Abstract
During plant sexual reproduction, pollen germination and tube growth require development under tight spatial and temporal control for the proper delivery of the sperm cells to the ovules. Pollen tubes are fast growing tip-polarized cells able to perceive multiple guiding signals emitted by the female organ. Adhesion of pollen tubes via cell wall molecules may be part of the battery of signals. In order to study these processes, we investigated the cell wall characteristics of in vitro-grown Arabidopsis (Arabidopsis thaliana) pollen tubes using a combination of immunocytochemical and biochemical techniques. Results showed a well-defined localization of cell wall epitopes. Low esterified homogalacturonan epitopes were found mostly in the pollen tube wall back from the tip. Xyloglucan and arabinan from rhamnogalacturonan I epitopes were detected along the entire tube within the two wall layers and the outer wall layer, respectively. In contrast, highly esterified homogalacturonan and arabinogalactan protein epitopes were found associated predominantly with the tip region. Chemical analysis of the pollen tube cell wall revealed an important content of arabinosyl residues (43%) originating mostly from (1→5)-α-l-arabinan, the side chains of rhamnogalacturonan I. Finally, matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis of endo-glucanase-sensitive xyloglucan showed mass spectra with two dominant oligosaccharides (XLXG/XXLG and XXFG), both being mono O-acetylated, and accounting for over 68% of the total ion signals. These findings demonstrate that the Arabidopsis pollen tube wall has its own characteristics compared with other cell types in the Arabidopsis sporophyte. These structural features are discussed in terms of pollen tube cell wall biosynthesis and growth dynamics.
Fertilization of flowering plants requires the delivery of the two sperm cells, carried by a fast growing tip-polarized pollen tube, to the egg cell. In plants with dry stigma and solid style such as Arabidopsis (Arabidopsis thaliana), this process begins with the deposition and specific adhesion of the pollen grains on the stigmatic tissue, subsequent hydration of the pollen grains, and germination of pollen tubes (Palanivelu and Preuss, 2000). Pollen tubes invade the papillae cell wall of the stigma, enter the short style, and grow through the apoplast of the specialized transmitting tract (TT) that is filled with a nutrient-rich extracellular matrix (Kandasamy et al., 1994; Lennon et al., 1998). During this invasive growth, pollen tubes are guided to the ovules via signals that need to pass through the cell wall to reach their membrane-associated or intracellular targets (Lord and Russell, 2002; Kim et al., 2003; Boavida et al., 2005; McCormick and Yang, 2005; Johnson and Lord, 2006). In plant species with wet stigma and hollow style such as lily (Lilium longiflorum), adhesion between the pollen tube wall and the TT epidermis extracellular matrix is important for the growth of the pollen tubes toward the ovules (Mollet et al., 2000, 2007; Park et al., 2000; Chae et al., 2007). In addition to being the interface between the tube cells and the surroundings (female sporophyte or culture medium), the pollen tube wall also controls the cell shape, protects the generative cells, and allows resistance against turgor pressure (Geitmann and Steer, 2006; Geitmann, 2010).
Most of our knowledge on cell wall polymers of higher plants comes from investigations on vegetative organs in which cells have diffuse growth. The cell wall is mainly composed of polysaccharides (cellulose, hemicellulose, pectin, and occasionally callose, depending on the tissue) and proteoglycans (e.g. extensin and arabinogalactan proteins [AGPs]) forming a complex network with processing enzymes.
Pectins are complex wall macromolecules with uncertain supramolecular organization (Vincken et al., 2003) consisting of homogalacturonan (HG) that can be methylesterified and acetylesterified, rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II), and xylogalacturonan (Carpita and McCann, 2000). HG is a polymer of repeated units of (1→4)-α-d-GalUA that can be cross-linked with calcium upon block-wise action of pectin methylesterases (PMEs) on methylesterified HG (Micheli, 2001). RG-II has the same homopolymer backbone as HG but is substituted with four different oligosaccharides composed of unusual sugars, such as apiose, aceric acid, and 3-deoxy-d-manno-2-octulosonic acid, of unknown function (for review, see Caffall and Mohnen, 2009). RG-I consists of the repeating disaccharide (1→4)-α-d-GalUA-(1→2)-α-l-Rha, with a wide variety of side chains attached to the rhamnosyl residues, ranging from monomers to large oligosaccharides such as (1→4)-β-d-galactan, (1→5)-α-l-arabinan, and/or type I arabinogalactan (Caffall and Mohnen, 2009).
Xyloglucan (XyG) is the major hemicellulosic polysaccharide of the primary wall of flowering plants. Classic XyG consists of a (1→4)-β-d-glucan backbone substituted with Xyl, Gal-Xyl, or Fuc-Gal-Xyl motifs, which correspond, according to the one-letter code proposed by Fry et al. (1993), to X, L, and F, respectively, G being the unsubstituted glucosyl residue of the glucan backbone. The main XyG fragments released after endo-glucanase treatment of the cell wall from wild-type Arabidopsis vegetative organs are generally XXXG, XXLG/XLXG, XXFG, and XLFG (Zablackis et al., 1995; Lerouxel et al., 2002; Nguema-Ona et al., 2006; Obel et al., 2009). In addition, O-acetylation of XyG can occur, most generally on the galactosyl residues, but its biological function is unknown (Cavalier et al., 2008). In the primary wall, XyG interacts with cellulose microfibrils via hydrogen bonds and participates in the control of cell expansion (Cosgrove, 1999).
AGPs and extensin belong to the Hyp-rich glycoproteins superfamily with very high levels of type II arabinogalactan glycosylation (Nothnagel, 1997; Showalter, 2001). These proteoglycans have been implicated in many aspects of plant development, including cell expansion, cell signaling and communication, embryogenesis, wound response, and pollen tube guidance (Wu et al., 1995; Nothnagel, 1997; Seifert and Roberts, 2007; Driouich and Baskin, 2008).
Despite the importance of pollen tubes for the delivery of the sperm cells to the egg, little is known about the underlying molecular mechanisms that regulate the mechanical interaction of pollen tubes with female floral tissues. There are very scarce data concerning the different components of the pollen tube cell wall. Past approaches to characterize the pollen tube cell wall are limited to a few plant genera, including Camellia (Nakamura and Suzuki, 1981), Lilium (Jauh and Lord, 1996; Mollet et al., 2002), Nicotiana (Rae et al.,1985; Li et al., 1995; Ferguson et al., 1998; Qin et al., 2007), Pinus (Derksen et al., 1999), and Zea (Rubinstein et al., 1995), and are mostly based on immunocytochemistry. These studies revealed that, depending on the species, the pollen tube cell wall contains epitopes that are found in the polymers described above, including HGs with varying levels of methylesterification, AGPs, extensin-like proteins, and low amounts of cellulose. Unlike most other plant cells, callose, a (1→3)-β-glucan, is predominant and is deposited in the wall back from the tip. Moreover, it is deposited at regular intervals to form callose plugs that maintain the tube cell in the apical expanding region of the tube and separate the viable from the degenerating region of the tube (for review, see Geitmann and Steer, 2006). Only a few reports have investigated the pollen tube of the model plant Arabidopsis. They have focused either on in vivo-grown or on in vitro-grown pollen tubes using monoclonal antibodies (MAbs) directed against a subset of cell wall epitopes present in HG, XyG, and AGPs (Lennon and Lord, 2000; Freshour et al., 2003; Pereira et al., 2006), but quantitative chemical analyses are lacking. This lack of information is most likely due to the fact that substantial amounts of pollen tube material are needed for chemical analysis, and a reproducible and efficient method for liquid culture of Arabidopsis pollen tubes had not been established until recently (Boavida and McCormick, 2007; Bou Daher et al., 2009).
Here, we report the composition and localization of different cell wall polymers of in vitro-grown wild-type Arabidopsis pollen tubes based on biochemical analyses coupled to immunocytochemical investigations both at light and transmission electron microscopy (TEM) levels using recently developed MAbs. Our results show distinct patterns of labeling (tip, whole tube, and shank of the tube) depending on the recognized epitope. The most striking observations are (1) the abundance of (1→5)-α-l-arabinan in the tube wall (greater than 40 mol % of Ara), mostly localized, with LM6 and LM13, in the outer wall layer of the tube and (2) an atypical XyG matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) profile with over 68% of the oligosaccharide fragments being O-acetylated.
RESULTS
In Vitro Pollen Tube Growth and β-Glucan Localization
In order to study the cell wall of Arabidopsis pollen tubes, sufficient material was necessary to perform the biochemical analysis. To achieve this, a large number of flowers, a good in vitro pollen germination rate, and sufficient pollen tube length were required. As shown in Figure 1A, high rates of pollen germination were obtained (67% ± 12%), with an average pollen tube length of 1,248 ± 374 μm and a growth rate of 0.46 ± 0.05 μm min−1 after 16 h of culture in liquid medium. Moreover, 16-h-old pollen tubes displayed typical tip organization (Fig. 1A, inset) without any plasmolysis event. These results showed that using these in vitro conditions, pollen tube length is approaching what we should expect in in vivo conditions given the distance between stigma and ovules. By comparison, 6-h-old pollen tubes were 4-fold shorter, with an average length of 307 ± 102 μm and an estimated growth rate of 1.6 ± 0.17 μm min−1.
Figure 1.
