Abstract
3, 3′-diindolylmethane (DIM) modulates estrogen metabolism and acts as an anti-androgen which down-regulates the androgen receptor and prostate specific antigen (PSA). We conducted a dose-escalation, phase I study of BioResponse (BR)-DIM with objectives to determine the maximum tolerated dose (MTD), toxicity profile, and phar-macokinetics (PK) of BR-DIM, and to assess its effects on serum PSA and quality of life (QoL). Patients and Methods: Cohorts of 3-6 patients received escalating doses of twice daily oral BR-DIM providing DIM at 75 mg, then 150 mg, 225 mg, and 300 mg. Toxicity was evaluated monthly. Serum PSA and QoL were measured at baseline, monthly during treatment, and at end of study. Results: 12 patients with castrate-resistant, non-metastatic, PSA relapse prostate cancer were treated over 4 dose cohorts; 2 patients (at 150 mg and 225 mg, respectively) underwent intra-patient dose escalation, by one dose level. After oral administration of the first dose of BR-DIM, the plasma exposure to DIM appeared dose proportional at doses ranging from 75 to 300 mg, with the mean Cmax and mean AUClast increasing from 41.6 to 236.4 ng/ml and from 192.0 to 899.0 ng/ml*h, respectively. Continued relatively stable systemic exposure to DIM was achieved following twice daily oral administration of BR-DIM. Minimal toxicity was observed. Two of the four patients treated at 300 mg had grade 3 asymptomatic hyponatremia (AH) discovered on routine blood work. The other 2 patients at this dose had no AH. Therefore, the maximum tolerated dose (MTD) was deemed to be 300 mgand the recommended phase II dose (RP2D) of BR-DIM was 225 mg twice daily. One patient without AH at 225 mg experienced a 50% PSA decline. One patient with BR-DIM dose of 225 mg had PSA stabilization. The other 10 patients had an initial deceleration of their PSA rise (decrease in slope), but eventually progressed based on continual PSA rise or evidence of metastatic disease. Ten patients completed monthly QoL reports for a mean of 6 months (range: 1-13). QoL measures emotional functioning may have held up somewhat better over time than their physical functioning. Conclusion: BR-DIM was well tolerated. Increasing systemic exposure to DIM was achieved with the increase of BR-DIM dose. Modest efficacy was demonstrated. Patients' QoL varied over time with length of treatment. Phase II studies are recommended at the dose of 225 mg orally twice daily.
Keywords: BR-DIM, DIM, PSA relapse, clinical trial, phase I, diindolylmethane, indole-3-carbinol
Introduction
Prostate cancer is the most common cancer diagnosed in men in the United States and is the second leading cause of cancer death in men [1]. Unfortunately, despite undergoing appropriate treatment for localized disease, approximately 40% of men will eventually experience disease recurrence and disease progression [2, 3]. Disease recurrence and progression are often identified by rising PSA, and not necessarily by clinical symptoms or radiologic evidence. With prostate cancer being detected and treated at earlier stages, the men experiencing biochemical recurrence, without clinical evidence of disease are a growing subset and present a challenge for clinical management.
Patients who experience biochemical recurrence with a PSA doubling time of less than 10 months are at higher risk for disease progression [4]. Other characteristics suggestive of rapid disease progression include post-treatment PSA > 0.4 ng/mL, two consecutive increases > 0.2 ng/mL, and high Gleason score. First line therapy available to these patients is androgen deprivation therapy (ADT) [5, 6]. Unfortunately, after a period of time, despite ADT, a majority of the men will experience PSA progression, without clinical or radiologic evidence of distant disease. This particular group of men presents a challenge to clinicians as there are no standard treatment options. A rising PSA along with lack of accepted standard treatment options cause great anxiety in both patients and physicians. The objective of treatment at this juncture is to delay the onset of distant metastatic disease, recognizing that most men in this group are clinically asymptomatic.
