Abstract
As interest in lignocellulosic biomass feedstocks for conversion into transportation fuels grows, the summative compositional analysis of biomass, or plant-derived material, becomes ever more important. The sulfuric acid hydrolysis of biomass has been used to measure lignin and structural carbohydrate content for more than 100 years. Researchers have applied these methods to measure the lignin and structural carbohydrate contents of woody materials, estimate the nutritional value of animal feed, analyze the dietary fiber content of human food, compare potential biofuels feedstocks, and measure the efficiency of biomass-to-biofuels processes. The purpose of this paper is to review the history and lineage of biomass compositional analysis methods based on a sulfuric acid hydrolysis. These methods have become the de facto procedure for biomass compositional analysis. The paper traces changes to the biomass compositional analysis methods through time to the biomass methods currently used at the National Renewable Energy Laboratory (NREL). The current suite of laboratory analytical procedures (LAPs) offered by NREL is described, including an overview of the procedures and methodologies and some common pitfalls. Suggestions are made for continuing improvement to the suite of analyses.
Keywords: Summative compositional biomass analysis, lignocellulose, cellulose, hemicellulose, biofuels, 72% sulfuric acid hydrolysis, Klason lignin, laboratory analytical procedure, LAP, NREL
Introduction
Lignocellulosic biomass is typically nonedible plant material composed primarily of the polysaccharides cellulose and hemicellulose. The third major component is lignin, a phenolic polymer that provides structural strength to the plant. Technology for producing biofuels (such as ethanol, butanol, or various hydrocarbons) and biobased chemicals from lignocellulosic material is experiencing significant advances in an effort to meet global energy and chemical needs (1, 2). Examples of lignocellulosic biomass materials considered as feedstocks for bioethanol production include crop residues such as corn stover and wheat straw, woody residues from forest thinning and paper production, cool- and warm-season grasses such as switchgrass and fescue, and crops such as sorghum.
Accurate feedstock compositional analysis enables evaluation of conversion yields and process economics due to changes in feedstock or process design. Accurate measurement of biomass carbohydrate content is of prime importance because it is directly proportional to ethanol yield (L Mg−1) in biochemical conversion processes (3). The minor components in biomass can include protein, ash, organic acids, and other nonstructural materials. Although these individual components may make up only a small fraction of the feedstock, their presence will become significant in the running of an industrial-scale biorefinery. Quantifying minor components also enables summative mass closure to account for all constituents in a given feedstock.
The purpose of this paper is to review the history and lineage of biomass compositional analysis methods based on a sulfuric acid hydrolysis. These methods have become the de facto procedure for biomass compositional analysis. Many analogous versions of sulfuric acid methods have been previously published. We describe them in this paper and relate how they have progressed over time and give special emphasis to changes to the hydrolysis conditions. However, we discuss improvements to the instrumental analysis of biomass-derived sugars as well.
The methods appear to have originated as wood lignin isolation procedures and evolved into summative compositional analysis methods for biomass feedstocks. The methods are labor intensive and require attention to detail. They are empirical, and differences in technique can affect the final results. The methods reviewed here utilize a strong sulfuric acid solution (typically 72 wt %) in a primary hydrolysis, followed by dilution with water and a secondary high-temperature (100−125 °C) hydrolysis. This procedure hydrolyzes the polymeric carbohydrates into soluble monosaccharides, leaving behind a lignin-rich residue that is vacuum-filtered and measured gravimetrically. The liberated sugars in the hydrolysate solution are measured as monomers to quantify the carbohydrate fraction of the sample. This hydrolysis procedure, when combined with a suite of other tests, provides a quantitative summative compositional analysis of lignocellulosic biomass, ideally accounting for 100% of the original material.
The composition of different feedstocks can vary greatly due to the complex and heterogeneous nature of biomass. The composition of corn stover, for example, has been shown to be variable. Factors such as harvest year, environment, and variety result in glucan, lignin, or xylan values ranging up to 10% on an absolute basis (4). Similar variations in component concentrations have been seen within the same corn plant between its anatomical fractions (see ref (5), p 1467). Feedstock variability will affect process economics; therefore, robust and accurate analysis methods are crucial to determining the chemical composition of feedstocks.
As we show below, researchers have applied these methods to measure the lignin and structural carbohydrate content of woody materials, estimate the nutritional value of animal feed, analyze the dietary fiber content of human food, compare potential biofuels feedstocks, and measure the efficiency of biomass-to-biofuels processes. Researchers have also worked to improve the methods by speeding the hydrolysis steps, using smaller sample aliquots, and using better sugar detection techniques. We trace changes to the biomass compositional analysis methods through time to the biomass methods currently used at the National Renewable Energy Laboratory (NREL). We also present our current suite of analytical methods (laboratory analytical procedures or LAPs) used at NREL for biomass compositional analysis. Included is a brief outline of each LAP, as well as major interferences or pitfalls. It is important to note that these outlines are not intended to be a complete description of the procedures; rather, they are intended only as an overview. Complete procedures are available in the Supporting Information or from NREL (6), and updates are posted to the Website when available.
Early Sulfuric Acid Wood Lignin Methods (prior to 1920)
The use of a two-stage sulfuric acid hydrolysis for the analysis of lignin dates to the turn of the 20th century, although the use of concentrated acid to release sugars from wood dates to the early 19th century (7). Klason, in 1906, is often credited as the first to use sulfuric acid to isolate lignin from wood (7−9). The method became named after Klason, and the insoluble residue from the test is known as “Klason lignin.” An English translation of a Klason paper, from this period (10), describes his attempt to determine the structure of spruce wood lignin. According to Brauns (7), Klason’s method originally used 72 wt % sulfuric acid; he later reduced this to 66 wt % to gelatinize the wood. He filtered the solids and subjected them to a second hydrolysis in 0.5 wt % hydrochloric acid.
Although Klason is generally credited as being the first to use sulfuric acid for lignin analysis, Sherrard and Harris (11) credit the use of sulfuric acid to Fleschsig in 1883, Ost and Wilkening in 1912, and König and Rump in 1913. According to Harris (12), Fleschsig, in 1883, dissolved cotton cellulose and converted it nearly quantitatively into sugars using strong sulfuric acid followed by dilution and heating. According to Browning (13), Ost and Wilkening introduced the use of 72 wt % sulfuric acid for lignin determinations in 1910. A translated paper by Heuser (14) credited König and Ost and Wilkening for the sulfuric acid lignin method. Dore (15) described several improved analytical methods (cellulose, lignin, soluble pentosans, mannan, and galactan) for the summative analysis of coniferous woods. The discrepancies in attribution may be due to differing definitions for the method cited (e.g., first to use acid to determine lignin, first to use sulfuric acid, first to use 72 wt % sulfuric acid, etc.) and to missed citations across continental distances in the early 20th century.
Wood Lignin Isolation Methods (1920−1940)
Table 1 summarizes the important changes to the hydrolysis methods, highlighting the specific analysis conditions and sugar detection techniques used by different groups. Researchers at the U.S. Department of Agriculture (USDA) Forest Products Laboratory (FPL) made numerous contributions to the wood lignin and later wood sugar methods. In 1922, Mahood and Cable (16) chose a 72 wt % sulfuric acid lignin method based on work by Ost and Wilkening. The authors analyzed sawdust after extracting it in an alcohol/benzene solvent. They used a 16 h, room temperature, 72 wt % primary hydrolysis step followed by dilution with water to 3 wt % acid for a 2 h, reflux boiling secondary hydrolysis. In 1928, Bray (17) published a collection of analytical methods for the analysis of pulps and pulp woods in the Technical Association of the Pulp and Paper Industry (TAPPI) section of the Paper Trade Journal. This is an early example of the relationship between literature methods and methods promulgated by standards organizations. In 1932, Sherrard and Harris (11) gave an extensive history of different lignin isolation methods plus added hot and cold water extractions to the sample preparation. They tested different primary hydrolysis conditions (70 wt % sulfuric acid, 10 °C) on isolated maple cellulose (not whole wood). Their conditions minimized carbohydrate charring into insoluble products that would contaminate large-scale lignin preparations. Ritter et al. (18), that same year, studied different hydrolysis temperatures and times and suggested modifications (2 h, 20 °C) to the primary hydrolysis from the Mahood and Cable method (16). This was found to give lower lignin values (i.e., less interferences) with a faster and more consistent primary hydrolysis. Ritter and Barbour (19), in 1935, suggested running a third 95% alcohol extraction, prior to the alcohol/benzene and water extractions, on some types of wood, such as redwood and white oak, to remove catechol tannins prior to lignin determination.
