Abstract
Xylem vulnerability to cavitation is a key parameter in the drought tolerance of trees, but little is known about the control mechanisms involved. Cavitation is thought to occur when an air bubble penetrates through a pit wall, and would hence be influenced by the wall's porosity. We first tested the role of wall-bound calcium in vulnerability to cavitation in Fagus sylvatica. Stems perfused with solutions of oxalic acid, EGTA, or sodium phosphate (NaPO4) were found to be more vulnerable to cavitation. The NaPO4-induced increase in vulnerability to cavitation was linked to calcium removal from the wall. In contrast, xylem hydraulic conductance was unaffected by the chemical treatments, demonstrating that the mechanisms controlling vulnerability to cavitation and hydraulic resistance are uncoupled. The NaPO4 solution was then perfused into stems from 13 tree species possessing highly contrasted vulnerability to cavitation. Calcium was found to be a major determinant of between-species differences in vulnerability to cavitation. This was evidenced in angiosperms as well as conifer species, thus supporting the hypothesis of a common mechanism in drought-induced cavitation.
In plants, long-distance sap transport occurs under negative pressures in xylem conduits. Sap flows between adjoining conduits through pits that form pores in the walls, and that facilitate the flow of water while preventing the passage of air bubbles. Under water stress conditions, xylem tensions increase and the conduits become vulnerable to cavitation. Cavitation provokes an air embolism that leads to a loss of hydraulic conductance, thus exacerbating plant water deficit.
Species resistance to cavitation has been intensively studied over the past two decades, and is now ranked among the traits with the highest functional and ecological significance. In woody species for instance, xylem vulnerability to cavitation correlates tightly with species-specific drought tolerance (Pockman and Sperry, 2000; Tyree et al., 2003; Maherali et al., 2004), with more xerophilous species proving less vulnerable to cavitation. Substantial variations have also been found between genotypes of a different species (Cochard et al., 2007; Dalla-Salda et al., 2009). This implies that this trait could potentially be used in breeding programs to identify more drought-tolerant species or genotypes. However, efforts in this direction are still strongly impeded by a lack of understanding of the molecular and genetic basis of cavitation resistance. Our work represents a first significant step toward resolving this challenging issue.
Understanding the fine mechanism of cavitation formation is a pivotal step toward identifying the key structures and the key genes coding for these structures, yet we currently have only partial insights. According to a hypothesis first formulated by Zimmermann (1983), water stress-induced cavitation is thought to occur when a tiny air bubble penetrates through a pit membrane, and would consequently be strongly influenced by the porosity of the membrane (Tyree and Sperry, 1988; Cochard, 2006). There is also experimental evidence for a role of the mechanical properties of the pit membrane in this cavitation process (Choat et al., 2004; Sperry and Hacke, 2004). Clearly, the structural, physical, and chemical properties of pit membranes are central to the determinism of cavitation.
Pit membranes are modified primary cell walls made of tightly interwoven cellulose microfibrils in a matrix of hydrated hemicelluloses and pectins. Pectins consist of a complex set of GalUA (GalA)-rich polysaccharides, and four pectic domains can be distinguished: homogalacturonan (HG), rhamnogalacturonan I, rhamnogalacturonan II, and xylogalacturonan (Willats et al., 2001). The high degree of structural complexity and heterogeneity across the pectin family is the result of both biosynthesis in the endomembrane system and the action of an array of wall-based pectin-modifying enzymes (Willats et al., 2001). HG units are synthesized in the Golgi apparatus and deposited in the cell wall in a form containing 70% to 80% methyl-esterified GalA residues (O'Neill et al., 1990; Mohnen, 1999). The removal of methyl ester groups by pectin methyl esterase (PME) within the cell wall matrix produces free carboxyl groups capable of being cross-linked by calcium cations in an “egg-box” structure (Grant et al., 1973; Pelloux et al., 2007). These calcium-dependent cross-linkages are dependent both on the degree and the distribution of methyl-esterified GalA units through the HG network (Willats et al., 2001). Calcium therefore plays a central role as it determines the supramolecular assembly of the pectic chains and the formation of a pectate gel.
Pectins capable of Ca2+ cross-linking are particularly common in bordered pit membranes (Chaffey et al., 1997; Hafren et al., 2000). Moreover, pectin-bound calcium influences wall elasticity (Ezaki et al., 2005; Proseus and Boyer, 2006; Derbyshire et al., 2007), and could therefore influence the stretching properties of the pit membranes and, consequently, the mechanism of cavitation. We tested the hypothesis that calcium plays a major role in the determinism of cavitation. This hypothesis was formulated long ago (Sperry and Tyree, 1988) but has not yet been thoroughly tested. We designed a series of experiments to demonstrate the specific role of calcium in this mechanism, and analyzed a large number of woody species to establish the role of calcium cross-linkage in across- and within-species variation in cavitation resistance. The data strongly support our hypothesis.