Chemotherapy is currently the standard treatment for patients with proven metastatic, castrate-resistant prostate cancer. Docetaxel based therapies have been evaluated in phase III clinical trials and reported to confer a survival advantage when compared to mitoxantrone-based regimens [7, 8]. The role of chemotherapy in patients who are castrate-resistant, but, without obvious metastatic disease is not well defined. The reluctance to initiate chemotherapy treatments in this setting is two-fold; difficulty in justifying treatment toxicity when rising PSA is the only reason for treatment and the unknown impact of chemotherapy on delaying disease progression. Additional therapeutic options for this group of men are urgently needed.
Increased consumption of cruciferous vegetables has been associated with a decreased risk in several cancers such as colon cancer, breast cancer and prostate cancer [9-12]. Several epidemiologic studies support the role of cruciferous vegetables such as broccoli, brussel sprouts and cauliflower, in lowering the incidence of prostate cancer [11, 13]. Two major active compounds in cruciferous vegetables are indole-3-carbinol (I3C) and its in vivo dimeric derivative, 3, 3′-diindolylmethane (DIM). Conversion of I3C into DIM significantly increases during cooking, making DIM the dominant active dietary indole from crucifers (cabbage, Brussels sprouts, broccoli, etc.) [13]. When added in vitro to cell culture media, I3C spontaneously dimerizes forming DIM in a quantitative and time-dependent fashion [14]. Dr. Sarkar and others have reported inhibition of cell growth and induction of G1 cell-cycle arrest in androgen-independent PC3 prostate cancer cells with I3C or DIM administration [15-17]. Other potential mechanisms of action of I3C/DIM in prostate cancer include induction of apoptosis by upregulation of BAX, downregulation of Bcl-2 and BCLXL, and inactivation of Akt and NF-kB. In androgen-sensitive LNCap prostate cancer cells, Le et al. reported studies supporting the role of DIM as a pure anti-androgen [17]. DIM down-regulated PSA expression at the transcriptional levels and exhibited no androgen receptor (AR) agonist activity. Both I3C and DIM inhibit prostate cancer cell growth and promote apoptosis, regardless of androgen dependent status [18]. When tested in an in vivo murine model of transplanted prostate cancer, 2.5 mg/kg/day DIM injected subcutaneously resulted in a 50% reduction in tumor mass associated with evidence of increased apoptosis [19].
BR-DIM is a dietary supplement which contains pharmaceutically pure DIM, microdispersed in spray-dried starch particles. Chronic animal feeding studies and Phase I human testing in normal volunteers has reported little toxicity associated with BR-DIM use [20, 21]. However, it remains important for us to establish the appropriate dose of BR-DIM, specifically in men with prostate cancer. We therefore conducted a phase I trial of BR-DIM in patients with non-metastatic, castrate-resistant prostate cancer with rising PSA to determine the maximum tolerated dose (MTD), dose limiting toxicity (DLT), recommended phase II dose (RP2D), pharmacokinetics of BR-DIM, and its effects on PSA and patients' quality of life.
Patients and methods
Patient selection
All patients were required to have histologically confirmed prostate adenocarcinoma with PSA-only failure after any local therapy without any evidence of metastasis (by bone scan or CT/MRI scan). Rising PSA at a minimum level of ≥ 5 ng/mL was confirmed by two separate values at least 1 week apart along with testosterone at castrate levels (< 50 ng/mL). All patients had adequate organ function, had ECOG performance status ≤ 3, and took no micronutrient supplements or dietary soy products during the study. One daily multivitamin was allowed. Current use of warfarin or proton pump inhibitors was not allowed. Patients with serious acute or chronic medical or psychiatric conditions were not eligible. This study was approved by the Wayne State University Institutional Review Board. All patients gave signed informed consent.
Treatment
BR-DIM capsules providing 75 mg DIM per capsule, with DIM content confirmed by independent laboratory HPLC assay, were supplied by BioResponse, LLC, Boulder, CO. BR-DIM was administered at a starting dose of 75 mg orally twice daily. Patients were instructed to take tablets twice daily with 8 ounces of water, with or without food. Patients recorded their pill intake on a study calendar. One treatment cycle consisted of 28 days of therapy. Patients continued on therapy until disease progression or unacceptable toxicity.