Table 1. Sulfuric Acid Hydrolysis Conditions Used by Selected Researchers for the Compositional Anlaysis of Wood and Biomass (Notable Changes to the Methods Are Bolded).
| primary hydrolysis |
secondary hydrolysis |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| author | year | substrate | drying method | sample particle size | extraction solvent(s) | extraction time | sample amount | H2SO4 concn | primary acid amt | temp | time | secondary acid concn | amt of water added for secondary | temp | time | sugar detection method |
| Mahood and Cable (16) | 1922 | eucalyptus and Western white pine | air-dried | 80−100 mesh | min boiling pt alcohol/benzene | 4 h | 2 g | 72% | 10 × sample wt | room temp | 16 h | 3% | n/a | reflux boil | 2 h | none |
| Sherrard and Harris (11) | 1932 | isolated cellulose from sugar maple | air-dried | 60−80 mesh | 1:2 alcohol benzene then hot and cold water | 50−60 h; several hours | 8 g | 70% | 80 cm3 | 10 °C | 16 h | 3% | 3 L | reflux boil | 4 h | none |
| Ritter et al. (18) | 1932 | sugar maple, white spruce, incense cedar, catalpa, mesquite |
air-dried | 60−80 or 80−100 mesh | alcohol-benzene, hot water | 4 h then 3 h | 2 g | 72% | 25 cm3 | 20 °C | 2 h | 3% | not described | reflux boil | 4 h | none |
| Ritter et al. (20) | 1933 | isolated cellulose from spruce | n/a | sawdust | n/a | n/a | 1 g | 72% | 20 cm3 | 8−45 °C | 2 or 6 h | 4% | not described | reflux boil | 4 h | reduced copper from nitric acid solution |
| Saeman et al. (21) | 1945 | 15 hardwoods and softwoods | air-dried | 30 mesh | not described | not described | 0.35 g cellulose equiv | 72% | 5 mL chilled (15 °C) | 30 °C | 45 min | 4% | 140 mL | autoclave 15 lb or reflux boil | 1 h of autoclaving or 4.5 h of boiling | reducing sugar |
| Saeman et al. (22) | 1945 | wood sugar hydrolysates | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | electrometric titration, reducing sugar, or yeast sorption |
| Saeman et al. (23) | 1954 | wood or wood pulps | air-dried | 30 mesh | not described | not described | 0.30 g of cellulose equiv | 72% | 3 mL | 30 ± 0.5 °C | 1 h | 4% | 84 mL | autoclave at 15 ± 1 psi | 1 h | paper chromatography |
| Sloneker (36) | 1971 | food, feed, feedlot wastes (corn) | 4 h at 100 °C | 60 or 80 mesh | not described | not described | 20−30 mg | 72% | 0.3 mL | 30 °C | 1 h | 1 N | 8.4 mL | autoclave at 120 °C | 1 h | gas−liquid chromatography of alditol acetate derivatives |
| Effland (35) | 1977 | wood | not described | through at least 20 mesh | ethanol-benzene, plus ethanol for high tannin woods | 25 cycles/min | 200 or 300 mg | 72% | 1 mL per 0.1 g of sample | 30 ± 0.5 °C | 1 h | not described | 28 mL for each mL of acid | autoclave at 120 °C or boil for 4 h | 1 h | TAPPI spectrophotometric (T 250 pm-75) |
| Slavin and Marlett (38) | 1983 | food and fecal samples | n/a | n/a | neutral detergent fiber | 1 h | ∼2 g | 72% | 10−15 g | 24 °C | 3 h | 2 N | 70−100 g | 100 °C | 2 h | HPLC |
| Pettersen et al. (48) | 1984 | 4 hardwoods, 2 softwoods | 105 °C dried | 40 mesh | some none, some 9:1 acetone/water, some added 1:1 toluene/ethanol | not described | ∼200 mg | 72% | not described | 30 °C | 1 h | 3% | not described | 120 °C | 1 h | HPLC vs paper chromatography |
| Theander and Westerlund (44) | 1986 | food | n/a | analysis on food flours | sonication 80% EtOH followed by light petroleum ether | 3× 15 min then 2 × 10 min | 150−250 mg | 72% or 12 M | 3 mL | 30 °C | 1 h | not described | 84 mL | autoclave at 125 °C | 1 h | gas−liquid chromatography of alditol acetate derivatives |
| Kaar et al. (55) | 1991 | wood | air-dried | comminuted wood | 2:1 benzene/95% EtOH, 95% ethanol, boiling water | ?, ?, 4 h | 0.35 g | 72% | 3 mL | 30 °C | 1 h | 4% | 84 mL | autoclave 120 °C in sealed bomb | 1 h | HPLC |
| Pettersen and Schwandt (50) | 1991 | wood | not described | 40 mesh | 2:1 benzene/EtOH or 9:1 acetone/ water, followed by 1:1 toluene/ethanol | not described | 50 mg | 72% | 0.5 mL | 30 °C | 1 h | 4% | not described | 120 °C | 1 h | anion chromatography |
| Milne et al. (59) | 1993 |
Populus deltoides, Pinus radiata, wheat straw, bagasse |
air-dried | −20/+74 mesh | 95% EtOH | 6 h | 250 mg | 72% | 3 mL | 30 °C | 1 h | n/a | 84 mL | 125 °C | 1 h | GC or HPLC |
Wood Sugar Quantification Methods (1930−1970)
Ritter et al. (20), in 1933, sought to determine the causes for the wide variability in reducing sugar yields (not lignin yields) seen by previous researchers. They tested different primary hydrolysis time and temperature conditions on isolated spruce cellulose (not whole wood) and found maximum yields at 6 h at 16 °C or 2 h at 35 °C. They tested 4 wt % sulfuric acid, rather than 3 wt %, for the secondary hydrolysis without explanation. Saeman et al. (21), in 1945, suggested changes to the Ritter et al. methods (18, 20) in an effort to speed analysis times while measuring the effect on reducing sugar yield rather than on lignin yield. The authors adjusted both hydrolysis steps to speed the analysis. They tested a 45 min, 30 °C, primary hydrolysis step and a 1 h autoclave secondary hydrolysis, instead of a 4 h boiling water reflux step. That same year, Saeman et al. (22) described three sugar detection techniques for analyzing wood acid hydrolysates. The authors reported an electrometric titration, a reducing sugar assay, or a yeast sorption (i.e., a standardized fermentation) method to measure the sugars released from lignin hydrolyses. Saeman et al. (23), in 1954, published an updated version of their earlier method (21) in the TAPPI journal. The authors added a paper chromatography sugar detection method and introduced the use of loss factors to correct the sugars for degradation during hydrolysis. Paper chromatography became the standard sugar separation and quantification method for nearly 20 years. A useful compendium of wood and wood pulp methods was written in 1967 by Moore and Johnson at the FPL (24).