RESULTS
Effect of Chemical Treatments on Fagus Vulnerability to Cavitation
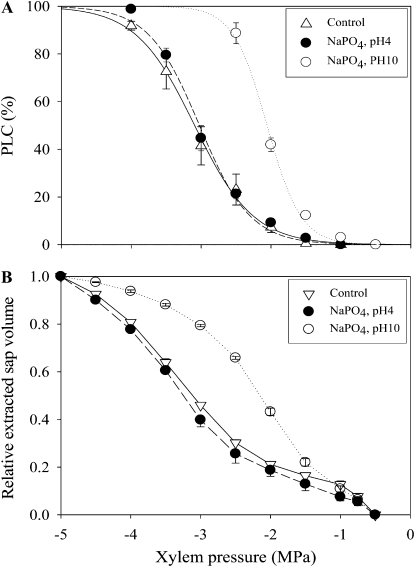
Fagus segments infiltrated with different chemical treatments exhibited highly contrasted cavitation curves. Whatever the treatment, the curves were always sigmoid and perfectly well fitted with Equation 1 (see “Materials and Methods”), as illustrated in Figure 1A showing three representative curves. Therefore, treatments were analyzed through their effects on the two parameters of Equation 1, i.e. the xylem pressure inducing 50% of loss of conductivity (P50) and the slope of the vulnerability curve at P50 (s; Table I). Xylem vulnerability was very substantially increased when stem segments were infiltrated with solutions of EGTA, oxalic acid, or sodium phosphate (NaPO4) at pH 10. These solutions have the property of chelating or precipitating with calcium cations. Efficacy differed across treatments, but this difference remained unchanged following a 5 to 50 mL increase in the volume of NaPO4 and oxalic acid solutions infiltrated into the segments (data not shown).
Figure 1.
Vulnerability to cavitation (A) and retention (B) curves of beech stems perfused with NaPO4 solutions. Fagus stems were perfused with a NaPO4 solution at pH 4 (black circles) or pH 10 (white circles). Then, the vulnerability (A) and retention (B) curves were established. As a control, a vulnerability curve was scored from stems perfused with a control (CaCl2 1 mm + KCl 10 mm, triangles up) and a retention curve was scored from nonperfused stems (triangles down). The data for the vulnerability and retention curves are means (±se) of three and four samples, respectively.
Table I. Effects of chemical treatments on xylem vulnerability to cavitation.
Fagus stems were perfused with the solutions indicated. Following perfusion with NaPO4 at pH 10, some stems were then perfused (arrows) with Tris-HCl at pH 10 containing or not 10 mm of CaCl2. Vulnerability curves were then established and the slope and P50 parameters were scored. Data are means (±se) of three to four samples. Two ANOVA analyses were performed to compare two data sets, the one bearing Roman letters and the other bearing Greek letters. Data sharing different letters are significantly different according to a Tukey's HSD test (P < 0.05).
| Perfused Solutions | Slope | P50 |
| MPa | ||
| Control | −63.0a, α (±7.8) | −3.09a, α (±0.03) |
| Water | −66.4a, α (±8.5) | −3.11a, α (±0.06) |
| NaPO4, pH 4 | −74.5a, α (±7.0) | −2.99ab, α (±0.13) |
| NaPO4, pH 10 | −110.0c (±26.5) | −2.03d (±0.08) |
| EGTA | −74.9a (±16.7) | −2.58bc (±0.14) |
| Oxalic acid, pH 2 | −106.9bc (± 9.4) | −2.33cd (±0.13) |
| Oxalic acid, pH 5 | −84.9ab (±11.9) | −2.76abc (± 0.19) |
| Tris-HCl, pH 6 | −69.2α (±6.8) | −3.06α (±0.01) |
| Tris-HCl, pH 8 | −59.2α (±8.7) | −3.00α (±0.02) |
| CaCl2 10 mm | −72.7α (±27.5) | −3.03α (±0.11) |
| NaPO4, pH 10 → Tris-HCl, pH 10 | −74.6α (±11.9) | −2.26β (±0.38) |
| NaPO4, pH 10 → Tris-HCl, pH 10 + CaCl2 | −83.5α (±27.8) | −3.06α (± 0.14) |
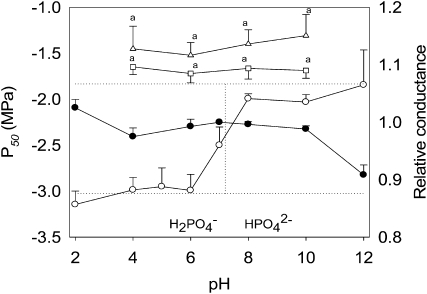
To further demonstrate that calcium played a role in this response, we treated segments with NaPO4 solutions buffered at pH = 2 to pH = 10. As only HPO42− anions can precipitate calcium cations, we hypothesized that only solutions at pH above pKaH2PO4−/HPO42− (7.2) would increase xylem vulnerability. The dependence of P50 on pH (Fig. 2) and the covariation of P50 with the proportion of HPO42− in the solutions strongly support this hypothesis. This pH response curve was not associated with pH per se, since there was no effect on xylem cavitation when segments were infiltrated with Tris-HCl at pH 6 or pH 8 (Table I). Likewise, this effect could not be explained by a modification of the surface tension of the solutions, since NaPO4 has a surface tension of 0.064 mN m−1 and 0.059 mN m−1 at pH 4 and pH 10, respectively. Furthermore, while treating Fagus segments with 10 mm CaCl2 had no effect on cavitation, the vulnerability of segments first perfused with NaPO4 at pH 10 reverted to control values following infiltration of 10 mm CaCl2 at pH 10 (Table I). This strongly supports the hypothesis that the effect of NaPO4 on xylem cavitation was due to calcium chelation. We are confident that these results were not biased by an effect of xylem conductance on cavitation (Gascó et al., 2006), for two reasons. First, the results were similar whether cavitation was measured via its effect on xylem conductance (Fig. 1A) or on xylem water content (Fig. 1B). Second, xylem hydraulic conductance was not modified by NaPO4 solutions at pH ranging from 4 to 10 (Fig. 2): There was only a slight increase and a slight decrease in conductance at pH 2 and pH 12, respectively.