Study design
The traditional “dose cohorts of 3+3 patients” design was utilized in this study. A maximum of 4 predefined dose levels were investigated: 75 mg twice daily (i.e., total dose of 150 mg/day); 150 mg twice daily (total dose 300 mg/day); 225 mg twice daily (total dose 450 mg/day); and 300 mg twice daily (total dose 600 mg/day). Cohorts of at least three patients were treated at each dose level providing no dose limiting toxicities (DLTs) occurred. The first two patients enrolled at any dose level were observed for at least 28 days and the third patient was observed for at least 14 days prior to opening the next dose level for enrollment. Intra-patient dose escalation was allowed if the patient experienced no toxicity worse than grade 1 NCI Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Patients were not allowed to be escalated to a dose level at which two previous patients experienced DLTs. Cohort size was expanded to at least 6 patients per dose level at the first instance of a first course DLT.
DLT was defined as any grade 3 or 4 toxicity according to the CTCAE. If 1 of 3 patients experienced a DLT in the first course at a dose level, an additional 3 patients were enrolled in the cohort. If only 1 of 6 patients experienced a DLT, then further escalation occurred until 2 of 6 patients experienced a DLT. At least six patients were studied at the dose level below that which resulted in greater than or equal to 2 DLTs in the first course. Patients were de-escalated to the next lower dose level if they experienced a DLT or if two previous patients had experienced DLT at the current dose level during the current or earlier course. The occurrence of a DLT in two patients during cycle 1 resulted in the determination of the previous dose level as the MTD or RP2D. The MTD for a patient was defined as the dose which resulted in CTCAE grade 2 toxicity or greater, and was the RP2D.
Study evaluation
Required studies at baseline included a history and physical examination, assessment of ECOG performance status, complete blood count (CBC), chemistry profile (sodium, potassium, calcium, BUN, creatinine, alkaline phosphatase, AST, ALT, total bilirubin), serum PSA and testosterone, and radiographic imaging studies (bone scan, CT/MRI scan). Baseline quality of life (QoL) questionnaires were administered as well. Starting on the first day of treatment, all patients were seen and evaluated on their first day of each cycle. CBC, chemistry, serum PSA and testosterone, and psychosocial questionnaires were obtained at the beginning of each cycle with radiographic imaging studies after every 2 cycles. Response was assessed by the Prostate Cancer Working Group Criteria [22].
Pharmacokinetics
Pharmacokinetic evaluation was performed in all treated patients. Blood samples (∼5 ml for each) were collected at 0 (baseline), 0.5, 1, 2, 3, 4, and 8 hours after the oral administration of the first dose of BR-DIM on Cycle 1 Day 1. In addition, trough blood samples were collected prior to the administration of the morning dose of BR-DIM on days 8 and 28. Within 1 h of the collection, the blood samples were centrifuged at 4°C, at 2000 g for 10 minutes, and plasma was collected immediately after centrifugation and transferred to the screw-cap polypropylene cryogenic tubes. The plasma samples were stored at -80°C until analysis.