Wood Lignin Methods Applied to Herbaceous Feedstocks (1930−1955)
Peterson et al. (25), in 1932, tested different primary hydrolysis temperatures (with a constant 18 h duration) on lignin measurements when applied to corn stalks and wood. Also in the 1930s, Norman and Jenkins (26, 27) and Norman (28) studied the causes of interferences with the lignin method applied to straws and some grasses. They found that certain carbohydrates, protein, or nitrogenous substances could coprecipitate with lignin. Dunning and Lathrop (29), in 1945, described a slightly modified hydrolysis method [based on Ritter et al. (18)] and applied it to an acid saccharification process on agricultural residues. Four years later, Dunning and Dallas (30) described a faster hydrolysis method that included a 55 °C, 5 min primary hydrolysis and a 25 min autoclave secondary hydrolysis. This method was used for control analyses supporting a semiworks pilot plant (∼1000 pounds per run) hydrolyzing agricultural residues. The authors describe factors used to correct values to corresponding Association of Official Analytical Chemists (AOAC) methods for pentosans, which suggests the methods were precise and fast, but not necessarily accurate. Ellis (31), also in 1949, described an AOAC interlaboratory study using mature timothy hay, immature Sudan grass, and sheep feces to test for lignin and gravimetric cellulose. They found the lignin method to be reasonably reproducible, but the gravimetric cellulose method to be more variable. In 1954, Harwood (32) analyzed grasses and clovers by prehydrolyzing them in 1 N (4.76 wt %) sulfuric acid to remove most of the pentosans. The remaining solids were hydrolyzed in 72 wt %/1 N sulfuric acid for the analysis of the cellulose-rich solids.
Applications to Food, Feed, and Dietary Fiber (1970−1990)
Prior to the 1970s, the sulfuric acid methods had been applied mainly to the analysis of wood [cf. Hardell and Theander (33), Musha and Goring (34), and Effland (35)]. Now these methods would be applied to different substrates. In 1971, Sloneker (36), of the USDA Northern Regional Research Laboratory, tested a miniaturized version of the Saeman hydrolysis (23) to the analysis of total aldoses in foods, feeds, and feedlot wastes. He added a new sugar detection method that used gas−liquid chromatography (GC) to quantify the hydrolyzed sugars, after derivatizing them to alditol acetates. Nearly a decade later, Krull and Inglett (37) described similar methods to analyze for neutral carbohydrates in agricultural residues. Slavin and Marlett (38), in 1983, tested high-performance liquid chromatography (HPLC) using a heavy metal cation exchange column for neutral sugars in acid-hydrolyzed neutral detergent fiber extracted food and fecal samples. The 72 wt % sulfuric acid hydrolysis methods were adapted for measurement of dietary fiber by various researchers [Southgate (39), Southgate et al. (40), Theander and Aman (41), Selvendran et al. (42), Englyst et al. (43), Theander and Westerlund (44), and Hoebler et al. (45)] for dietary fiber analyses in human nutrition research, and these methods were later adapted for biomass analyses.
Early Applications to Biofuels Feedstocks (1980−1998)
In 1984, Grohmann et al. (46) described the hydrolysis of wheat straw using less severe hydrolysis conditions. They found low glucose yields (∼50%) on Sigmacell 50 using the regular Moore and Johnson method (24), and they attributed the low yield to undetected sulfonated glucose. In contrast, later research at NREL showed good component closure on five feedstocks (5) and again on 15 southern hardwood and softwood species (47) using the original Moore and Johnson method (24). That same year, Pettersen et al. (48) of FPL compared the analysis of wood hydrolysate sugars by HPLC with paper chromatography, based on Saeman’s method (23). The authors found some statistically significant differences between the methods, but they were judged to be small enough to make the HPLC method useful. Pettersen (49), also in 1984, published wood composition data collected from 1927 to 1968 and briefly described the FPL analytical methods used to generate the data. In 1991, Pettersen and Schwandt (50) used anion chromatography and pulsed amperometric detection (PAD) to analyze wood sugar solutions. This was followed in 1998 with a paper by Davis (51) describing an updated anion chromatography/PAD method with improved peak resolution and sample throughput.
In 1985, Theander, from the Swedish University of Agricultural Sciences in Uppsala, applied the sulfuric acid dietary fiber methods to plant materials for use as biofuels feedstocks (52). In 1991, he added 80% ethanol to extract lignocellulosic materials prior to sulfuric acid hydrolysis (53). This method, known as the Uppsala method, was tested in a 1995 collaborative study (54) for total dietary fiber in food samples. Kaar et al. (55), in 1991, developed “a rapid, reliable summative analysis method” for the analysis of wood. The authors performed the autoclave step in sealed bombs, thus retaining the volatile acetic acid and 2-furaldehyde, and analyzed the hydrolysates by HPLC. The authors measured hydroxymethylfurfural (HMF), furfural, acetic acid, levulinic acid, and uronic acids in the hydrolysates and added these amounts into their glucan and xylan components, instead of measuring sugar loss factors. Kaar and Brink applied their summative method to nine North American woods (56) and closed mass balance (99.1 ± 0.5 wt %).
NREL Contributions to Biomass Methods (1990−2007)
In the 1990s, NREL researchers applied the sulfuric acid methods for biofuels research. Milne et al. (57), in 1990, wrote a book containing a comprehensive collection of biomass analysis methods. Researchers from NREL led a 1992 International Energy Agency (IEA) round robin study (58−60) to test biomass analysis methods on four feedstocks for biofuels production. More than 20 laboratories worldwide analyzed these samples to quantify the variability of the method (based on the Uppsala method (53)). Results from this study determined the reported compositions of the four National Institute of Standards and Technology (NIST) biomass feedstock Reference Materials (RMs). These four RMs [sugar cane bagasse (RM 8491), Eastern cottonwood (RM 8492), Monterey pine (RM 8493), and wheat straw (RM 8494)] are available through NIST (61). Both HPLC and GC [based on Blakeney’s (62) alditol acetate GC procedure] sugar detection methods were used to quantify carbohydrates, and the results were surprisingly similar, considering different HPLC column types were used. A 1994 article by Ehrman and Himmel at NREL (63) described method adjustments for biofuels conversion process intermediate samples. The authors reported that pretreated slurries, alkaline pretreatment samples, and fermentation slurries need to be separated into solid and liquor fractions for separate analysis. This was supported by Hsu and Nguyen (64). Ehrman wrote a useful 1996 book chapter (65) reviewing the available analytical methods for feedstocks, process intermediates, and end products. Decker et al. (5) wrote a comprehensive 2007 book chapter describing all facets of biomass research at NREL (fermentation, biomass analysis, enzymatic saccharification, chemical deconstruction catalysts, and thermochemical conversion).
Contemporary Contributions to Biomass Methods (1995−2010)
Recently proposed modifications to the sulfuric acid method include a 1997 paper from Thammasouk et al. (66) describing the effects of water or ethanol extractions on the compositions of switchgrass, corn stover, and fescue. Generally, extracted biomass had lower lignin or glucan values compared to the native biomass. These extractions were assumed to have removed interferences and led to more accurate lignin or glucan values. A decade later, Foyle et al. (67) compared different hydrolysis methods for the analysis of waste paper and wheat straw and settled on a modified version of the Grohmann et al. method (46). Also that same year, Moxley and Zhang (68) reported a modified quantitative saccharification method. They suggested that a separate 1% acid hydrolysis corrected for loss with an associated 1% sugar recovery standard is more appropriate to correct the labile sugars, xylose and arabinose. They did not present complete summative component closure data that would help confirm this hypothesis. In 2007, Chen et al. (69) analyzed the components found in water extractives from corn stover. Hames wrote a 2009 book chapter (70) describing compositional analysis methods applicable to biomass samples. Dien wrote a 2010 book chapter (71) discussing sulfuric acid biomass compositional analytical methods and their use for yield calculations needed to compare pretreatment experiments. Chen et al. (72), in 2010, analyzed the water-extractable components found in switchgrass.
Methods Promulgated by Standards Organizations
Two main versions of the sulfuric acid hydrolysis method emerge from this literature review, differing mainly in sample amount and secondary hydrolysis heating technique. The first method is by Ritter et al. (18), known at FPL as the “standard” method (2 g of sample, 25 mL of 72% acid for 2 h at 20 °C primary hydrolysis, and 4 h in reflux boiling at 3% acid secondary hydrolysis). The second method is by Saeman et al. (23), known at FPL as the “modified” method (0.3 g of sample, 3 mL of 72% acid for 1 h at 30 °C primary hydrolysis, and 1 h in autoclave at 4% acid hydrolysis). These two methods were shown to give equivalent results, for gravimetric lignin only, by Moore and Johnson (24) and by Effland (35).
