Abstract
CD4+CD25bright regulatory T cells (Treg) play an important role in cancer-mediated immunosuppression. We and others have previously shown that prostaglandin E2 (PGE2) and transforming growth factor beta (TGF-β) induce CD4+CD25brightFOXP3+Treg. Based on these studies, we investigated the requirement for PGE2 in Treg induction by TGF-β. TGF-β stimulation of human CD4+ T cells induced COX-2-dependent production of PGE2. PGE2-neutralizing antibody treatment significantly reduced the suppressive function of TGF-β-induced Treg (TGF-β-Treg) in vitro. TGF-β concentration measured in the plasma of non-small cell lung cancer (NSCLC) patients directly correlated with the frequency of circulating CD4+CD25brightFOXP3+T cells. Flow cytometry analysis showed increased FOXP3 expression in circulating CD4+CD25+HLA-DR- cells of lung cancer patients compared to control subjects. Immunohistochemical analysis revealed co-expression of TGF-β, COX-2, and FOXP3 in serial sections from resected lung tumor tissues. All together these observations suggest interplay between TGF-β and COX-2 in the induction of Treg activities. Interrupting TGF-β and PGE2 signaling may be important in therapeutic interventions that aim to limit Tregfunction in lung cancer.
Keywords: T regulatory cells, PGE2, TGF-β, non- small cell lung cancer, CD4+T cells
Introduction
Lung cancer is the leading cause of cancer-related mortality in the United States [1]. There is limited understanding of how lung cancers evade host immunity [2]. Increased trafficking of suppressor cells to the tumor site and tumor-induced differentiation of CD4+ T cells into CD4+CD25bright Treg have been well documented, and both may contribute to cancer-mediated immunosuppression [2-4].
Treg are required for resolution of immune responses and maintenance of self-tolerance [5]. Treg can be induced by exposing CD4+ T cells to antigens or polyclonal activators in the presence of immunosuppressive cytokines, notably TGF-β or IL-10 [5-7]. Woo et al. reported an increased frequency of CD4+CD25bright T cells with a regulatory phenotype in peripheral blood lymphocytes (PBL) of lung cancer patients [8, 9]. Similar findings have been reported in other malignancies [10,11]. Additional studies have demonstrated an enrichment of Treg among tumor infiltrating lymphocytes (TILs) within the primary tumor and draining lymph nodes of patients with lung cancer and other neoplasms [12-14]. Accumulation of Treg in tumor-associated tissues has been associated with recurrence in early stage NSCLC [15]. These findings suggest that Treg are operative in the inhibition of local anti-tumor immunity and promotion of cancer progression [13-15]. Thus, therapies aimed at targeting Treg function may improve anti-tumor immune responses [16,17].
Immunosuppressive cytokines, such as TGF-β, induce regulatory properties in conventional CD4+ T cells [18,19], contribute to maintenance of lymphocyte homeostasis [20-22], and play a role in tumorigenesis [23]. TGF-β is highly expressed in the lungs of some individuals at risk for lung cancer [24]. Moreover, abnormally high levels of TGF-β and loss of negative regulatory signaling in response to TGF-β have been described in lung cancer and were associated with enhanced tumorigenicity and reduced survival [25-27].
There is growing evidence to suggest an integral relationship between aberrant inflammation (COX-2/PGE2 pathway) and cancer-mediated immunosuppression (TGF-β induction of Treg) [28]. We have previously described a novel pathway in which PGE2 stimulates the development of Treg in vitro and in vivo, suggesting tumor-derived COX-2 promotion of the Treg phenotype [29,30]. Here, we investigated the contribution of COX-2/PGE2 network in TGF-β induced Treg in patients with NSCLC.
Materials and methods
Generation of Treg and stimulation of cell cultures
Human CD4+ T cells were purified from enriched buffy-coat from healthy volunteers using the CD4+ T cell isolation kit (Miltenyi Biotech, Auburn, CA) and the AutoMACS cell sorter (Miltenyi Biotech), following the manufacturer's instructions. Briefly, PBL, obtained by Ficoll gradient separation, were depleted of monocytes by 2 h adherence to tissue culture flasks and incubated with CD4-conjugated microbeads (20μl/106 cells) for 15 minutes at 4° C prior to CD4+ T cell selection using the AutoMACS cell sorter. Alternatively, monocyte-depleted PBL were utilized as a source of CD4+ T cells. To generate human Treg in vitro, purified CD4+ T cells were pre-incubated for 6 days in X-Vivo 15 medium (BioWhittaker, Walkersville, MD) + 10% FBS + 1% human AB serum (Gemini, Woodland, CA) with TGF-β1 (1-5ng/ml, Peprotech, Rocky Hill, NY) in the presence of soluble anti-CD3 (1μg/ml, eBioscience, San Diego, CA) and anti-CD28 (2μg/ml, eBioscience), as previously described [29]. Alternatively, phorbol myristate acetate (PMA) (20ng/ml, Sigma, St. Louis, MO) and ionomycin (1μg/ml, Sigma) were utilized for co-stimulation. In dose-response experiments, purified CD4+ T cells or monocyte-depleted PBL were stimulated with escalating concentration of TGF-β (1-10ng/ml) in the presence of co-stimulation, as described above. In some experiments, CD4+ T cells were pre-incubated with celecoxib (1μM, Pfizer, New York, NY) for 2 h prior to stimulation with TGF-β (1-3ng/ml) for 24-48 h.