The concentrations of DIM in human plasma samples were determined by a validated high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) method. Briefly, to 50 μl human plasma, 100 μl of 2% formic acid in methanol (containing 1 μg/mL internal standard zileuton) was added to precipitate proteins. The mixture was vortex-mixed for 1 minute, and centrifuged at 14,000 g at 4°C for 10 minutes. The supernatant was collected and 10 μl was injected into the LC-MS/MS system. Chromatographic analysis was performed using a Waters model 2690 separation system (Milford, MA, USA). The analytes were separated on a Waters X-Terra MS column (150 mm × 2.1 mm i.d.) using a mobile phase consisting of methanol/0.45% formic acid (70:30, v/v), and isocratic flow at 0.2 mL/min. The analytes were monitored by a Waters Micromass triple quadrupole mass spectrometer (Milford, MA, USA) using an electrospray probe in the positive ionization mode operating at a cone voltage of 23V for DIM and 13V for the internal standard zileuton. Samples were introduced into the ionization source through a heated nebulized probe (350°C). The collision energy was set at 30eV and 9eV for DIM and zileuton, respectively. The transitions at 129.9 > 76.8 and 237.1 > 161.1 were monitored for DIM and zileuton, respectively. The linear calibration curve was set over the DIM plasma concentration range of 10 to 5, 000 ng/mL. The lower limit of quantitation (LLOQ) was determined at 10 ng/mL for DIM in human plasma. The intra- and inter-day accuracy and precision were within the generally accepted criteria for bioanalytical method (<15%).
The pharmacokinetic parameters for individual patients were estimated using noncompartmental analysis with the computer software program WinNonlin version 5 (Pharsight Corporation, Mountain View, California). The maximum plasma concentration (Cmax), the time of occurrence for the Cmax (Tmax), and the trough plasma concentration (Cmin) were obtained by visual inspection of the plasma concentration-time curves after the oral administration. The total area under the plasma concentration-time curve from time zero to the last measurable time point (AUC0-t) was calculated using the linear and logarithmic trapezoidal method for ascending and descending plasma concentrations, respectively.
Quality of life
QoL was measured in the patients who completed treatment with BR-DIM and those who were withdrawn due to treatment failure. This information was assessed to document the long-term impact of treatment with BR-DIM on QoL outcomes in prostate cancer patients. Patients completed a sociodemographic questionnaire at baseline. QoL was assessed at baseline and on the first day of every treatment cycle using the EORTC QLQ-30 scale, which measures patients' Physical Functioning and Emotional Functioning [24].
Statistical methods
Except for the pharmacokinetics parameter estimation, only descriptive statistics and statistical graphics (e.g., dot plots, spaghetti plots, and line plots) were employed. Pharmacokinetics data were displayed using dot plots, PSA values over time via spaghetti plots, and median QoL scores via line plots.
Results
Patient characteristics
A total of 12 patients with castrate-resistant, non-metastatic, PSA relapse prostate cancer patients were treated over 4 dose cohorts; 2 patients (at 150 mg and 225 mg, respectively) underwent intra-patient dose escalation, by one dose level (Table 1). The median age was 77 years (range, 62 - 91) (Table 1). 58% of patients were African-American. The median baseline PSA was 29.7 (range, 3.5 - 89.7). The median number of treatment cycles was 4.4 (range, 1.1-20.1).
Table 1.
Patient characteristics (N=12)
| Age: | |
| median (years) | 77 |
| range | 62 - 91 |
| Race: Caucasian | 5 (42%) |
| African-American | 7 (58%) |
| Baseline PSA: | |
| median (ng/ML) | 29.7 |
| range | 3.5 - 89.7 |
| No. of BR-DIM treatment cycles: | |
| median | 4.4 |
| range | 1.1 - 20.1 |
| Length of BR-DIM treatment: | |
| median (months) | 4.0 |
| range | 1.0 - 18.5 |
| Reason off BR-DIM treatment: | |
| Progressive disease | 11 (92%) |
| Refused further treatment | 1 (8%) |
Dose escalation
The first three patients on dose level 1 (75 mg orally twice daily) and dose level 2 (150 mg twice daily) experienced no DLTs. The patients treated on dose level 3 (225 mg twice daily) also experienced no toxicity. At dose level 4 (300 mg twice daily), two of 4 patients had grade 3 asymptomatic hyponatremia (AH) discovered on routine blood work. The two other patients (one from original dose level 3 who was intra-patient dose escalated) experienced no AH. Therefore, the maximum tolerated dose (MTD) and the RP2D was 225 mgtwice daily.
