Abstract
Anthocyanins are important health-promoting phytochemicals that are abundant in many fleshy fruits. Bilberry (Vaccinium myrtillus) is one of the best sources of these compounds. Here, we report on the expression pattern and functional analysis of a SQUAMOSA-class MADS box transcription factor, VmTDR4, associated with anthocyanin biosynthesis in bilberry. Levels of VmTDR4 expression were spatially and temporally linked with color development and anthocyanin-related gene expression. Virus-induced gene silencing was used to suppress VmTDR4 expression in bilberry, resulting in substantial reduction in anthocyanin levels in fully ripe fruits. Chalcone synthase was used as a positive control in the virus-induced gene silencing experiments. Additionally, in sectors of fruit tissue in which the expression of the VmTDR4 gene was silenced, the expression of R2R3 MYB family transcription factors related to the biosynthesis of flavonoids was also altered. We conclude that VmTDR4 plays an important role in the accumulation of anthocyanins during normal ripening in bilberry, probably through direct or indirect control of transcription factors belonging to the R2R3 MYB family.
Naturally occurring polyphenols in plants are likely to make a major contribution to human health and therefore life expectancy (Renaud and Delorgeril, 1992). Fruits are some of the most accessible dietary sources of polyphenols, of which anthocyanins are recognized for their high antioxidant capacity and reported positive effects on blood vessels (Kähkonen et al., 2003; Vinson et al., 2005; Dragsted et al., 2006). Furthermore, these secondary metabolites are thought to have positive anticancer effects (Butelli et al., 2008) and to promote brain function (Williams et al., 2008). Anthocyanins generate the characteristic red, blue, and purple pigments in many flowers and in fruits such as bilberry (Vaccinium myrtillus), black currant (Ribes nigrum), blackberry (Rubus fruticosus), and strawberry (Fragaria spp.).
Bilberry is one of the richest natural sources of anthocyanins, the ripe fruit typically containing 29 mg g−1 dry weight (Lätti et al., 2008). In many fruits, these colored compounds accumulate only in the skin, while in bilberry they occur throughout the fruit flesh. At least 15 different anthocyanidin glycosides contribute to the anthocyanin profile of the fruit, which also contains high levels of the flavonols quercetin and myricetin in addition to hydroxycinnamic acids (Jaakola et al., 2002, 2004; Riihinen et al., 2008).
The biosynthetic pathway in plants leading to anthocyanins is well established, and transcription factors that directly regulate multiple steps of the expression of genes in this metabolic pathway are known (Grotewold, 2006). This information has recently been used to generate tomatoes (Solanum lycopersicum) containing high concentrations of anthocyanins (Butelli et al., 2008). In bilberry fruit, ripening-related gene expression has been studied in relation to flavonoid biosynthesis (Jaakola et al., 2002). Many berry species accumulate anthocyanins in their fruits only during the ripening phase, but while anthocyanins are markers of ripening, the molecular circuits connecting their accumulation to the ripening process are poorly understood. A major goal is to identify and understand how those molecular circuits involved with anthocyanin biosynthesis are interlinked with the control of ripening and then to harness this information to breed novel fruit varieties with enhanced nutritional and health benefits.
The regulation of ripening in fleshy fruits has been most extensively studied and is best understood in tomato (Giovannoni, 2007; Seymour et al., 2008). Tomato and bilberry differ in their ripening behavior. Tomatoes are climacteric fruits where ripening is under the control of ethylene, while bilberry is a nonclimacteric fruit where ethylene appears to have little influence on ripening. Both these fruits, however, are classified as berries, and there is increasing evidence that fleshy fruit species may share common mechanisms of ripening control related to developmental cues that act above and beyond the influence of ethylene (Giovannoni, 2004). Information on the identity of these ripening-control genes has now become apparent through the study of nonripening mutants of tomato. A small number of single gene mutants have been identified that almost completely abolish ripening, including ripening inhibitor (rin) and Colorless non-ripening (Cnr; Giovannoni, 2007). The gene at the tomato rin locus is a member of the MADS box SEPALLATA (SEP) subfamily, LeMADS-RIN (Vrebalov et al., 2002). MADS box genes are normally associated with floral development, but LeMADS-RIN is necessary for ripening. The gene at the Cnr locus encodes a SQUAMOSA promoter-binding protein (SBP box) transcription factor (Manning et al., 2006) that is likely to interact with the promoters of the SQUAMOSA (SQUA) subfamily of MADS box genes (Lännenpää et al., 2004). SQUA MADS box genes are commonly associated with floral development and especially the regulation of floral meristem identity (Simpson et al., 1999; Theissen, 2001; Scott et al., 2002). This class of transcription factors has also been shown, however, to have other functions. They have been implicated in the regulation of tuber formation in vegetative meristems of potato (Solanum tuberosum; Rosin et al., 2003b), and the FRUITFULL gene (FUL) in Arabidopsis (Arabidopsis thaliana) mediates cell differentiation during fruit development (Dinneny et al., 2005) as well as floral meristem identity (Mandel and Yanofsky, 1995). In tomato, TDR4, which is a likely FUL ortholog, shows enhanced expression during fruit ripening (Eriksson et al., 2004), although it has yet to be assigned a function.
Elevated expression of SEP- and SQUA-class MADS box genes during ripening has now been observed in a wide range of climacteric and nonclimacteric fruits, including strawberry, banana (Musaspp.), and grape (Vitis vinifera; Vrebalov et al., 2002; Rosin et al., 2003a; Liu et al., 2009). LeMADS-RIN and LeSBP-CNR are necessary for normal ripening in tomato, with the latter likely controlling the expression of the SQUA gene TDR4. Therefore, these genes and other related transcription factors can be seen as part of the high-level regulatory network controlling the ripening process. Their links to downstream effectors that bring about changes in color, texture, and flavor, however, are poorly understood.
The aim of this work was to investigate the regulatory mechanisms controlling anthocyanin accumulation in bilberry, and especially the higher level regulatory network linking anthocyanin biosynthesis to ripening. We have cloned a bilberry homolog of the tomato TDR4 gene, which we name VmTDR4. Transcripts of this gene were found to have a strong ripening-related pattern of expression. In this article, we report on VmTDR4 expression in ripening bilberry and how inhibition of VmTDR4 expression substantially reduces the accumulation of anthocyanins in the fruit. Our data also suggest that the VmTDR4 gene may act directly or indirectly on transcription factors belonging to the R2R3 MYB family, the known regulators of flavonoid biosynthesis.