Figure 2.
Effect of NaPO4 solutions at different pH on both xylem hydraulic conductance and vulnerability to cavitation. For vulnerability to cavitation measures, stems from Fagus (circles), Salix (triangles), or Betula (squares) were perfused with a NaPO4 solution (pH from 2–12) and then the P50 value (white symbols) was determined. Data are means (±se) of three to four samples. For conductance measures, Fagus stems were perfused with a solution of KCl 10 mm and CaCl2 1 mm and conductance was scored under a pressure of 4 kPa. The stems were then perfused with a NaPO4 solution (pH from 2–12), and conductance (black circles) was scored and expressed relative to initial conductance. Data are means (±se) of five samples. Data sharing different letters are significantly different according to a Tukey's HSD test (P < 0.05).
Across- and Within-Species Effects of NaPO4 Treatments on Xylem Cavitation
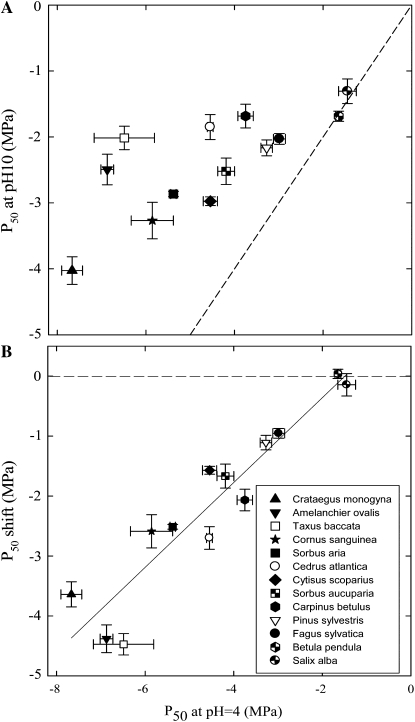
These results show that alkaline NaPO4 solutions have the strongest effect on xylem cavitation, with an abrupt transition at pH above pKa. Therefore, to test for the implication of calcium in the variation of xylem vulnerability to cavitation across tree species, we perfused stem segments with NaPO4 at pH 10 and with NaPO4 at pH 4 as a control. The P50 value of stem segments perfused with NaPO4 at pH 4 (Fig. 3) were in agreement with those found in the literature (Vogt, 2001; Cochard et al., 2005; Cochard, 2006). Eleven of the 13 species tested showed an increase in vulnerability to cavitation when perfused with NaPO4 at pH 10 (Fig. 3A). Only two species, Salix alba and Betula pendula, remained unaffected by NaPO4 treatments from pH 4 to pH 10 (Fig. 2). Figure 3B shows the relation between the P50 of control stems (measured at pH 4) and the shift in P50 measured after treatment at pH 10. The y axis can be interpreted as the proportion of species cavitation resistance due to the presence of calcium in the walls. Clearly, species less vulnerable to cavitation display a higher share of calcium-dependant cavitation resistance.
Figure 3.
Role of xylem calcium in vulnerability to cavitation in different woody species. For each species, stems were perfused with NaPO4 solutions at pH 4 or pH 10, and the P50 values were scored. Each dot represents the data for one angiosperm species (black symbols) or for one coniferophyte species (white symbols). The top section (A) shows P50 values at pH 4 versus P50 values at pH 10. The bottom section (B) shows the P50 values at pH 4 versus the shift in P50 values calculated as P50 pH4 − P50 pH10. Data are means (±se) of three to four samples. The solid line is a regression on the mean values, with r2 = 0.88 and P50 pH4 − P50 pH10 = 0.707 P50 pH4 + 1.055 (P < 0. 001). The dashed line is the function P50 pH4 = P50 pH10.
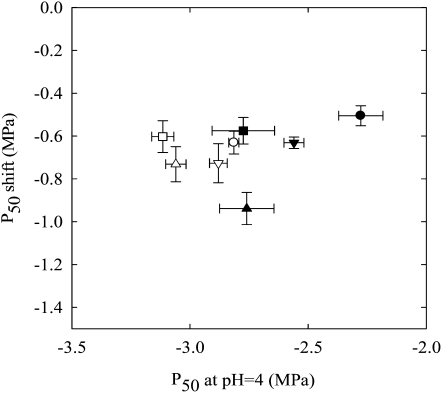
Fagus segments from shoots grown in full-sunlight conditions were significantly more cavitation resistant than shoots grown in shade (Fig. 4). Perfusion with NaPO4 at pH 10 induced a similar increase in vulnerability to cavitation in both types of stems, but the difference due to light exposure was not changed. Moreover, we found no significant difference between trees in P50 shift values.