Toxicity
The most common grade 2 toxicities reported were diarrhea and hyperglycemia with no significant changes observed with other laboratory values (Table 2). The main DLT was asymptomatic grade 3 AH in 2 of 4 treated at a dose level of 300 mgtwice daily. The first patient is a 94 year old Caucasian male who had no preexisting evidence of hyponatremia who continued to experience intermittent grade 1-3 AH even after discontinuation of BR-DIM. The second patient is an elderly African American male who had no preexisting evidence of hyponatremia, but had bouts of chronic abdominal problems. After discontinuation of this study due to rising PSA only, the patient continued to also experience intermittent grade 1-3 AH. There was one patient who experienced bowel necrosis, but this was deemed to be unrelated to BR-DIM.
Table 2.
Toxicity
| Dose mg/bid | Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|---|
| 75 | |||||
| Hyperglycemia | 1 | 0 | 0 | 0 | |
| Pruritis | 1 | 0 | 0 | 0 | |
| Increased creatinine | 2 | 0 | 0 | 0 | |
| Fatigue | 1 | 0 | 0 | 0 | |
| 150 | |||||
| Hyponatremia | 0 | 0 | 0 | 0 | |
| Hyperglycemia | 2 | 0 | 0 | 0 | |
| Increased creatinine | 2 | 0 | 0 | 0 | |
| Anemia | 2 | 0 | 0 | 0 | |
| Urinary Frequency | 1 | 0 | 0 | 0 | |
| Urinary Incontinence | 1 | 0 | 0 | 0 | |
| 225 | |||||
| Hyperglycemia | 3 | 1 | 0 | 0 | |
| Diarrhea | 0 | 2 | 0 | 0 | |
| Flatulance | 3 | 0 | 0 | 0 | |
| Dyspepsia | 1 | 0 | 0 | 0 | |
| Increased creatinine | 1 | 0 | 0 | 0 | |
| 300 | |||||
| Hyponatremia | 0 | 0 | 2 | 0 | |
| Hyperglycemia | 2 | 0 | 0 | 0 | |
| Flatulance | 1 | 0 | 0 | 0 | |
| Dizziness | 1 | 0 | 0 | 0 | |
| Constipation | 1 | 0 | 0 | 0 |
Pharmacokinetics
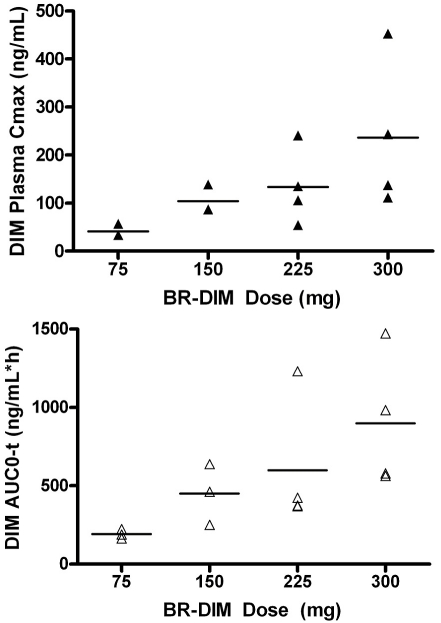
The pharmacokinetic parameters of DIM following a single-dose oral administration of BR-DIM for individual patients are summarized in Table 3. Following the oral administration of the first dose of BR-DIM, DIM was rapidly absorbed with the Tmax occurring at 2 to 4 h. The systemic exposure to DIM was increased with the BR-DIM dose escalation (Figure 1). Specifically, after oral administration of BR-DIM at the dose of 75, 150, 225, and 300 mg, DIM achieved a mean Cmax of 42, 104, 134, and 236 ng/ml, respectively, and a mean AUC0-t of 192, 450, 599, and 899 ng/ml*h, respectively (Table 3 and Figure 1). The observed systemic exposure (Cmax and AUC) of DIM following oral administration of a single dose of BR-DIM in the cancer patients was in agreement with that reported previously in healthy subjects [21]. Trough plasma samples were obtained from 13 and 9 patients on day 8 and day 28, respectively. The trough plasma concentrations of DIM in individual patients and the mean trough levels (with % Coefficient of Variation [%CV]) at each BR-DIM dose level are presented in Table 4. These data suggested that continued relatively stable systemic exposure to DIM was achieved following twice daily oral administration of BR-DIM.