These literature methods, described above, have been adapted and promulgated at various times by different standards organizations, although they can be traced to these two main hydrolysis methods. Table 2 shows the relationships among biomass methods as promulgated by different organizations and their relationship to the original literature methods. Related methods from different organizations are shown on the same lines. Browning (8) reports the Ritter method (18) as the basis for the TAPPI T-13 m method “Lignin in Wood” (later combined with T-222 “Acid-Insoluble Lignin in Wood and Pulp”) and the American Society for Testing and Materials (ASTM) D1106 “Standard Test Method for Acid-Insoluble Lignin in Wood”. The Saeman hydrolysis (23) method appears to be the basis for the TAPPI T-249 method “Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gas−Liquid Chromatography” and the ASTM D1915-63 “Standard Test Method for Chromatographic Analysis of Chemically Refined Cellulose” (later replaced with ASTM D5896-96 “Standard Test Method for Carbohydrate Distribution of Cellulosic Material”).
Table 2. Related Biomass Methods Promulgated by Different Standards Organizations (Related Methods Are Located on the Same Line).
| Forest Products Lab | TAPPI (73) |
ASTM (74) |
NREL (6) | ||||
|---|---|---|---|---|---|---|---|
| author | method no. | title | method no. | title | method no. | title | title |
| Ritter et al. (18) (standard method) | T 13 os 54; later T 222 om-06 | Lignin in Wood (original); Acid-Insoluble Lignin in Wood and Pulp (later) | D 1106-96 (2007) | Standard Test Method or Acid-Insoluble Lignin in Wood | n/a | n/a | n/a |
| Saeman et al. (23) (modified method) | T 249 cm-00 | Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gas−Liquid Chromatography | ASTM D1915-63 (1989) withdrawn, replaced by D5896 | Standard Test Method for Chromatographic Analysis of Chemically Refined Cellulose (withdrawn 1996) | E 1721 | Standard Test Method for Determination of Acid-Insoluble Residue in Biomass | Determination of Structural Carbohydrates and Lignin in Biomass |
| ASTM D5896-96(2007) | Standard Test Method for Carbohydrate Distribution of Cellulosic Material | E 1758 | Determination of Carbohydrates in Biomass by High Performance Liquid Chromatography | ||||
NREL Adaptations of Wood Hydrolysis Methods to Herbaceous Feedstocks
The current biomass analysis methods used by NREL are derived from the Saeman hydrolysis (23) as described by Moore and Johnson (24) or TAPPI T-249. We adapted the ethanol extraction method from the Uppsala method (53) and the sealed hydrolysis tubes and HPLC method described by Kaar et al. (55). NREL has posted this suite of methods online (6), and we describe the methods below. To increase their industrial relevance and distribute these methods more widely, NREL submitted, in 1995, a suite of biomass methods for dissemination through the ASTM Committee E48 on Biotechnology. These hydrolysis methods were collaboratively reviewed and issued as ASTM 1721 “Standard Test Method for Determination of Acid-Insoluble Residue in Biomass” and ASTM 1758 “Determination of Carbohydrates in Biomass by High Performance Liquid Chromatography”. The methods promulgated by TAPPI (73) and ASTM (74) are available on the Web.
Considerable progress has been made at NREL in the development of wet chemical and instrumental methods for analysis of biomass. We have assembled a suite of methods that gives nearly complete component closure of feedstocks and biomass conversion process intermediates. Modifications to these methods by NREL have resulted in analytical methods that can be applied to more than just woody materials, including methods optimized for certain agricultural residues and grasses. Herbaceous feedstocks, for example, can contain considerably more water-extractable components (30 wt % or more) than woody biomass. This requires the addition of an initial water extraction step prior to the traditional ethanol extraction of the feedstock. Sucrose quantification in the water extract was added to measure this nonstructural carbohydrate. We compared alternate methods of extraction, including traditional Soxhlet extractions and the Dionex accelerated solvent extraction (ASE) system. We found the ASE used smaller sample amounts and significantly increased sample throughput. The NREL calculation of feedstock-specific extinction coefficients and corresponding wavelengths minimized the interference of sugar degradation products. Amino acid profiles of the raw feedstock, coupled with total nitrogen determinations before and after water/ethanol extraction, have also been studied to determine the contribution of structural protein to the composition of herbaceous feedstock.
Alternate Biomass Sugar Analysis Methods
We have used HPLC as the default method to detect carbohydrates in biomass hydrolysates. The HPLC method has the advantage of simple sample preparation (no derivatization) and a simple isocratic HPLC separation with water as mobile phase and a refractive index detector (RID). We use lead cation (Pb2+) exchange columns, which give near-baseline resolution for the common biomass sugars (glucose, xylose, galactose, arabinose, and mannose). At NREL, we have also run GC carbohydrate methods. These have the advantage of baseline sugar resolution, although they require a multistep derivatization step prior to analysis. Additionally, we have sought more ideal chromatography methods that would combine baseline separation of the carbohydrates, fast injection cycle times, and greater ease of use. We have tested alternate HPLC columns, evaporative light scattering detection (ELSD), anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD), and capillary electrophoresis (CE), but we have not adopted them for routine use.
Description of Current NREL Biomass Analytical Methods (LAPs)
We have developed a system for coordinating the incremental tasks necessary to achieve complete biomass compositional analysis, utilizing our suite of LAPs and calculation spreadsheets. Included in this section is a brief outline of each LAP, as well as major interferences or pitfalls. These outlines are not intended to be complete descriptions of the procedures. Complete procedures are available in the Supporting Information or from NREL (6), and updates are posted to the Website when available.
The LAPs have been optimized for complete analysis of wood and corn stover. Applied correctly, they generally work well on woody feedstocks and herbaceous materials such as switchgrass, sorghum, and miscanthus. Feedstocks not mentioned here typically require additional methods to measure components not included in the LAP suite. Creating a tailored suite of LAPs to a particular feedstock should result in nearly 100% summative mass closure, assuming that the LAPs are appropriate for the feedstock type. These methods have not been optimized for the characterization of potential feedstocks such as municipal solid waste, corn fiber, or algae.
Intricate calculations are needed to determine the component concentrations from raw data. To address this issue, we have developed a series of calculation spreadsheets. These spreadsheets are available for multiple biomass types including woody materials, herbaceous feedstocks such as corn stover, and process intermediates. Each spreadsheet calculates the component closure on a dry weight basis and automatically applies the calculations from the LAPs in a coherent fashion.
LAP “Summative Mass Closure”
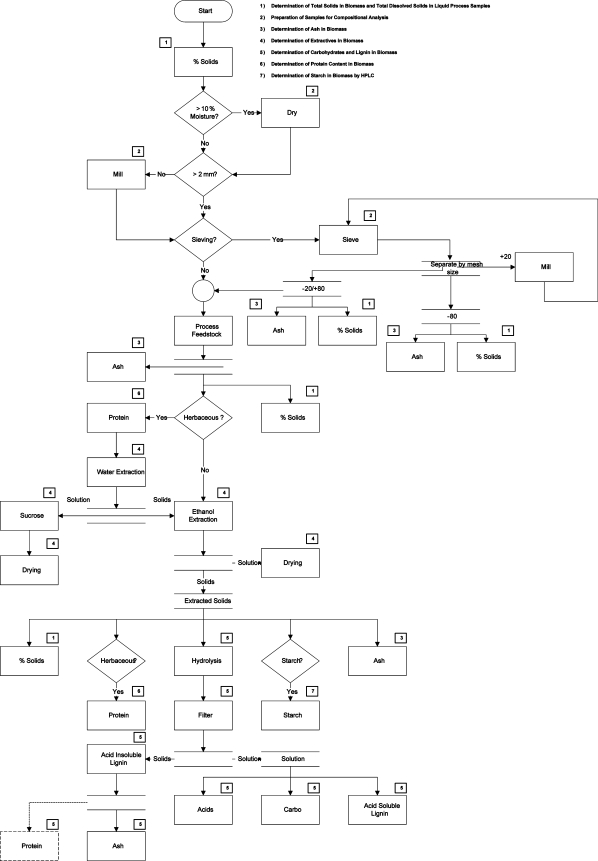
This summative mass closure LAP provides an overview of each biomass analysis LAP, including the methodology behind the procedures, critical points in the methods, and pitfalls in the analyses. It also includes a detailed flowchart of analyses (Figure 1) to assist in capturing all of the appropriate constituents in a feedstock. An example decision tree is provided in the LAP that describes the necessary decisions at each step in the analysis.
