably yields ROS, damage to mitochondria are suspected to be the progenitors of oxidative stress, and therefore mitochondria themselves are thought to be integral to AD pathobiology.
Specific deficiencies in mitochondrial enzymes have been reported in AD. These include enzymes involved in the TCA cycle, such as ketoglutarate dehydrogenase complex and pyruvate dehydrogenase complex, as well as those involved in the electron transport chain of oxidative phosphorylation, such as cytochrome oxidase.[50-56] As these complexes orchestrate the oxidation/reduction reactions necessary for oxidative phosphorylation (and the generation of adenosine triphosphate (ATP)), any such deficiency will produce a malfunctioning metabolism. Sporadic mutations in mtDNA have also been reported in certain regions of AD at a much higher rate than in age-matched controls, and in some cases, mtDNA mutations are completely unique to AD cases.[9,57] Moreover, calcium homeostasis is altered in AD, and mitochondrial impairment is the proposed reason for its dysregulation.[58] Altogether, mounting evidence supports the role of mitochondrial disturbance in the pathogenesis of AD, and recent studies show that mitochondrial dynamics lie at the heart of these disturbances.
AD neurons are known to contain significantly lower percentages of normal mitochondria as well as relatively high percentages of mitochondria with broken cristae, when compared with age-matched controls.[9] Significant changes in mitochondrion size and number were also found in vulnerable AD neurons.[59] These specific trends provide a potential cause for the aforementioned mitochondrial disturbances and, notably, are associated with defects in fission/fusion dynamics and the corresponding proteins.[9]
In fibroblast cells, for example, altered dynamics was confirmed in AD in a series of studies by Wang et al.[9] While fibroblast cells are very different to the hippocampal neurons that are devastated in AD, these data nonetheless provide important insights into the specifics of dynamic alterations in the mitochondria of AD cells. Fibroblast mitochondria were significantly longer when compared with the short and rounded mitochondria of age-matched normal human fibroblasts, and were often joined together irregularly in a mitochondrial network.[60] Further investigation indicated that DLP-1 mutations were the likely cause of the shift in mitochondrial dynamics toward fusion: (i) DLP-1 levels were significantly decreased, while OPA-1 levels were unchanged; (ii) DLP-1 knockdown, as well as the expression of dominant negative DLP-1 in normal human fibroblasts, caused mitochondrial changes similar to those seen in sporadic AD (sAD) fibroblasts; and (iii) DLP-1 overexpression rescued abnormal morphology in sAD fibroblasts.[60] The distribution of mitochondria themselves was also altered in sAD fibroblasts when compared with controls: the organelles were mainly concentrated in the perinuclear area and were sparse in remote and peripheral regions of the cells. Notably, this phenomenon had little effect on the functioning of the fibroblast cell; however, in the vastly polarized environments characteristic of neurons, such alterations in mitochondria distribution would be detrimental.
Indeed, mitochondrial fission/fusion proteins have been demonstrated to exist at altered levels and in altered distributions in AD hippocampal pyramidal neurons compared with age-matched controls.[61] Specifically, AD neurons had reduced DLP-1, OPA-1 and Mfn1/2 protein levels and increased Fis1 levels. These proteins, furthermore, were aggregated in neuronal soma and not in the neuronal processes, indicating abnormal mitochondrial distribution. Interestingly, synaptic dysfunction, an early and robust correlate of AD-associated cognitive defects,[62] may be the direct result of this altered mitochondrial distribution. Whereas under normal conditions mitochondria are abundantly located at synaptic terminals[63] due to the intense energy demands of an active neuron (which is engaged in synaptic transmission but also requires calcium buffering), in Drosophila containing a mutant Milton protein (which is involved in mitochondria transport via binding to kinesin heavy chain), loss of mitochondria at axon terminals led to synaptic dysfunction in photoreceptor cells.[64] Similarly, loss of Miro protein (which is involved in mitochondria morphology regulation) resulted in mitochondria accumulation in neuronal cell bodies at neuromuscular junctions with subsequent synaptic dysfunction.[65,66] DLP-1 mutations in Drosophila also produced impaired calcium buffering and dysfunction in the mobilization of reserve pool vesicles, and this effect was primarily due to the elongation of mitochondria and their resulting failure to populate the distal axon.[67] Finally, the plasticity of spines and synapses was demonstrated to be perturbed in cells after an alteration of DLP-1 or OPA-1 that yielded a lack of dendritic mitochondria.[68]
Clearly, alterations in mitochondria dynamics and distribution are profound in AD neurons. While there is no certainty as to their origins, evidence implicates the gradual accumulation of oxidative damage over the course of decades in the generation of malfunctioned mitochondria, and increasing data identifies such abnormalities as the primary mediator in neurodegeneration.