Table 3.
BR-DIM PK parameters after the oral administration of the first dose of BR-DIM on Cycle 1, Day 1
| Patient # | Dose (mg) | Tmax (h) | Cmax (ng/ml) | AUC0-t (ng/ml h) | Mean Cmax (CV%) (ng/mL) | Mean AUC0-t (CV%) (ng/mL*h) |
|---|---|---|---|---|---|---|
| 1 | 75 | 3.0 | 57 | 224 | 42 (32%) | 192(16%) |
| 2 | 75 | 4.0 | 34 | 189 | ||
| 3 | 75 | 3.0 | 34 | 163 | ||
| 4* | 150 | 3.0 | 139 | 638 | 104(29%) | 450 (43%) |
| 5* | 150 | 3.0 | 87 | 251 | ||
| 6 | 150 | 4.0 | 87 | 462 | ||
| 7 | 225 | 4.0 | 241 | 1231 | 134 (59%) | 599 (70%) |
| 4* | 225 | 4.0 | 54 | 374 | ||
| 5* | 225 | 2.0 | 106 | 370 | ||
| 8 | 225 | 4.0 | 135 | 423 | ||
| 9 | 300 | 3.0 | 452 | 1472 | 236 (66%) | 899 (48%) |
| 10 | 300 | 2.0 | 138 | 578 | ||
| 11 | 300 | 2.0 | 244 | 984 | ||
| 12 | 300 | 3.0 | 112 | 563 |
Figure 1.
DIM maximum plasma concentration (Cmax) (a) and area under the plasma concentration-time curve (AUC0-t) as a function of BR-DIM dose. The symbols (▴ and Δ) represent the individual values for Cmax and AUC0-t; the dash lines represent the mean value at each dose level.
Table 4.
Trough plasma levels of DIM on Days 8 and 28 followingoral administration of BR-DIM twice daily
| Patient # | Dose (mg) | Day | Trough DIM (ng/ml) | Mean trough (%CV) (ng/ml) |
|---|---|---|---|---|
| 1 | 75 | 8 | 9.9 | 12.6(71%) |
| 2 | 75 | 8 | 22.5 | |
| 3 | 75 | 8 | 5.3 | |
| 4 | 150 | 8 | 22.1 | 35.4 (34%) |
| 5 | 150 | 8 | 27.2 | |
| 6 | 150 | 8 | 45.3 | |
| 7 | 225 | 8 | 117.0 | 48.3 (96%) |
| 8 | 225 | 8 | 33.9 | |
| 9 | 225 | 8 | 29.2 | |
| 10 | 225 | 8 | 13.2 | |
| 11 | 300 | 8 | 43.4 | 32.6 (40%) |
| 12 | 300 | 8 | 18.1 | |
| 13 | 300 | 8 | 36.3 | |
| 1 | 75 | 28 | 8.4 | 17.7 (46%) |
| 2 | 75 | 28 | 23.7 | |
| 3 | 75 | 28 | 20.9 | |
| 4 | 150 | 28 | 7.0 | 24.7 (86%) |
| 5 | 150 | 28 | 19.1 | |
| 6 | 150 | 28 | 48.1 | |
| 10 | 225 | 28 | 39.6 | 39.6 (ND) |
| 11 | 300 | 28 | 13.7 | 17.2 (ND) |
| 12 | 300 | 28 | 20.7 |
Response
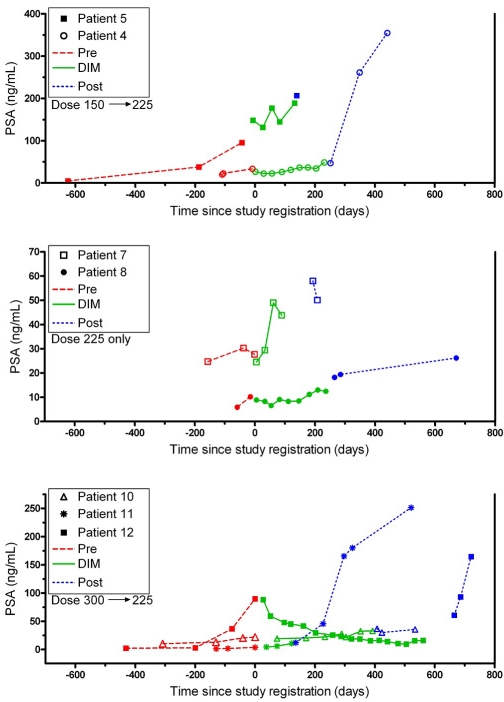
The individual PSA levels over time are shown in Figure 2 for the 7 patients who ended their BR-DIM treatment at the RP2D of 225 mg twice daily. One patient without AH at 225 mg dose level showed a > 50% PSA response (PR). A patient at the 225 mg dose level with intermittent BR-DIM dosing due to AH had PSA stabilization. The other 10 patients had an initial deceleration of their PSA rise (decrease in slope), but eventually progressed based on continual PSA rise or evidence of metastatic disease. The one patient (ID # 4) in the original 150 mg cohort who was intra-patient dose escalated experienced a PSA drop of 11.2 ng/mL (33%), from a baseline PSA level of 33.7 to a nadir of 22.5 ng/mL. One patient in the original 300 mg cohort (ID # 12) was on study for 18.5 months and experienced a dramatic PSA decrease of 80.3 ng/mL (90%), from a baseline PSA level of 89.7 to a nadir of 9.4 ng/mL.