Figure 4.
Role of xylem calcium in the light-induced increase in vulnerability to cavitation. Full sunlight-grown (white symbols) and shade-grown (black symbols) stems were sampled on four beech trees (four different symbols). They were perfused with NaPO4 solutions at pH 4 or pH 10, and the P50 values were scored. The section shows P50 values at pH 4 versus the shift in P50 values calculated as P50 pH4 − P50 pH10. Data are means (±se) of three to four samples. According to a repeated ANOVA analysis, the P50 pH4 means was significantly different between trees and between sunlight- and shade-grown stems, with P < 0.01. However, the means of P50 shift were not significantly different between trees (P = 0.226) and between full sunlight- and shade-grown stems (P = 0.899).
DISCUSSION
Our study demonstrates that calcium plays a major role in cavitation resistance in trees, and highlights the fundamental implication of wall pectins in this mechanism. Wall calcium was also found to explain most of the differences in cavitation resistance across a large spectrum of species ranging from angiosperms to coniferophytes.
Removing Calcium from Vessel Wall Increases Xylem Vulnerability to Cavitation in Fagus
To our knowledge, Sperry and Tyree (1988) were the first to investigate the mechanisms of xylem vulnerability to cavitation. They found that oxalic acid and calcium, but not oxalic acid alone, increased vulnerability to cavitation in Acer saccharum stems, leading them to hypothesize a role for calcium in this process. To validate this hypothesis, we used several calcium-modulating treatments that have already been employed to demonstrate the role of external calcium in cell growth (Proseus and Boyer, 2006) and defense against pathogens (Walters and Murray, 1992; Hill et al., 1998). Our results demonstrate that EGTA, oxalic acid, and inorganic phosphate (at pH > 7) increased xylem vulnerability to cavitation. The effects were neither additive nor synergistic between treatments (data not shown), suggesting that they all have the same action, i.e. removing calcium from the wall. However, this action was achieved to different degrees across treatments. This difference was not related to perfusion time, but may be explained by a different capacity for the molecules to access to the calcium-binding sites in the pit membrane (Shomer et al., 2003). The fact that treatment efficacy correlates inversely with molecule size supports this hypothesis.
We focused more closely on the action of NaPO4 on cavitation in this study for two reasons. First, the strongest effect on vulnerability to cavitation was obtained using treatments at pH > 7 (Figs. 1 and 2). Second, the pKa for this molecule (7.2) is close to typical sap pH values, which enabled us to test its action while minimizing other pH effects on wall structure that may occur at very low or very high pH values. At pH > pKa, HPO42− precipitates with calcium cations to form CaHPO4 2H2O (dicalcium phosphate dihydrate), whereas at pH < pKa H2PO4− is unable to bind calcium. The striking correlation we found between the change in vulnerability and the concentration of H2PO4− anions in the solutions strongly supports the hypothesis of a role played by calcium removal from the wall in the increase of vulnerability to cavitation. We further demonstrated that this action was not linked to pH variations per se, nor to a modification in surface tension (Sperry and Tyree, 1988; Cochard et al., 2009), nor to a bias due to a change in xylem conductance (Gascó et al., 2006). Finally, the finding that the increase in vulnerability to cavitation by perfusion of NaPO4 at pH 10 was reversed by perfusion of calcium demonstrates that calcium, and not any other bivalent cations, was implicated in the cavitation process per se. Moreover, Stiller and Sperry (2002) showed that the cavitation fatigue, an increased sensitivity to cavitation as a result of a prior cavitation, can be partly restored when perfusing sunflower (Helianthus annuus) stems with CaCl2. This result supports a role of calcium in vulnerability to cavitation. However, the cavitation fatigue is probably a more complex process since perfusing sunflower stems with KCl also partly restored the cavitation fatigue.
Calcium Explains Across-Species Differences in Vulnerability to Cavitation
Another important finding of our study is that calcium explains a major proportion of across-species variations in cavitation resistance. Our experiments were performed on stems sampled from a single tree per species to minimize the genetic variation. We are confident that our results are to a large extent representative of the behavior of each species, for two reasons. First, the P50 values we report here are similar to previous reports on the same species. Second, the effect of calcium for several beech trees was very consistent and repeatable (Fig. 4). Nevertheless, the intraspecific variability of this calcium response should be investigated in more detail in the future. Clearly, species more resistant to cavitation were more affected by the chemical sequestration of calcium. In contrast, in Betula and Salix, which are among the most vulnerable species measured so far, calcium removal had no effect on their vulnerability to cavitation. From the regression in Figure 3B, we estimated that 70% of the between-species difference in P50 can be explained by the presence of calcium in the wall. We hypothesize that calcium-mediated between-species differences in vulnerability to cavitation are dependent on the HG content in the pit membrane and its methyl-esterification pattern, and consequently on PME activity. The enzyme PME determines the number of sites where pectin chains are potentially cross-linked by calcium, and may thus strongly impact the mechanical properties of pit membranes (Pelloux et al., 2007). Clearly, further studies are needed on the methyl-esterification pattern of pit membranes and on PME activity during vessel formation to substantiate our hypothesis.