3. Oxidative Stress in AD
The abundant evidence for mitochondrial malfunctions in AD, coupled with the immense impact of mitochondria on cell vitality and function, implicate the metabolic organelle as a primary mediator of the neurodegeneration in AD. While much has yet to be elucidated, the ‘age-induced mitochondrial cascade’ of neurodegenerative events seems a likely key operator in AD pathogenesis.
There is no doubt that oxidative stress is an instigating factor in AD onset and progression. Current research, in fact, suggests it to be the primary producer of pathology traditionally descriptive of AD, and much evidence corroborates this theory. Our two-hit hypothesis, for example, describes a system whereby a long-term, pathological condition, such as oxidative stress in late-onset AD, instills a degree of damage upon the affected cell such that it must enter a compensatory ‘steady state’ to maintain basic functions.[8] Indeed, oxidative markers generally precede all of those associated with typical hallmarks of AD, such as NFT formation and Aβ aggregation. Specifically, 8-hydroxyguanosine (8-OHG), a marker of oxidative damage, appears decades prior to Aβ senile plaques, and AβPP-mutant Tg2576 transgenic mice demonstrate oxidative damage preceding that due to Aβ aggregation.[69-76] This repeated oxidative stress eventually overwhelms the cell's antioxidant machinery, producing cumulative alterations in vital macromolecules: indeed, lipid peroxidation, nitration and nucleic acid oxidation are all prevalent in AD and AD models.[8]
As such, because the new ‘oxidative steady state’ represents the cell under repeated and consistent stress, vulnerabilities to damage produced by other aberrant mechanisms, such as mitotic re-entry and aggregation of non-soluble Aβ, become the progenitors of the cell's death (because it cannot fight these alterations in its weakened state).[8] In fact, oxidative stress itself seems to be the direct precursor of several AD pathologies. Evidence demonstrates that Aβ secretion, for example, initially occurs as an antioxidant response to elevated ROS. Aβ has been shown to have antioxidant activity and to protect lipoproteins from oxidation in cerebrospinal fluid and plasma (via metal sequestration),[77-80] and AD cases with the most extensive Aβ deposition show the lowest levels of 8-OHG while neurons lacking such AD pathology have significantly higher levels of 8-OHG.[81] Tau hyperphosphorylation and the formation of NFTs have similarly been demonstrated as a possible compensation for overwhelming concentrations of ROS.[82,83] Neurons with NFTs have significantly lower 8-OHG levels despite obvious oxidative damage (i.e., the presence of advanced glycation endproducts or lipid peroxidation),[81] and phosphorylation of tau has been demonstrated to antagonize apoptosis by stabilizing β-catenin.[84] Therefore, a chronic oxidative stress situation, as initiated by altered mitochondrial dynamics, may in fact elicit the hallmark pathologies of AD.[85]
Interestingly, while the hallmark pathologies of AD seem to originate in response to excessive cellular oxidative damage, their presence eventually inflicts burden on the associated cells and only intensifies the cascade of events that leads to neurodegeneration. Specifically, once secreted, Aβ becomes subject to oxidation itself, and its resulting dityrosine cross-linkages make the peptide insoluble and thus more likely to aggregate.[80,86] Aβ, in its aggregated form, then has a detrimental effect on the cell because it elicits further oxidative stress[80,81] and inflicts damage on mitochondrial functioning. The latter process has been demonstrated in M17 cells overexpressing mutant AβPP (these cells exhibited more than a 4-fold decrease in the rate of mitochondrial fusion[87]), and studies further indicate that Aβ overproduction induces mitochondria fragmentation, dysfunction, heightened ROS production and reduced ATP generation.[60] These detriments to the cell directly stem from oxidative damage, and as we discuss next, it is the mitochondria that are responsible for the accumulation of oxidative stress.