Figure 1.
Flowchart of analyses illustrating the path of a biomass feedstock through the LAP suite, including decisions necessary to customize the methods for different biomass types.
LAP “Preparation of Samples for Compositional Analysis”
The LAP for biomass sample preparation describes the drying, size reduction, optional particle size selection, and representative sampling necessary to obtain suitable samples for analysis. Biomass samples typically arrive from the field containing soil or other debris and significant moisture content. Proper sample preparation will minimize interferences in subsequent compositional analyses; all NREL biomass compositional analysis procedures assume that the samples have been prepared to meet these specifications.
Drying and size reduction are the first sample preparation considerations. We present several options for drying technique, including air-drying, 45 °C convection oven-drying, and lyophilization. Samples need to be dried under mild conditions (<45 °C) to preserve the biomass material and prevent microbial degradation. The technique chosen should be compatible with sample type and ambient laboratory conditions. Knife-milling the dry sample to a ≤2 mm particle size will prevent the incomplete hydrolysis of the carbohydrates during acid hydrolysis steps.
Sieving of a sample is sometimes necessary to accurately analyze a biomass feedstock, but it may interfere with representative sampling. Sieving was originally developed for the analysis of very homogeneous materials, such as wood samples. However, in herbaceous feedstocks, fines (−80 mesh) can contain a disproportionately large percentage of inorganic materials as compared to the bulk sample. Because certain anatomical fractions segregate disproportionally into the fines fraction, removal of the fines may cause an overall change in composition. In such cases sieving is not recommended to ensure the integrity of the sample.
LAP “Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples”
Due to the high variability in moisture content in biomass feedstocks, results of all biomass compositional analyses are reported on a dry weight basis, meaning that the weight contribution of moisture has been mathematically removed. This is crucial for comparing biomass samples and process intermediates on a consistent basis. This LAP describes the methods used to measure the total solids or moisture present in a solid or slurry biomass sample, as well as methods to determine dissolved solids in a liquor sample. In this LAP, a traditional 105 °C convection oven-drying procedure is described, along with an alternative solids determination using an automatic infrared moisture analyzer. It is not suitable for biomass samples that chemically change upon heating, such as acid- or alkaline-pretreated biomass samples.
LAP “Determination of Extractives in Biomass”
Nonstructural materials, components easily extracted with water or solvents, in biomass may contribute significantly, up to 30% or more, to the mass closure and will interfere with the subsequent characterization of carbohydrates and lignin. Herbaceous feedstocks tend to contain more nonstructural materials than woody feedstocks, and these methods were adjusted to better analyze herbaceous materials. This procedure covers the determination of soluble nonstructural materials in a biomass feedstock sample, including a two-step extraction process to remove water-soluble and ethanol-soluble material, and the determination of water-extractable sucrose, if necessary. The procedure describes two alternative methods for extraction. Traditional Soxhlet extraction is the industry standard for extraction and is robust for many types of biomass. The use of a Dionex ASE greatly reduces the amount of time and solvent needed for each extraction, but it may not be suitable for all biomass types.
The extractives-free material is used for subsequent analyses, and the measured extractives percentages are used to convert compositions from an extractives-free basis to an as-received, dry weight basis. This method is not suitable for samples that have been chemically or physically altered, such as by pretreatment, as this may change the solubility of other biomass constituents and bias the result high.
Extraction with ethanol is required for all biomass feedstock types to ensure the removal of waxy materials that coprecipitate during filtration of the acid hydrolysate, causing difficult filtration and elevated lignin values. When woody feedstocks are analyzed, ethanol extraction alone is generally sufficient to remove interfering extractable material. For herbaceous materials or biomass types with measurable amounts of free sugars in the sample, a water extraction is required before ethanol extraction. Water-soluble materials may include inorganic material, nitrogenous material, sugar acids, and nonstructural sugars (69), which interfere with structural carbohydrate analysis. This nonstructural material is difficult to quantify independently due to the large number of individual compounds, each at a relatively small concentration. Sucrose is the exception, and early removal and analysis of the nonstructural sucrose allow for better quantification of the structural glucan present in a feedstock.
LAP “Determination of Ash in Biomass”
Inorganic materials are present in both whole and extracted biomass samples. In addition to contributing significantly to total mass closure, inorganic material may interfere with acid hydrolysis. Structural ash is inorganic material that is bound in the physical structure of the biomass, whereas extractable ash is inorganic material that can be removed by washing or extraction. Extractable ash may partially be the result of field soil adhering to the biomass. To quantify both structural and extractable ashes, the analysis is performed on both whole and extracted materials. This method uses combustion in a furnace at 575 °C, with or without preignition, to determine the amount of inorganic material in biomass.
LAP “Determination of Protein Content in Biomass”
Measurement of protein in biomass is performed indirectly by measurement of nitrogen content and use of a nitrogen-to-protein conversion multiplier (N factor). Furthermore, protein will interfere with lignin measurements in subsequent analyses. Quantification of the protein will allow this interference to be mathematically minimized. Because nitrogen-containing compounds can be removed during the extraction process, protein analysis is performed on both whole and extracted materials.
This LAP covers the calculation of nitrogen-to-protein conversion factors. The nitrogen content of biomass is generally measured by combustion or Kjeldahl methods. An N factor of 6.25 is often used for animal feeds and other materials. The practice of using 6.25 as an N factor is based on an assumption that protein in a given material contains 16% nitrogen (100/16 = 6.25) (75−77). N factors for biomass feedstocks are likely to be different from 6.25, so measuring an N factor for a given substrate will enable more accurate mass closure. The NREL protein LAP describes the determination of a more accurate N factor based on the technique described by Mosse et al. (78, 79).
LAP “Determination of Structural Carbohydrates and Lignin in Biomass”
Carbohydrates and lignin make up the majority of biomass composition. The analytical hydrolysis procedure uses a two-step acid hydrolysis, 72% sulfuric acid followed by autoclave incubation in 4% sulfuric acid in a sealed vessel, to fractionate the biomass into forms that are more easily quantified. The LAP describes lignin quantification and carbohydrate analysis, including preparation of standards, hydrolysate neutralization, HPLC method setup, and acetyl analysis. It is a “micro” method, using 300 mg of sample.
During the hydrolysis, lignin fractionates into acid-insoluble material and acid-soluble material, which are measured separately. The acid-insoluble material, measured gravimetrically, is considered to be high molecular weight lignin and is corrected for ash and may need to be corrected for nitrogeneous material. Acid-soluble lignin is low molecular weight lignin solubilized in the acidic hydrolysis solution. The LAP describes the measurement of acid-soluble lignin by UV−vis spectroscopy, but it does not detail the determination of the extinction coefficient for feedstocks. A short list of extinction coefficients for common feedstocks is included in the LAP.
Structural carbohydrates, those bound into the structure of the biomass, are hydrolyzed into monomeric sugars, which are soluble in the hydrolysis solution and easily quantified by HPLC. Hydrolysis of the oligomeric sugars to monomers produces a variety of degradation products, and a loss factor is applied to make appropriate corrections. Because obtaining native oligomeric forms of the sugars common to biomass is problematic, it is assumed that monomeric sugars act as suitable models of this degradation. Monomeric sugars are hydrolyzed concurrently during the secondary hydrolysis step, and this degradation is used to account for the loss of carbohydrates to degradation products in the sample. Acetic acid is measured by HPLC to obtain acetyl content.