RESULTS
VmTDR4 Is a Bilberry Homolog of the Tomato TDR4 and Arabidopsis FUL Genes
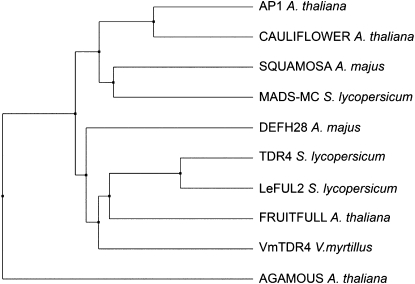
The aim of this work was to better understand the molecular events controlling ripening in bilberry, a fruit that accumulates high levels of health-promoting anthocyanins. In preliminary experiments, we attempted to isolate bilberry orthologs of the tomato ripening regulatory genes CNR and TDR4. We were able to clone a TDR4-like gene, VmTDR4 (accession no. FJ418852). The full-length VmTDR4 cDNA is related by sequence homology to FUL from Arabidopsis and TDR4/FUL-like genes from tomato; VmTDR4 showed 72% identity to tomato TDR4 at the nucleotide level and 70% identity at the amino acid level. A phylogenetic analysis (Fig. 1) indicated a close relationship between protein sequences from Arabidopsis, tomato, and bilberry. In Arabidopsis, the FUL gene has pleiotrophic effects, being involved in regulating the formation of the lignified dehiscence zone in the fruit as well as floral meristem identity (Dinneny et al., 2005). The role of TDR4 in tomato fruits is unknown.
Figure 1.
Phylogenetic analysis of the relationship between SQUA-class MADS box genes. Full-length protein sequences were aligned using MAFFT version 5 (Katoh et al., 2005), and an average distance tree was constructed based on percentage identity between sequences in Jalview 2.4 (Clamp et al., 2004) of Arabidopsis (AGAMOUS, NP_567569; AP1, NP_177074; CAULIFLOWER, Q39081; FUL, NP_568929), tomato (MADS-MC, AAM15774; TDR4, AAM33098; LeFUL2, AY306156), Antirrhinum (SQUA, CAA45228; DEFH28, AAK72467), and bilberry (VmTDR4, FJ418852).
VmTDR4 Is Expressed in Bilberry Fruit Tissues, and the Expression Pattern Is Temporally and Spatially Related to Anthocyanin Biosynthesis
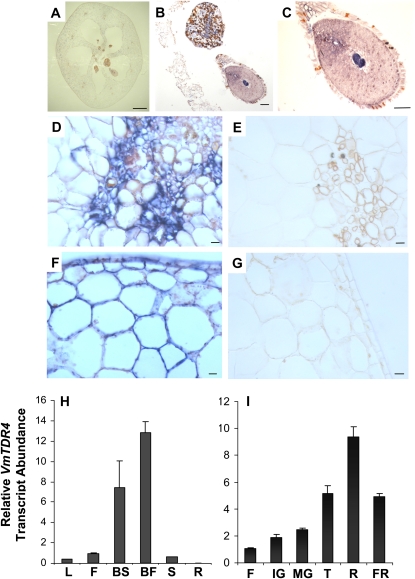
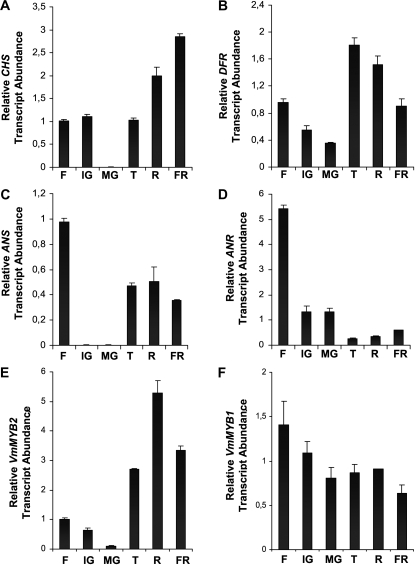
In situ hybridization experiments revealed that VmTDR4 transcripts are present in various tissues of the unripe fruit (Fig. 2, A–G). These included pericarp cells, the skin and developing embryo tissues, and especially the vascular regions of the placenta. Ripe fruits were unsuitable for in situ experiments due to problems with effective fixation of the material. VmTDR4 expression was investigated in ripe fruit and other organs by quantitative reverse transcription (QRT)-PCR (Fig. 2, H and I). Transcripts were detected mainly in the fruit and floral tissues, with the highest levels in the ripe flesh of the berry (Fig. 2H). Limited expression was detected in leaves, and none was detected in the rhizome. The expression pattern of VmTDR4 during berry development and ripening is shown in Figure 2I. VmTDR4 transcripts continued to accumulate until just before the fruit were fully ripe and just prior to anthocyanins reaching their highest levels (Jaakola et al., 2002). The expression patterns of several genes known to be involved in anthocyanin biosynthesis (Jaakola et al., 2002) are shown in Figure 3, in addition to novel MYB transcription factors VmMYB1 and VmMYB2. Chalcone synthase (CHS; accession no. AY123765), dihydroflavonol 4 reductase (DFR; accession no. AY123767), and anthocyanin synthase (ANS; accession no. AY123768) all showed a ripening-related pattern with similarities to that of VmTDR4 (Fig. 3, A–D). The levels of anthocyanin reductase (ANR; accession no. FJ666338) are low during the ripening phase, reflecting the flux toward colored anthocyanin pigments and away from proanthocyanidins (Fig. 3D; see also pathway in Fig. 4). We have recently cloned R2R3-MYB family transcription factors from ripening bilberry fruit, including VmMYB1 (accession no. GU904211) and VmMYB2 (accession no. GU227356). The expression profile of the VmMYB2 gene was very similar to VmTDR4, being strongly ripening related and reaching its highest level before the fully ripe stage (Fig. 3E). In contrast, VmMYB1 expression was at its highest level during early fruit development (Fig. 3F).
Figure 2.
In situ hybridization and expression profiles for VmTDR4 in bilberry. A to G, In situ hybridization. Bluish color indicates the expression of VmTDR4 in samples hybridized with the antisense probe. A, Cross section of whole bilberry fruit. B, Placenta and developing seed, antisense probe. C, Closeup of developing seed, antisense probe. D, Vascular tissue, antisense probe. E, Vascular tissue, sense probe. F, Epidermis and pericarp, antisense probe. G, Epidermis and pericarp, sense probe. Bars = 1 mm in A, 10 μm in B and C, and 1 μm in D to G. H and I, Determination of VmTDR4 transcript abundance by QRT-PCR. H, VmTDR4 transcript levels in bilberry leaf (L), flower (F), ripe berry skin (BS), ripe berry flesh (BF), mature seed (S), and rhizome (R). I, VmTDR4 transcript levels during bilberry fruit development and ripening in flowers (F), immature green (IG), mature green (MG), turning (T), ripe (R), and fully ripe (FR) fruits. Data are means ± se (n = 3).
Figure 3.
Determination of transcript abundance of genes at different stages of bilberry fruit development and ripening. A, CHS. B, DFR. C, ANS. D, ANR. E, VmMYB2. F, VmMYB1. Stages are flower (F), immature green (IG), mature green (MG), turning (T), ripe (R), and fully ripe (FR) fruit. Data are means ± se (n = 3).
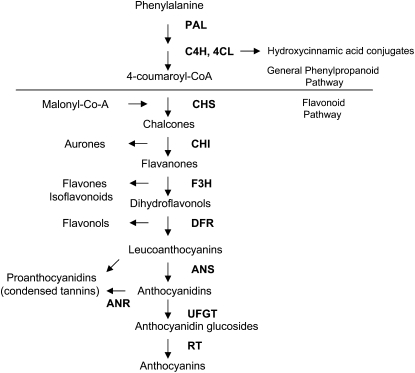
Figure 4.