4. Mitochondrial Mediation of Oxidative Stress and Neurodegeneration: an Attractive Culprit
Mitochondria are the centres of energy production in the cell, and, over the course of aging, the metabolic machinery they possess inevitably produce ROS. As stated in section 2, key enzyme complexes in both the TCA cycle and the electron transport chain function as oxidizing/reducing agents, and dysfunction in one of these complexes results in aberrant electron transfer and the generation of oxidative free radicals. Ketoglutarate dehydrogenase, cytochrome oxidase and pyruvate dehydrogenase have all been shown to elicit ROS in vitro; in particular, in the electron transport chain, superoxide radical (O2−) and hydrogen peroxide (H2O2) are predominantly produced.[81] Furthermore, the sheer number of oxidation/reduction reactions that take place within a given neuron statistically guarantees the production of a large number of ROS on a daily basis: the average non-neuronal cell uses 1013 O2 molecules per day in metabolic processes, and approximately 1% of these molecules (i.e., 1011) become O2−.[81] As the brain utilizes 20% of the body's oxygen supply despite constituting only 2–3% of its mass,[81] the ROS generation in the brain is likely much higher than 1011 molecules per day. Although the cell contains antioxidant mechanisms, this astounding number of radicals eventually overwhelms these mechanisms, leading to a cascade of mtDNA mutation and oxidative damages characteristic of the ‘oxidative steady state’.
Notably, mtDNA damage and general mitochondrial malfunction takes years to accumulate; the integrity of these organelles is strictly maintained via the delicate balance of fission and fusion. As stated in section 1, fusion provides defective mitochondria with replenished supplies of mtDNA and mitochondrial proteins, and fission allows the sequestration and elimination of irreversibly damaged mitochondria and mitochondrial proteins.[28] However, despite these efforts, mutations in vital mitochondrial proteins eventually cross a threshold beyond which mitochondrial dysfunction propagates uncontrollably.[28,65] Once mutations in mitochondrial dynamics become manifest, the cell's mitochondrial safety net quickly dissipates and the cascade of neurodegeneration begins.
Research has shown that mutations in over 80% of mtDNA genes elicit abnormal mitochondria morphology, including fragmentation and elongation.[88-90] In addition, short exposure of mitochondria to ROS causes mitochondrial imbalances in fission and fusion as a result of mtDNA mutation.[91] Mfn1/2-null cells and OPA-1 deficient cells also have excessively fragmented mitochondria and demonstrate greatly reduced endogenous and uncoupled respiratory rates. The latter phenomena are due to attenuation of electron transport rates in complexes I, III and IV.[19,23] Similarly, Purkinje cells of Mfn2 knockout mice demonstrate aberrant electron transport activity,[16] and genetic inhibition of DLP-1 causes a reduced rate of ATP synthesis due to decreased complex IV activity and inefficient oxidative phosphorylation.[24,28] Fission/fusion imbalance as a result of mtDNA mutation can also disrupt calcium homeostasis,[92,93] and both excessive fragmentation and elongation of mitochondria lead to increases in ROS produced in the cell, with excessive deposition of iron.[22,25,94-96]
Once excessive fragmentation or elongation takes place within the cell (as a result of accumulating oxidative damages and corresponding mtDNA mutations), the sequestration mechanisms for defective mitochondria become incapable of controlling the accumulation of these damages. As the defective mitochondria directly produce neuronal insults, such as oxidative free radicals and reduced metabolism, a vicious cycle ensues in which ROS generation, oxidative steady state compensation and mitochondrial deterioration upregulate each other. It is this feedback cascade that ultimately proves fatal to the cell. Mitochondrial malfunctions thus seem to be the primary medium through which age produces neurodegeneration.