This hydrolysis has several potential interferences. It is suitable only for samples that do not contain extractives, as extractives may partition irreproducibly between soluble and insoluble fractions, resulting in an erratic and biased lignin value. This procedure has been optimized for a specific particle size range, as discussed in the sample preparation section above. Samples with ash contents above 10 wt % may not be suitable for this procedure, as the sample may contain minerals that will neutralize a fraction of the added acid. Samples with moisture content above 10 wt % may not be suitable for this procedure, as the excess moisture will dilute the added acid. Samples containing protein may bias the acid-insoluble lignin high unless the protein is accounted for in the gravimetric determination of acid-insoluble material. This procedure is not suitable for samples containing added acid, base, or other catalyst.
Samples containing high starch contents pose an additional challenge, as starch is also hydrolyzed to glucose using these sulfuric acid methods. Because the structural carbohydrates are analyzed as monomers, it is not known which polymer type gave rise to each monomeric sugar. The starch content of most seed-free herbaceous or woody feedstocks is low, so the measured glucose is assumed to come from cellulose. For feedstocks with high starch values, a separate enzymatic starch analysis is performed. The starch results are subtracted from the sulfuric acid glucan (cellulose + starch) value.
Suggested Future Improvements to Biomass Methods
We have found the NREL LAP methods generate useful compositional data on woody and herbaceous feedstocks. For more information about the uncertainty of these methods, see the following paper in this issue (80). Changes to the suite of analyses are continually sought to improve quantitation and component closure, increase sample throughput, and reduce analytical complexity. We see three general areas for future work to improve these biomass analysis methods.
First, there is a need for new or improved methods to better characterize existing components or to detect components that are not captured using current procedures. This would help close the analytical component nearer to 100 wt % and provide a check that could reveal over- or undercounting of the other components. A method to specifically quantitate lignin would be preferential over the current gravimetric method, which may be prone to interferences. The acid-insoluble residue method is the “trash bin” of the analyses because any unhydrolyzed solids end up being counted with lignin. A paper by Hatfield and Fukushima describes several analytical lignin methods, although they report different results for the different methods (81). In addition, a small but useful correction could be made if there was a method to measure protein that coprecipitates with acid-insoluble lignin. About one-fifth of the lignin in biomass is solubilized during analytical hydrolysis. UV spectroscopy is used to measure this acid-soluble lignin (ASL), but determining a suitable extinction coefficient for accurate Beer’s law calculations remains problematic. This method is prone to interferences from other non-lignin components that absorb in the UV region (e.g., HMF and furfural). Other methods such as nuclear magnetic resonance (NMR), selective adsorption, or HPLC may be used to determine ASL content more specifically. Uronic acids are not routinely measured in the NREL suite of analyses. A portion of them are present as sugar conjugates after analytical hydrolysis (e.g., 4-O-methylglucuronoxylose). Whereas HPLC looks promising for the uronic acid analysis, the lack of commercial standards makes quantification difficult. As described above, the use of monomeric sugars to correct for sugar losses from polymers during hydrolysis is imperfect. Finding a better method for measuring the sugars degraded during hydrolysis would improve the carbohydrate analysis. Water-soluble material can be as much as 30% of the dry weight composition of some herbaceous feedstocks. Some work has been recently published on the composition of water-soluble materials in corn stover (69). Expanding this work to other feedstocks would be useful.
The second major area for improvement is a need to increase the throughput and efficiency of these methods to enable more cost-effective and faster analyses. One alternative would be to automate the two-stage hydrolysis that is the core of the carbohydrate and lignin measurement procedure. We would also benefit from faster methods for the analysis of ash in feedstock and acid-insoluble residue. Current HPLC methods for the analysis of monosaccharides from biomass can take more than 45 min of run time per sample. New HPLC stationary phases could improve the throughput and resolution of biomass sugars. Improvements toward baseline separation of biomass sugars and faster separation times are desired. Improved analytical sensitivity would improve the quantification of the “minor” biomass sugars (galactose, arabinose, and mannose). Other instrumental techniques may allow for better and faster measurement while retaining the current method’s ease of use.
The third area for potential improvement is to validate the performance of the above methods on a wider variety of biomass substrates, altering the methods or developing new methods when needed. This includes materials from industrial residues (e.g., corn fiber and municipal solid waste) and emerging biofuel feedstocks. These tests may reveal the need to analyze for additional components to completely account for the different components found in these feedstocks.
Additionally, although not reviewed in this paper, it is worth noting for the interested reader that near-infrared (NIR) spectroscopy coupled with multivariate calibration methods have been developed to characterize biomass (82, 83). Spectroscopic scanning is faster and technically easier for the generation of compositional data. A well-calibrated NIR model takes on the variability of the underlying analysis method; therefore, good data quality from the wet chemical procedures is needed to have good data quality from NIR.
Abbreviations Used
ASE, accelerated solvent extraction; ASTM, American Society for Testing and Materials; FPL, Forest Products Laboratory; IEA, International Energy Agency; LAP, laboratory analytical procedure; NIST, National Institute of Standards and Technology; NREL, National Renewable Energy Laboratory; AOAC, Association of Official Analytical Chemists; TAPPI, Technical Association of the Pulp and Paper Industry; USDA, U.S. Department of Agriculture.
Acknowledgments
We thank our colleagues (past and present) who have helped us refine the LAPs, including Tina Ehrman, Bonnie Hames, David Johnson, Stuart Black, Fannie Posey-Eddy, Bill Michener, David Crocker, and Deb Hyman. We thank Al Berger, Ed Wolfrum, and Mike Himmel for fruitful discussions of the method lineage. We also thank Al Darzins, Deb Hyman, Rui Katahira, Daniel Inman, and Dan Schell for their helpful review of the manuscript.
Supporting Information Available
Current versions of the NREL LAPs. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the U.S. Department of Energy Office of the Biomass Program.
Supplementary Material
References
- Himmel M. E.; Ding S.-Y.; Johnson D. K.; Adney W. S.; Nimlos M. R.; Brady J. W.; Foust T. D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 2007, 315 (5813), 804–807. [DOI] [PubMed] [Google Scholar]
- Ragauskas A. J.; Williams C. K.; Davison B. H.; Britovsek G.; Cairney J.; Eckert C. A.; Frederick W. J. Jr.; Hallett J. P.; Leak D. J.; Liotta C. L.; Mielenz J. R.; Murphy R.; Templer R.; Tschaplinski T. The path forward for biofuels and biomaterials. Science 2006, 311 (5760), 484–489. [DOI] [PubMed] [Google Scholar]
- Aden A.; Ruth M.; Ibsen K.; Jechura J.; Neeves K.; Sheehan J.; Wallace B.; Montague L.; Slayton A.; Lukas J.. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing Co-Current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover; National Renewable Energy Laboratory; Harris Group: Golden, CO, and Seattle, WA, June 2002. [Google Scholar]
- Templeton D. W.; Sluiter A. D.; Hayward T. K.; Hames B. R.; Thomas S. R. Assessing corn stover composition and sources of variability via NIRS. Cellulose 2009, 16 (4), 621–639. [Google Scholar]
- Decker S. R.; Sheehan J.; Dayton D. C.; Bozell J. J.; Adney W. S.; Hames B.; Thomas S. R.; Bain R. L.; Czernik S.; Zhang M.; Himmel M. E.. Biomass conversion. In Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology, 11th ed.; Kent J. A., Ed.; Springer Verlag: New York, 2007; Vol. 2, pp 1449−1548. [Google Scholar]
- NREL Laboratory Analytical Procedures; http://www.nrel.gov/biomass/analytical_procedures.html (Dec 2009).