A schematic presentation of the flavonoid biosynthetic pathway. Enzyme abbreviations not defined in the text are as follows: PAL, Phe ammonia-lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumaroyl:CoA ligase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; UFGT, UDP Glc-flavonoid 3-O-glucosyl transferase; RT, rhamnosyl transferase.
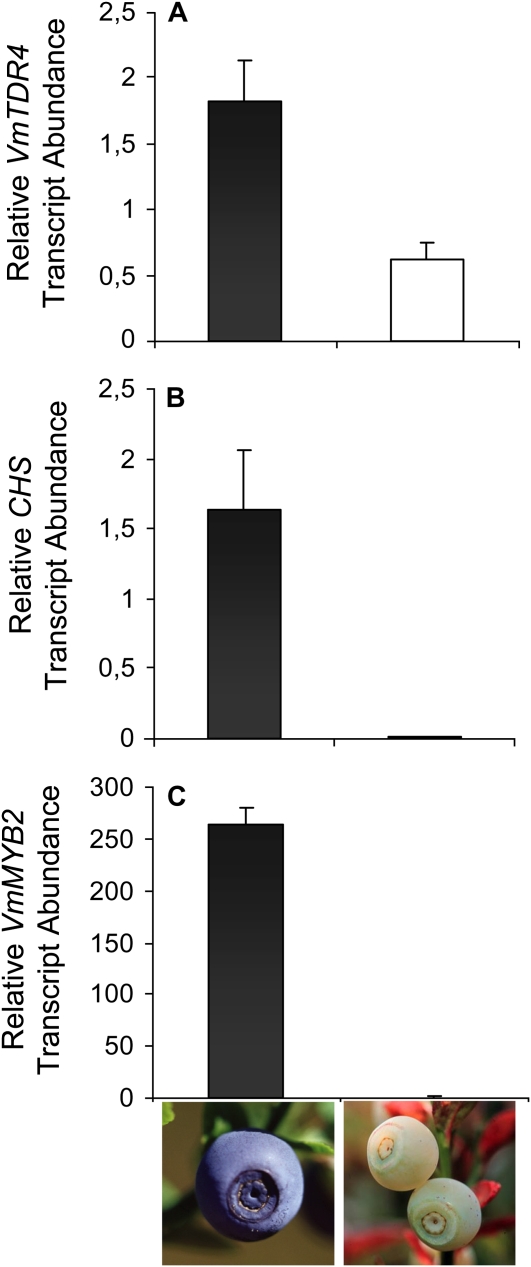
A natural bilberry fruit color mutant, white bilberry, which ripens with a white fleshy pericarp, has been described (Jaakola et al., 2002). The mutant fruits have greatly reduced levels of anthocyanins and decreased expression of anthocyanin biosynthetic genes compared with the wild type (Jaakola et al., 2002). QRT-PCR analyses of VmTDR4, VmMYB2, and CHS in white bilberry showed that at the same stage of ripening (ripe fruits) genes are down-regulated in the fruits of the white mutant compared with wild-type controls (Fig. 5). Collectively, the expression data from wild-type and mutant bilberry fruits are suggestive of a relationship between VmTDR4 expression and anthocyanin biosynthesis, but they do not, of course, provide a functional link.
Figure 5.
Gene expression in wild-type and white mutant bilberry. Determination of VmTDR4 and CHS transcript abundance by quantitative PCR in wild-type (black bars) and white mutant (white bars) bilberry fruits. Data are means ± se (n = 3).
Functional Analysis of VmTDR4 in Bilberry Using Virus-Induced Gene Silencing
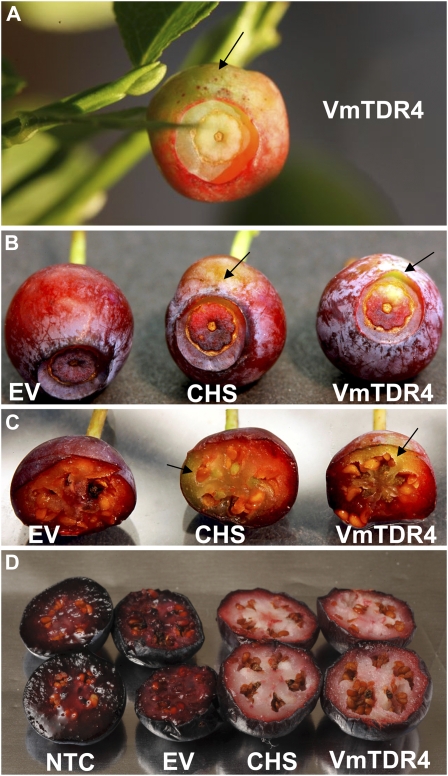
To further investigate the function of VmTDR4 in relation to flavonoid biosynthesis in bilberry, we used virus-induced gene silencing (VIGS) with a CHS construct as a positive control, following the lead of other studies involving fruits where anthocyanins are the principal pigments affecting fruit color (Hoffmann et al., 2006). This is the first report, to our knowledge, of VIGS in this species. Fragments of the VmTDR4 and CHS genes were amplified by PCR and cloned into the VIGS vectors (see “Materials and Methods”). Inoculations with the VmTDR4 sequence resulted in fruits with altered pigmentation, which included green sectors (Fig. 6). Fruits were photographed during the ripening process at 10 d (Fig. 6A) and 32 d (Fig. 6, B and C) after injections. A similar experiment was undertaken in a second year, whereby fruits were collected 42 d after the injections when they were fully ripe (Fig. 6D). Each gene construct was tested on 100 individual berries. In the VmTDR4 and CHS treatments, between 16% and 20% of berries showed sectors with abnormal pericarp color development (Fig. 6, A–C). Cross sections of fruits at the fully ripe stage revealed a substantial reduction in red pigmentation in the pericarp in fruit from both the VmTDR4 and CHS treatments (Fig. 6D). All untreated fruits or those injected with the empty vector (negative control) ripened normally, developing a dark red/black pigmentation in the pericarp and skin. Green sectors were completely absent.
Figure 6.
Images of bilberry fruits during development and ripening after injection of VmTDR4- and CHS-silencing constructs. Bilberry fruit are shown at 10 d (A), 32 d (B and C), and 42 d (D) following injections with VmTDR4 and CHS VIGS vectors in comparison with the nontreated control (NTC) and empty vector control (EV) fruits. Fruit in D are fully ripe.