5. Potential Treatment Strategies
The profound influences of brain mitochondria in the pathogenesis of AD afford a new perspective on potential therapeutics for the disease. Indeed, recent research has focused on mitochondrial repair strategies and mitochondrial antioxidants to prevent and control the neurodegenerative cascade for which the metabolic organelles are responsible. Mitochondrial antioxidant therapy has been found to be most effective in producing pathological changes without any adverse effects.[97-99] In particular, the restoration and rescue of mitochondrial complexes can be achieved via selective mitochondrial antioxidant treatment.[100]
Studies of the brains of aged rats have specifically demonstrated the protective effects of such mitochondrial antioxidants. The antioxidants acetyl-L-carnitine (ALCAR) and R-α-lipoic acid (LA) reduced oxidative stress and mitochondrial abnormalities and restored cognitive function in rat parenchyma cells,[98,100-102] and such targeting of mitochondrial oxidative stress also improved the overall cognitive ability of aged rats and dogs.[102-106]
Furthermore, administration of ALCAR 0.5% and LA 0.2% greatly reduced mitochondrial damage in hippocampal neurons of aged rats.[98] Neuronal cell bodies showed fewer giant mitochondria (i.e. less fusion abnormalities) compared with age-matched controls, and treated mitochondria lacked ultrastructural abnormalities and appeared intact or with minimal damage. ALCAR/LA administration was followed by statistically significant decreases in the prevalence of damaged mitochondria (p < 0.001), increases in normal, intact mitochondria (p < 0.02) and significant improvements in the differences in damaged mitochondria between old and young rats. In addition, dietary supplementation of these antioxidants in young and old rats indicated that more significant treatment effects can be achieved with early supplementation.[98] Thus, there appear to be significant benefits associated with mitochondrial dysfunction treatment, and it is to be hoped that increased investigation will see efficient and reliable therapies become a clinical reality.
6. Conclusions
The effects of mitochondria and mitochondrial balances within the cell are immense. As accumulating evidence confirms, mitochondrial behaviour in the cell is dynamic and complicated, and maintaining the exact balance in their processing is absolutely necessary for full cell functioning. In our proposed ‘age-induced mitochondrial cascade’ (figure 1), this balance is eventually tipped by aggregation of oxidizing agents that are the natural result of cellular respiration. Although this rate-limiting step takes years, perhaps decades, to become harmful, it nonetheless seems to be an inevitable part of the aging process, and presents substantial concern for the aged community. Fortunately, though, antioxidant therapies are demonstrating significant benefits for damaged and potentially damaged mitochondria, and the results of studies of these agents indicate an overall improvement in cognitive ability and function. Although much investigation is necessary in the future, there is certainly a light at the end of the neurodegenerative tunnel that will hopefully relieve society of the burden of AD.