- Brauns F. E.The chemistry of lignin. In Wood Chemistry, Wise L. E., Jahn E. C., Eds.; American Chemical Society: Washington, DC, 1952; Vol. 1, pp 409−539. [Google Scholar]
- Browning B. L.Determination of lignin. In Methods of Wood Chemistry; Interscience Publishers: New York, 1967; Vol. 2, pp 785−823. [Google Scholar]
- Adams G. A.Lignin determination (Chapter 55). In Methods in Carbohydrate Chemsitry; Whistler R. L, Ed.; Academic Press: New York, 1965; Vol. V, pp 185−187. [Google Scholar]
- Klason P. Contributions to a more exact knowledge of the chemical composition of spruce wood, part I. Pap. Trade J. 1922, 74 (18), 45–51. [Google Scholar]
- Sherrard E. C.; Harris E. E. Factors influencing properties of isolated wood lignin. Ind. Eng. Chem. 1932, 24 (1), 103–106. [Google Scholar]
- Harris E. E.Wood yydrolysis. In Wood Chemistry; Wise L. E., Jahn E. C., Eds.; American Chemical Society: Washington, DC, 1952; Vol. 2, pp 852−932. [Google Scholar]
- Browning B. L.The determination of lignin. In Wood Chemistry, Wise L. E., Jahn E. C., Eds.; American Chemical Society: Washington, DC, 1952; Vol. 2 pp 1214−1237. [Google Scholar]
- Heuser E. Determination of lignin. Paper 1921, 27, 24–26. [Google Scholar]
- Dore W. H. The proximate analysis of coniferous woods. J. Ind. Eng. Chem. 1920, 12 (5), 476–479. [Google Scholar]
- Mahood S. A.; Cable D. E. The chemistry of wood. J. Ind. Eng. Chem. 1922, 14 (10), 933–934. [Google Scholar]
- Bray M. W. Chemical analysis of pulps and pulp woods. Pap. Trade J. 1928, 87, 59–68. [Google Scholar]
- Ritter G. J.; Seborg R. M.; Mitchell R. L. Factors affecting quantitative determination of lignin by 72% sulfuric acid method. Ind. Eng. Chem. Anal. Ed. 1932, 4 (2), 202–204. [Google Scholar]
- Ritter G. J.; Barbour J. H. Effect of pretreatments of wood on the lignin determination. Ind. Eng. Chem. Anal. Ed. 1935, 7 (4), 238–240. [Google Scholar]
- Ritter G. J.; Mitchell R. L.; Seborg R. M. Some factors that influence the conversion of cellulosic materials to sugar. J. Am. Chem. Soc. 1933, 55 (7), 2989–2991. [Google Scholar]
- Saeman J. F.; Bubl J. L.; Harris E. E. Quantitative saccharification of wood and cellulose. Ind. Eng. Chem. Anal. Ed. 1945, 17 (1), 35–37. [Google Scholar]
- Saeman J. F.; Harris E. E.; Kline A. A. Analysis of wood sugars. Ind. Eng. Chem. Anal. Ed. 1945, 17 (2), 95–99. [Google Scholar]
- Saeman J. F.; Moore W. E.; Mitchell R. L.; Millet M. A. Techniques for the determination of pulp constituents by quantitative paper chromatography. Tappi J. 1954, 37 (8), 336. [Google Scholar]
- Moore W. E.; Johnson D. B.. Procedures for the Chemical Analysis of Wood and Wood Products; Forest Products Laboratory, U.S. Department of Agriculture: Madison, WI, 1967. [Google Scholar]
- Peterson C. J.; Walde A. W.; Hixon R. M. Effect of temperature on sulfuric acid method for lignin. Ind. Eng. Chem. Anal. Ed. 1932, 4 (2), 216–217. [Google Scholar]
- Norman A. G.; Jenkins S. H. The determination of lignin: errors introduced by the presence of certain carbohydrates. Biochem. J. 1934, 28 (6), 2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. G.; Jenkins S. H. The determination of lignin: errors introduced by the presence of proteins. Biochem. J. 1934, 28 (6), 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. G. The determination of lignin: acid pretreatment and the effect of the presence of nitrogenous substances. Biochem. J. 1937, 31 (9), 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning J. W.; Lathrop E. C. Saccharification of agricultural residues. Ind. Eng. Chem. 1945, 37 (1), 24–29. [Google Scholar]
- Dunning J. W.; Dallas D. E. Analytical procedures for control of saccharification operations. Anal. Chem. 1949, 21 (6), 727–729. [Google Scholar]
- Ellis G. H. Report on lignin and cellulose in plants. J. Assoc. Off. Agric. Chem. 1949, 32, 287–291. [Google Scholar]
- Harwood V. D.Analytical studies on the carbohydrates of grasses and clovers. V. Development of a method for the estimation of cell-wall polysaccharides. J. Sci. Food Agric. 1954, 5 (June), 270−275. [Google Scholar]
- Hardell H. L.; Theander O. Quantitative determination of carbohydrates in cellulosic materials − losses as sulphates. Sven. Papperstidn.-Nord. Cellul. 1970, 73 (9), 291–293. [Google Scholar]
- Musha Y.; Goring D. A. I. Klason and acid-soluble lignin content of hardwoods. Wood Sci. 1974, 7 (2), 133–134. [Google Scholar]
- Effland M. J. Modified procedure to determine acid-insoluble lignin in wood and pulp. Tappi J. 1977, 60 (10), 143–144. [Google Scholar]
- Sloneker J. H. Determination of cellulose and apparent hemicellulose in plant tissue by gas−liquid chromatography. Anal. Biochem. 1971, 43 (2), 539–546. [DOI] [PubMed] [Google Scholar]
- Krull L. H.; Inglett G. E. Analysis of neutral carbohydrates in agricultural residues by gas−liquid chromatography. J. Agric. Food Chem. 1980, 28 (5), 917–919. [DOI] [PubMed] [Google Scholar]
- Slavin J. L.; Marlett J. A. Evaluation of high-performance liquid chromatography for measurement of the neutral saccharides in neutral detergent fiber. J. Agric. Food Chem. 1983, 31 (3), 467–471. [DOI] [PubMed] [Google Scholar]
- Southgate D. A. Determination of carbohydrates in foods. 2. Unavailable carbohydrates. J. Sci. Food Agric. 1969, 20, 331–335. [DOI] [PubMed] [Google Scholar]
- Southgate D. A. T.; Hudson G. J.; Englyst H. Analysis of dietary fiber − choices for the analyst. J. Sci. Food Agric. 1978, 29 (11), 979–988. [DOI] [PubMed] [Google Scholar]
- Theander O.; Aman P. Studies on dietary-fibers. 1. Analysis and chemical characterization of water-soluble and water-insoluble dietary-fibers. Swed. J. Agric. Res. 1979, 9 (3), 97–106. [Google Scholar]
- Selvendran R. R.; March J. F.; Ring S. G. Determination of aldoses and uronic acid content of vegetable fiber. Anal. Biochem. 1979, 96 (2), 282–292. [DOI] [PubMed] [Google Scholar]
- Englyst H.; Wiggins H. S.; Cummings J. H. Determination of the non-starch polysaccharides in plant foods by gas−liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [DOI] [PubMed] [Google Scholar]
- Theander O.; Westerlund E. A. Studies on dietary fiber. 3. Improved procedures for analysis of dietary fiber. J. Agric. Food Chem. 1986, 34 (2), 330–336. [Google Scholar]
- Hoebler C.; Barry J. L.; David A.; Delortlaval J. Rapid acid-hydrolysis of plant-cell wall polysaccharides and simplified quantitative-determination of their neutral monosaccharides by gas-liquid chromotography. J. Agric. Food Chem. 1989, 37 (2), 360–367. [Google Scholar]
- Grohmann K.; Himmel M.; Rivard C.; Tucker M.; Baker J.; Torget R.; Graboski M. Chemical−mechanical methods for the enhanced utilization of straw. Biotechnol. Bioeng. 1984, 137–157. [Google Scholar]
- Vinzant T. B.; Ponfick L.; Nagle N. J.; Ehrman C. I.; Reynolds J. B.; Himmel M. E. SSF comparison of selected woods from southern sawmills. Appl. Biochem. Biotechnol. 1994, 611–626. [Google Scholar]