Figure 2.
PSA Response Curves for 7 patients. The actual PSA values are plotted overtime for each of the 7 patients who ended their BR-DIM treatment at the RP2D of 225 mg twice daily. The different line types identify three time periods: pre-, during, and post-therapy with BR-DIM. The upper graph is for the 2 patients (ID # 4, 5) who started at 150 mg twice daily; the middle graph is for the 2 patients (# 7, 8) who started at 225 mg twice daily; and the lower graph is for the 3 patients (# 10, 11, 12) who started at 300 mg twice daily. Patient ID numbers in Figure 2 match those in Table 3. To accommodate the high variability in PSA values while still providing adequate visibility in their lower range, and to keep PSA in its original units for easier visual interpretation, different scalings on each of the three Y-axes were utilized.
Quality of Life
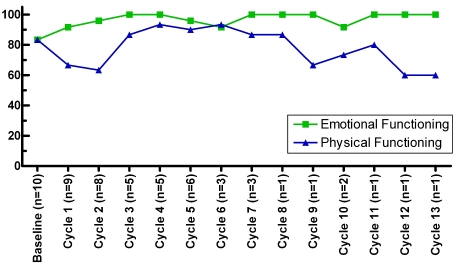
Ten patients completed QoL questionnaires for up to 13 cycles. The mean number of cycles was 6 (SD=3.81); median was 5.5 cycles (range: 1-13). QoL measures of Emotional Functioning and Physical Functioning varied greatly within and across the patient population over time while on treatment (Figure 3). Although our ability to assess QoL data beyond treatment Cycle 7 was limited, results suggest that the patients' Emotional Functioning may have held up somewhat better over time than their Physical Functioning.
Figure 3.
QoL scores over time. Median scores over time for Emotional Functioning and for Physical Functioning are shown for patients who completed QoL questionnaires.
Discussion
Prostate cancer patients who are castrate resistant without any clinical or radiologic evidence of metastatic disease, but who have biochemical PSA relapse comprise a challenging group to manage. There are no standard of care treatments, but men are understandably anxious because of their rising PSA values and the progression to obvious metastatic disease is an impending event. Developing novel non-toxic treatments that can potentially reduce the PSA levels and/or delay progression to obvious metastases for this group of men is important.