Full sunlight-exposed beech stems were significantly less vulnerable to cavitation that shade-exposed stems, in agreement with previous results (Cochard et al., 1999; Barigah et al., 2006). However, the phenotypic plasticity in vulnerability to cavitation was not due to calcium. This clearly demonstrates that cavitation resistance in trees is conferred by several independent mechanisms that have yet to be elucidated. Structural features such as pit membrane thickness, porosity, or mechanics (Jansen et al., 2009) are other important determinants of cavitation resistance. The structure of the xylem conduits could also influence vulnerability to cavitation. For example, a correlation was found between vulnerability to cavitation and the average area of the pit overlap between connecting vessels (Wheeler et al., 2005), although this correlation was not found when comparing three Acer species (Christman et al., 2009). Although pit structure varies dramatically across species (Sano, 2005; Jansen et al., 2007; Schmitz et al., 2007), and despite the high heterogeneity in pectin structures (Willats et al., 2001), the fact remains that in-pit calcium appears to be a major contributor to across-species resistance to cavitation.
Role of Calcium in Pit Membrane Functions
Pit membrane not only influences the passage of water between conduits, it also controls the passage of air bubbles from an embolized vessel to a sap-filled vessel (Choat et al., 2008). Thus, it has been hypothesized that a higher resistance to cavitation should be associated with a lower hydraulic conductivity (Zimmermann 1983; Sperry and Hacke, 2004). This tradeoff between xylem efficiency and safety was proposed to be linked to pit membrane porosity (Choat and Pittermann, 2009), both functions then depending on the same mechanisms. Here, we bring evidence that the two parameters are largely uncoupled. Indeed, removing calcium from the wall increased xylem vulnerability to cavitation but had no effect on xylem hydraulic conductance. In support of this uncoupling, we recently showed that increasing sap ionic concentration induced changes in hydraulic conductivity but had no effect on vulnerability to cavitation (Cochard et al., 2010). A modification in sap ionic strength can make pectins shrink or swell depending on species (Zwieniecki et al., 2001; Cochard et al., 2010). These swelling properties of pectins impact on the size of microchannels in the pit membranes through which water moves, and hence on resistance to water flow. However these structural modifications do not induce any change in vulnerability to cavitation. The pores in pit membranes are not actually large enough to be responsible for air seeding at realistic air pressures (Wheeler, 1983; Shane et al., 2000; Choat et al., 2003). One explanation for this discrepancy is that the pits have a different physical structure when water flows through a pit compared to during the process of cavitation. When sap flows through a pit, there is a very small pressure difference across the pit that is thus in a relaxed state. During air seeding, the pit membrane is exposed to a larger pressure difference, and the membrane is forced to undergo substantial stretching or local deformations. This stretched state would allow air seeding to occur through enlarged pores in the pit membrane. We propose that calcium controls vulnerability to cavitation through the stretching properties of the pit membrane. In support of this, intermolecular links of pectin chains through calcium bridges are known to influence cell wall rigidity (Ezaki et al., 2005; Proseus and Boyer, 2006; Derbyshire et al., 2007). Moreover, studies involving modified PME expression indicate that PME plays a role in wall stiffening and in the inhibition of wall plasticity (Wen et al., 1999; Hasunuma et al., 2004; Bosch et al., 2005).
It has been suggested that cavitation fatigue could result from the rupture or the loosening of pit membranes during cavitation events (Hacke et al., 2001). Therefore, we can speculate that calcium could also play a critical role in this process and its repair (Stiller and Sperry, 2002). This hypothesis awaits further investigations on different species, since cavitation fatigue was shown to vary greatly across species (Hacke et al., 2001).
The strong effect of calcium sequestration on conifer cavitation resistance was a surprising result of our study. Pit structure in conifers differs greatly from pit structure in angiosperms with their central thickening (tori) surrounded by a web-like structure (margo). When the pit is in relaxed state, the margo allows the water flow with a low resistance. When a tracheid embolizes, the pit membranes are deflected to the inside of the pit border and the tori block the pit aperture, preventing the spreading of air bubbles (Cochard, 2006). Several hypothetical mechanisms have been proposed to explain the mechanisms of cavitation in conifers (Cochard, 2006). We recently showed that cavitation in conifers was triggered by the capillary failure of an air-water meniscus (Cochard et al., 2009), like in angiosperms species. However, the place of air seeding remains unknown, and two hypotheses have been proposed: (1) the seal between tori and pit walls or (2) pores in the tori. Here, calcium was shown to be a major determinant of vulnerability to cavitation for both angiosperm and conifer species, suggesting a common mechanism in the two plant groups. We assume that the difference in vulnerability to cavitation between conifer species was mainly attributed to the pectin-bound calcium in the tori, and that cavitation would occur through pores in the tori.