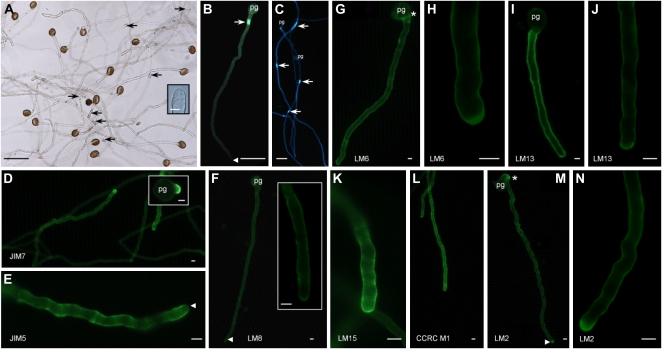
In vitro pollen tube growth, β-glucan staining, and immunolocalization of Arabidopsis pollen tube wall epitopes. A, Sixteen-hour-old in vitro pollen tubes grown in liquid medium (arrows show callose plugs). The inset is a closeup of a 16-h-old in vitro pollen tube tip. B, Cytochemical staining of β-glucan (callose) with aniline blue showing a callose plug (arrow) and a lack of staining in the pollen tube tip region (arrowhead). C, Cytochemical staining with calcofluor white showing callose plugs (arrows) and the localization of β-glucans in the wall. D to N, Immunofluorescence labeling of cell wall polymer epitopes at the surface of pollen tubes. D to F, Arabidopsis pollen tube wall epitopes probed with anti-pectin MAbs JIM7 (D), JIM5 (E), and LM8 (F), specific for highly and low methylesterified HG and xylogalacturonan, respectively. D, The JIM7 tag showed a strong signal at the tip in a well-developed pollen tube and emerging tube tip from the pollen grain (inset). E, Pollen tube stained with JIM5 showing a very weak intensity of labeling at the tip (arrowhead) compared with the wall back from the tip. F, LM8 labeled evenly the entire pollen tube wall. The insert is a closeup of an in vitro pollen tube tip. G to J, Detection of (1→5)-α-l-arabinan epitopes with LM6 (G and H) and LM13 (I and J). Labeling was evenly distributed along the entire tube wall, with a strong signal at the pollen tube tip. Note a collar-like structure (asterisk) labeled at the emergence of the pollen tube from the pollen grain in G. K and L, Immunofluorescence staining of the nongalactosylated (XXXG motif) and fucosylated XyG with LM15 (K) and CCRC-M1 (L), respectively. Labeling with both MAbs was not evenly detected in the wall and displayed periodic deposition of the epitopes containing polysaccharides during pollen tube growth. M and N, LM2, which recognizes β-d-GlcpUA-(1→3)-α-d-GalpUA-(1→2)-l-Rha moieties of AGPs, labeled weakly the whole tube, with the strongest signal at the tip. In M, a more intense labeling (asterisk) was observed at the emergence of the tube from the pollen grain. Pollen tubes were grown for 6 h except in A and C (16 h). pg, Pollen grain. Bars = 50 μm (A–C) and 5 μm (A inset and D–N).
Cytochemical staining of β-glucans with aniline blue (for callose) and calcofluor white (mainly callose and cellulose) was performed in order to visualize the distribution of these β-glucans along the tube wall and within the tube. Aniline blue staining was not detectable in the tip region and was mainly localized in the wall, back from the tip, and within the tube as callose plugs that are periodically synthesized (Fig. 1B; Table I). In contrast, staining with calcofluor white was uniform along the whole tube and the tip. The bright regions within the tubes revealed several plugs (Fig. 1C). At the ultrastructural level, the Arabidopsis pollen tube wall back from the tip appeared as a bilayered structure consisting of a fibrillar outer and a weakly electron-dense inner wall layer (Fig. 2A). Immunogold localization of callose resulted in dense labeling in the inner wall layer back from the tip (Supplemental Fig. S1A).
Table I. Summary of cytochemical staining and immunolocalization of cell wall polymers in in vitro-grown Arabidopsis pollen tubes.
Data are based on observations at the fluorescence microscopy (FM) and electron microscopy (EM) levels. −, No labeling detected (at over 500-ms exposure time); ±, weak labeling detected; +, strong labeling detected. iw, Inner wall layer; na, not applicable; nd, not determined; ow, outer wall layer.
| Target | Probe | Back from the Tip |
Tip |
|
| FM Level | EM Level | FM Level | ||
| β-Glucan | Calcofluor white | + | na | + |
| Callose | Aniline blue | + | na | − |
| Anti-callose | nd | iw | nd | |
| Extensin | LM1 | − | nd | − |
| AGP | LM2 | ± | nd | + |
| MAC207 | ± | nd | + | |
| JIM13 | − | nd | − | |
| HG | JIM5 | + | nd | ± |
| JIM7 | ± | nd | + | |
| Galactan | LM5 | ± | nd | ± |
| Arabinan | LM6 | + | ow | + |
| LM13 | + | nd | + | |
| Xylogalacturonan | LM8 | + | nd | + |
| XyG | CCRC-M1 | + | iw/ow | + |
| LM15 | + | iw/ow | + | |
| Controls | − | − | − | |
Figure 2.
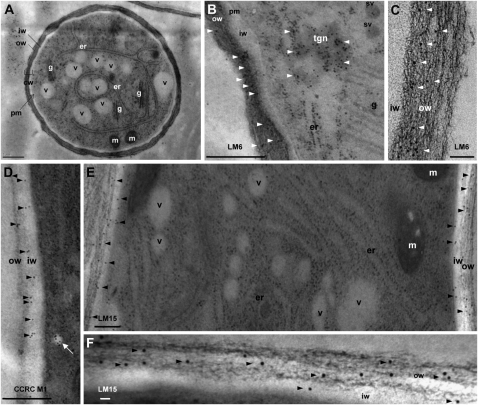
Electron micrographs showing the ultrastructure (A) and immunogold labeling of cell wall epitopes (B–F) of high-pressure frozen/freeze-substituted Arabidopsis pollen tube grown in vitro for 6 h. A, Cross section of a pollen tube showing the cell wall (cw) composed of two distinct layers: a fibrillar outer wall (ow) and a weakly electron-dense inner wall (iw). Well-preserved organelles are also clearly distinguishable, including endoplasmic reticulum (er), Golgi stacks (g), mitochondria (m), and vacuoles (v). pm, Plasma membrane. B and C, Immunogold labeling of (1→5)-α-l-arabinan epitopes with LM6. Gold particles (arrowheads) are mostly localized in the outer wall layer. In B, possible trans-Golgi network (tgn) and secretory vesicles (sv) labeled with LM6 are seen. D, Immunogold labeling of fucosylated XyG motif recognized by CCRC-M1 in the inner and outer wall layers. Note the presence of gold particles in vesicles in the vicinity of the plasma membrane (white arrow). E and F, Immunogold labeling of nonfucosylated XyG motif (XXXG) with LM15. Gold particles (arrowheads) are visible in the inner and mainly in the outer walls. Bars = 1 μm (A), 0.5 μm (B, D, and E), and 100 nm (C and F).
Immunolocalization of Arabidopsis Pollen Tube Cell Wall Epitopes
Immunolabeling Pattern of Pectin Domains
Probing with JIM7 and JIM5, which recognize highly and partially methylesterified HGs, revealed that JIM7 epitopes were dominantly localized in the tip region of the pollen tubes (Table I; Fig. 1D). Similar observations were made at tube tips emerging from pollen grains (Fig. 1D, inset). In contrast, JIM5 staining was observed mostly back from the tip with brighter ring-like deposits, presumably originating from successive temporary slowdowns in the growth of the tubes (Fig. 1E). LM8, specific for xylogalacturonan epitopes, uniformly labeled the whole pollen tube wall (Fig. 1F, inset). (1→5)-α-l-Arabinan epitopes, localized with LM6 and LM13, were clearly detected in the entire pollen tube wall (Fig. 1, G and I), with stronger fluorescent signal at the pollen tube tip (Fig. 1, H and J). A ring-like structure was also noticeable as a collar at the emergence of the pollen tube from the pollen grain (Fig. 1G). Immunogold labeling of epitopes recognized by LM6 was mostly found in the fibrillar outer wall layer (Fig. 2, B and C). A strong concentration of gold particles was also observed over densely stained vesicles, possibly associated with the trans-Golgi network (Fig. 2B). Epitopes recognized by LM6 were also detected in the intine wall of the pollen grain (Supplemental Fig. S1B).
Immunolabeling of XyG Motifs
To investigate the distribution of XyG, pollen tubes were probed with the MAbs LM15 and CCRC-M1, which recognize nongalactosylated (XXXG motif) and fucosylated XyG domains, respectively. As shown in Figure 1, K and L, both MAbs bound to the entire pollen tube wall.
TEM observations showed that fucosylated XyG epitopes probed with the MAb CCRC-M1 were mainly found in the inner pollen tube wall (Fig. 2D), whereas the nongalactosylated XyG epitopes tagged with LM15 were detected in both the inner and outer layers of the wall (Fig. 2, E and F; Table I). Immunolabeling of pollen grains with the LM15 and CCRC-M1 MAbs resulted in a strong detection of XyG epitopes, mostly in the intine wall and at the vicinity of the plasma membrane (Supplemental Fig. S1, C and D).
Immunolabeling of AGPs
Epitopes of AGPs recognized by the MAb LM2 were found slightly labeled throughout the pollen tube wall, with a stronger signal in the tip region (Fig. 1, M and N; Table I). Similar results were observed with MAC207, whereas JIM13 did not label the tube cell wall (Table I).
Immunolabeling at the fluorescence microscopy level was carried out on 6- and 16-h-old pollen tubes and did not show any difference in labeling pattern between the two time points (data not shown). Controls for immunofluorescent labeling did not show any fluorescence of the pollen tubes and showed a weak autofluorescence of the pollen grain exine (data not shown). Controls for immunogold detection did not exhibit any significant nonspecific gold particles on the resin, pollen tubes, and pollen grains (Supplemental Fig. S1, E–H). A summary of immunolocalization data on the Arabidopsis pollen tube wall is shown in Table I.