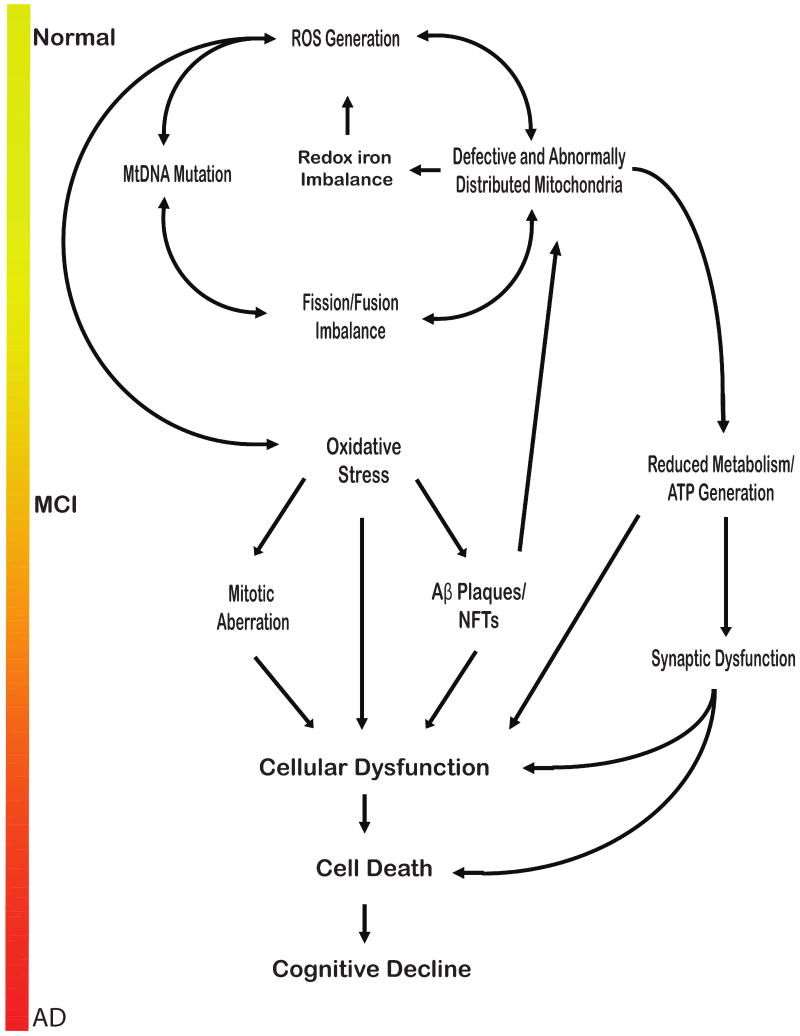
Fig. 1.
The age-induced mitochondrial cascade of neurodegeneration. The reactive oxidative species (ROS) inevitably generated in the respiration process accumulate within the cell over years, gradually damaging mitochondrial DNA (mtDNA) and related mitochondrial proteins. Eventually, an imbalance in mitochondrial dynamics (i.e., fission/fusion) occurs and initiates a detrimental cycle of further ROS generation, increased mitochondrial damage and dysfunction, and cell death. It is through this sequence of events that the neurodegeneration typical of Alzheimer's disease (AD) occurs. Aβ = amyloid β; ATP = adenosine triphosphate; MCI = mild cognitive impairment; NFT = neurofibrillary tangle.
Acknowledgments
Work in the authors' laboratories is supported by the National Institutes of Health (AG031852) and the Alzheimer's Association (IIRG-07-60196). Mark Smith and Xiongwei Zhu have acted as consultants to and received honoraria from Pfizer and Medivation. The other authors have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Serretti A, Olgiati P, De Ronchi D. Genetics of Alzheimer's disease: a rapidly evolving field. J Alzheimers Dis. 2007 Aug;12(1):73–92. doi: 10.3233/jad-2007-12108. [DOI] [PubMed] [Google Scholar]
- 3.Rogaeva E, Kawarai T, George-Hyslop PS. Genetic complexity of Alzheimer's disease: successes and challenges. J Alzheimers Dis. 2006;9(3 Suppl):381–7. doi: 10.3233/jad-2006-9s343. [DOI] [PubMed] [Google Scholar]
- 4.Gustaw-Rothenberg KA, Siedlak SL, Bonda DJ, et al. Dissociated amyloid-β antibody levels as a serum biomarker for the progression of Alzheimer's disease: a population-based study. Exp Gerontol. 2010;45:47–52. doi: 10.1016/j.exger.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iqbal K, Zaidi T, Thompson CH, et al. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol (Berl) 1984;62(3):167–77. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- 6.Grundke-Iqbal I, Iqbal K, Tung YC, et al. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancuso M, Orsucci D, Siciliano G, et al. Mitochondria, mitochondrial DNA and Alzheimer's disease: what comes first? Curr Alzheimer Res. 2008 Oct;5(5):457–68. doi: 10.2174/156720508785908946. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Lee HG, Perry G, et al. Alzheimer disease, the two-hit hypothesis: an upcollab. Biochim Biophys Acta. 2007 Apr;1772(4):494–502. doi: 10.1016/j.bbadis.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Su B, Zheng L, et al. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem. 2009 May;109 1:153–9. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blass JP. The mitochondrial spiral: an adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–83. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 11.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 12.Bleazard W, McCaffery JM, King EJ, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nature Cell Biology. 1999 Sep;1(5):298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sesaki H, Jensen RE. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol. 1999 Nov 15;147(4):699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008 Jan 23;27(2):433–46. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng X, Kanki T, Fukuoh A, et al. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem. 2005 Dec;138(6):673–8. doi: 10.1093/jb/mvi169. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007 Aug 10;130(3):548–62. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Frank S, Gaume B, Bergmann-Leitner ES, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001 Oct;1(4):515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Jeong SY, Karbowski M, et al. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004 Nov;15(11):5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005 Jul 15;280(28):26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 20.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006 Jul 25;16(14):R551–60. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 21.Parone PA, James DI, Da Cruz S, et al. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006 Oct;26(20):7397–408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Detmer SA, Ewald AJ, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003 Jan 20;160(2):189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007 Mar 1;120(Pt 5):838–48. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 25.Parone PA, Da Cruz S, Tondera D, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3(9):e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006 Jun 30;125(7):1241–52. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Knott AB, Perkins G, Schwarzenbacher R, et al. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008 Jul;9(7):505–18. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su B, Wang X, Zheng L, et al. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:135–42. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnova E, Griparic L, Shurland DL, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001 Aug;12(8):2245–56. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James DI, Parone PA, Mattenberger Y, et al. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003 Sep 19;278(38):36373–9. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 31.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004 Dec 15;117(Pt 26):6535–46. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 32.Zuchner S, Mersiyanova IV, Muglia M, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004 May;36(5):449–51. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 33.Cipolat S, Martins de Brito O, Dal Zilio B, et al. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004 Nov 9;101(45):15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007 Jul 27;282(30):21583–7. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 35.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007 Oct;8(10):939–44. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004 Feb 17;14(4):340–5. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Karbowski M, Neutzner A, Youle RJ. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol. 2007 Jul 2;178(1):71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meuer K, Suppanz IE, Lingor P, et al. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007 Apr;14(4):651–61. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- 39.Taguchi N, Ishihara N, Jofuku A, et al. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007 Apr 13;282(15):11521–9. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 40.Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15803–8. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han XJ, Lu YF, Li SA, et al. CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol. 2008 Aug 11;182(3):573–85. doi: 10.1083/jcb.200802164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho DH, Nakamura T, Fang J, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009 Apr 3;324(5923):102–5. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frazier AE, Kiu C, Stojanovski D, et al. Mitochondrial morphology and distribution in mammalian cells. Biol Chem. 2006 Dec;387(12):1551–8. doi: 10.1515/BC.2006.193. [DOI] [PubMed] [Google Scholar]
- 44.Smirnova E, Shurland DL, Ryazantsev SN, et al. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998 Oct 19;143(2):351–8. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griparic L, van der Wel NN, Orozco IJ, et al. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004 Apr 30;279(18):18792–8. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- 46.Spinazzi M, Cazzola S, Bortolozzi M, et al. A novel deletion in the GTPase domain of OPA1 causes defects in mitochondrial morphology and distribution, but not in function. Hum Mol Genet. 2008 Nov 1;17(21):3291–302. doi: 10.1093/hmg/ddn225. [DOI] [PubMed] [Google Scholar]
- 47.Kann O, Kovacs R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007 Feb;292(2):C641–57. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Lee HG, Casadesus G, et al. Oxidative imbalance in Alzheimer's disease. Mol Neurobiol. 2005;31(1-3):205–17. doi: 10.1385/MN:31:1-3:205. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa O, Zhu X, Perry G, et al. Mitochondrial abnormalities and oxidative imbalance in neurodegenerative disease. Sci Aging Knowledge Environ. 2002 Oct 16;2002(41):pe16. doi: 10.1126/sageke.2002.41.pe16. [DOI] [PubMed] [Google Scholar]
- 50.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105(8-9):855–70. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekaran K, Giordano T, Brady DR, et al. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994 Jul;24(1-4):336–40. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 52.Cottrell DA, Blakely EL, Johnson MA, et al. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001 Jul 24;57(2):260–4. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- 53.Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000 May-Jun;21(3):455–62. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 54.Nagy Z, Esiri MM, LeGris M, et al. Mitochondrial enzyme expression in the hippocampus in relation to Alzheimer-type pathology. Acta Neuropathol (Berl) 1999 Apr;97(4):346–54. doi: 10.1007/s004010050997. [DOI] [PubMed] [Google Scholar]