PGE2 and COX-2 EIA
PGE2 production was measured in culture-conditioned media from CD4+ T cells stimulated as described above, using a specific enzyme immunoassay (PGE2 EIA kit, Cayman Chemical, Ann Arbor, MI) according to manufacturer's instructions. The sensitivity of this assay was 15.6pg/ml. COX-2 expression in the cell lysates was analyzed using the TiterZyme® human COX -2 enzyme immunometric assay (EIA) kit (Assay Designs, Ann Arbor, MI) according to manufacturer's instructions. Briefly, non-stimulated or stimulated [soluble CD3 (1ug/ml, eBioscience) and CD28 (2ug/ml, eBioscience) antibodies] magnetic bead-purified CD4+ T cells (2.5×106) were incubated with or without TGF-β (3ng/ml) for 48 h. Cells were harvested and cell lysate was prepared, as previously described [18]. The sensitivity of the COX-2 EIA was 0.249ng/ml.
In vitro proliferation assay
We measured T cell proliferation by 5′bromo-2′-deoxyuridine (BrdU) ELISA (Roche Applied Science, Indianapolis, IN), as previously described [29], with some modifications. Briefly, magnetic bead-purified CD4+ T cells were cultured with (1-3ng/ml) or without TGF-β (diluent medium) for 6 days to generate functional Treg [18]. TGF-β was washed off and cells were utilized in a standard proliferation assay. Untreated and TGF-β-treated CD4+ autologous T cells were cultured at a 1:1 ratio in 96-well plates coated with anti-CD3 (lμg/ml, eBioscience) antibody. In some experiments, IL-2 (100 U/ml, Proleukin, biological activity 18×106 International Units (IU)/1.1mg, Chiron Corporation, Emeryville, CA) was added to the proliferation assay. To neutralize PGE2 in the culture-conditioned media, anti-PGE2 antibody (1μg/ml, provided by J. Portanova, Searle, Saint Louis, MO) was added, with a mouse IgG isotype antibody (1μg/ml, Sigma) serving as the control. After five days incubation at 37° C and 5% CO2, the cells were pulsed with BrdU (100μM) and were assessed for BrdU incorporation 4 h later. Results are expressed as optical density (OD) at 405 nm.
Patients and controls
PBL were obtained from 26 lung cancer patients from the Jonsson Comprehensive Cancer Center (JCCC), University of California, Los Angeles (UCLA) and from 26 healthy volunteers from the UCLA Blood Bank. Each individual signed an informed consent form approved by the UCLA Institutional Review Board (IRB). Table 1 shows the clinical-pathologic characteristics of each patient. Mononuclear cells (MNC) were isolated by density gradient sedimentation on Ficoll-Hipaque (Amersham Biosciences, Uppsala, Sweden), as previously described [29]. The mononuclear cell fraction was collected from the interface, washed twice in RPMI 1640 (Cellgro, Me-diatech, Herndon, VA) and cryopreserved in 10% DMSO (Sigma) and 90% AB serum (Gemini) at -80°C until use. Blood samples were collected in Vacutainer CPT MDSU 8 ml tubes (BD Biosci-ence) containing 0.1ml of 1M sodium citrate anticoagulant (Fisher Scientific, Pittsburgh, PA) and plasma was separated by centrifugation at 1500xg at room temperature (RT) for 20 min, followed by immediate cryopreservation at -80° C.
Table 1.
Patient characteristics (n=26)
| Age | 42-80 |
|---|---|
| Mean ± SD | 64.1 ±11 |
| Gender | |
| Male | 12 |
| Female | 14 |
| Ethnicity | |
| Caucasian | 24 |
| Asian | 1 |
| Hispanic | 1 |
| Tumor stage | |
| Ia | 10 |
| Ib | 2 |
| IIa | 1 |
| IIb | 2 |
| IIIb | 2 |
| IV | 9 |
| Tumor subtype | |
| AC1 | 11 |
| AC/BAC2 | 9 |
| Carcinoid | 1 |
| NA3 | 4 |
Adenocarcinoma
Bronchoalveolar carcinoma
Data not available.
Five-color flow cytometry
We utilized 5-color flow cytometry to analyze CD4+ T cells. Briefly, cryopreserved PBL specimens from patients and controls were thawed and allowed to recover for 2 h in adult bovine serum (Omega Scientific, Tarzana, CA) at 37°C. For surface staining, cells (106) were labeled with fluorescein isothiocyanate (FITC)-conjugated CD25 (IgG2a, Miltenyi Biotech), HLA-DR PE-Texas red (ECD) (IgG1, Immunotech, Marseille, France), CD4+ peridinin-chlorophyll-protein complex (PerCP) (IgG1, BD Bioscience) and CD45RO allophycocyanin (APC) (IgG2a, BD Bioscience) for 20 min at RT in FACS buffer (PBS+2% fetal calf serum) (Cellgro and Gemini, respectively). Cells were fixed and permeabilized with 1× Fix/Perm buffer (1ml, eBioscience) for 1 h at 4°C. For intracellular staining, cells were incubated with phycoerithrin (PE)-conjugated anti-human FOXP3 antibody (20μl/106, clone PCH101, eBioscience) in the presence of 2% rat serum (Gemini) in permeabilization buffer for 30 min at 4°C. CD4-PerCP antibody and specific manufacturer-recommended isotype controls for the fluorochromes FITC, PE, APC and ECD were also utilized to establish the parameters of specific staining. Cell acquisition was performed using a BD FACSAria™ cell sorter (BD Biosciences Immunocytometry System, San Jose, CA). All events were acquired within a pre-set lymphocyte gate based upon the forward and side scatter properties of the target cell population. We acquired a minimum of 500,000 events within this pre-set T cell region to reach statistical significance. FACS DiVa version 2.1 (BD Biosciences) was utilized to analyze the percentage of CD25+ FOXP3+ HLA-DR+ T cells within both CD4+CD45RO+ and CD4+CD45RO− populations.