Contribution of Pollen Grain Wall versus Pollen Tube Wall for Biochemical Analysis
We estimated the relative abundance of the pollen tube cell wall versus the pollen grain cell wall by measuring the cell wall thickness based on TEM observations, pollen germination rates, and pollen tube length (Supplemental Table S1). The estimated ratio of the pollen grain cell wall on the pollen tube cell wall was about 1:7.4. Similarly, we also quantified, by gas chromatography (GC), the amount of carbohydrates present in nongerminated pollen grains and 16-h-old germinated pollen tubes isolated from 520 flowers (Supplemental Fig. S2). Monosaccharide composition of the pollen grain and the pollen tube cell walls was similar, and the amount of carbohydrates contained in the pollen grain cell wall was 8-fold lower than the amount of carbohydrates contained in the pollen tube cell wall. These data indicate that the quantity of pollen grain cell wall material would interfere only weakly in the cell wall analysis and were used as a baseline for the monosaccharide composition of Arabidopsis pollen tubes.
Monosaccharide Composition of the Arabidopsis Pollen Tube Wall
Monosaccharide analysis of Arabidopsis pollen tube cell wall (Table II) displayed a high level of Glc (19.8 mol %), originating probably mostly from callose and to a lesser extent from XyG polymers. The most abundant monosaccharide, Ara (43.6 mol %), and Gal (8.4 mol %) may come from AGP glycans and/or the pectic RG-I domain. Indeed, both sugars composing the backbone of RG-I (i.e. Rha and GalUA) were present in the extract in equimolar amounts (approximately 5 mol %). The remaining GalUA (approximately 5 mol %) composes undoubtedly the pectic HG backbone. XyG polymers generally consist of Xyl and Fuc residues that are also found in this analysis, although in low amounts (1.2 mol % Fuc and 6.2 mol % Xyl), suggesting that XyG is not a major cell wall component of the pollen tube cell wall.
Table II. Monosaccharide composition and linkage analysis of total pollen tube cell wall and pectin-enriched (ammonium oxalate) and hemicellulose-enriched (KOH) extracts.
| Carbohydrate Analysis | Pollen Tube Wall | Oxalate Extract | KOH Extract |
| Neutral sugara (%) | – | 78.5 | 97.6 |
| Uronic acidb (%) | – | 21.5 | 2.4 |
| Monosaccharide compositionb | |||
| Ara | 43.6 ± 4.5 | 24.7 ± 1.9 | 23 ± 7.4 |
| Fuc | 1.2 ± 0.2 | 1.0 ± 0.5 | 0.9 ± 0.7 |
| Gal | 8.4 ± 1.0 | 18.3 ± 0.6 | 14 ± 3.2 |
| Glc | 19.8 ± 5.1 | 19.3 ± 2.8 | 40.1 ± 6.6 |
| GalUA | 10.9 ± 0.7 | 21.5 ± 1.4 | 6.8 ± 0.2 |
| GlcUA | 2.2 ± 3.0 | 3.4 ± 0.8 | 1.5 ± 0.4 |
| Rha | 5.3 ± 0.8 | 4.2 ± 0.2 | 2.4 ± 0.7 |
| Man | 2.2 ± 1.5 | 1.7 ± 3.0 | 4.9 ± 0.1 |
| Xyl | 6.2 ± 1.2 | 5.8 ± 0.6 | 6.5 ± 0.4 |
| Linkage analysisc | |||
| t-Araf | – | 27.4 | 8.5 |
| 5-Araf | – | 28.2 | 14.6 |
| 2,5-Araf | – | 3.7 | 2.2 |
| 3,5-Araf | – | 3.9 | 2.7 |
| t-Xyl | – | 1.4 | 1.1 |
| 2-Xyl | – | 1 | 2.4 |
| t-Gal | – | 1.7 | 6 |
| 3-Gal | – | 2 | 1.2 |
| 4-Gal | – | 1.5 | nd |
| 6-Gal | – | 4 | 1.8 |
| 3,6-Gal | – | 5.6 | 7.3 |
| 4,6-Gal | – | 1.2 | 2.2 |
| 3-Glc | – | 10 | 27.6 |
| 4-Glc | – | 8.4 | 15.4 |
| 2,3-Glc | – | nd | 7 |
Determined by the phenol sulfuric and m-hydroxydiphenyl colorimetric assays.
Determined by GC and expressed as mol %. Values are means ± sd from two independent experiments.
Determined by GC-MS of partially methylated alditol acetates and expressed as percentage of total area of the identified peaks. t-Araf denotes 1,4-di-O-acetyl-1-deuterio-2,3,5-tri-O-methyl-d-arabinitol, etc. –, Not determined; nd, not detected.
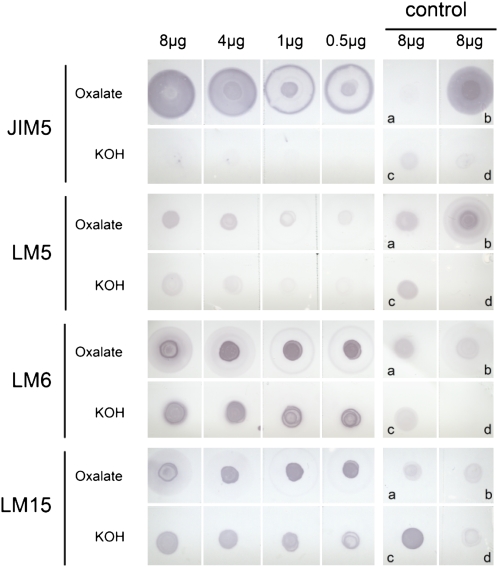
The sequential extraction of the Arabidopsis pollen tube cell wall with ammonium oxalate and KOH aimed at yielding pectin- and hemicellulose-enriched fractions. Both extracts were analyzed for monosaccharide composition by GC and linkage/substitution sites by GC-mass spectrometry (MS; Table II). The oxalate extract showed similar levels of uronic acid content by colorimetric assay (21.5 mol %) and GC (24.9 mol %) analyses. This extract was enriched in GalUA (21.5 mol %), indicating the presence of the pectin domains HG and highly branched RG-I, as shown by the detection of 4.2 mol % Rha, 24.7 mol % Ara, and 18.3 mol % Gal. These data are supported by the immunodot assay (Fig. 3), showing a strong interaction of the oxalate extract with JIM5 for partially methylesterified HG and LM6 for (1→5)-α-l-arabinan. This labeling was consistent with the presence of 28.2% 5-Araf (Table II). Labeling with LM5 was weak, which suggests a low amount of (1→4)-β-d-galactan. This observation was confirmed by the detection of only 1.5% 4-linked galactosyl residues (Table II). Instead, galactosyl residues detected in the monosaccharide analysis originate probably from type II arabinogalactans, as shown by the detection of 3-Gal, 6-Gal, and 3,6-Gal (Table II).
Figure 3.
Immunodot blot assay of pectin-enriched (oxalate extract) and hemicellulose-enriched (KOH extract) fractions isolated from Arabidopsis pollen tube cell wall. Oxalate and KOH extracts (8, 4, 1, and 0.5 μg) were probed with JIM5 for partially methylesterified HG, LM5 for (1→4)-β-d-galactan, LM6 for (1→5)-α-l-arabinan, and LM15 for nongalactosylated XyG (XXXG). Controls (8 μg) are as follows: a, arabinan from sugar beet; b, pectin with 8.6% methylesterification from citrus; c, XyG from tamarind seed; d, gum arabic from acacia. [See online article for color version of this figure.]
In the KOH extract, levels of HG (GalUA) and RG-I backbone (Rha and GalUA) decreased significantly compared with the oxalate extract, but the remaining RG-I domains were probably highly substituted by arabinans and/or arabinogalactans, as both arabinosyl and galactosyl residues counted for 37 mol % of the total sugars (Table II). The dot-blot immunoassay (Fig. 3) revealed a significant reduction of the signal in the KOH extract with JIM5 compared with the oxalate extract, indicating that KOH treatment had substantially removed methylester groups from the partially methylesterifed HG, leaving a low amount of nonesterified HG. In contrast, labeling for (1→5)-α-l-arabinan with LM6 was still strong (Fig. 3) and was confirmed with the detection of 14.6% 5-Araf (Table II). The different components of XyG (i.e. Glc, Xyl, Gal, and Fuc) were also present in the KOH extract (Table II), with a large increase in the Glc level (40.1 mol %) compared with the oxalate extract. As revealed by methylation analysis, the latter originated from XyG (2.2% 4,6-Glc), starch, and/or amorphous cellulose (15.4% 4-Glc) but dominantly from callose with the presence of 3-Glc (27.6%) and possibly 2,3-Glc (7%), which may suggest that callose can be substituted on the C2 of the glucosyl residues (Table II). The MAb LM15 reacted strongly with both oxalate and KOH extracts (Fig. 3), suggesting that XyG was present in both extracts.
MALDI-TOF MS Analysis of Pollen Tube Cell Wall XyG Fragments
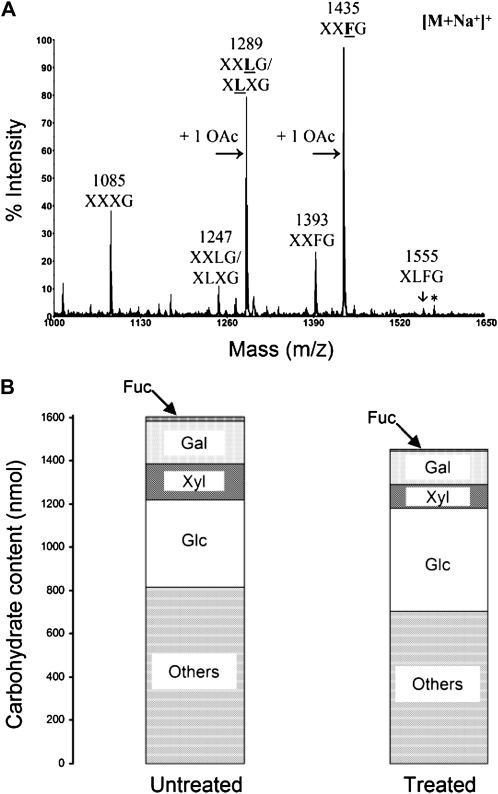
Analysis by MALDI-TOF MS of XyG fragments released after XyG endo-glucanase treatment of Arabidopsis pollen tube cell wall (Fig. 4A) showed the typical ions, XXXG, XLXG/XXLG, and XXFG (14.7%, 5.7%, and 8.4%, respectively), found in XyG (Table III). However, these oligosaccharides were not the main motifs. Instead, the two principal ions identified (XLXG/XXLG and XXFG) present a shift of mass-to-charge ratio = 42, characteristic of an O-acetyl group, branching presumably the galactosyl residue. These two O-acetylated fragments count for over 68% of the total oligosaccharide released. Overall, galactosylated and fucosylated XyG oligosaccharides showed a relative abundance of 85% and 52%, respectively. By comparison, the main XyG fragment released after endo-glucanase treatment of Arabidopsis leaf cell wall was XXXG (Supplemental Fig. S3) and the total level of O-acetylated fragments was 33%. Moreover, based on the signal intensity, the ratio of Fuc to Xyl can be estimated as 1:5.7, similar to what was found in the compositional analysis of the pollen tube wall extract (1:5.2), which suggests that other Xyl-containing polymers such as xylan may not be present or in very low amount in the pollen tube cell wall. Finally, monosaccharide analysis of the endo-glucanase-resistant pollen tube cell wall residue (Fig. 4B) revealed, despite a clear decrease in the carbohydrate content of Xyl, Gal, and Fuc compared with the untreated cell wall material, that all the XyGs have not been completely degraded by the enzyme, perhaps due to their tight association with other cell wall components.