- 55.Parker WD, Jr, Mahr NJ, Filley CM, et al. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology. 1994 Jun;44(6):1086–90. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- 56.Parker WD, Jr, Parks J, Filley CM, et al. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994 Jun;44(6):1090–6. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 57.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10726–31. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keller JN, Guo Q, Holtsberg FW, et al. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998 Jun 15;18(12):4439–50. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001 May 1;21(9):3017–23. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Su B, Fujioka H, et al. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008 Aug;173(2):470–82. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Su B, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in susceptible neurons of Alzheimer disease. Alzheimers Dement. 2008;4 2:T645. [Google Scholar]
- 62.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002 Oct 25;298(5594):789–91. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 63.Sheehan JP, Swerdlow RH, Miller SW, et al. Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer's disease. J Neurosci. 1997 Jun 15;17(12):4612–22. doi: 10.1523/JNEUROSCI.17-12-04612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stowers RS, Megeath LJ, Gorska-Andrzejak J, et al. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002 Dec 19;36(6):1063–77. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 65.Melov S. Modeling mitochondrial function in aging neurons. Trends Neurosci. 2004 Oct;27(10):601–6. doi: 10.1016/j.tins.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 66.Guo X, Macleod GT, Wellington A, et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005 Aug 4;47(3):379–93. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 67.Verstreken P, Ly CV, Venken KJ, et al. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005 Aug 4;47(3):365–78. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 68.Li Z, Okamoto K, Hayashi Y, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004 Dec 17;119(6):873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Perry G, Smith MA. Is oxidative damage central to the pathogenesis of Alzheimer disease? Acta Neurol Belg. 1998 Jun;98(2):175–9. [PubMed] [Google Scholar]
- 70.Nunomura A, Perry G, Pappolla MA, et al. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000 Nov;59(11):1011–7. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 71.Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001 Aug;60(8):759–67. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 72.Pratico D, Uryu K, Leight S, et al. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001 Jun 15;21(12):4183–7. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997 Apr 24;336(17):1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 74.Stewart WF, Kawas C, Corrada M, et al. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997 Mar;48(3):626–32. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 75.Odetti P, Angelini G, Dapino D, et al. Early glycoxidation damage in brains from Down's syndrome. Biochem Biophys Res Commun. 1998 Feb 24;243(3):849–51. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- 76.Smith MA, Hirai K, Hsiao K, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998 May;70(5):2212–5. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 77.Kontush A, Berndt C, Weber W, et al. Amyloid-beta is an antioxidant for lipoproteins in cerebrospinal fluid and plasma. Free Radic Biol Med. 2001 Jan 1;30(1):119–28. doi: 10.1016/s0891-5849(00)00458-5. [DOI] [PubMed] [Google Scholar]
- 78.Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998 May 22;273(21):12817–26. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 79.Cuajungco MP, Goldstein LE, Nunomura A, et al. Evidence that the beta-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of abeta by zinc. J Biol Chem. 2000 Jun 30;275(26):19439–42. doi: 10.1074/jbc.C000165200. [DOI] [PubMed] [Google Scholar]