Real Time PCR for FoxP3
For quantitative real time analysis of FoxP3 mRNA expression, PBL were isolated by Ficoll gradient centrifugation, as described above. RNA was extracted and cDNAwas prepared with a kit from Invitrogen (Carlsbad, CA) according to the manufacturer's instructions. Human FoxP3 mRNA was quantified using the SYBR Green quantitative PCR kit (Finnzymes, Espoo, Finland) in the iCycler thermocycler (Bio Rad, Hercules, CA). Amplification was carried out in a 20 μl reaction volume for 40 cycles of 15 seconds at 95°C, 20 seconds at 60°C and 30 seconds at 72°C. Samples were run in triplicate, and their relative expression was determined by normalizing values for each target to human β-actin and then comparing this normalized value to the normalized expression in a reference sample to calculate a fold change. The primers for FoxP3 were: forward 5′-CAA GTT CCA CAA CAT GCG AC3′; reverse 5′-ATT GAG TGT CCG CTG CTT CT -3′. FoxP3 primers were synthesized by Integrated DNA Technologies (Coralville, IA). For β-actin, the primers were: forward 5′-GATGAGATTGGC ATGGCTTT-3′; reverse 5′-CACCTTCACCGTTCCAGTTT-3′.
TGF-β ELISA
Because TGF-β1 is the main isoform secreted by most immune cells, we utilized the human TGF-β1 ELISA kit (RayBio, Norcross, GA) to measure TGF-β1 in citrated plasma of lung cancer patients and healthy controls, following the manufacturer's protocol. To activate latent TGF-β1 to the immune reactive form, plasma samples were first acidified with 2.5M acetic acid and 10M urea (1:1) for 10 min at RT and then neutralized with equal volume of 1.2M NaOH and 0.5M HEPES (pH 7.76). The detection limit of the TGF-β1 ELISA is > 80pg/ml. TGF-β1 is referred as TGF-β throughout the manuscript.
Immunohistochemistry for TGF-β, COX-2, and FOXP3 expression
Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded tissue sections of resected lung tumors obtained from the UCLA Lung Cancer SPORE tissue bank. Only stage I and stage II patients were included (n=12). Expression of TGF-β, COX-2 and FOXP3 was examined by single staining of serial sections. Briefly, tissue sections (4μm-thick) were de-paraffinized in xylene and rehydrated through graded alcohol and deionized water. For antigen retrieval, the sections were heated in 50mM Tris buffer containing 2mM EDTA (pH 9.0) for 20 min in a steamer and washed with PBS (Cellgro). Endogenous peroxidases were inactivated by incubation with methanol containing 3% hydrogen peroxide (Sigma) for 10 min, followed by a wash in PBS. To block nonspecific binding, the sections were treated with normal horse serum (Vector Laboratories, Bur-lingame, CA) for 30 min at RT. For FOXP3 staining, the tissue sections were incubated with mouse anti-human FOXP3 monoclonal antibody (Dr. Alison H. Banham, University of Oxford, UK) diluted 1:50 in PBS for 40 min at RT. These specimens were rinsed and incubated with horse anti-mouse IgG-biotin (7.5μg/ml, Vector Laboratories) for 40 min at RT. Samples were then incubated with avidin-HRP (Vector Laboratories) diluted 1:1000 in PBS for 30 min at RT, washed, and treated with 3,3′ diaminobenzidine (DAB substrate kit, Vector Laboratories) for brown color development. For COX-2, a tissue section from the same patient was incubated with goat anti-human COX-2 polyclonal IgG (1.0μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 37°C, followed by incubation with horse anti-goat IgG-biotin (7.5μg/ml, Vector Laboratories) and by incubation with Vectastain ABC-alkaline phosphates kit (Vector Laboratories) for red color development. For TGF-β, a tissue section from the same patient was incubated with mouse anti-human TGF-β1 monoclonal antibody (Abcam, Cambridge, MA) diluted 1:2000 in PBS for 1 h at RT, incubated with horse anti-mouse IgG-biotin (7.5μg/ml) for 40 min at RT, followed by incubation with avidin-HRP diluted to 1:1000 in PBS for 40 min. Samples were washed and treated with DAB substrate kit for brown color development.