Figure 4.
Analysis of Arabidopsis pollen tube XyG. A, MALDI-TOF mass spectrum of endo-glucanase-generated XyG fragments from the cell wall of 16-h-grown Arabidopsis pollen tubes. The structures of the XyG fragments are shown according to the nomenclature proposed by Fry et al. (1993). Underlined and boldface structures represent O-acetylated side chains (+ 1 OAc). The asterisk indicates the signal of the XLFG fragment with K+ adduct ion instead of Na+. B, Monosaccharide composition of 16-h-grown Arabidopsis pollen tube cell wall before (untreated) and after (treated) endo-glucanase treatment. Only the monosaccharides composing XyG are shown. “Others” include Ara, GalUA, GlcUA, Man, and Rha.
Table III. Relative quantification of XyG oligosaccharides obtained after endo-glucanase digestion of Arabidopsis pollen tube wall.
| Massa | Compositionb | Structurec | Relative Abundanced |
| 1,085 | Hex4Pent3 | XXXG | 14.7 ± 3.3 |
| 1,247 | Hex5Pent3 | XLXG/XXLG | 5.7 ± 0.6 |
| 1,289 | Hex5Pent3OAc1 | XLXG/XXLG + 1 OAc | 27.6 ± 3.9 |
| 1,393 | Hex5Pent3Dox1 | XXFG | 8.4 ± 0.5 |
| 1,435 | Hex5Pent3Dox1OAc1 | XXFG + 1 OAc | 41.2 ± 6.6 |
| 1,555 | Hex6Pent3Dox1 | XLFG | 2.5 ± 0.1e |
Mass of the fragments [M+Na+]+.
Hex, Hexose; Pent, pentose; OAc, O-acetyl substituent; Dox, deoxyhexose.
Based on XyG oligosaccharide structures found in Pauly et al. (2001).
Values are expressed as percentage and are means ± sd from MALDI spectra obtained after endo-glucanase digestion from three different Arabidopsis pollen tube cell wall extracts.
Relative abundance of this oligosaccharide corresponds to the total of the K+ and Na+ adduct fragments.
DISCUSSION
Since the Arabidopsis Genome Initiative (2000), increasing genomic, transcriptomic, and proteomic data on Arabidopsis have become available (Becker et al., 2003; Honys and Twell, 2004; Tung et al., 2005; Qin et al., 2009; Zou et al., 2009). The use of male and female gametophyte or sporophyte mutants has allowed major insight into pollen formation, pollen tube growth, guidance, and signaling (Rhee et al., 2003; Dong et al., 2005; Escobar-Restrepo et al., 2007), but the biosynthesis, the remodeling, and the overall role of the cell wall of the tip-growing pollen tube cell are far from being fully understood. Using the in vitro pollen tube method developed by Boavida and McCormick (2007), we obtained high levels of pollen germination, ranging from 55% to 79%, with pollen tubes that can expand over 1.5 mm after 16 h of culture. Based on this, we decided to investigate one of the main components, the cell wall, of this tip-growing cell by means of immunolocalization at light and electron microscopy levels and analytical polysaccharide biochemistry. Our results showed that (1) the pectin wall is enriched in HG and (1→5)-α-l-arabinan and (2) O-acetylation of XyG fragments released by endo-glucanase is high compared with other sporophytic tissues.
Most of the precursor studies on the pollen tube wall of the so-called “pollen tube model plants” (i.e. Nicotiana or Lilium) employed MAbs recognizing the HG pectin domains (JIM5 and JIM7) and a subset of carbohydrate epitopes of the cell surface AGPs (mainly JIM13, MAC207, LM2, and JIM8). To date, only scarce reports on the pollen tube wall have been published using other well-defined MAbs such as anti-XyG (CCRC-M1; Freshour et al., 2003) and, to our knowledge, none with the more recently produced MAbs on pollen tube wall.
Arabidopsis Pollen Tube Wall Is Composed of an Inner Layer Enriched in Callose and an Outer Fibrillar Layer
TEM observations of in vitro rapidly frozen Arabidopsis pollen tubes showed the typical two wall layers at the shank of the tip (a fibrillar outer and a weakly electron-dense inner wall layer), as observed in in vitro-grown tobacco (Nicotiana tabacum) pollen tube (Li et al., 1995; Ferguson et al., 1998) and in in vivo-grown pollen tube from lily (Roy et al., 1997) and Arabidopsis (Lennon and Lord, 2000; Derksen et al., 2002). Our data and those of others have shown that callose is the main component of the inner wall layer but is not detectable at the tip, whereas cellulose is weakly detected (Ferguson et al., 1998).
Arabidopsis Pollen Tube Wall Contains a Subset of AGPs Recognized by LM2
We found that AGPs were labeled in the whole tube, with a stronger signal at the tip with LM2, whereas JIM13 labeling was almost absent. In contrast, JIM13 labeled lily pollen tube at the tip (Mollet et al., 2002) and uniformly or in a ring-like deposition along the tube of tobacco (Li et al., 1992; Qin et al., 2007). Pereira et al. (2006) also noted a lack of labeling of Arabidopsis pollen tube with JIM13, unlike MAC207, which labels uniformly the whole tube (Coimbra et al., 2008). These divergent data depend on the MAb used [despite the apparent identical epitope, β-d-GlcpUA-(1→3)-α-d-GalpUA-(1→2)-l-Rha, recognized by MAC207 and JIM13] and the species investigated. These differences may suggest that the carbohydrate moieties of AGPs are species and/or cell type specific (vegetative and sperm cells) or that the cell wall organization is different in these species, which may alter the accessibility of the epitopes.
HG Pectin Domains Are Present in Different Locations in Arabidopsis Pollen Tube Wall
Immunofluorescent labeling with JIM5 and JIM7 shows a pattern similar to that observed in pollen tubes from families possessing a solid style, such as the Solanaceae (potato [Solanum tuberosum], tobacco, petunia [Petunia hybrida]), Oleaceae (jasmine [Jasminum species]), and Poaceae (corn [Zea mays]; Li et al., 1994; Qin et al., 2007). A dominant localization of highly methylesterified HG, recognized by JIM7, was present at the tip, and a periodic patterning of low methylesterified HG epitopes, labeled with JIM5, was visible behind the tip region. Pollen tube wall contains 10 mol % GalUA, half of it being presumably dedicated to HG building, which explains the weak labeling by JIM5 along the wall compared with other species (Jauh and Lord, 1996; Qin et al., 2007), even though reduced accessibility of the MAb to the epitopes may occur due to other polymers. In fact, the pectin-enriched extract isolated from the pollen tube wall showed a strong interaction with JIM5 (Fig. 3). Highly methylesterified HG and other pectic motifs are deposited at the growing tip by Golgi-derived vesicles and deesterified via PMEs in the wall back from the tip, to allow calcium cross-linking of the carboxyl groups (Geitmann and Steer, 2006). In the tobacco pollen tube, two PME isoforms have been localized in the Golgi, secretory vesicles, and at the outer surface of the plasma membrane, where they may deesterify HG (Li et al., 2002). Two Arabidopsis mutants, vanguard1 (Jiang et al., 2005) and Atppme1 (Tian et al., 2006), defective in two different pollen-specific PMEs, showed slight reduction (about 20%) of overall PME activity, reduced growth compared with wild-type pollen tubes, but different pollen tube phenotypes. vanguard1 pollen tubes were unstable in vitro, resulting in tip bursting, and in vivo, vanguard1 plants showed reduced male fertility. In contrast, Atppme1 pollen tubes showed an in vitro branching pattern with numerous tips but did not show male sterility in vivo. These reports suggest that these mutant pollen tubes have modified cell wall mechanical properties, despite a lack of either immunolocalization or biochemical data showing a modification in the methylesterification level of the HG motif. It is noteworthy to point out, as suggested by Jiang et al. (2005), that these PMEs may also have a role in modifying the female TT wall to facilitate pollen tube progression through the female tissue. AtPPME1 and VANGUARD1 are among a group of 15 other pollen-specific genes that are separated in two groups (I and II) and encode putative PMEs containing either a catalytic domain only (five genes) or a catalytic domain and a putative PME inhibitor (PMEI) domain (10 genes; Chen and Ye, 2007; Pelloux et al., 2007). More studies on these important protein members are required to understand the fine-tuning of demethylesterification of HG and to determine the interaction between PME and PMEI and their roles in modifying the pollen tube mechanics and sensing (Bosch et al., 2005). Low esterified HGs have been implicated in important physiological processes, such as cell attachment in vegetative cells or organs (Bouton et al., 2002; Leboeuf et al., 2005; Durand et al., 2009) and, associated with a stigma/style Cys-rich adhesin, a secreted plant lipid transfer protein (LTP), in lily pollen tube adhesion (Mollet et al., 2000; Park et al., 2000). Several LTPs are present in the TT of the Arabidopsis pistil along the pollen tube path (Tung et al., 2005). Recently, the LTP5, produced in Arabidopsis pollen tube and in the pistil TT, has been proposed to play important roles in maintaining cell polarity at the tube tip and adhesion-mediated guidance perhaps by interaction with pectins (Chae et al., 2009).
