Abstract
Rice (Oryza sativa) as a staple food, provides a major source of dietary selenium (Se) for humans, which essentially requires Se, however, the molecular mechanism for Se uptake is still poorly understood. Herein, we show evidence that the uptake of selenite, a main bioavailable form of Se in paddy soils, is mediated by a silicon (Si) influx transporter Lsi1 (OsNIP2;1) in rice. Defect of OsNIP2;1 resulted in a significant decrease in the Se concentration of the shoots and xylem sap when selenite was given. However, there was no difference in the Se concentration between the wild-type rice and mutant of OsNIP2;1 when selenate was supplied. A short-term uptake experiment showed that selenite uptake greatly increased with decreasing pH in the external solution. Si as silicic acid did not inhibit the Se uptake from selenite in both rice and yeast (Saccharomyces cerevisiae) at low pHs. Expression of OsNIP2;1 in yeast enhanced the selenite uptake at pH 3.5 and 5.5 but not at pH 7.5. On the other hand, defect of Si efflux transporter Lsi2 did not affect the uptake of Se either from selenite or selenate. Taken together, our results indicate that Si influx transporter OsNIP2;1 is permeable to selenite.
The essential trace mineral, selenium (Se), is of fundamental importance to human health (Rayman, 2000). Deficiency of Se is associated with health disorders including oxidative stress-related conditions, reduced fertility and immune functions, and an increased risk of cancers (Rayman, 2002; Whanger, 2004). It has been estimated that about 500 to 1,000 million people are suffering from Se deficiency in the world (Combs, 2001). On the other hand, Se is also toxic at higher concentrations (Terry et al., 2000). The window between Se deficiency and toxicity for humans is narrow (Zhu et al., 2009).
Plants are the main sources of dietary Se (Rayman, 2008), although the essentiality of this element for higher plants has not been recognized. Therefore, controlling Se uptake of plants from the environment (soils) will be important to decrease healthy risk of both toxicity and deficiency. However, it is still poorly understood how plants take up Se from the roots. Selenate and selenite are two main inorganic Se forms available for plant uptake in soils and their prevalence depends on the redox potential and pH. Selenate can be taken up via sulfate transporters in plants due to the chemical similarity between selenate and sulfate (Terry et al., 2000; Sors et al., 2005). This has been supported by several studies. For example, sulfate competitively inhibits selenate uptake in barley (Hordeum vulgare; Leggett and Epstein, 1956). The gene Sultr1;2 involved in sulfate uptake of roots has been identified in Arabidopsis (Arabidopsis thaliana) using selenate as a toxic analog of sulfate (Shibagaki et al., 2002; Kassis et al., 2007). The Se uptake from selenate was greatly enhanced in the sulfur-starved plants probably due to up-regulation of sulfate transporter gene in wheat (Triticum aestivum; Li et al., 2008).
By contrast, little is known about the uptake mechanisms of selenite in plants. Shrift and Ulrich (1969) and Arvy (1993) reported that selenite is taken up by plant roots through passive diffusion. However, a recent study with wheat showed that selenite uptake is an active process because the uptake was significantly inhibited by a metabolic inhibitor CCCP (Li et al., 2008). Terry et al. (2000) concluded that there was no evidence that the selenite uptake is mediated by membrane transporter. However, Li et al. (2008) reported that selenite uptake is at least partly to be mediated by phosphate transporters based on evidence that phosphorus deficiency enhanced selenite uptake in wheat. This is also supported by earlier works, which reported that increasing phosphate concentration decreased selenite uptake in different plant species (Broyer et al., 1972; Hopper and Parker, 1999). However, convincing evidence on how selenite is taken up by the roots is still lacking.
Rice (Oryza sativa) is a staple food for nearly half of the world's population. Therefore, rice provides a major source of dietary intake of Se in many countries (Rayman, 2008). In paddy soil, Se is present in the form of selenite. A physiological study showed that selenite uptake by rice roots was inhibited by HgCl2 and AgNO3, suggesting that aquaporin is implicated in the uptake of selenite (Zhang et al., 2006). However, the exact mechanism for selenite uptake has not been understood. Recently, a silicon (Si) influx transporter OsNIP2;1 (Lsi1) belonging to the nodulin 26-like intrinsic membrane protein (NIP) subfamily of aquaporins, has been identified in rice (Ma et al., 2006). Further studies showed that this transporter is also permeable to arsenite (Ma et al., 2008) and methylated arsenic (As; Li et al., 2009) in rice roots. Si, As, and Se belong to metalloids, showing some similar properties. These facts lead us to hypothesize that OsNIP2;1 is also able to transport selenite. In this study, we used both rice mutants defective in OsNIP2;1 function and yeast (Saccharomyces cerevisiae) expression system and showed convincing evidence that OsNIP2;1 is permeable to selenite. We also found that different from Si and arsenite, Lsi2, an efflux transporter of Si (Ma et al., 2007), is not involved in the transport of selenite.