Statistical analysis
Mixed-effects analysis of variance (ANOVA) was used to compare outcome (e.g. PGE2 and COX-2) levels between experimental groups. These models contained terms for the experimental parameters (dose and time) as well as the interaction effect between these parameters. To account for the technical and experimental replicates of the assays, we included random effects terms for both in the ANOVA models. In cases where the experimental parameters were significant, we performed post-hoc comparisons between individual experimental conditions using two-tailed unpaired t-tests. Due to unequal replicate counts, we used the coefficients estimated in the ANOVA models to derive estimates for the group means and standard errors for each experimental condition across all replicates (Figure 1). The t-test was used to compare two-group experiments (Figures 3A, 3C, 3D and Table 2). Simple linear regression was used to assess the relationship between TGF-β and CD4 percentage (Figure 3E). Statistical analyses were performed in SAS version 9 (SAS Institute, NC) and Graph Pad Prism 4 (version 4.0c) (San Diego, CA) software.
Figure 1.
TGF-β induces PGE2 in human activated CD4+ T cells. Human CD4+ T cells were obtained as described in Materials and Methods. (A) CD4+ T cells were stimulated with escalating doses of TGF-β for 48 h, as indicated. (B) CD4+ T cells were stimulated with TGF-β (1ng/ml) for 24, 48, or 120h in the presence of anti-CD3 (lμg/ml) and anti-CD28 (2μg/ml). PGE2 was measured in the culture-conditioned medium by specific EIA at 48 h (A) or at the time point indicated (B). TGF-β induces COX-2 in non-activated and TCR-activated CD4+ T cells. (C) Purified CD4+ T cells (1×106) were stimulated with TGF-β (3ng/ml) for 48 h. COX-2 was measured in the cell lysates by specific EIA. COX-2 is expressed as ng/106 CD4+ T cells. Celecoxib inhibits TGF-β-induced PGE2 in CD4+ T cells. (D) CD4+ T cells were pre-treated with celecoxib (1μM) for 2 h prior to stimulation with TGF-β (1-3ng/ml) for 48 h. PGE2 was measured in the culture-conditioned medium by EIA. PGE2 is expressed as pg/106 CD4+ T cells. Asterisks indicate significant values compared to the controls. One representative experiment of five is shown.
Figure 3.
Increased frequency of circulating CD4+CD25brightT cells in NSCLC patients (A) 26 NSCLC patients and 26 healthy controls were analyzed for the presence of circulating CD4+CD25bright FOXP3+T cells by flow cytometry, as described in Materials and Methods. Results indicate the percentage of CD4+CD25brightT cells detected in the periphery. Asterisk indicates statistical significance compared to the control group (p=0.003). Characterization of CD4+CD25brightT cells by five- color flow cytometry (B) PBL from NSCLC and control subjects were incubated with fluorescent–labeled monoclonal antibodies for CD4, CD25, CD45RO and HLA-DR prior to fixation and permeabilization, as described in Materials and Methods. Cells were then stained for intracellular FoxP3. Representative density plots from one healthy donor (B1) and one lung cancer patient (B2) are shown. Increased FoxP3 transcript in lung cancer patients compared to control subjects (C) FoxP3 mRNA expression was quantified in PBL of NSCLC patients and healthy controls by real time PCR and normalized to endogenous β-actin, as described in Materials and Methods. Average fold increase ± SEM in FoxP3 expression in lung cancer patients (n=11) relative to controls subjects (n=10) is reported, representative of one experiment of three, performed in duplicate. Asterisk indicates statistical significance compared to control (p=0.037). Plasma TGF-β is increased in NSCLC patients compared to healthy donors and correlates with the high frequency of CD4+CD25brightT cells in PBL (D) Immunoreactive TGF-β in the plasma of NSCLC patients and control subjects (n=18 per group) was detected by specific ELISA. Values refer to ng TGF-β per ml plasma. Scatter plot analysis shows the distribution of TGF-β production in lung cancer and control subjects. Each value was obtained from triplicate ELISA determinations, and one representative experiment of three is shown. Asterisk indicates statistical significance compared to control (p=0.011). (E) Linear regression analysis correlated the frequency of circulating CD4+CD25brightT cells with TGF-β protein levels in the plasma of NSCLC patients
Table 2.
Characterization of circulating CD4+ T cells subsets by 5-color flow cytometry
| CD45RO+CD25bright | CD45RO-CD25dim | |||
|---|---|---|---|---|
| PBL | HLA-DR+ | HLA-DR− | HLA-DR+ | HLA-DR− |
| Mean % positive ± SEM (Mean Fluorescent Intensity FOXP3 ± SEM) | ||||
| NSCLC patients | 20 ± 3 (9,477 ± 608) | 80 ± 3 (6,851 ± 357)* | 9 ± 2 (19,794 ± 10,299) | 90 ± 2 (3,030 ± 325)** |
| Control subjects | 17 ± 3 (8,265 ± 1,025) | 83 ± 3 (5,579 ± 261) | 11 ± 3 (11,452 ± 6,463) | 88 ± 0.7 (1,854 ± 271) |
p≤0.006 compared to control;
p≤0.007 compared to control
Results
F-β induces PGE2 production in human PBL and CD4+ T cells in a COX-2-dependent manner
To determine the contribution of COX-2/PGE2 to TGF-β Treg activity, we first analyzed the time-and concentration-dependent production of PGE2 by human CD4+ T cells from healthy donors following stimulation with TGF-β. PGE2 production by PBL was most effectively enhanced by TGF-β stimulation at 0.5 and 1ng/ml (Figure 1A). Maximal PGE2 production was observed 48 h following stimulation with TGF-β (Figure 1B). In addition, TGF-β (1-3ng/ml) induced COX-2 expression in both non-activated and CD3/CD28-stimulated CD4+ T cells (Figure 1C). Celecoxib (luM) significantly inhibited the capacity of TGF-β to induce PGE2 production by CD4+ T cells (Figure 1D).