Arabidopsis Pollen Tube Wall Is Enriched in Pectin Arabinan
In addition to HG motifs, Arabidopsis pollen tube wall contains highly branched RG-I with arabinan. Our results based on immunolocalization using LM6 and LM13, both recognizing (1→5)-α-l-arabinan epitopes, and biochemical analysis indicate, first, a strong and evenly distributed labeling in the shank of the tube with a slight increase of the signal in the tip region. Second, the most striking point is the high level of arabinosyl residues present in the pollen tube wall, oxalate, and KOH extracts, which counts for over 40 mol % of the total analyzed sugar. Chemical analysis of the pollen tube cell wall from tobacco (Rae et al., 1985) revealed also a high level of Ara (between 15.4 and 26.4 mol % depending on the hydrolysis method), mostly 5-linked and to a lesser extent 5,2-linked. Moreover, tobacco pollen tube wall displayed a low level of galactosyl residues and uronic acids (Rae et al., 1985). Similarly, Lilium, Camellia, and Tulipa pollen tube wall also showed high levels of arabinosyl residues (Nakamura and Suzuki, 1981). Golgi vesicles from Camellia pollen tubes were also enriched in Ara, Gal, and uronic acid (Hasegawa et al., 1998). Altogether, our data suggest that Arabidopsis pollen tube wall contains a short RG-I backbone harboring long chains of (1→5)-α-l-arabinans in the outer wall layer. The significance of this common feature (high level of arabinan) between the pollen tubes originating from nonrelated species is not known but may suggest an important role of this polymer in pollen tube biology. The role of arabinan side chains of RG-I is not clear. Indeed, mutation in the ARABINAN DEFICIENT1 gene, a putative arabinosyltransferase involved in the biosynthesis of pectic arabinan in Arabidopsis, showed high reduction of arabinan side chains of RG-I without any compensation by other polymers. However, no obvious phenotype was detected, suggesting that a loss of arabinan in RG-I does not affect vegetative growth (Harholt et al., 2006). On the other hand, plants exhibiting mutations in two Reversibly Glycosylated Peptides (RGP1 and RGP2) that may be involved in cell wall biosynthesis showed a strong defect in the inner pollen wall and pollen lethality (Drakakaki et al., 2006). Interestingly, rice RGPs show strong amino acid sequence identity (approximately 80%), with UDP-arabinopyranose mutase implicated in UDP-Arap and UDP-Araf interconversion (Konishi et al., 2007). We might speculate that RGP may be part of the arabinan biosynthesis network, but further biochemical studies are required to validate this, as many other molecules contain arabinosyl residues such as arabinoxylans and arabinogalactans. In Commelina communis, arabinan side chains of RG-I have been implicated in guard cell opening and closing. The authors suggested that they may prevent HG polymers from forming tight associations (Jones et al., 2003). A similar effect may occur between the pollen tube wall and the TT cell wall. Finally, pectic arabinans have also been implicated in cell attachment (Iwai et al., 2001; Orfila et al., 2001; Leboeuf et al., 2004, 2005; Peňa and Carpita, 2004). Adhesion of pollen tubes to the TT cells may be an important cue for guiding the tube cell within the female tissue, as was shown in the hollow style plant, lily (Mollet et al., 2007). Investigation of mutant pollen defective in arabinosyltransferase will undoubtedly give insight into the function of arabinan side chains of RG-I in pollen/pollen tube biology.
Arabidopsis Pollen Tube Wall Endo-Glucanase-Sensitive XyG Is Highly Acetylated
MAbs directed against two different XyG epitopes labeled the whole tube mostly in the outer wall layer and the tip. Oligosaccharide fragments containing these two epitopes (XXXG and XXFG) were also detected with MALDI-TOF MS after pollen tube cell wall digestion. Interestingly, XyG analysis from vegetative organs (leaf, stem, or root) revealed consistently that the main fragments are XXXG and XXFG (Zablackis et al., 1995; Pauly et al., 2001; Lerouxel et al., 2002). Arabidopsis pollen tube XyG shows distinct mass spectra with two major fragments detected (XLXG/XXLG and XXFG), both being substituted by one acetyl group, presumably linked to the galactosyl residue. The signal intensity of these two fragments consistently accounted for over 68%. Recent investigations of XyG from leaf tissues isolated after laser microdissection has allowed a more detailed analysis showing subtle modification of XyG (Obel et al., 2009). As an example, the level of acetylation of XLFG fragments in the mesophyll tissue was significantly higher than in the vascular tissue, but the overall acetylation level in these tissues did not exceed 35% to 40% (Obel et al., 2009). Together, these findings indicate that subtle changes in the acetylation level of XyG can occur, but the function of these hydrophobic groups in cell wall biology is still unknown.
XyGs are known to interact with cellulose microfibrils. Despite the low amount of cellulose in the pollen tube wall (Doblin et al., 2001), cellulose is present at the tip and plays an important role in stabilizing the pollen tube tip wall. Cellulase treatment on growing pollen tubes resulted in tip swelling and eventually bursting (Aouar et al., 2010). Cellulose at the tip region may also serve as an interacting partner for XyG cross-linking. Acetylation of XyG does not seem to play a major role in the interaction with cellulose. In vitro experiments have shown that native (acetylated) and deacetylated XyG were able to cross-link cellulose microfibrils in a similar manner (Whitney et al., 2006). In contrast, the high molecular mass of the XyG (880 kD) and the presence of galactosyl residues appeared to be necessary to promote the interaction with cellulose. A lack of Gal in the XyG resulted in the self-association of XyG polymers instead of the interaction between XyG and cellulose (Whitney et al., 2006). Similarly, Arabidopsis mutants with XyG lacking xylosyl, galactosyl, or fucosyl residues showed reduction of tensile strength (Peňa et al., 2004) or abnormal bulging in the tip-growing root hairs, possibly due to impaired cellulose-XyG assembly (Nguema-Ona et al., 2006; Zabotina et al., 2008). A similar phenotype was also noticed in plants exhibiting double mutations in the two XyG xylosyltransferase 1 and 2 genes (XXT1 and XXT2) that are apparently lacking XyG (Cavalier et al., 2008). In the moss Physcomitrella, AGPs were found to be enriched in 3-O-methyl-rhamnosyl residues compared with AGPs from more evolved plant species. This suggests a possible hydrophobic interaction of AGPs, which for a long time had been considered to have large capacity for interacting with water (Fu et al., 2007). Altogether, acetyl groups of XyG may promote hydrophobic interactions with other molecules within the pollen tube cell wall but also with other female components of the TT extracellular matrix during the intrusive growth of pollen tubes. Moreover, acetylation may modulate water uptake due to the nonpolar property of these groups, but this requires further investigation.
In summary, this study provides a solid foundation for the use of Arabidopsis pollen tubes in cell wall biology and for the investigation of the male gametophyte cell elongation with mutants impaired in proteins implicated in the biosynthesis of specific cell wall polymers (XyG, HG, RG-I, RG-II, and AGPs), cell wall deposition, and cell wall remodeling. It may also help to dissect the mechanisms controlling the interaction between the pollen tube wall with the female counterpart. The challenge in this field is to overcome the generally observed phenotypes, such as arrest of pollen formation, lack of pollen viability, absence of pollen tube germination or growth resulting in reduced male fertility, when mutations are located in important male gametophyte genes (Lalanne et al., 2004; Drakakaki et al., 2006; Iwai et al., 2006; Delmas et al., 2008; Boavida et al., 2009). Chemical genetic screens on in vitro-grown pollen tubes may be an alternative. Nevertheless, studies on the two distinct features of Arabidopsis pollen tube wall (high levels of pectic arabinan side chains and XyG acetylation) require closer attention and investigation to better understand their possible roles in pollen tube growth, signaling, guidance, and adhesion.
MATERIALS AND METHODS
Plant Materials
Arabidopsis (Arabidopsis thaliana ecotype Columbia) seeds stored at 4°C were spread on the surface of sterile soil and cultured in a growth chamber with a photoperiod of 16 h of light/8 h of dark at 20°C during the light phase and at 16°C in the dark phase with 60% humidity and daily watering. Only recently opened flowers were collected.
In Vitro Pollen Tube Growth
Pollen was grown in vitro in a liquid medium according to the method described by Boavida and McCormick (2007). Briefly, flowers (40 per 1.5-mL tube) were submerged in 1 mL of germination medium (GM) containing 5 mm CaCl2 2H2O, 0.01% (w/v) H3BO3, 5 mm KCl, 1 mm MgSO4 7H2O, and 10% (w/v) Suc, pH 7.5. Tubes were shaken with a vortex to release the pollen grains from the anthers. Flowers were removed with a pair of tweezers, and the pollen suspension was then pelleted down at 3,200g for 6 min. New GM (250 μL) was added to the pellet, and pollen grains were transferred into glass vials (14 × 45 mm) and grown in a growth chamber in the dark at 22°C. For large-scale culture, pollen from 200 flowers was grown in glass vials (25 × 50 mm) in 1.25 mL of GM. Using this harvesting method, the number of pollen grains collected per flower was estimated at 291 ± 63.3 (n = 10 independent experiments). Pollen tubes were grown for 6 h for TEM sample preparation, 6 and 16 h for immunolabeling at the fluorescence microscopy level, and 16 h for biochemical analysis. Before any further manipulation, pollen germination and pollen tube growth were assessed with an inverted microscope.