CONCLUSION
This study showed that removing calcium from the wall induced a sharp increase in xylem vulnerability to cavitation, without any effect on xylem hydraulic conductance. Calcium was responsible for a major proportion of the between-species difference in vulnerability to cavitation, in angiosperms as well as in conifers. These results open new avenues for insight into the mechanisms underpinning cavitation resistance. To date, research has focused on the relationship between vulnerability to cavitation and xylem anatomy or pit structure (Choat and Pittermann, 2009). Our results suggest that research should also focus on the chemical composition of pectins and on the enzymes controlling this composition. The secret of cavitation resistance in plants is probably hidden in the great chemical complexity of pectins (Willats et al., 2001).
MATERIALS AND METHODS
Plant Material
The series of experiments designed to demonstrate the specific role of calcium on cavitation were conducted on stems from a 100-year-old beech tree (Fagus sylvatica) taken from the Allagnat forest in the center of France (45°45.23′ N, 2°56.26′ E, 1,000 m above sea level). To evaluate the role of calcium on the within-species variation of cavitation, we analyzed shoots growing in contrasted light conditions (sunlit and shaded) from four other mature beech trees located in the same forest. To infer the implication of calcium on differences in cavitation across a large spectrum of species, we selected 10 woody angiosperms (Amelanchier ovalis, Betula pendula, Carpinus betulus, Cornus sanguinea, Crataegus monogyna, F. sylvatica, Salix alba, Sorbus aria, Sorbus aucuparia, and Cytisus scoparius) and three coniferophytes (Cedrus atlantica, Pinus sylvestris, and Taxus baccata). The species were located in the vicinity of the INRA-Crouël campus in Clermont-Ferrand (France). The selected stems were 0.5 to 1 cm in diameter and grown in full sunlight-exposed conditions. To avoid intraspecific variations, stems were sampled on one tree for each species. We collected 0.5-m-long stem samples in the morning, enclosed them in a black airtight plastic bag to reduce water loss through transpiration, and brought them to the laboratory for hydraulic analyses. Measurements of xylem hydraulic conductance were carried out on the same day. For vulnerability to cavitation, samples were wrapped in humid paper, bagged, and stored at 5°C until analysis.
Xylem Vulnerability to Cavitation
Xylem vulnerability to cavitation was assessed using the Cavitron technique (Cochard, 2002; Cochard et al., 2005) on 0.28-m-long stem samples. The technique uses the centrifugal force to increase the water tension in a xylem segment and, at the same time, measures the decrease in hydraulic conductance. The curve of percentage loss of xylem conductance (PLC) versus xylem water tension indicates the sample's vulnerability to cavitation. Vulnerability curves were determined for three to four samples for each treatment. If a segment was infiltrated with a specific solution before measurement, the same solution was used during centrifugation. Xylem pressure (P) was first set to a reference pressure and the sample maximal conductance (Kmax) was determined. Xylem pressure was then reset to a more negative pressure and the new sample conductance K was determined. The percent loss of conductance was then computed as PLC = 100 × (1 − K/Kmax). The procedure was repeated for increasingly negative pressures dropped at −0.25 or −0.5 MPa step increments until PLC reached at least 90%. Following Pammenter and Vander Willigen (1998) and Cochard et al. (2007), a sigmoid function was fitted to each curve:
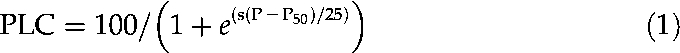 |
(1) |
where P50 is the pressure causing a 50% loss in hydraulic conductivity and s is the slope of the curve at this point (MPa−1).
Effect of Chemical Treatments on Cavitation
Chemical treatments were applied just prior to the assays on vulnerability to cavitation. Samples were infiltrated under vacuum by connecting their terminal part to a vacuum pump while their base was immersed in a flask containing an excess of solution until at least 5 mL had perfused through the samples. Eighteen different solutions were tested in this study. All the solutions were prepared with ultrapure water. We tested: ultrapure water and water plus 10 mm KCl and 1 mm CaCl2 (two control solutions); 10 mm CaCl2; 10 mm EGTA; 10 mm oxalic acid at pH 2 and pH 5; 10 mm NaPO4 at pH 2, 4, 5, 6, 7, 8, 10, and 12 (prepared by mixing 10 mm Na3PO4 and 10 mm H3PO4); 10 mm Tris-HCl at pH 6, 8, and 10; and a solution containing 10 mm Tris-HCl and 10 mm CaCl2. Fagus segments were treated with all these solutions. Salix and Betula segments were treated with 10 mm NaPO4 solutions at pH 4, 6, 8, and 10. All the other species we treated with 10 mm NaPO4 solutions at pH 4 and 10.