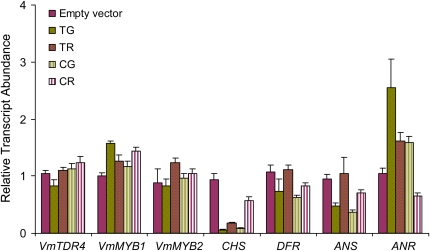
Expression levels of VmTDR4, CHS, DFR, ANS, ANR, VmMYB1, and VmMYB2 were examined in VmTDR4 and CHS VIGS-treated fruits by QRT-PCR (Fig. 7). RNA was isolated just prior to the fully ripe stage at 32 d post injection (Fig. 6, B and C), as this is the point at which anthocyanin-related gene expression reaches a peak. In VIGS experiments, the effects are often localized to small tissue sectors; therefore, gene expression was compared between the “green” and “red” sectors. It is difficult to compare absolute values with empty vector-treated material directly because individual fruits on the same plant can ripen at slightly different rates. However, the expression of genes related to flavonoid biosynthesis in both untreated and empty vector treatments was consistent with transcript levels observed during normal ripening in bilberry.
Figure 7.
Effects of VmTDR4- and CHS-silencing constructs on bilberry gene expression during ripening. Relative expression is shown for VmTDR4, VmMYB1, VmMYB2, and flavonoid biosynthetic genes CHS, DFR, ANS, and ANR in green and red sectors of bilberry fruits 32 d after injection of VIGS vectors. VmTDR4-silenced fruit green sector (TG), VmTDR4-silenced fruit red sector (TR), CHS-silenced fruit green sector (CG), CHS-silenced fruit red sector (CR), and empty vector-injected fruits are indicated. Data are means ± se (n = 3).
In pTV00-VmTDR4-treated fruit, the levels of VmTDR4 expression were approximately 25% lower in the green than in the red sectors (Fig. 7), and this difference was significant (P ≤ 0.005). This initially suggested an uncoupling between VmTDR4 expression and reduced pigment accumulation in the green sectors, where a more dramatic reduction in VmTDR4 levels might have been expected. However, beneath the skin in the green sectors of the fruits, partial color development occurred in the fleshy pericarp tissue (Fig. 6C). While VIGS may lead to “patchy” ripening effects and make data interpretation more challenging, it is an effective method for functional analysis in a species where analysis of stable transformants could take several years. The small amount of material in the sectors was insufficient for both RNA and metabolomic analysis, so the latter was performed on whole fully ripe berries (Fig. 6D; see below), where the most dramatic effects of the VIGS were seen. In contrast with the VmTDR4-injected fruits, VmTDR4 expression was not suppressed in the pTV00-CHS-treated material, although these fruits also developed green sectors. This is consistent with VmTDR4 being upstream of CHS in a regulatory network. In pTV00-CHS-treated fruits, CHS expression was markedly down-regulated in the green sectors, as might be predicted, which indicates effective silencing of this gene with the CHS construct. However, CHS expression was also lower in green compared with red sectors from pTV00-VmTDR4-infected fruits. CHS expression in both treatments is relatively low, and this may reflect some silencing of the target genes throughout the fruit by the VIGS treatment. Alternatively, as the VIGS method causes transient silencing, it is possible that at the time samples were collected (32 d after injections), the silencing effect was already weaker with respect to its effect on VmTDR4, but silencing of VmTDR4 had longer lasting effects on the suppression of CHS mRNA levels.
We also examined the expression of novel bilberry MYBs and several genes known to be involved in the anthocyanin pathway (Fig. 7). VmMYB2 expression was significantly (P ≤ 0.005) lower in the pTV00-VmTDR4 green sectors as compared with red sectors, indicating a link between VmTDR4 and VmMYB2 expression and development of the red anthocyanin pigmentation. There was, however, no significant difference in VmMYB2 expression in the red or green sectors of the CHS VIGS fruit, indicating that the effects of CHS on pigment accumulation are downstream of these two genes. Interestingly, the expression of VmMYB1 was significantly higher (P ≤ 0.005) in the pTV00-VmTDR4 green sectors as compared with the red sectors. VmMYB1 shares the closest sequence-level homology with FaMYB1, which is a known suppressor of anthocyanin biosynthesis in strawberry fruit (Aharoni et al., 2001). The expression of DFR, ANS, and ANR was also examined. DFR and ANS, which are below CHS in the pathway leading to anthocyanins (Fig. 4), showed significant (P ≤ 0.05) reductions in their levels of expression in green sectors in both pTV00-VmTDR4 and pTV00-CHS VIGS fruits. In contrast, ANR expression was higher in the green sectors from both VIGS treatments. ANR catalyzes the synthesis of flavan-3-ols such as (−)-epicatechins, a step in the pathway prior to proanthocyanidins, which are abundant in bilberry fruit tissues prior to the accumulation of anthocyanins.
We wanted to obtain metabolite data along with information from the transcriptomics, but this proved difficult with the small sectors from the VIGS fruits at 32 d after injection. However, we were able to repeat the VIGS experiments in a second season with similar results, and this time fully ripe bilberry samples were collected 42 d after injection, when distinct effects on pigment accumulation could be seen throughout the fruit (Fig. 6D). This provided sufficient tissue for metabolite analysis. Furthermore, as the fruit were fully ripe and the effect was apparent throughout the fruits, they could also then be compared with whole fully ripe empty vector or untreated fruits. The metabolite data (Table I) demonstrated that the principal effect in VmTDR4- and CHS-silenced fruits was a 2- to 3-fold reduction in levels of anthocyanins compared with the empty vector or nontreated fruits. Levels of quercetin derivatives were low in all samples, and despite levels in the VmTDR4- and CHS-silenced fruits being elevated, these values are within the normal variation of these compounds in fully ripe bilberry fruits, unlike the anthocyanin fraction. The biosynthesis of quercetin is highest in the early stages of fruit development, and the quercetin contents are 3- to 4-fold lower in the ripe bilberries compared with the unripe fruits (Jaakola et al., 2002). The VIGS treatments were made during berry ripening and after the peak in quercetin content. It is possible that quercetin derivatives seen in the VIGS fruit accumulated prior to this stage. The empty vector fruits showed evidence of an increase in the early parts of the phenylpropanoid pathway (e.g. p-coumaric acid) and in cyanidin that was not apparent in the VmTDR4- and CHS-treated fruits. This may be the result of defense-related responses by the fruits, arising from the presence of the viral vector in the absence of constructs that modulate the expression of genes in the flavonoid pathway. Collectively, these data provide strong evidence for a role for VmTDR4 in the regulation of flavonoid biosynthesis in bilberry fruits.
Table I. Phenolic-related metabolite changes in bilberry VIGS fruits.