Cytochemical Staining
Calcofluor white (0.01%, w/v) and decolorized aniline blue (0.1%, w/v) in 100 mm K3PO4, pH 11 (Johnson-Brousseau and McCormick, 2004), were used to localize β-glucans (cellulose and callose) and callose, respectively.
MAbs
JIM13, MAC207, and LM2 MAbs recognize a carbohydrate moiety of AGPs (Yates et al., 1996). Extensins were probed with LM1 (Smallwood et al., 1995). The MAbs JIM5 and JIM7 recognize different levels of esterification of HG regions of pectins (Clausen et al., 2003). (1→4)-β-d-Galactans were probed with LM5 (Jones et al., 1997) and (1→5)-α-l-arabinans with LM6 (Willats et al., 1998) and LM13 (Verhertbruggen et al., 2009). The MAbs directed against XyG were CCRC-M1, which recognizes an α-Fuc-(1→2)-β-Gal epitope (Puhlmann et al., 1994), and LM15, which binds to the XXXG motif (Marcus et al., 2008). MAbs were kindly provided by P. Knox (University of Leeds) and M. Hahn (Complex Carbohydrate Research Center, University of Georgia) or purchased at Plant Probes. Finally, callose was localized with a mouse anti-(1→3)-β-glucan (Biosupplies Australia).
Immunolocalization of Arabidopsis Pollen Tube Wall Epitopes
Pollen tubes in GM were mixed (v/v) with a fixation medium containing 100 mm PIPES buffer, pH 6.9, 4 mm MgSO4 7H2O, 4 mm EGTA, 10% (w/v) Suc, and 5% (w/v) formaldehyde and incubated for 90 min at room temperature. Pollen tubes were rinsed three times by centrifugation with 50 mm PIPES buffer, pH 6.9, 2 mm MgSO4 7H2O, and 2 mm EGTA and three times with phosphate-buffered saline (100 mm potassium phosphate, 138 mm NaCl, and 2.7 mm KCl, pH 7.4) supplemented or not with 3% fat-free milk. Primary antibodies were diluted at 1:5 or 1:10 as described previously (Mollet et al., 2002) with phosphate-buffered saline (with or without 3% milk). Pollen tubes were rinsed with the buffer and incubated overnight at 4°C in the dark with the secondary antibody combined with fluorescein isothiocyanate (FITC; Sigma) diluted at 1:50 with the appropriate buffer for 3 h at 30°C. For JIM, LM, and MAC antibody detection, goat anti-rat IgG (whole molecule)-FITC was used; for CCRC antibody detection, sheep anti-mouse IgG (whole molecule)-FITC was used. Controls were carried out by incubation of the pollen tubes with the secondary antibody only.
Microscope Observation and Acquisition of Pollen Tube Images
Pollen tubes were observed under Nomarski differential interference contrast optics or fluorescence illumination on a Leica DLMB microscope equipped with FITC (absorption, 485–520 nm; emission, 520–560 nm wavelength) or calcofluor white filter sets. Images were acquired with a Leica DFC300FX camera.
Pollen count and germination rate were estimated with a Nageotte chamber. A pollen grain was considered germinated if the pollen tube length was greater than the pollen grain diameter. Pollen tube length was measured from the image using the program ImageJ (Abramoff et al., 2004). At least 350 pollen tubes were measured in three independent experiments.
Electron Microscopy Preparation of in Vitro-Grown Pollen Tubes
High-Pressure Freezing/Freeze Substitution Sample Preparation
Centrifuged 6-h-old pollen tubes were transferred into the cavity of gold cupules (200 μm in depth and 1.2 mm in diameter) coated with soybean (Glycine max) lecithin (100 mg mL−1 in chloroform). Excess medium was removed using a filter paper. Then, samples were frozen using a high-pressure freezing EM-PACT (Leica) according to a maximum cooling rate of 10,000°C s−1, incoming pressure of 7.5 bars, and working pressure of 4.8 bars. Cupules containing frozen samples were stored in liquid nitrogen until the freeze substitution procedure was initiated.
After high-pressure freezing, samples were transferred to a freeze substitution automate (EM-AFS; Leica) precooled to −140°C. Freeze substitution conditions followed a modified procedure from D. Studer (personal communication). Substitution media were composed of 2% osmium in anhydrous acetone. Samples were substituted at −90°C for 72 h. The temperature was gradually raised (2°C h−1) to −60°C and stabilized during 12 h, then gradually raised (2°C h−1) to −30°C (12 h) and gradually raised again (2°C h−1) to 0°C for 2 h. Samples were washed at room temperature with fresh anhydrous acetone. Infiltration was done at +4°C in acetone-Spurr resin (2:1, 1:1, 1:2, 8 h each step) and with pure resin for at least 2 d. Polymerization was performed at 60°C for 16 h. Using an ultracut EM-UC6 (Leica), thin sections (90 nm) were mounted on formvar-coated nickel grids.
Immunogold Labeling and TEM Observation
Grids were rehydrated in a Tris-buffered saline (TBS) + bovine serum albumin (BSA) 0.2% buffer and blocked in a TBS/BSA 0.2%/milk 3% solution for 30 min. After three brief rinses in TBS/BSA 0.2% solution, grids were incubated 3 h at 25°C in primary antibodies: nondiluted for LM15 and CCRC-M1 or diluted (LM6, 1:2; anti-callose, 1:100) in TBS/BSA 0.2% buffer. Then, grids were washed (six times for 5 min each) in TBS/BSA 0.2% and incubated for 1 h at 25°C in a 1:20 secondary antibody (goat anti-rat for LM6 and LM15, goat anti-mouse for CCRC-M1 and anti-callose) conjugated to 10-nm gold particles (British Biocell International). Finally, grids were washed (six times for 5 min each) in a TBS + BSA 0.2% buffer, 1 min in TBS, 10 min in TBS + glutaraldehyde 2%, 5 min in TBS, and then two times for 5 min each in double deionized water.
The sections were stained with 0.5% (w/v) uranyl acetate in methanol for 10 min in the dark, rapidly rinsed 10 times with water and stained with lead citrate for 10 min, and briefly rinsed 10 times with water.
Grids were observed at 80 kV with a TEM apparatus (Tecnai 12 Bio-Twin; Philips), and images were acquired with an Erlangshen ES500W camera.
Cell Wall Extraction of Arabidopsis Pollen Tubes
Sixteen-hour-old pollen tubes from pollen grains from 8,500 flowers collected manually were pooled after addition of 3 volumes of 95% ethanol to the GM. This experiment was performed three times to allow three independent replicates. Pollen tubes were centrifuged at 5,000g and rinsed three times with 70% ethanol to remove salts and Suc from the GM. The insoluble material (3 × 12 mg) was ground and then treated three times with 70% ethanol at 70°C for 15 min followed by incubation with a mixture of chloroform:methanol (1:1, v/v). The remaining insoluble material was then dried to yield the cell wall fraction (3 × 10 mg). As a baseline control, cell wall from nongerminated pollen grains was extracted. A similar extraction method was applied to Arabidopsis mature rosette leaves to yield a leaf wall extract, except that an acetone incubation was added after the chloroform:methanol treatment for pigment removal. This extract was used to compare the XyG fragments analyzed by MALDI-TOF MS with the pollen tube cell wall extract.
As described by Ray et al., (2004), a pectin-enriched fraction was obtained by extraction with boiling ammonium oxalate 0.5% (w/v) for 1 h. After centrifugation, the supernatant was dialyzed against double deionized water and freeze dried. The ammonium oxalate-insoluble residue was then treated with 4 m KOH supplemented with 20 mm NaBH4 at room temperature for 12 h. The alkaline-soluble fraction was acidified to pH 5.5 with acetic acid, dialyzed against water, and freeze dried to yield a hemicellulose-enriched fraction.
Analysis of Cell Wall Polysaccharides
Photometric Assays
Total uronic acid and neutral sugars were estimated with the m-hydroxydiphenyl assay (Blumenkrantz and Asboe-Hansen, 1973) and the phenol sulfuric method (Dubois et al., 1956) with Gal and GalUA as standards. Estimation of uronic acid and neutral sugar contents was calculated after correction of the interference of GalUA with the phenol sulfuric assay and that of Gal with the m-hydroxydiphenyl assay.
Immunodot Blot Assays
Ammonium oxalate and KOH extracts (8, 4, 1, and 0.5 μg) were blotted onto nitrocellulose membranes. Immunodot blots were processed according to Jauh and Lord (1996). Arabinan from sugar beet (Beta vulgaris; Megazyme), gum arabic from acacia (Fisher), pectin with 8.6% O-methylation from citrus fruits (Sigma), and XyG from tamarind seeds (Tamarindus indica; Megazyme) were used as controls. MAb binding was revealed with a goat anti-rat IgG (whole molecule) antibody conjugated with alkaline phosphatase (Sigma) diluted 1:1,000 and developed with the nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate kit (Promega).
GC-MS
Monosaccharide compositions of the total cell wall and pectin- and hemicellulose-enriched fractions were determined by gas liquid chromatography according to York et al. (1985) using inositol as an internal standard. Briefly, each fraction (500 μg) was treated with 1 m methanolic-HCl at 80°C for 16 h, and the free monosaccharides were converted to their methyl glycosides. After silylation at 110°C for 20 min, samples were dried, dissolved in cyclohexane, and analyzed using a GC 3800 Varian GC system equipped with a DB1 capillary column and a flame ionization detector. A temperature program optimized for separation of the most common cell wall monosaccharides (Ara, Fuc, Gal, GalUA, Glc, GlcUA, Man, Rha, Xyl) was used. Data were analyzed and integrated using Varian GC Star Workstation software, with the quantity of each monosaccharide was corrected according to its response factor.