PGE2-neutralizing antibody reduces the immunosuppressive activity of TGF-β-Treg
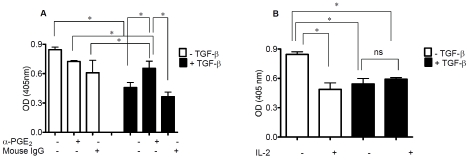
The functional implications of these findings were assessed by generating TGF-β-Treg from purified CD4+ T cells from healthy donors [18]. The suppressive activity of TGF-β-Treg on autologous T cell proliferation was assessed in the presence or absence of neutralizing anti-PGE2 antibody. TGF-β-Treg significantly inhibited the proliferation of autologous T cells (Figure 2A). Importantly, the addition of anti-PGE2 antibody significantly diminished this TGF-β-Treg inhibitory function (Figure 2A). These findings suggest that PGE2 may contribute to TGF-β-Treg suppression of T cell proliferation. Because of the fundamental role of IL-2 and the IL-2 receptor, CD25, in Treg biology and function [31,32], we evaluated the impact of IL-2 on TGF-β-Treg suppressive activity in vitro. Exogenous IL-2 (100 IU) added to the proliferation assay did not revert the suppressive activity of TGF-β-Treg; indeed IL-2 inhibited the proliferative capacity of autologous T cells (Figure 2B).
Figure 2.
Anti-PGE2 reduces TGF-β-induced Tregfunction. (A) Human purified CD4+ T cells were cultured with or without TGF-β (1-3ng/ml) for 5 days in the presence of soluble antibodies directed against CD3 (1μg/ml) and CD28 (2μg/ml). On day 5, purified autologous CD4+ T cells (105) were admixed with cultured CD4+ T cells (+/− TGF-β stimulation, as indicated) at a 1:1 ratio in a standard proliferation assay. Anti-PGE2 (1μg/ml) or isotype control mouse IgG antibody (1μg/ml) was added at the beginning of the assay. Cell proliferation was measured by BrdU incorporation in the re-sponder T cells after 72 h. Values refer to the optical density (OD) of BrdU incorporation into proliferating CD4+ T cells. IL-2 does not revert the inhibition of CD4+ T cell proliferation in response to TGFβ-induced Treg. (B) Purified CD4+ T cells were pre-treated with IL-2 (100U/ml), where indicated, 24 h prior to generation of TGF-β-Treg and cell proliferation was assessed as above. Asterisks denote statistical significance. Representative experiments of three are shown.
Increased frequency of circulating CD4+CD25bright T cells in NSCLC patients compared to healthy controls
The COX-2/PGE2 pathway has been implicated in promoting the Treg phenotype [29,30,33]. We analyzed circulating Treg in both NSCLC patients and healthy subjects by flow cytometry. The patient characteristics, tumor stage, and tumor histology are reported in Table 1. The frequency of circulating CD4+CD25bright T cells expressing FOXP3 was significantly elevated in NSCLC patients compared to healthy subjects (Figure 3A), in agreement with previous findings [8,9]. The mean fluorescence intensity (MFI) for FOXP3 expression was similar in CD4+CD25bright T cells from both control and cancer groups (2222 ± 7 and 2021 ± 7, respectively). Because specific subsets of memory Treg may be selectively activated in cancer [3,34], we analyzed the expression of Major Histocompatibility Complex (MHC) class II in naïve and memory CD4+CD25brightand CD4+CD25dim T cells from the PBL of healthy individuals and NSCLC patients. On a percentage basis, none of the indicated T cell subsets were differentially expressed in the control subjects versus lung cancer patients. Both CD45RO+ and CD45RO− T cells from lung cancer and healthy donors expressed similar levels of HLA-DR (17-20% and 9-11%, respectively). Interestingly, the MFI of FOXP3 was increased only in the HLA-DR− subsets of CD45RO+CD25brightand CD45RO−CD25dimT cells of lung cancer patients compared to the control subjects (6851 ± 357 versus 5579 ± 261 and 3030 ± 325 versus 1854 ± 271, respectively) (Table 2). Figure 3B shows a representative density plot from a healthy donor (B1) and a cancer patient (B2).
Increased FoxP3 transcript in NSCLC patients compared to control subjects
Mouse and human genetic studies have demonstrated a fundamental role for FoxP3 in the regulation of Treg development and function [35,36]. Mutations in the FoxP3 gene were linked to autoimmune manifestations observed in the Scurfy mouse and in humans affected by immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) disease, and other conditions characterized by lack of functional Treg [37]. However, FoxP3 is not always expressed in every type of Treg [5-7], and discrepancies between protein and gene expression profiles have been reported [38]. Thus, we analyzed FoxP3 mRNA from individual PBL samples by RT-PCR analysis. FoxP3 mRNA levels were 2-fold higher in PBL from lung cancer patients compared to control subjects (Figure 3C), consistent with the increased frequency of CD4+CD25brightFOXP3+ T cells detected in the peripheral blood of the NSCLC patients (Figure 3A).