Determination of phenolics present in ripe bilberry fruit 42 d after injection of VIGS VmTDR4 and CHS constructs. HPLC analysis was undertaken on pooled samples of three individual fruits in each treatment. All values are provided in μg mg−1 dry weight, according to the protocol described in “Materials and Methods.” Data are means ± se. Values in the same column that are significantly different (P < 0.001) from the empty vector treatment are shown in boldface.
| Tissue Sample | Chlorogenic Acid | p-Coumaric Acid | Quercetin Derivatives | Delphinidin | Cyanidin |
| Untreated fruits | 200 ± 38.0 | 126 ± 10.0 | 0.23 ± 0.02 | 78.7 ± 4.5 | 55.0 ± 3.1 |
| Empty vector | 280 ± 36.0 | 276 ± 12.0 | 0.2 ± 0.1 | 95.0 ± 10.6 | 67.7 ± 5.0 |
| VmTDR4 VIGS | 208 ± 22.0 | 172 ± 10.0 | 0.3 ± 0.04 | 33.8 ± 2.7 | 14.8 ± 0.6 |
| CHS VIGS | 196 ± 16.0 | 136 ± 0.06 | 0.9 ± 0.20 | 42.5 ± 1.8 | 33.8 ± 2.7 |
DISCUSSION
Some of the key genes involved in the molecular regulation of fruit ripening have been revealed through the study of nonripening mutants in tomato, such as rin, Cnr, Nr, and nor (Giovannoni, 2007; Seymour et al., 2008). However, the links between these regulators and their downstream effectors that bring about the changes in color, texture, and flavor are poorly understood.
In many fleshy fruit species, flavonoids including anthocyanins are the major colored pigments synthesized during ripening. Regulation of flavonoid biosynthesis occurs via coordinated transcriptional control of enzymes in the biosynthetic pathway by the interaction of DNA-binding R2R3 MYB transcription factors and MYC-like basic helix-loop-helix and WD40-repeat proteins (Stracke et al., 2001; Broun, 2005; Koes et al., 2005; Ramsay and Glover, 2005; Grotewold, 2006). It is also known that other factors, such as light, temperature, and nutrients, play a role in flavonoid accumulation (Peng et al., 2008). A major missing part of the network is the association between the regulatory genes controlling fruit ripening and the downstream pathways that lead to the synthesis of important secondary products such as anthocyanins. In this work, we report a functional link between a SQUA MADS box transcription factor and the control of flavonoid biosynthesis in bilberry fruit.
VmTDR4 Is a SQUA MADS Box Gene Involved in Anthocyanin Biosynthesis in Ripening Bilberry
A role for MADS box transcription factors in regulating anthocyanin biosynthesis in a range of plant tissues has been reported previously. A sweet potato (Ipomoea batatas) SQUA transcription factor, IbMADS10, showed a pattern of expression that was tightly correlated with anthocyanin levels in pigmented tissues, especially in the red roots. Also, when ectopically expressed in sweet potato callus, it induced anthocyanin accumulation (Lalusin et al., 2006). IbMADS10 shares significant sequence homology with DEFH28 from Antirrhinum majus (Müller et al., 2001), a likely ortholog of VmTDR4 from bilberry, TDR4 from tomato, and FUL from Arabidopsis (Fig. 1). Furthermore, a link between the expression of a MADS box gene and the accumulation of flavonoids has also been reported in Arabidopsis for seed coat pigmentation. The TT16/ABS MADS domain protein is required for normal development and pigmentation of the seed coat and is postulated to modulate anthocyanin biosynthesis by interacting directly with an R2R3-MYB DNA-binding domain protein (Nesi et al., 2001; Debeaujon et al., 2003).
In bilberry fruits, high levels of anthocyanins accumulate in the skin and flesh during ripening, and these changes are correlated with the expression of the SQUA MADS box gene, VmTDR4. The in situ hybridization experiments and QRT-PCR indicated that VmTDR4 expression was associated with numerous different tissues in the unripe fruit, including the vascular tissues, and with anthocyanin biosynthesis in ripe fruits. Interestingly, VmTDR4 expression is low in a white mutant of bilberry that ripens normally. These observations are suggestive of a link between VmTDR4 and flavonoid biosynthesis but do not establish any functional relationship.
Functional analysis of the VmTDR4 gene in bilberry was undertaken by VIGS. Down-regulation of VmTDR4 in the fruit suppressed the expression of several flavonoid biosynthesis genes and inhibited anthocyanin biosynthesis. VmTDR4 and VmMYB2 were not down-regulated in the CHS VIGS fruits, indicating that their effects are upstream of this gene. The down-regulation of VmTDR4 also suppressed the expression of VmMYB2, a bilberry R2R3-MYB family member transcription factor, the expression of which is closely associated with accumulation of anthocyanins during the fruit development. The partial sequence we obtained for VmMYB2 shares 62% to 63% amino acid identity with the R2R3 region of Arabidopsis AtPAP1 and AtPAP2 and 61.5% identity with VvMYBA1, an ortholog of AtPAP genes of grapevine berry (Supplemental Fig. S1). However, VmMYB2 shares closest identity with VvMYBPA1 (86%) and VvMYB5b (73%; Bogs et al., 2007; Deluc et al., 2008). Moreover, another R2R3-MYB family member, VmMYB1, was isolated from bilberry fruits recently. VmMYB1 shares 78% identity with FaMYB1, a negative regulator and suppressor of anthocyanin biosynthesis from strawberry (Aharoni et al., 2001). The QRT-PCR analysis of VmMYB1 shows that transcripts are more abundant at the early stages of bilberry fruit development and in the green sectors of the VmTDR4 VIGS berries. These results indicate that VmTDR4 functions via known regulators of flavonoid biosynthesis, including R2R3-MYB family members. The metabolic analyses of VIGS-treated fruits revealed that the levels of anthocyanins were 2- to 3-fold lower in VmTDR4- and CHS-silenced fruits, whereas the contents of hydroxycinnamic acids or flavonols were not affected. The lack of an effect on hydroxycinnamic acids may be because they are produced in an earlier part of the phenylpropanoid pathway and prior to the regulatory step affected by VmTDR4. Also, flavonols such as quercetin are generated at the beginning of bilberry fruit ripening (Fig. 4), at a stage before the VIGS injections were made. It is still unclear if VmTDR4 only regulates anthocyanin biosynthesis or if it also is involved in the regulation of other flavonoid subgroups. From grapevine, it is known that different R2R3-MYB family members can control separately the biosynthesis of anthocyanins, flavonols, and proanthocyanins (Bogs et al., 2005, 2007; Azuma et al., 2008; Czemmel et al., 2009).
In the VmTDR4-suppressed VIGS fruits, transcript levels of ANR, leading to the synthesis of proanthocyanidins, were elevated. In many species, proanthocyanidins are produced at the early phases of fruit development, and it has been suggested that they act as defensive and astringent compounds to provide protection against fungal pathogens and predation of unripe fruits (Harborne, 1997; Jaakola et al., 2002; Bogs et al., 2005). In the skin of red grape, the flavonoid pathway switches to the production of anthocyanins instead of proanthocyanidins at the onset of ripening, and this phenomenon is controlled by MYB transcription factors (Bogs et al., 2007). In bilberry, the content of proanthocyanidins and the flavonol quercetin are higher at the early stages of fruit development. At the onset of ripening, the level of proanthocyanidins declines whereas quercetin remains at a constant but low level during the ripening phase. The flavonol myricetin is synthesized in bilberry skin and flesh at the same time as the accumulation of anthocyanins (Jaakola et al., 2002). Therefore, the higher levels of ANR transcripts in VmTDR4-silenced bilberry fruit sections could suggest a delay in ripening in the sectors. However, QRT-PCR analysis of another ripening-related MADS box transcription factor, VmTAGL1, which is expressed at a higher level in earlier stages of bilberry fruit development, did not show differences between the green and red sectors of the VmTDR4- or CHS-silenced fruits (data not shown). An alternative metabolic explanation could be that lack of anthocyanidin intermediates in the VmTDR4-silenced sectors stimulates ANR transcription.