Preparation and linkage analysis of the partially methylated alditol acetates of pectin- and hemicellulose-enriched fractions were performed according to Smith et al. (1994), except that the ultrasonic bath was performed for 1 h instead of 15 min, dichloromethane was used instead of chloroform, and a stream of air was used to dry samples instead of nitrogen. The resulting partially methylated alditol acetates were separated by GC (Hewlett-Packard 6890) on an Optima 5-MS capillary column (30 m i.d., 0.25 mm; Macherey Nagel) and analyzed by electron-impact MS using an Autospec mass spectrometer (Micromass) equipped with an Opus 3.1 data system.
Preparation of XyG Oligosaccharides
A total of 0.5 mg of pollen tube cell wall or leaf cell wall extract was incubated under agitation for 16 h at 37°C with 500 μL of endo-(1→4)-β-d-glucanase (5 units; EC 3.2.1.4; Megazyme) prepared in 10 mm ammonium acetate buffer, pH 5.0. Glucanase-resistant material was removed by centrifugation after the addition of ethanol to reach a final concentration of 80%. The ethanol-soluble XyG oligosaccharides were concentrated by evaporation under a stream of air.
MALDI-TOF MS Analysis of XyG-Derived Oligosaccharides
MALDI-TOF mass spectra were acquired on a Voyager DE-Pro MALDI-TOF instrument (Applied Biosystems) equipped with a 337-nm nitrogen laser. MS was performed in the reflector delayed extraction mode using 2,5-dihydroxybenzoic acid (Sigma) as matrix. The matrix, freshly dissolved at 5 mg mL−1 in 70:30 acetonitrile:0.1% trifluoroacetic acid, was mixed with the water-solubilized oligosaccharides at a ratio of 1:1 (v/v). These spectra were recorded in positive mode using an acceleration voltage of 20,000 V with a delay time of 100 ns and above 50% of the laser energy. They were externally calibrated using commercially available mixtures of peptides and proteins (Applied Biosystems). In this study, the spectra were calibrated using des-Arg-1-bradykinin (904.4681 D), angiotensin I (1,296.6853 D), Glu-1-fibrinopeptide B (1,570.6774 D), and ACTH18-39 (2,465.1989 D). Laser shots were accumulated for each spectrum in order to obtain an acceptable signal-to-noise ratio (sum of 10 spectra of 1,000 shots per spectrum).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Immunogold labeling of cell wall epitopes in Arabidopsis pollen tube, hydrated pollen grain, and controls.
Supplemental Figure S2. Monosaccharide composition and carbohydrate content of the cell wall of nongerminated pollen grains and 16-h in vitro-grown pollen tubes isolated from 520 flowers.
Supplemental Figure S3. MALDI-TOF MS of XyG fragments of Arabidopsis cell wall leaf released by endo-glucanase treatment.
Supplemental Table S1. Estimation of the amount of pollen grain wall versus pollen tube cell wall.
Supplementary Material
Acknowledgments
A. Geitmann (University of Montreal) is greatly acknowledged for her comments on and proofreading of the manuscript. We are also grateful to F. Richard, L. Chevalier, and M.L. Follet-Gueye for their expertise in high-pressure freezing/freeze substitution sample preparation and technical assistance with the TEM at PRIMACEN (Institut Fédératif de Recherche Multidisciplinaire sur les Peptides 23) and to C. Loutelier-Bourhis (Institut de Recherche en Chimie Organique Fine) for the GC-MS analysis, both part of the University of Rouen. Finally, R. Ngouala Finassi is greatly acknowledged for his help in flower harvest and pollen tube wall immunolocalization.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42 [Google Scholar]
- Aouar L, Chebli Y, Geitmann A. (2010) Morphogenesis of complex plant cell shapes: the mechanical role of crystalline cellulose in growing pollen tubes. Sex Plant Reprod 23: 15–27 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijo JA. (2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol 133: 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Boavida LC, Shuai B, Yu HJ, Pagnussat GC, Sundaresan V, McCormick S. (2009) A collection of Ds insertional mutants associated with defects in male gametophyte development and function in Arabidopsis thaliana. Genetics 181: 1369–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida LC, Vieira AM, Becker JD, Feijò JA. (2005) Gametophyte interaction and sexual reproduction: how plants make a zygote. Int J Dev Biol 49: 615–632 [DOI] [PubMed] [Google Scholar]
- Bosch M, Cheung AY, Hepler PK. (2005) Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138: 1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Daher F, Chebli Y, Geitmann A. (2009) Optimization of conditions for germination of cold-stored Arabidopsis thaliana pollen. Plant Cell Rep 28: 347–357 [DOI] [PubMed] [Google Scholar]
- Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F, Lahaye M, Höfte H, Truong HN. (2002) QUASIMODO1 encodes a putative membrane-bound glycosyltranferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14: 2577–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Carpita NC, McCann MC. (2000) The cell wall. Buchanan BB, Gruissem W, Jones R, , Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, 52–109 [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, Yamauchi K, Reinecke A, Freshour G, Zabotina OA, Hahn MG, Burgert I, Pauly M, et al. (2008) Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Kieslich CA, Morikis D, Kim SC, Lord EM. (2009) A gain-of-function mutation of Arabidopsis Lipid Transfer Protein 5 disturbs pollen tube tip growth and fertilization. Plant Cell 21: 3902–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae K, Zhang K, Zhang L, Morikis D, Kim S, Mollet JC, de la Rosa N, Tan K, Lord EM. (2007) A relationship between structural features and lily pollen tube adhesion activity of SCA (stigma/style cysteine-rich adhesin) isoforms. J Biol Chem 282: 33845–33858 [DOI] [PubMed] [Google Scholar]
- Chen LQ, Ye D. (2007) Roles of pectin methylesterases in pollen tube growth. J Integr Plant Biol 49: 94–98 [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338: 1797–1800 [DOI] [PubMed] [Google Scholar]
- Coimbra S, Jones B, Pereira LG. (2008) Arabinogalactan proteins (AGPs) related to pollen tube guidance into the embryo sac in Arabidopsis. Plant Signal Behav 3: 455–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (1999) Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol 50: 391–417 [DOI] [PubMed] [Google Scholar]
- Delmas F, Séveno M, Northey JGB, Hernould M, Lerouge P, McCourt P, Chevalier C. (2008) The synthesis of the rhamnogalacturonan II component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J Exp Bot 59: 2639–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J, Knuiman B, Hoedemaekers K, Guyon A, Bonhomme S, Pierson ES. (2002) Growth and cellular organization of Arabidopsis pollen tubes in vitro. Sex Plant Reprod 15: 133–139 [Google Scholar]
- Derksen J, Li YQ, Knuiman B, Geurts H. (1999) The wall of Pinus sylvestris L. pollen tubes. Protoplasma 208: 26–36 [Google Scholar]
- Doblin MS, De Melis L, Newbigin E, Bacic A, Read SM. (2001) Pollen tubes of Nicotiana alata express two genes from different β-glucan synthase families. Plant Physiol 125: 2040–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Kim ST, Lord EM. (2005) Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 138: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G, Zabotina O, Delgado I, Robert S, Keegstra K, Raikhel N. (2006) Arabidopsis reversibly glycosylated polypeptides 1 and 2 are essential for pollen development. Plant Physiol 142: 1480–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A, Baskin TI. (2008) Intercourse between cell wall and cytoplasm exemplified by arabinogalactan proteins and cortical microtubules. Am J Bot 95: 1491–1497 [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350–356 [Google Scholar]
- Durand C, Vicré-Gibouin M, Follet-Gueye ML, Duponchel L, Moreau M, Lerouge P, Driouich A. (2009) The organization pattern of root border-like cells of Arabidopsis thaliana is dependent on cell wall homogalacturonan. Plant Physiol 150: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. (2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Ferguson C, Teeri TT, Siika-aho M, Read SM, Bacic A. (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206: 452–460 [Google Scholar]
- Freshour G, Bonin CP, Reiter WD, Albersheim P, Darvill AG, Hahn MG. (2003) Distribution of fucose-containing xyloglucans in cell walls of the mur1 mutant of Arabidopsis. Plant Physiol 131: 1602–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau JP, Seitz HU, Kato Y, Pérez Lorences E, Maclachlan GA, et al. (1993) An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant 89: 1–3 [Google Scholar]
- Fu H, Yadav MP, Nothnagel EA. (2007) Physcomitrella patens arabinogalactan proteins contain abundant terminal 3-O-methyl-L-rhamnosyl residues not found in angiosperms. Planta 226: 1511–1524 [DOI] [PubMed] [Google Scholar]
- Geitmann A. (2010) How to shape a cylinder: pollen tube as a model system for the generation of complex cellular geometry. Sex Plant Reprod 23: 63–71 [DOI] [PubMed] [Google Scholar]
- Geitmann A, Steer M. (2006) The architecture and properties of the pollen tube cell wall. Malhó R, , The Pollen Tube. Plant Cell Monographs, Vol 3 Springer-Verlag, Berlin, 177–200 [Google Scholar]
- Harholt J, Jensen JK, Sørensen SO, Orfila C, Pauly M, Scheller HV. (2006) ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol 140: 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Nakamura S, Kakizoe S, Sato M, Nakamura N. (1998) Immunocytochemical and chemical analyses of Golgi vesicles isolated from the germinated pollen of Camellia japonica. J Plant Res 111: 421–429 [Google Scholar]
- Honys D, Twell D. (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Hokura A, Oishi M, Chida H, Ishii T, Sakai S, Satoh S. (2006) The gene responsible for borate cross-linking of pectin rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc Natl Acad Sci USA 103: 16592–16597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Ishii T, Satoh S. (2001) Absence of arabinan in the side chains of the pectic polysaccharides strongly associated with cell walls of Nicotiana plumbaginifolia non-organogenic callus with loosely attached constituent cells. Planta 213: 907–915 [DOI] [PubMed] [Google Scholar]
- Jauh GY, Lord EM. (1996) Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199: 251–261 [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Lord EM. (2006) Extracellular guidance cues and intracellular signaling pathways that direct pollen tube growth. Malhó R, , The Pollen Tube. Plant Cell Monographs, Vol 3 Springer-Verlag, Berlin, pp 223–242 [Google Scholar]