Effects of NaPO4 Solutions on Xylem Hydraulic Conductance and Xylem Water Retention Curves
A variation in hydraulic conductance of pits joining adjacent xylem vessels is known to impact vulnerability curves (Gascó et al., 2006). Indeed, an increase of pit conductance would increase the lateral flows that bypass an embolized vessel, and thus it would lower the loss of conductivity. To confirm that the observed effect of NaPO4 solutions on vulnerability curves was caused by the process of cavitation per se, we conducted two more experiments on beech segments. First, we analyzed the effect of NaPO4 on xylem hydraulic conductance (K). We used a XYL'EM apparatus (Bronkhorst) to measure K on 25-cm-long stems. The segments were first were perfused at 0.15 MPa for 10 min with a control solution (10 mm KCl and 1 mm CaCl2) and K was determined under low pressure (4 kPa). The procedure was repeated until K stabilized (Kc). Then, the control solution was replaced with a 10 mm NaPO4 solution at pH 2, 4, 6, 7, 8, 10, or 12 and KpH was determined as indicated earlier. The change in xylem hydraulic conductance caused by the NaPO4 solution was expressed as the ratio KpH/Kc. Five stem samples were used for each phosphate solution tested. Second, we determined xylem water retention curves as described by Cochard et al. (2010). Xylem water retention curves show the relative variation in xylem water content with xylem pressure. Stem segments were prepared and perfused with a control solution or with NaPO4 solutions at pH 4 or pH 10, as described above. The bark was entirely removed to reduce branch symplasmic water content. The segments were then installed in a Cavitron with the two ends immersed 1 cm into the same solution in two intact (no holes) plastic reservoirs. Xylem pressure was decreased stepwise, which released water from the segment and increased the water level in each reservoir. Equilibrium was typically obtained in under 2 min, and the water levels in the two reservoirs tended to equilibrate through sample conductance. The water levels in the two reservoirs were then averaged. Retention curves were constructed with three segments for each treatment.
Statistical Analyses
We proceeded with ANOVA to investigate the effects of treatments on vulnerability to cavitation and on specific hydraulic conductivity. If the effects were significant, mean values were compared with the Tukey's Honestly Significant Difference (HSD) test, with significance set at P < 0.05. All the measured and derived data were subjected to statistical analysis using the Statgraphics plus 4.1 software package.
References
- Barigah TS, Ibrahim T, Bogard A, Faivre-Vuillin B, Lagneau LA, Montpied P, Dreyer E. (2006) Irradiance-induced plasticity in the hydraulic properties of saplings of different temperate broad-leaved forest tree species. Tree Physiol 26: 1505–1516 [DOI] [PubMed] [Google Scholar]
- Bosch M, Cheung AY, Hepler PK. (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138: 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffey NJ, Barnett JR, Barlow PW. (1997) Visualization of the cytoskeleton within the secondary vascular system of hardwood species. J Microsc 187: 77–84 [DOI] [PubMed] [Google Scholar]
- Choat B, Ball M, Luly J, Holtum J. (2003) Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiol 131: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Cobb AR, Jansen S. (2008) Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol 177: 608–625 [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Zwieniecki MA, Smets E, Holbrook NM. (2004) Changes in pit membrane porosity due to deflection and stretching: the role of vestured pits. J Exp Bot 55: 1569–1575 [DOI] [PubMed] [Google Scholar]
- Choat B, Pittermann J. (2009) New insights into bordered pit structure and cavitation resistance in angiosperms and conifers. New Phytol 182: 557–560 [DOI] [PubMed] [Google Scholar]
- Christman MA, Sperry JS, Adler FR. (2009) Testing the ‘rare pit’ hypothesis for xylem cavitation resistance in three species of Acer. New Phytol 182: 664–674 [DOI] [PubMed] [Google Scholar]
- Cochard H. (2002) A technique for measuring xylem hydraulic conductance under high negative pressures. Plant Cell Environ 25: 815–819 [Google Scholar]
- Cochard H. (2006) Cavitation in trees. C R Phys 7: 1018–1026 [Google Scholar]
- Cochard H, Casella E, Mencuccini M. (2007) Xylem vulnerability to cavitation varies among poplar and willow clones and correlates with yield. Tree Physiol 27: 1761–1767 [DOI] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Ameglio T. (2005) Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiol Plant 124: 410–418 [Google Scholar]
- Cochard H, Herbette S, Hernandez E, Holtta T, Mencuccini M. (2010) The effects of sap ionic composition on xylem vulnerability to cavitation. J Exp Bot 61: 275–285 [DOI] [PubMed] [Google Scholar]
- Cochard H, Holtta T, Herbette S, Delzon S, Mencuccini M. (2009) New insights into the mechanisms of water-stress-induced cavitation in conifers. Plant Physiol 151: 949–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Lemoine D, Dreyer E. (1999) The effects of acclimation to sunlight on the xylem vulnerability to embolism in Fagus sylvatica L. Plant Cell Environ 22: 101–108 [Google Scholar]
- Dalla-Salda G, Martinez-Meier A, Cochard H, Rozenberg P. (2009) Variation of wood density and hydraulic properties of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) clones related to a heat and drought wave in France. For Ecol Manage 257: 182–189 [Google Scholar]
- Derbyshire P, McCann MC, Roberts K. (2007) Restricted cell elongation in Arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki N, Kido N, Takahashi K, Katou K. (2005) The role of wall Ca2+ in the regulation of wall extensibility during the acid-induced extension of soybean hypocotyl cell walls. Plant Cell Physiol 46: 1831–1838 [DOI] [PubMed] [Google Scholar]