RESULTS
Defect of OsNIP2;1 Affects Selenite Uptake, But Not Selenate Uptake by Rice
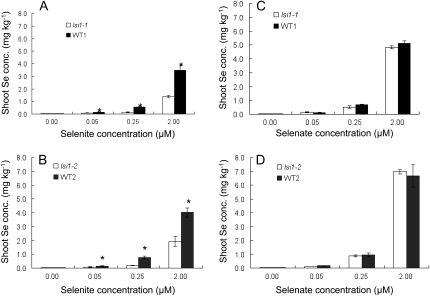
To investigate whether Si influx transporter OsNIP2;1 (Lsi1) is involved in the uptake of selenite or selenate, we compared Se accumulation between two independent mutants defective in Si influx transport and their respective wild-type rice at different concentrations of selenite or selenate. lsi1-1 and lsi1-2 differed in the mutation position of OsNIP2;1 gene (Supplemental Fig. S1). When exposed to selenite, lsi1-1 accumulated less than half Se in the shoots compared with the wild-type rice at all Se concentrations tested (Fig. 1A). Similar trend was observed between lsi1-2 and its wild-type rice (Fig. 1B). However, when exposed to selenate, no difference in the shoot Se concentration was found between mutant and wild-type rice at all concentrations (Fig. 1, C and D). Comparison between selenate and selenite showed that both wild-type rice accumulated higher Se from selenate than that from selenite (Fig. 1).
Figure 1.
Concentration-dependent accumulation of Se in wild-type rice and two mutants exposed to selenite [Se(IV)] or selenate [Se(VI)]. Rice seedlings (20-d-old) of WT1 (Oochikara) and lsi1-1 mutant (A and C), WT2 (Nipponbare), and lsi1-2 mutant (B and D) were exposed to a nutrient solution containing different concentrations of selenite (A and B) or selenate (C and D) for 1 d. The pH of solution buffered with 5 mm MES was 5.4. The Se concentration in the shoots was determined. Data are means ± sd (n = 3). The asterisks above the columns indicate statistically significant differences between wild-type rice and its mutant (P < 0.05 by independent-samples t test).
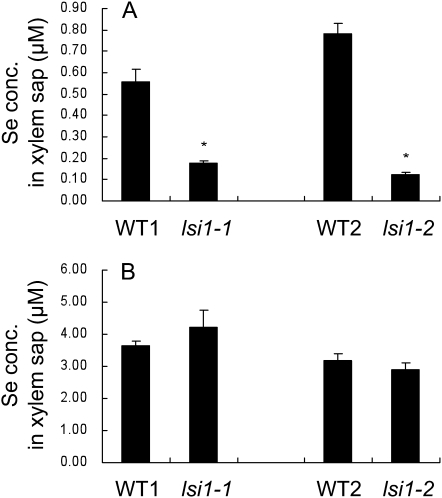
The concentration of Se in the xylem sap was also compared between two wild-type rice and two independent mutants (lsi1-1 and lsi1-2). When exposed to selenite, both wild-type rice showed several times higher Se than their corresponding mutants in the xylem sap (Fig. 2A). However, there was no difference in the Se concentration between two wild-type rice and their corresponding mutants when selenate was supplied (Fig. 2B). The Se concentration in the xylem sap of both wild-type rice was higher when selenate was supplied than when selenite was supplied, which is in agreement with the Se concentration in the shoots (Fig. 1).
Figure 2.
Concentration of Se in xylem sap of rice. Seedlings (21-d-old) of two wild-type rice (WT1, Oochikara; WT2, Nipponbare) and two independent mutants (lsi1-1 and lsi1-2) were exposed to a nutrient solution containing 2.5 μm selenite (A) or selenate (B) for 1 d and then xylem sap was collected for 1 h after decapitation. The initial pH of the solution was 5.6. Data are means ± sd (n = 3). The asterisks above the columns indicate statistically significant differences between wild-type rice and its mutant (P < 0.05 by independent-samples t test).