Role of TDR4-Like Genes in Other Plant Species
Elegant work in the model plant Arabidopsis has demonstrated that a range of MADS box and other transcription factors are involved in regulating the development of their dry fruits. This includes the expression of FUL, a SQUA gene that is primarily responsible for proper valve development (Dinneny et al., 2005). In the fleshy fruits of tomato, the TDR4 gene, which shares sequence homology with FUL, shows a strong ripening-related pattern of expression (Eriksson et al., 2004), but its function is not known. Studies we have undertaken to reveal the role of TDR4 in tomato fruits were inconclusive, with no obvious phenotypes where the gene was down-regulated in RNA interference lines (G.B. Seymour and M. Poole, unpublished data). This is probably due to the presence of other TDR4-like genes in the tomato genome (Hileman et al., 2006). However, we observed that tomato TDR4 induced anthocyanin biosynthesis when it was expressed ectopically in Arabidopsis siliques (Supplemental Fig. S2). These effects are consistent with those observed for VmTDR4 in bilberry in that they indicate a link between SQUA genes and the regulation of the phenylpropanoid pathway. Furthermore, in Arabidopsis, the endogenous FUL gene, which is strongly related to TDR4 and VmTDR4 by sequence homology, is necessary for the expression of the MYB transcription factor PAP2 under the condition of nitrogen starvation (Supplemental Fig. S3). This MYB is known to be involved in the regulation of anthocyanin biosynthesis (Borevitz et al., 2000).
The data reported in this article provide strong evidence that VmTDR4 plays an important role in the control of anthocyanin biosynthesis in bilberry, acting directly or indirectly through MYB transcription factors to control carbon flux through the phenylpropanoid pathway. Further work is required to determine the precise molecular links between the regulatory factors involved in this process. However, this study provides, to our knowledge, the first evidence for a functional association between SQUA MADS box genes and anthocyanin biosynthesis during ripening in a fleshy fruit species.
MATERIALS AND METHODS
Plant Material
The flowers and fruits of wild bilberry (Vaccinium myrtillus), growing in a natural forest stand in Oulu (65°01′ N, 25°28′ E), Finland, were collected at six different ripening stages, from flower to ripe fruit. Samples were immediately frozen in liquid nitrogen and stored at −70°C until analyzed. Arabidopsis (Arabidopsis thaliana) Landsberg erecta and Columbia seeds were obtained from the European Arabidopsis Stock Centre at the University of Nottingham. ful mutant lines were donated by Prof. M. Yanofsky (University of California San Diego).
Cloning of VmTDR4 and TDR4
Total RNA was isolated from bilberry fruit samples with the method described by Jaakola et al. (2002). The quality of the isolated RNA was verified by measuring the absorbance spectrum with NanoDrop N-1000 spectrophotometer (NanoDrop Technologies) and on a 1% (w/v) ethidium bromide-stained agarose gel.
Degenerate primers VmTDR4f (5′-GTGATGCWGAGGTTGSTTTGA-3′) and VmTDR4r (5′-AGCWGGTTRTTTTGCTCCTGC-3′) for VmTDR4 were designed for isolating the VmTDR4 based on homology of the MADS box genes from tomato (Solanum lycopersicum; TDR4) and Arabidopsis (FUL). The full-length sequence of VmTDR4 was cloned using the SMART RACE cDNA Amplification Kit (Clontech) with the gene-specific primers gspTDRf (5′-TGGAACCTGGAATACCCGAAGCTCA-3′) and gspTDRr (5′-TGAAGCTCTCTCAGGGTCAAGGTGTCA-3′). The full-length cDNA sequence was deposited in GenBank under accession number FJ418852.
Phylogenetic Estimation
Full-length protein sequences were aligned using MAFFT version 5 (Katoh et al., 2005), and an average distance tree was constructed based on percentage identity between sequences in Jalview 2.4 (Clamp et al., 2004).
Quantification of Transcript Abundance
Transcript accumulation of VmTDR4, VmMYB2, VmMYB1, and the flavonoid pathway genes (CHS, DFR, ANS, and ANR) was detected using the DyNAmo Capillary SYBR Green qPCR Kit (Finnzymes). Glyceraldehyde-3-phosphate dehydrogenase (AY123769) was used as a control gene for relative quantification. QPCR analyses were performed with a LightCycler 2.0 instrument and software (Roche). The PCR conditions were 95°C for 10 min, followed by 45 cycles of 95°C for 10 s, 60°C for 20 s, and 72°C for 10 s. Concerning the transcript abundance of VmTDR4 and VmMYB2, a t test was used to determine if the relative quantity was significantly lower (P ≤ 0.05) in green versus red parts of the VIGS-treated fruits based on data from three to four technical replicates. We separated those visible sectors of VmTDR4- or CHS-silenced fruits with a scalpel.
In Situ Hybridization
Unripe bilberry fruits were cut into cubes of 5 mm2 and fixed in 4% (w/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde in 0.1 m sodium phosphate buffer (pH 7.0) overnight at 4°C. The samples were rinsed in 0.1 m sodium phosphate buffer (pH 6.8) and dehydrated in a graded series of ethanol up to absolute. The ethanol was replaced by a series of xylene (25%, 50%, 75%, and 100%, v/v), after which the samples were gradually infiltrated with paraffin (Merck). Paraffin-embedded samples were sectioned to a thickness of 8 μm with a microtome (Microm HM 325). The sections were spread on glass slides previously coated with 2% (v/v) 3-aminopropyltriethoxysilane (Sigma) in acetone and dried overnight at 40°C. Two 20-min incubations in xylene were used for removing paraffin from the samples. For in situ hybridizations, samples were rehydrated in a graded ethanol series up to water.