- Gascó A, Nardini A, Gortan E, Salleo S. (2006) Ion-mediated increase in the hydraulic conductivity of laurel stems: role of pits and consequences for the impact of cavitation on water transport. Plant Cell Environ 29: 1946–1955 [DOI] [PubMed] [Google Scholar]
- Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. (1973) Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 32: 195–198 [Google Scholar]
- Hacke UG, Stiller V, Sperry JS, Pittermann J, McCulloh KA. (2001) Cavitation fatigue: embolism and refilling cycles can weaken the cavitation resistance of xylem. Plant Physiol 125: 779–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafren J, Daniel G, Westermark U. (2000) The distribution of acidic and esterified pectin in cambium, developing xylem and mature xylem of Pinus sylvestris. IAWA J 21: 157–168 [Google Scholar]
- Hasunuma T, Fukusaki E, Kobayashi A. (2004) Expression of fungal pectin methylesterase in transgenic tobacco leads to alteration in cell wall metabolism and a dwarf phenotype. J Biotechnol 111: 241–251 [DOI] [PubMed] [Google Scholar]
- Hill AE, Grayson DE, Deacon JW. (1998) Suppressed germination and early death of Phytophthora infestans sporangia caused by pectin, inorganic phosphate, ion chelators and calcium-modulating treatments. Eur J Plant Pathol 104: 367–376 [Google Scholar]
- Jansen S, Choat B, Pletsers A. (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96: 409–419 [DOI] [PubMed] [Google Scholar]
- Jansen S, Sano Y, Choat B, Rabaney D, Lens F, Dute RR. (2007) Pit membranes in tracheary elements of Rosaceae and related families: new records of tori and pseudotori. Am J Bot 91: 503–514 [DOI] [PubMed] [Google Scholar]
- Maherali H, Pockman WT, Jackson RB. (2004) Adaptative variation in the vulnerability of woody plants to xylem cavitation. Ecology 85: 2184–2199 [Google Scholar]
- Mohnen D. (1999) Biosynthesis of Pectins and Galactomannans, Vol 3. Elsevier Science, Amsterdam [Google Scholar]
- O'Neill MA, Albersheim P, Darvill A. (1990) The Pectic Polysaccharides of Primary Cell Walls, Vol 2. Academic Press, London [Google Scholar]
- Pammenter NW, Vander Willigen C. (1998) A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol 18: 589–593 [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rusterucci C, Mellerowicz EJ. (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Pockman W, Sperry J. (2000) Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am J Bot 87: 1287–1299 [PubMed] [Google Scholar]
- Proseus TE, Boyer JS. (2006) Calcium pectate chemistry controls growth rate of Chara corallina. J Exp Bot 57: 3989–4002 [DOI] [PubMed] [Google Scholar]
- Sano Y. (2005) Inter- and intraspecific structural variations among intervascular pit membranes as revealed by field-emission scanning electron microscopy. Am J Bot 92: 1077–1084 [DOI] [PubMed] [Google Scholar]
- Schmitz N, Jansen S, Verheyden A, Kairo JG, Beeckman H, Koedam N. (2007) Comparative anatomy of intervessel pits in two mangrove species gowing along a natural salinity gradient in Gazi Bay, Kenya. Ann Bot (Lond) 100: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Canny MJ. (2000) Architecture of branch-root junctions in maize: structure of the connecting xylem and the porosity of the pit membranes. Ann Bot (Lond) 85: 613–624 [Google Scholar]
- Shomer I, Novacky AJ, Pike SM, Yermiyahu U, Kinraide TB. (2003) Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol 133: 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Hacke UG. (2004) Analysis of circular bordered pit function—I. Angiosperm vessels with homogenous pit membranes. Am J Bot 91: 369–385 [DOI] [PubMed] [Google Scholar]
- Sperry JS, Tyree MT. (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88: 581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller V, Sperry JS. (2002) Cavitation fatigue and its reversal in sunflower (Helianthus annuus L.). J Exp Bot 53: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Tyree MT, Engelbrecht BM, Vargas G, Kursar TA. (2003) Desiccation tolerance of five tropical seedlings in panama: relationship to a field assessment of drought performance. Plant Physiol 132: 1439–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. (1988) Do woody plant operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiol 88: 574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt UK. (2001) Hydraulic vulnerability, vessel refilling, and seasonal courses of stem water potential of Sorbus aucuparia L. and Sambucus nigra L. J Exp Bot 52: 1527–1536 [DOI] [PubMed] [Google Scholar]
- Walters DR, Murray DC. (1992) Induction of systemic acquired resistance to rust in Vicia faba by phosphate and EDTA: effects of calcium. Plant Pathol 41: 444–448 [Google Scholar]
- Wen F, Zhu Y, Hawes MC. (1999) Effect of pectin methylesterase gene expression on pea root development. Plant Cell 11: 1129–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EA. (1983) Intervascular pit membranes in Ulmus and Celtis native to the United States. IAWA J 4: 79–88 [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N. (2005) Inter-vessel pitting and cavitation in woody rosaceae and other vesselled plants: a basis for a safety versus a efficiency trade-off in xylem transport. Plant Cell Environ 28: 800–812 [Google Scholar]
- Willats WG, McCartney L, Mackie W, Knox JP. (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47: 9–27 [PubMed] [Google Scholar]
- Zimmermann MH. (1983) Xylem Structure and Ascent of Sap. Springer-Verlag, New York [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. (2001) Hydrogel control of xylem hydraulic resistance in plants. Science 291: 1059–1062 [DOI] [PubMed] [Google Scholar]