- Pettersen R. C.; Schwandt V. H.; Effland M. J. An analysis of the wood sugar assay using HPLC − a comparison with paper-chromatography. J. Chromatogr. Sci. 1984, 22 (11), 478–484. [Google Scholar]
- Pettersen R. C. The chemical-composition of wood. Adv. Chem. Ser. 1984, No. 207, 57–126. [Google Scholar]
- Pettersen R. C.; Schwandt V. H. Wood sugar analysis by anion chromatography. J. Wood Chem. Technol. 1991, 11 (4), 495–501. [Google Scholar]
- Davis M. W. A rapid modified method for compositional carbohydrate analysis of lignocellulosics by high pH anion-exchange chromatography with pulsed amperometric detection (HPAEC/PAD). J. Wood Chem. Technol. 1998, 18 (2), 235–252. [Google Scholar]
- Theander O.Chemical investigations in the Swedish agrobioenergy project. In Energy from Biomass: 3rd E.C. Conference; Palz W., Coombs J., Hall D. O., Eds.; Elsevier Applied Science Publishers: London, U.K., 1985; pp 1044−1048. [Google Scholar]
- Theander O. Chemical-analysis of lignocellulose materials. Anim. Feed Sci. Technol. 1991, 32 (1−3), 35–44. [Google Scholar]
- Theander O.; Aman P.; Westerlund E.; Andersson R.; Petersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J. AOAC Int. 1995, 78 (4), 1030–1044. [PubMed] [Google Scholar]
- Kaar W. E.; Cool L. G.; Merriman M. M.; Brink D. L. The complete analysis of wood polysaccharides using HPLC. J. Wood Chem. Technol. 1991, 11 (4), 447–463. [Google Scholar]
- Kaar W. E.; Brink D. L. Summative analysis of 9 common North-American woods. J. Wood Chem. Technol. 1991, 11 (4), 479–494. [Google Scholar]
- Milne T. A.; Brennan A. H.; Glenn B. H.. Sourcebook of Methods of Analysis for Biomass and Biomass Conversion Processes; Elsevier Applied Science: New York, 1990. [Google Scholar]
- Chum H. L.; Johnson D. K.; Agblevor F. A.; Evans R. J.; Hames B. R.; Milne T. A.; Overend R. P. In Status of the IEA Voluntary Standards Activity Round Robins on Whole Wood and Lignins; International Conference on Advances in Thermochemical Biomass Conversion, Interlaken, Switzerland, 1992; Bridgwater A. V., Ed.; Blackie Academic and Professional: Interlaken, Switzerland, 1992; pp 1701−1716. [Google Scholar]
- Milne T. A.; Chum H. L.; Agblevor F.; Johnson D. K. Standardized analytical methods. Biomass Bioenergy 1992, 2 (1−6), 341–366. [Google Scholar]
- Agblevor F.; Chum H. L.; Johnson D. K.. Compositional analysis of NIST biomass standards from the IEA feedstock round robin. In Energy from Biomass and Wastes XVI; Klass D. L., Ed.; Institute of Gas Technology: Orlando, FL, 1992; pp 395−421. [Google Scholar]
- NIST Standard Reference Materials; http://ts.nist.gov/measurementservices/referencematerials/index.cfm (Dec 2009).
- Blakeney A. B.; Harris P. J.; Henry R. J.; Stone B. A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113 (2), 291–299. [Google Scholar]
- Ehrman C. I.; Himmel M. E. Simultaneous saccharification and fermentation of pretreated biomass − improving mass-balance closure. Biotechnol. Tech. 1994, 8 (2), 99–104. [Google Scholar]
- Hsu T.; Nguyen Q. Analysis of solids resulted from dilute-acid pretreatment of lignocellulosic biomass. Biotechnol. Tech. 1995, 9 (1), 25–28. [Google Scholar]
- Ehrman C. I.Methods for the chemical analysis of biomass process streams. In Handbook on Bioethanol: Production and Utilization; Wyman C. E., Ed.; Taylor & Francis: Washington, DC, 1996. [Google Scholar]
- Thammasouk K.; Tandjo D.; Penner M. H. Influence of extractives on the analysis of herbaceous biomass. J. Agric. Food Chem. 1997, 45 (2), 437–443. [Google Scholar]
- Foyle T.; Jennings L.; Mulcahy P. Compositional analysis of lignocellulosic materials: evaluation of methods used for sugar analysis of waste paper and straw. Bioresour. Technol. 2007, 98 (16), 3026–3036. [DOI] [PubMed] [Google Scholar]
- Moxley G.; Zhang Y. H. P. More accurate determination of acid-labile carbohydrates in lignocellulose by modified quantitative saccharification. Energy Fuels 2007, 21 (6), 3684–3688. [Google Scholar]
- Chen S. F.; Mowery R. A.; Scarlata C. J.; Chambliss C. K. Compositional analysis of water-soluble materials in corn stover. J. Agric. Food Chem. 2007, 55 (15), 5912–5918. [DOI] [PubMed] [Google Scholar]
- Hames B. R.Biomass compositional analysis for energy applications. In Biofuels, Methods in Molecular Biology; Mielenz J. R., Ed.; Humana Press: Totowa, NJ, 2009; Vol. 581. [DOI] [PubMed] [Google Scholar]
- Dien B. S.Mass balances and analytical methods for biomass pretreatment experiments. In Biomass to Biofuels: Strategies for Global Industries; Vertes A. A., Qureshi N., Blaschek H. P., Yukawa H., Eds.; Wiley: New York, 2010. [Google Scholar]
- Chen S.-F.; Mowery R. A.; Svecik R. S.; Scarlata C. J.; Chambliss C. K. Compositional analysis of water-soluble materials in switchgrass. J. Agric. Food Chem. 2010, 58 (6), 3251–3258. [DOI] [PubMed] [Google Scholar]
- TAPPI Standards & TIPs; http://www.tappi.org/s_tappi/sec.asp?CID=2&DID=3 (Dec 2009).
- ASTM Standards; http://www.astm.org/Standard/index.shtml (Dec 2009).
- Jones D. Factors for converting percentages of nitrogen in foods and feeds into percentages of proteins. U.S. Dept. Agric. Circ. 1931, 183, 21. [Google Scholar]
- Tkachuk R. Nitorgen-to-protein conversion factors for cereals and oilseed meals. Cereal Chem. 1969, 46, 419–423. [Google Scholar]
- Yeoh H. H.; Wee Y. C. Leaf protein contents and nitrogen-to-protein conversion factors for 90 plant-species. Food Chem. 1994, 49 (3), 245–250. [Google Scholar]
- Mosse J.; Huet J. C.; Baudet J. The amino-acid composition of wheat-grain as a function of nitrogen-content. J. Cereal Sci. 1985, 3 (2), 115–130. [Google Scholar]
- Mosse J. Nitrogen to protein conversion factor for 10 cereals and 6 legumes or oilseeds − a reappraisal of its definition and determination − variation according to species and to seed protein-content. J. Agric. Food Chem. 1990, 38 (1), 18–24. [Google Scholar]
- Templeton D. W.; Scarlata C. J.; Sluiter J. B.; Wolfrum E. J.. Compositional analysis of lignocellulosic feedstocks. 2. Method uncertainties. J. Agric. Food Chem. 2010, doi: 10.1021/jf100807b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield R.; Fukushima R. S. Can lignin be accurately measured?. Crop Sci. 2005, 45 (3), 832–839. [Google Scholar]
- Wolfrum E. J.; Sluiter A. D. Improved multivariate calibration models for corn stover feedstock and dilute-acid pretreated corn stover. Cellulose 2009, 16 (4), 567–576. [Google Scholar]
- Hames B. R.; Thomas S. R.; Sluiter A. D.; Roth C. J.; Templeton D. W. Rapid biomass analysis − new tools for compositional analysis of corn stover feedstocks and process intermediates from ethanol production. Appl. Biochem. Biotechnol. 2003, 105, 5–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.