Using primers 5′-CACCTTGACCCTGAGAGAGC-3′ and 5′-GTCCACCTTGGTTTTGTTGC-3′, a 218-bp fragment from VmTDR4 was amplified from bilberry fruit cDNA by PCR with DyNazyme II DNA polymerase (Finnzymes) under standard PCR conditions. The PCR fragment was gel purified using the Montage DNA Gel Extraction KIT (Millipore) and ligated into pGEM-T Easy vector (Promega). Digoxigenin (DIG)-labeled sense and antisense probes were prepared from the linearized plasmid by in vitro transcription with SP6 or T7 RNA polymerase using the DIG RNA Labeling Kit (Roche) according to the manufacturer's instructions. Before hybridization, rehydrated tissue sections were treated with proteinase K (1 μg mL−1 in Tris-HCl and 50 mm EDTA, pH 7.5) for 30 min at 37°C followed by dehydration in a graded ethanol series up to absolute. For the hybridization, 100 μL of hybridization mixture (0.1 μg mL−1 DIG-labeled antisense or sense probe, 50% [v/v] deionized formamide [Sigma], 0.3 m NaCl, 10 mm Tris-HCl [pH 7.5], 1 mm EDTA, 1× Denhardt's solution [Sigma], 150 μg mL−1 tRNA [Roche], 500 μg mL−1 polyadenylic acid [Sigma], 10% dextran sulfate, and 0.06 m dithiothreitol) was dispersed on the sections, and the hybridization was carried out at 50°C overnight. After hybridization, slides were washed in 2× SSC (300 mm NaCl, 30 mm sodium citrate, pH 7.0), in 1× SSC at 37°C and 0.5× SSC at 37°C, for 10 min in each. Excess RNA probes were removed in RNase A treatment at 37°C for 60 min following by four 15-min washes in 10 mm Tris-HCl, 500 mm NaCl, and 1 mm EDTA and one 30-min incubation in 2× SSC. For localization of the hybridized transcripts, slides were washed in 100 mm Tris-HCl, 150 mm NaCl, and 0.3% (v/v) Triton X-100 for 5 min and blocked with 2% (w/v) blocking reagent (Roche) for 30 min, followed by a 2-h incubation at room temperature with a 1:750 dilution of anti-(DIG-alkaline phosphatase) conjugate (Roche) and four 10-min washes in the same buffer. For color development, slides were washed for 5 min in 100 mm Tris-HCl, pH 9.5, 100 mm NaCl, and 50 mm MgCl2 and immersed in 5-bromo-4-chloro-3-indolyl phosphate and blue tetrazolium chloride (Roche) overnight in the same buffer. The next day, slides were washed in water, dehydrated in a graded series of ethanol up to absolute, and air dried before sealing with coverslips.
VIGS
The pBINTRA6 and pTV00 vectors were obtained from David Baulcombe and PBL at the Sainsbury Laboratory. For pTV00-VmTDR4 construction, a 150-bp fragment of the VmTDR4 gene was PCR amplified from bilberry fruit cDNA using primers (forward, 5′-CTCGGATCCGGTGGACAAAGTTCATCC-3′; reverse, 5′-GCTAAGCTTCGGCGGCATCAAAGTGTT-3′). The resulting PCR product was cloned into pTV00 to form pTV00-VmTDR4. For pTV00-VmCHS construction, a 100-bp fragment of the VmCHS (AY123765) gene was PCR amplified from bilberry fruit cDNA using primers (forward, 5′-CTCGGATCCAAGATCACCCACTCAGTCTTTTG-3′; reverse, 5′-GCTAAGCTTGCTTCACGGAGGGACGGAGCC-3′). The resulting PCR product was cloned into pTV00 to form pTV00-VmCHS. The pTV00-VmTDR4 and pTV00-VmCHS vectors were transformed into Agrobacterium tumefaciens strain GV3101 for the inoculations.
Plant infiltration was performed as described previously (Ratcliff et al., 2001). The Agrobacterium strains GV3101 containing pTV00-VmTDR4 or pTV00-VmCHS and C58c1 containing pBINTRA6 were grown at 28°C in liquid Luria-Bertani medium including antibiotics (50 μg mL−1 kanamycin, 5 μg mL−1 tetracycline, and 50 μg mL−1 rifampicillin, pH 5.6). After 24 h, the cells were harvested by centrifugation and resuspended in the infiltration buffer (10 mm MgCl2 with 200 μm acetosyringone and 10 mm MES, pH 5.6) to a final optical density at 600 nm of approximately 1 and shaken for 2 h (+28°C) before mixing in a 1:1 ratio. The Agrobacterium mix containing either pBINTRA6 + pTV00-VmTDR4 or VmCHS vectors was injected in bilberry fruits that were in the middle of fruit development with a 1-mL syringe and needle. As a control, only Agrobacterium with pBINTRA6 or infection buffer was injected in the fruits.
Metabolite Analysis
Contents of anthocyanins, flavonols, and hydroxycinnamic acids from fully ripe bilberry fruits 35 to 42 d following VIGS injections with VmTDR4, CHS, or empty vector constructs were determined. Phenolics were extracted from homogenized freeze-dried material (20–80 mg) with methanol (1 mL) containing salicylic acid as an internal standard (10 μg). The mixture was heated to 90°C for 60 min; after cooling, the suspension was centrifuged at 3,000g for 5 min; the supernatant was removed, and extracts were passed through a 0.2-μm syringe-fitting filter before HPLC analysis. An Agilent 1100 HPLC solvent delivery system was used to separate phenolics on a reverse-phase C18 column (250 mm × 4.6 mm, 5 μm; Hichrom). The mobile phase consisted of 2% water in methanol acidified with 0.015% HCl by volume (A) and acetonitrile (B). The initial gradient conditions used were 95% A, 5% B for 10 min, followed by a linear gradient to 50% B over 30 min. An online photo diode array detector enabled detection and identification from characteristic UV/visible light spectra. Authentic standards were used to confirm the identity of the phenolics. Relative quantification was carried out by comparison of integrated peak areas with the internal standard at the λmax of the phenolics detected. Previously, the identity of the phenolics had been confirmed with liquid chromatography-electrospray ionization-mass spectrometry using identical conditions except that formic acid was used instead of HCl.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers FJ418852 (VmTDR4), AY123765 (CHS), AY123767 (DFR), AY123768 (ANS), FJ666338 (ANR), GU904211 (VmMYB1), and GU227356 (VmMYB2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Alignment of R2 and R3 DNA-binding regions of VmMYB2 with Arabidopsis PAP1, PAP2, ATMYB113, and ATMYB12, grape VvMYBA1, VvMYBPA1, and VvMYB5b, and maize (Zea mays) C1 anthocyanin regulator.
Supplemental Figure S2. Effects of ectopic expression of tomato TDR4 in Arabidopsis.
Supplemental Figure S3. Determination of AtPAP2 transcript abundance by QPCR of cDNA in wild-type and ful mutant leaves in response to growth with and without nitrogen (N; error bars indicate se; n = 3).
Supplemental Materials and Methods S1.
Supplementary Material
Acknowledgments
We thank Cathie Martin at the John Innes Centre and Jacquie De Silva at Unilever for useful discussions during the preparation of this article. Also, Chris Gerrish at Royal Holloway is thanked for assistance in the analysis of the phenolics.