Plasma TGF-β is increased in NSCLC patients and correlates with their increased frequency of circulating CD4+CD25brightT cells
Several malignancies, including lung cancer, produce high levels of TGF-β [25,26,39], and, similar to PGE2, exogenous TGF-β promotes the development of Treg [18,19]. Thus, we measured TGF-β in the plasma of NSCLC patients and healthy donors and evaluated whether plasma TGF-β and frequency of circulating Treg were correlated (n=18). As shown in Figure 3D, plasma TGF-β concentrations were increased in NSCLC patients compared to healthy individuals and were significantly correlated with the frequency of circulating CD4+CD25brightT cells of NSCLC patients (r2=0.43, p≤ 0.003, Figure 3E).
Co-expression of TGF-β, COX-2 and FOXP3 in resected lung cancer
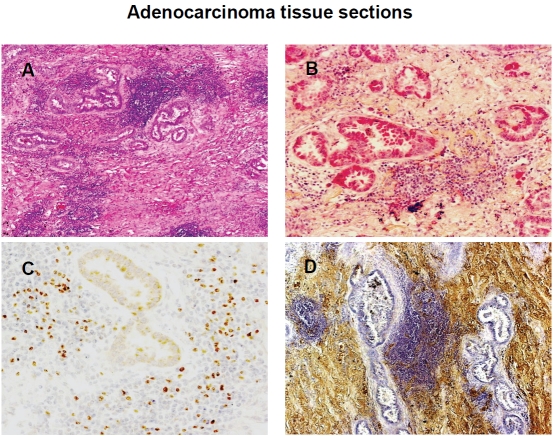
As we previously reported, PGE2 induces CD4+CD25brightFOXP3+ Treg with characteristics of both naturally and acquired Treg function [29,30]. Similarly, TGF-β drives conventional CD4+ T cells toward the development of acquired Treg phenotypes [18,19]. In addition, TGF-β has been shown to increase COX-2 expression and PGE2 production in several cell types [21-23]. Based on these findings, we investigated the interplay between TGF-β and COX-2/PGE2 in Treg induction at the tumor site. Herein, we evaluated TGF-β, COX-2, and FOXP3 expression by immunohistochemical analysis of NSCLC tumor biopsies (n=16). Figure 4 shows representative tissue sections of lung adenocarcinoma from the same patient. In Figure 4A, a hematoxylin and eosin (H&E) stained section of lungadenocarcinoma shows blue-stained areas that represent a prominent lymphoid infiltrate (100×). In Figure 4B, the red-stained areas represent positive cytoplasmic staining for COX-2 expression (200×). In Figure 4C, FOXP3 is detected as brown nuclear staining within lymphocyte-enriched areas of the neoplasm (200×). In Figure 4D, brown-stained areas represent positive staining for TGF-β within the connective tissue (200×). These observations along with our previous findings support the interplay between TGF-β and COX-2 in the induction of Treg activities within the lung tumor microenvironment.
Figure 4.
Co-expression of TGF-β, COX-2, and FOXP3 in resected NSCLC. Expression of TGF-β, COX-2 and FOXP3 was assessed by immunohistochemical analysis of paraffin-embedded lung cancer tissue sections, as described in Materials and Methods. (A) Hematoxylin and eosin (H&E) stained sections of adenocarcinoma revealed a prominent lymphoid infiltrate (blue-stained regions, 100×). (B) Red-stained areas represent positive cytoplasmic staining for COX-2. (200×); (C) Brown-stained areas represent positive nuclear staining for FOXP3 detected in lymphoid cells within the neoplasm (200×). (D) Brown-stained areas represent positive staining for TGF-β detected in the connective tissue (200×). One representative section from one of 12 patients is shown.
Discussion
While Treg serve an essential maintenance role in self-tolerance, increased number of circulating Treg has been reported in several malignancies and correlated with poor outcome [3,13-15]. As such, Treg represent a potential therapeutic target in cancer therapy [41]. However depletion strategies may not be sufficient, since Treg populations can be replenished by conversion of conventional CD4+CD25× T cells into CD4+CD25+ Treg by exposure to TGF-β or PGE2 whose interplay in Treg induction is the object of the present investigation [4,18,19,29,30].
Several malignancies secrete the immunosup-pressive cytokine TGF-β [25,39,42], and tumor-derived TGF-β can convert naturally occurring CD4+ T cells into CD4+CD25brightFOXP3+ Treg in a mouse model of pancreatic cancer [43]. TGF-β has also been shown to induce COX-2/PGE2 expression in numerous epithelial cell types [21-23], and we have reported that tumor-derived COX-2/PGE2 favor the development of a Treg phenotype in human and mouse CD4+ T cells [29,30].
COX-2, the rate-limiting enzyme for the production of pro- and anti-inflammatory prostaglandins, plays an important role in cancer-related inflammation and progression [33,44]. Aberrant or increased expression of COX-2 and its major metabolite, PGE2, are found in several malignancies including NSCLC in which they promote metastatic development [33,44]. While COX-2/PGE2 and TGF-β are implicated in tumorigenesis via different mechanisms [33,39,42,44], both are capable of generating peripherally induced Treg [18,19,29,30].