- 80.Atwood CS, Smith MA, Martins RN, et al. Neuroinflammatory environments promote amyloid-β deposition and posttranslational modification. In: Wood PL, editor. Neuroinflammation: mechanisms and management. 2nd. Totowa, New Jersey: Humana Press Inc.; 2003. pp. 249–66. [Google Scholar]
- 81.Petersen RB, Nunomura A, Lee HG, et al. Signal transduction cascades associated with oxidative stress in Alzheimer's disease. J Alzheimers Dis. 2007 May;11(2):143–52. doi: 10.3233/jad-2007-11202. [DOI] [PubMed] [Google Scholar]
- 82.Castegna A, Aksenov M, Thongboonkerd V, et al. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002 Sep;82(6):1524–32. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 83.Paola D, Domenicotti C, Nitti M, et al. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000 Feb 16;268(2):642–6. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- 84.Li HL, Wang HH, Liu SJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3591–6. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su B, Wang X, Nunomura A, et al. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008 Dec;5(6):525–32. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masters CL, Simms G, Weinman NA, et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Su B, Siedlak SL, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19318–23. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Vos KJ, Allan VJ, Grierson AJ, et al. Mitochondrial function and actin regulate dynamin-related protein 1-dependent mitochondrial fission. Curr Biol. 2005 Apr 12;15(7):678–83. doi: 10.1016/j.cub.2005.02.064. [DOI] [PubMed] [Google Scholar]
- 89.Sandebring A, Thomas KJ, Beilina A, et al. Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE. 2009;4(5):e5701. doi: 10.1371/journal.pone.0005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ichishita R, Tanaka K, Sugiura Y, et al. An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem. 2008 Apr;143(4):449–54. doi: 10.1093/jb/mvm245. [DOI] [PubMed] [Google Scholar]
- 91.Jendrach M, Mai S, Pohl S, et al. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion. 2008 Sep;8(4):293–304. doi: 10.1016/j.mito.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Frieden M, James D, Castelbou C, et al. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004 May 21;279(21):22704–14. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 93.Szabadkai G, Simoni AM, Chami M, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004 Oct 8;16(1):59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 94.Lee S, Jeong SY, Lim WC, et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007 Aug 3;282(31):22977–83. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 95.Castellani RJ, Moreira PI, Liu G, et al. Iron: the redox-active center of oxidative stress in Alzheimer disease. Neurochem Res. 2007 Oct;32(10):1640–5. doi: 10.1007/s11064-007-9360-7. [DOI] [PubMed] [Google Scholar]
- 96.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009 Jul;1790(7):589–99. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer's disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aliev G, Liu J, Shenk JC, et al. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–33. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shenk JC, Liu J, Fischbach K, et al. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer's disease. J Neurol Sci. 2009 Aug 15;283(1-2):199–206. doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Long J, Gao F, Tong L, et al. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009 Apr;34(4):755–63. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu J, Head E, Gharib AM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):1876–81. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu J, Atamna H, Kuratsune H, et al. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002 Apr;959:133–66. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu J, Head E, Kuratsune H, et al. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann N Y Acad Sci. 2004 Nov;1033:117–31. doi: 10.1196/annals.1320.011. [DOI] [PubMed] [Google Scholar]
- 105.Ames BN, Liu J. Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci. 2004 Nov;1033:108–16. doi: 10.1196/annals.1320.010. [DOI] [PubMed] [Google Scholar]
- 106.Milgram NW, Araujo JA, Hagen TM, et al. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007 Nov;21(13):3756–62. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]