Epidemiologic, pre-clinical, and clinical data strongly suggest that cruciferous vegetables and their active components including in vivo derivatives of I3C, particularly dimeric 3, 3′-diindolylmethane (DIM), may indeed be active in prostate cancer. However, crystalline DIM is highly insoluble in both water and oil. Microdis-persion formulation technology provides enhanced oral bioavailability for DIM from BR-DIM, compared to highly insoluble crystalline DIM [22]. BR-DIM contains pharmaceutically pure DIM, microdispersed in spray-dried starch particles. Prior to supplementing prostate cancer patients with BR-DIM in larger studies, it is critical to first establish the appropriate dose as well as BR-DIM's potential effects on PSA. One of the constant criticisms of nutriceutical compounds and their role in prostate cancer is the lack of available evidence to justify appropriate dosing and toxicity management, as often demanded and required in the development of cytotoxic or biologic therapies. Without appropriate pharmacokinetics and standard toxicity evaluation for most nutriceutical compounds, physicians have difficulty in recommending such agents with sufficient confidence.
Reed et al. reported their results of using BR-DIM in healthy subjects, including men in which a single dose of BR-DIM at 200 mg was well tolerated [21]. Their pharmacokinetic data show a linear dose-exposure relationship with data supporting a twice daily dosing as was conducted in our study. BR-DIM was well tolerated by our 12 prostate cancer patients, who experienced minimal toxicities and reasonable quality of life. The two patients with asymptomatic AH continued to have asymptomatic AH even after discontinuation of BR-DIM, suggesting that this grade 3 toxicity may not be related to drug. After conducting this Phase I study with a standard dose escalation design, the MTD and the RP2D of BR-DIM was 225 mg twice daily. Additional studies utilizing BR-DIM in prostate cancer are warranted.
Acknowledgments
We gratefully acknowledge each of the 12 patients who participated in this Phase I study, and Daryn Smith, M.S. who provided statistical computing support. We acknowledge contributions from Michael A. Zeligs, M.D and BioRe-sponse, LLC, Boulder, CO, who provided technical support and production of BR-DIM, and qualified capsules.
This study was supported by the Fund for Cancer Research and Detroit Medical Center Institute for Oncology and Allied Diseases. Partially supported by NIH grants CA-62487 and Cancer Center Support Grant CA-22453.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50:93–99. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Cannon GM, Jr, Walsh PC, Partin AW, Pound CR. Prostate-specific antigen doubling time in the identification of patients at risk for progression after treatment and biochemical recurrence for prostate cancer. Urology. 2003;62(Suppl 1):2–8. doi: 10.1016/j.urology.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341:1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Gil T, Collette L, Pierart M. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 9.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–117. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 10.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Kristal AR. Diet and trend in prostate-specific antigen: inferences for prostate cancer risk. J Clin Oncol. 2002;20:3570–3571. doi: 10.1200/JCO.2002.20.17.3570. [DOI] [PubMed] [Google Scholar]
- 13.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 14.Li Y, Li X, Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar FH, Rahman KM, Li Y. Bax translocation to mitochondria is an important event in inducing apoptotic cell death by indole-3-carbinol (I3C) treatment of breast cancer cells. J Nutr. 2003;133:2434S–2439S. doi: 10.1093/jn/133.7.2434S. [DOI] [PubMed] [Google Scholar]
- 17.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. J Biol Chem. 2003;278:21136–21145. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 18.Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in huma prostate cancer cell line, PC3, by 3,3′-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3′-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004;61:153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- 20.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, Mayo MS, Crowell JA, Hurwitz A. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3′-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 21.Reed GA, Sunega JM, Sullivan DK, Gray JC, Mayo MS, Crowell JA, Hurwitz A. Single-dose pharmacokinetics and tolerability of absorption-enhanced 3,3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:2619–2624. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]