In the present study, we found that TGF-β can induce competent Treg from human PBL through a mechanism dependent upon COX-2/PGE2 signaling. Specifically, TGF-β stimulated PGE2 production and COX-2 expression in both PBL and purified CD4+ T cells, and pharmacologic inhibition of COX-2 suppressed the TGF-β-induced PGE2 production. PBL or CD4+ T cells stimulated with TGF-β exhibited a Treg phenotype in vitro evidenced by their capacity to inhibit the proliferation of autologous CD4+ T cells. Inhibition of PGE2 significantly reduced the TGF-β-Treg immu-nosuppressive activity, suggesting the involvement of COX-2/PGE2 in the inhibitory function of TGF-β-Treg. Interestingly, high concentrations of IL-2 did not prevent the in vitro suppression of T cell proliferation mediated by TGF-β-Treg, but it did hamper the overall proliferation of autologous T cells. It is possible that IL-2 may have recruited Treg precursors present in the T cell population and encouraged their suppressive function [31,32]. Alternatively, IL-2 may operate by sensitizing activated T cells to undergo apoptosis [45].
Clearly, our results indicate that TGF-β can drive purified CD4+ T cells towards a regulatory phenotype through the COX-2-induced production of PGE2. This observation may have important implications. First, it offers us an explanation why NSCLC, and maybe other tumors, rely greatly on TGF-β, COX-2 and PGE2. While the signaling between these players may go in both directions, positively feeding forwards and backwards the neoplastic process, it ultimately results in tumor progression and down-regulation of the immune response [33,42,44]. Our findings also emphasize that any given T cell phenotype may not be as static as previously thought, but rather environmental factors can shape the T cell repertoire/balance in the periphery [46]. Baecher-Allan et al. [33] identified a functionally distinct population of mature Treg based on expression of HLA-DR: 1) HLA-DR+ Treg acting via an early contact-dependent mechanism associated with FoxP3 induction, and 2) HLA-DR− Treg characterized by increased production of IL-10 and IL-4 and induction of late suppression of T cell proliferation. In the present study, FOXP3 was expressed at significantly elevated levels in the HLA-DR− population of both naive and memory CD4+CD25dim and CD4+ CD25brightT cell subsets in NSCLC patients compared to healthy donors. We hypothesize that HLA-DR- CD25bright FOXP3+ T cells may initially function via IL-10-mediated-suppression, then, following conversion into HLA-DR+ CD25bright FOXP3+T cells, via a cell-contact dependent mechanism. Functional studies are under way to elucidate the significance and the immunologic implications of these HLA-DR negative Foxp3+ T cell subsets.
The interplay between TGF-β, COX-2 and PGE2 is further illustrated by our clinical findings which showed increased frequency of peripheral CD4+CD25bright T cells in NSCLC patients which was correlated to high plasma levels of TGF-β and co-localization of COX-2, TGF-β and FOXP3 in resected NSCLC tissues.
Our findings support the evidence implicating a pathogenic interaction between dysregulated inflammation (COX-2/PGE2 pathway) and cancer-mediated immunosuppression (TGF-β induction of Treg). We hypothesize that elevated levels of TGF-β in the plasma or peri-tumoral stroma of NSCLC patients may promote the generation of Treg in part through COX-2/PGE2 signaling. Additional studies are warranted to clarify the interaction between TGF-β and COX-2/PGE2 in the induction of adaptive Treg. Defining the factors that contribute to Treg induction and understanding Treg suppression mechanisms may facilitate the development of new therapeutic strategies in lung cancer.
Acknowledgments
Flow cytometry was performed at the UCLA Jonsson Comprehensive Cancer for AIDS Research Flow Cytometry Core Facility that is supported by the National Institutes of Health awards CA-16042 and AI-28697, Jonsson Comprehensive Cancer Center, UCLA AIDS Institute, and David Geffen School of Medicine at UCLA.
This study was supported by the National Institutes of Health Grants R01 CA111851, UCLA Lung Cancer SPORE P50 CA90388, 5K12CA076095, and 1K23CA131577; UC Tobacco Related Research Program; Merit Review Research Funds from the Department of Veteran Affairs; Ronald Binder Memorial Fund for Lung Cancer Research; STOP Cancer; and Jonsson Cancer Foundation.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Dubinett S, Sharma S, Huang M, Mao JT, Batra R. Cancer and Immune Dysfunction. In: Finke J, Bukowski R, editors. Totowa, NJ: Human Press Inc; 2004. pp. 335–350. Immunotherapy at the Crossroads: How tumors evade immunity and what can be done. [Google Scholar]
- 3.Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Sem Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-β. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 5.Shevach EM. Certified professionals: CD4+C25+ suppressor T cells. J. Exp. Med. 2001;193:F41–46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–6327. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 8.Woo E Y, Chu C S, Goletz T J, Schlienger K, Yeh H, Koukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late- stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 9.Woo E Y, Chu C S, Goletz T J, Schlienger K, Carrol RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–4276. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 10.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Canc Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 11.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 12.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immuno-suppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 13.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Edvemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 14.