- Johnson-Brousseau SA, McCormick S. (2004) A compendium of methods useful for characterizing Arabidopsis pollen mutants and gametophytically expressed genes. Plant J 39: 761–775 [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100: 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Seymour GB, Knox JP. (1997) Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1-4)-β-d-galactan. Plant Physiol 113: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, Nasrallah JB, Nasrallah ME. (1994) Pollen-pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development 120: 3405–3418 [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM. (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100: 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T, Takeda T, Miyazaki Y, Ohnishi-Kameyama M, Hayashi T, O'Neill MA, Ishii T. (2007) A plant mutase that interconverts UDP-arabinofuranose and UDP-arabinopyranose. Glycobiology 17: 345–354 [DOI] [PubMed] [Google Scholar]
- Lalanne E, Honys D, Johnson A, Borner GH, Lilley KS, Dupree P, Grossniklaus U, Tweel D. (2004) SETH1 and SETH2, two components of the glycosylphosphatidylinositol anchor biosynthetic pathway, are required for pollen germination and tube growth in Arabidopsis. Plant Cell 16: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboeuf E, Guillon F, Thoiron S, Lahaye M. (2005) Biochemical and immunohistochemical analysis of pectic polysaccharides in the cell walls of Arabidopsis mutant quasimodo 1 suspension-cultured cells: implications for cell adhesion. J Exp Bot 56: 3171–3182 [DOI] [PubMed] [Google Scholar]
- Leboeuf E, Thoiron S, Lahaye M. (2004) Physico-chemical characteristics of cell walls from Arabidopsis thaliana microcalli showing different adhesion strengths. J Exp Bot 55: 2087–2097 [DOI] [PubMed] [Google Scholar]
- Lennon KA, Lord EM. (2000) The in vivo pollen tube cell of Arabidopsis thaliana. I. Tube cell cytoplasm and wall. Protoplasma 214: 45–56 [Google Scholar]
- Lennon KA, Roy S, Hepler PK, Lord EM. (1998) The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex Plant Reprod 11: 49–59 [Google Scholar]
- Lerouxel O, Choo TS, Seveno M, Usadel B, Faye L, Lerouge P, Pauly M. (2002) Rapid structural phenotyping of plant cell wall mutants by enzymatic oligosaccharide fingerprinting. Plant Physiol 130: 1754–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Bruun L, Pierson ES, Cresti M. (1992) Periodic deposition of arabinogalactan epitopes in the cell wall of pollen tubes of Nicotiana tabacum L. Planta 188: 532–538 [DOI] [PubMed] [Google Scholar]
- Li YQ, Chen F, Linskens HF, Cresti M. (1994) Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7: 145–152 [Google Scholar]
- Li YQ, Faleri C, Geitmann A, Zhang HQ, Cresti M. (1995) Immunogold localization of arabinogalactan proteins, unesterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum L. Protoplasma 189: 26–36 [Google Scholar]
- Li YQ, Mareck A, Faleri C, Moscatelli A, Liu Q, Cresti M. (2002) Detection and localization of pectin methylesterase isoforms in pollen tubes of Nicotiana tabacum L. Planta 214: 734–740 [DOI] [PubMed] [Google Scholar]
- Lord EM, Russell SD. (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18: 81–105 [DOI] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WGT, Knox JP. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S, Yang H. (2005) Is there more than one way to attract a pollen tube? Trends Plant Sci 10: 260–263 [DOI] [PubMed] [Google Scholar]
- Micheli F. (2001) Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Mollet JC, Faugeron C, Morvan H. (2007) Cell adhesion, separation and guidance in compatible plant reproduction. Annu Plant Rev 25: 69–90 [Google Scholar]
- Mollet JC, Kim S, Jauh GY, Lord EM. (2002) Arabinogalactan proteins, pollen tube growth, and the reversible effects of Yariv phenylglycoside. Protoplasma 219: 89–98 [DOI] [PubMed] [Google Scholar]
- Mollet JC, Park SY, Nothnagel EA, Lord EM. (2000) A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Suzuki H. (1981) Sugar composition of pollen grain and pollen tube cell walls. Phytochemistry 20: 981–984 [Google Scholar]
- Nguema-Ona E, Andème-Onzighi C, Aboughe-Angone S, Bardor M, Ishii T, Lerouge P, Driouich A. (2006) The reb1-1 mutation of Arabidopsis: effect on the structure and localization of galactose-containing cell wall polysaccharides. Plant Physiol 140: 1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothnagel EA. (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174: 195–291 [DOI] [PubMed] [Google Scholar]
- Obel N, Erben V, Schwarz T, Kuhnel S, Fodor A, Pauly M. (2009) Microanalysis of plant cell wall polysaccharides. Mol Plant 2: 922–932 [DOI] [PubMed] [Google Scholar]
- Orfila C, Seymour GB, Willats WG, Huxham IM, Jarvis MC, Dover CJ, Thompson AJ, Knox JP. (2001) Altered middle lamella homogalacturonan and disrupted deposition of (1→5)-α-l-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol 126: 210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D. (2000) Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol 10: 517–524 [DOI] [PubMed] [Google Scholar]
- Park SY, Jauh GY, Mollet JC, Eckard KJ, Nothnagel EA, Walling LL, Lord EM. (2000) A lipid transfer-like protein is necessary for lily pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M, Qin Q, Greene H, Albersheim P, Darvill G, York WS. (2001) Changes in the structure of xyloglucan during cell elongation. Planta 212: 842–850 [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ. (2007) New insights into pectin methylesterase structure and function. Trends Plant Sci 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Peňa MJ, Carpita NC. (2004) Loss of highly branched arabinans and debranching of rhamnogalacturonan I accompany loss of firm texture and cell separation during prolonged storage of apple. Plant Physiol 135: 1305–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peňa MJ, Ryden P, Madson M, Smith AC, Carpita NC. (2004) The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiol 134: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LG, Coimbra S, Oliveira H, Monteiro L, Sottomayor M. (2006) Expression of arabinogalactan protein genes in pollen tubes of Arabidopsis thaliana. Planta 223: 374–380 [DOI] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AG, Hahn MG. (1994) Generation of monoclonal antibodies against plant cell wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1,2)-linked fucosyl-containing epitope. Plant Physiol 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Chen D, Zhao J. (2007) Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma 231: 43–53 [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae AL, Harris PJ, Bacic A, Clarke AE. (1985) Composition of the cell walls of Nicotiana alata Link et Otto pollen tubes. Planta 166: 128–133 [DOI] [PubMed] [Google Scholar]
- Ray B, Loutelier-Bourhis C, Lange C, Condamine E, Driouich A, Lerouge P. (2004) Structural investigation of hemicellulosic polysaccharides from Argania spinosa: characterisation of a novel xyloglucan motif. Carbohydr Res 339: 201–208 [DOI] [PubMed] [Google Scholar]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. (2003) Microspore separation in the quartet3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol 133: 1170–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Eckard KJ, Lancelle S, Hepler PK, Lord EM. (1997) High-pressure freezing improves the ultrastructural preservation of in vivo grown lily pollen tubes. Protoplasma 20: 87–98 [Google Scholar]
- Rubinstein AL, Márque J, Cervera MS, Bedinger PA. (1995) Extensin-like glycoproteins in the maize pollen tube wall. Plant Cell 7: 2211–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Showalter AM. (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Martin H, Knox JP. (1995) An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 196: 510–522 [DOI] [PubMed] [Google Scholar]
- Smith K, Davies M, Hounsells E. (1994) Structural profiling of oligosaccharides of glycoproteins. Methods Mol Biol 32: 143–155 [DOI] [PubMed] [Google Scholar]
- Tian GW, Chen MH, Zaltsman A, Citovsky V. (2006) Pollen-specific pectin methylesterase involved in pollen tube growth. Dev Biol 294: 83–91 [DOI] [PubMed] [Google Scholar]
- Tung CW, Dwyer KG, Nasrallah ME, Nasrallah JB. (2005) Genome-wide identification of genes expressed in Arabidopsis pistils specifically along the path of pollen tube growth. Plant Physiol 138: 977–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Verhoef R, Schols HA, McCleary BV, McKee L, Gilbert HJ, Knox JP. (2009) Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J 59: 413–425 [DOI] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen R, McCann MC, Ulvskov P, Voragen AGJ, Visser RGF. (2003) If homogalacturonan were a side chain of rhamnogalacturonan I: implications for cell wall architecture. Plant Physiol 132: 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney SEC, Wilson E, Webster J, Bacic A, Reid JSG, Gidley MJ. (2006) Effects of structural variation in xyloglucan polymers on interactions with bacterial cellulose. Am J Bot 93: 1402–1414 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Marcus SE, Knox JP. (1998) Generation of a monoclonal antibody specific to (1-5)-alpha-L-arabinan. Carbohydr Res 308: 149–152 [DOI] [PubMed] [Google Scholar]
- Wu H, Wang H, Cheung AY. (1995) A floral transmitting tissue specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]
- Yates EA, Valdor JF, Haslam SM, Morris HR, Dell A, Mackie W, Knox JP. (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 6: 131–139 [DOI] [PubMed] [Google Scholar]
- York W, Darvill A, McNeil M, Stevenson TT, Albersheim P. (1985) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol 118: 3–40 [Google Scholar]
- Zablackis E, Huang J, Müller B, Darvill AG, Albersheim P. (1995) Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol 107: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabotina OA, van de Ven WTG, Freshour G, Drakakaki G, Cavalier D, Mouille G, Hahn MG, Keegstra K, Raikhel NV. (2008) Arabidopsis XXT5 gene encodes a putative α-1,6-xylosyltransferase that is involved in xyloglucan biosynthesis. Plant J 56: 101–115 [DOI] [PubMed] [Google Scholar]
- Zou J, Song L, Zhang W, Wang Y, Ruan S, Wu WH. (2009) Comparative proteomic analysis of Arabidopsis mature pollen and germinated pollen. J Integr Plant Biol 51: 438–455 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.