References
- Aharoni A, De Vos CH, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O'Connell AP. (2001) The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. Plant J 28: 319–332 [DOI] [PubMed] [Google Scholar]
- Azuma A, Kobayashi S, Nobuhito M, Shiraishi M, Yamada M, Ueno T. (2008) Genomic and genetic analysis of MYB-related genes that regulate anthocyanin biosynthesis in grape berry skin. Theor Appl Genet 117: 1009–1019 [DOI] [PubMed] [Google Scholar]
- Bogs J, Downey M, Harvey J, Ashton A, Tanner G, Robinson S. (2005) Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol 139: 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Jaffe F, Takos A, Walker A, Robinson S. (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143: 1347–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borevitz J, Xia Y, Blount J, Dixon R, Lamb C. (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12: 2383–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broun P. (2005) Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr Opin Plant Biol 8: 272–279 [DOI] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EG, Hall RD, Bovy AG, Luo J, et al. (2008) Enrichment of tomato fruit health-promoting anthocyanins by expression of selected transcription factors. Nat Biotechnol 26: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Clamp M, Cuff J, Searle S, Barton G. (2004) The Jalview Java alignment editor. Bioinformatics 20: 426–427 [DOI] [PubMed] [Google Scholar]
- Czemmel S, Stracke R, Weisshaar B, Cordon N, Harris NN, Walker AR, Robinson SP, Bogs J. (2009) The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol 151: 1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L. (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15: 2514–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluc L, Bogs J, Walker AR, Ferrier T, Decendit A, Merillon JM, Robinson SP, Barrieu F. (2008) The transcription factor VvMYB5 contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol 147: 2041–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny J, Weigel D, Yanofsky M. (2005) A genetic framework for fruit patterning in Arabidopsis thaliana. Development 132: 4687–4696 [DOI] [PubMed] [Google Scholar]
- Dragsted L, Krath B, Ravn-Haren G, Vogel U, Vinggaard A, Jensen P, Loft S, Rasmussen S, Sandstrom B, Pedersen A. (2006) Biological effects of fruit and vegetables. Proc Nutr Soc 65: 61–67 [DOI] [PubMed] [Google Scholar]
- Eriksson E, Bovy A, Manning K, Harrison L, Andrews J, De Silva J, Tucker G, Seymour G. (2004) Effect of the Colorless non-ripening mutation on cell wall biochemistry and gene expression during tomato fruit development and ripening. Plant Physiol 136: 4184–4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2004) Genetic regulation of fruit development and ripening. Plant Cell (Suppl) 16: S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. (2007) Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol 10: 283–289 [DOI] [PubMed] [Google Scholar]
- Grotewold E. (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57: 761–780 [DOI] [PubMed] [Google Scholar]
- Harborne J. (1997) Phytochemistry of fruits and vegetables: an ecological overview. Tomas-Barberan F, , Phytochemistry of Fruits and Vegetables. Oxford University Press, New York, pp 335–367 [Google Scholar]
- Hileman LC, Sundstrom JF, Litt A, Chen M, Shumba T, Irish VF. (2006) Molecular and phylogenetic analyses of the MADS-box gene family in tomato. Mol Biol Evol 23: 2245–2258 [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Kalinowski G, Schwab W. (2006) RNAi-induced silencing of gene expression in strawberry fruit (Fragaria x ananassa) by agroinfiltration: a rapid assay for gene function analysis. Plant J 48: 818–826 [DOI] [PubMed] [Google Scholar]
- Jaakola L, Määttä K, Pirttilä A, Törrönen R, Kärenlampi S, Hohtola A. (2002) Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol 130: 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola L, Määttä-Riihinen K, Kärenlampi S, Hohtola A. (2004) Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 218: 721–728 [DOI] [PubMed] [Google Scholar]
- Kähkonen MP, Heinämaki J, Ollilainen V, Heinonen M. (2003) Berry anthocyanins: isolation, identification and antioxidant activities. J Sci Food Agric 83: 1403–1411 [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33: 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10: 236–242 [DOI] [PubMed] [Google Scholar]
- Lalusin A, Nishita K, Kim S, Ohta M, Fujimura T. (2006) A new MADS-box gene (IbMADS10) from sweet potato (Ipomoea batatas (L.) Lam) is involved in the accumulation of anthocyanin. Mol Gen Genomics 275: 44–54 [DOI] [PubMed] [Google Scholar]
- Lännenpää M, Jänönen I, Hölttä-Vuori M, Gardemeister M, Porali I, Sopanen T. (2004) A new SBP-box gene BpSPL1 in silver birch (Betula pendula). Physiol Plant 120: 491–500 [DOI] [PubMed] [Google Scholar]
- Lätti A, Riihinen K, Kainulainen P. (2008) Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland. J Agric Food Chem 56: 190–196 [DOI] [PubMed] [Google Scholar]
- Liu J, Xu B, Hu L, Li M, Su W, Wu J, Yang J, Jin Z. (2009) Involvement of banana MADS-box transcription factor gene in ethylene-induced fruit ripening. Plant Cell Rep 28: 103–111 [DOI] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky F. (1995) The Arabidopsis AGL8 MADS box gene is expressed in inflorescence meristems and is negatively regulated by APETALA1. Plant Cell 7: 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Tor M, Poole M, Hong Y, Thompson A, King G, Giovannoni J, Seymour G. (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38: 948–952 [DOI] [PubMed] [Google Scholar]
- Müller B, Saedler H, Zachgo S. (2001) The MADS-box gene DEFH28 from Antirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J 28: 169–179 [DOI] [PubMed] [Google Scholar]
- Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13: 2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng MS, Hudson D, Schofield A, Tsao R, Yang R, Gu HL, Bi YM, Rothstein SJ. (2008) Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis which is controlled by the NLA gene. J Exp Bot 59: 2933–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay N, Glover B. (2005) MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10: 63–70 [DOI] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez A, Baulcombe D. (2001) Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Renaud S, Delorgeril M. (1992) Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 339: 1523–1526 [DOI] [PubMed] [Google Scholar]
- Riihinen K, Jaakola L, Kärenlampi S, Hohtola A. (2008) Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum x V angustifolium). Food Chem 110: 156–160 [DOI] [PubMed] [Google Scholar]
- Rosin FM, Aharoni A, Salentijn EMJ, Schaart JG, Boone MJ, Hannabel DJ. (2003a) Expression patterns of a putative homolog of AGAMOUS, STAG1 from strawberry. Plant Sci 165: 959–968 [Google Scholar]
- Rosin FM, Hart JK, Van Onckelen H, Hannapel DJ. (2003b) Suppression of a vegetative MADS box gene of potato activates axillary meristem development. Plant Physiol 131: 1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AT, Hofer JMI, Murfet IC, Sollinger JD, Singer SR, Knox MR, Ellis THN. (2002) PROLIFERATING INFLORESCENCE MERISTEM, a MADS-box gene that regulates floral meristem identity in pea. Plant Physiol 129: 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour G, Poole M, Manning K, King G. (2008) Genetics and epigenetics of fruit development and ripening. Curr Opin Plant Biol 11: 58–63 [DOI] [PubMed] [Google Scholar]
- Simpson GG, Gendall T, Dean C. (1999) When to switch to flowering. Annu Rev Cell Dev Biol 15: 519–550 [DOI] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshar B. (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Theissen G. (2001) Development of floral organ identity: stories from the MADS house. Curr Opin Plant Dev 4: 75–85 [DOI] [PubMed] [Google Scholar]
- Vinson J, Zubik L, Bose P, Samman N, Proch J. (2005) Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr 24: 44–50 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. (2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (Rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Williams CM, Mohsen M, Vauzour D, Rendeiro C, Butler LT, Ellis JA, Whiteman M, Spencer JPE. (2008) Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic Biol Med 45: 295–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.