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubetret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25 high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi M-B, Harpole DH, Jr, Patz EF., Jr Tumor infiltrating FOXP3+ regulatory T cells are associated with recurrence in pathologic stage I NSCLC. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 16.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12:4794–4803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 17.Randolph A, Fathman CG. CD4+CD25+ regulatory T cells and their therapeutic potential. Ann Rev Med. 2006;57:1.1–1.22. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 18.Fantini MC, Becker C, Monteleone G, Pallone F, Galle P R, Neurath MF. Cutting Edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 19.Pyzik M, Piccirillo CA. TGF-beta1 modulates Foxp3 expression and regulatory activity in distinct CD4+ T cell subsets. J Leuk Biol. 2007;82:335–346. doi: 10.1189/jlb.1006644. [DOI] [PubMed] [Google Scholar]
- 20.Li MO, Wan YY, Sanjabi S, Robertson A-KL, Flavell RA. TGF-β regulation of immune responses. Ann Rev Immun. 2005;24:4.1–4.48. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 21.Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, Beauchamp RD. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J Biol Chem. 2000;275:6628–35. doi: 10.1074/jbc.275.9.6628. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Yang SC, Sharma S, Luo J, Cui X, Peebles KA, Huang M, Sato M, Ramirez RD, Shay JW, Minna JD, Dubinett SM. EGFR Signaling is Required for TGF-beta1 Mediated COX-2 Induction in Human Bronchial Epithelial Cells. Am J Respir Cell Mol Biol. 2007;37:578–588. doi: 10.1165/rcmb.2007-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roman CD, Morrow J, Whitehead R, Beauchamp RD. Induction of cyclooxygenase-2 and invasiveness by transforming growth factor-beta (1) in immortalized mouse colonocytes expressing oncogenic Ras. J Gastrointest Surg. 2002;6:304–9. doi: 10.1016/s1091-255x(01)00041-5. [DOI] [PubMed] [Google Scholar]
- 24.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, Okada Y, Yamasawa F, Nakahara K, Umeda A. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163:1476–83. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 25.Barthelemy-Brichant N, David JL, Bosquee L, Bury T, Seidel L, Albert A, Bartsch P, Baugnet-Mahieu L, Deneufbourg JM. Increased TGF-β1 plasma level in patients with lung cancer: potential mechanisms. Eur J Clin Invest. 2002;32:193–198. doi: 10.1046/j.1365-2362.2002.00956.x. [DOI] [PubMed] [Google Scholar]
- 26.Kong FM, Washington MK, Jirtle RL, Anscher MS. Plasma transforming growth factor-b1 reflects disease status in patients with lung cancer after radiotherapy: a possible tumor marker. Lung Cancer. 1996;16:47–59. doi: 10.1016/s0169-5002(96)00611-3. [DOI] [PubMed] [Google Scholar]
- 27.Anumanthan G, Halder SK, Osada H, Takahashi T, Massion PP, Carbone DP, Datta PK. Restoration of TGF-β signaling reduces tumorigenicity in human lung cancer. Br J Cancer. 2005;93:1157–67. doi: 10.1038/sj.bjc.6602831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neil JR, Johnson KM, Nemenoff RA, Schieman WP. COX-2 inactivates Smad signaling and enhances EMT-stimulated by TGF-beta through a PGE2 -dependent mechanism. Carcinogenesisis. 2008;29:2227–35. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baratelli F, Ying L, Zhu L, Yang SC, Heuze-Vourc'h N, Reckamp K, Zeng G, Sharma S, Dubinett SM. PGE2 modulates CD25+ and CD25− CD4+ T regulatory cell function through induction of FOXP3 expression. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RJ, Dubinett SM. Tumor COX-2/PGE2 dependent promotion of FOXP3 expression and CD4+CD25+ T Regulatory cell activities in lung cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 31.Létourneau S, Krieg C, Pantaleo G, Boyman O. J Allergy Clin Immunol IL-2 and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J Allergy Clin Immunol. 2009;123:758–62. doi: 10.1016/j.jaci.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubinett SM, Sharma S, Huang M, Dohadwala M, Pold M, Mao J. Cyclooxygenase–2 in lung cancer. In: Dannenberg AJ, Dubois RN, editors. Basel: Karger; 2003. pp. 138–162. COX-2. [DOI] [PubMed] [Google Scholar]
- 34.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler SF. FOXP3 of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 36.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. FOXP3-dependent programme of regulatory T cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 37.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T –cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Morgan ME, Van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR. Expression of Foxp3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Dumont N, Arteage CL. The tumor microenvironment: a potential activator of the tumor suppressive and promoting actions of TGF-beta. Differentiation. 2002;70:574–82. doi: 10.1046/j.1432-0436.2002.700910.x. [DOI] [PubMed] [Google Scholar]
- 40.Reckamp KL, Krysan K, Morrow JD, Milne GL, Newman RA, Tucker C, Elashoff RM, Dubinett SM, Figlin RA. A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced Non-Small Cell Lung cancer. Clin. Cancer Res. 2006;12:3381–3388. doi: 10.1158/1078-0432.CCR-06-0112. [DOI] [PubMed] [Google Scholar]
- 41.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 42.Wong SF, Lai LC. The role of TGF-beta in human cancer. Path. 2001;33:85–92. [PubMed] [Google Scholar]
- 43.Moo-Young TA, Larson JW, Belt BA, Tan MC, Hawkins WG, Eberlein TJ, Goedegebuure PS, Linehan DC. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother. 2009;32:12–21. doi: 10.1097/CJI.0b013e318189f13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Met Rev. 2007 doi: 10.1007/s10555-007-9096-5. DOI 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 45.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol. Invest. 2004;33:109–142. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 46.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]