Effect of pH on Selenite Uptake by Rice
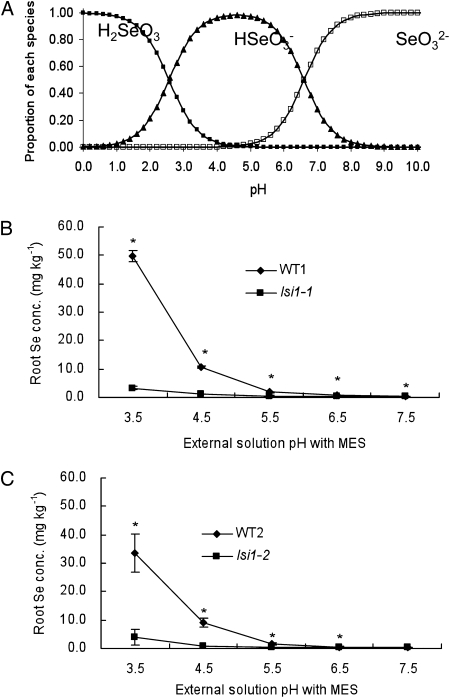
Selenite is a diprotic weak acid with pKa1 and pKa2 of 2.57 and 6.60, respectively (Zhang et al., 2006). This means that selenite will exist as H2SeO3, HSeO3−and SeO32−depending on pH (Fig. 3A). We therefore investigated the effect of pH on selenite uptake in two wild-type rice and two independent mutants (lsi1-1 and lsi1-2). A short-term (30 min) experiment showed that the Se uptake from selenite decreased with increasing external pH in both wild-type rice (Fig. 3, B and C). The difference in the Se uptake between the wild-type rice and the mutant was largest at pH 3.5 and gradually became smaller with pH increase (Fig. 3, B and C), although the significant difference in the Se uptake was also observed between the wild-type rice and the mutant at higher pHs (Fig. 3, B and C).
Figure 3.
Effect of pH on Se uptake in rice. A, Proportion of each species of selenite at different pHs, which is calculated based on Curiplot. B and C, Root Se concentration. Seedlings (14-d-old) of two wild-type rice (WT1, Oochikara; WT2, Nipponbare) and two independent mutants (lsi1-1 and lsi1-2) were exposed to a nutrient solution containing 2.5 μm selenite at different pHs with 5 mm MES for 30 min. Se concentration in the roots was determined. Data are means ± sd (n = 3). The asterisks indicate statistically significant differences between wild-type rice and its mutant at the same pH (P < 0.05 by independent-samples t test).
Interaction between Uptake of Selenite and Silicic Acid
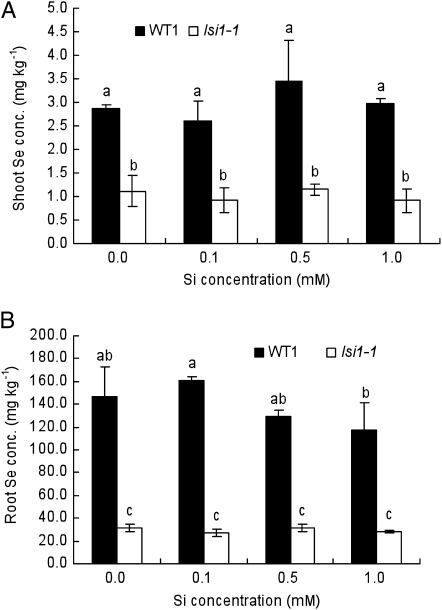
Because OsNIP2;1 was initially identified as a transporter for silicic acid (Ma et al., 2006), we investigated the effect of silicic acid on selenite uptake in both wild-type rice and mutant lsi1-1. The Se concentration in the roots and shoots was higher in the wild-type rice than lsi1-1 at all Si concentrations (Fig. 4, A and B). In these experiments, the concentration of Si was 40 to 400 times higher than that of selenite, but the presence of silicic acid did not greatly affect the selenite uptake in both wild-type rice and lsi1-1 (Fig. 4, A and B).
Figure 4.
Effect of Si on Se accumulation in rice. Seedlings (14-d-old) of both wild-type rice (WT1, Oochikara) and mutant (lsi1-1) were exposed to a nutrient solution containing 2.5 μm selenite in the presence of different concentrations of Si for 1 d. The initial pH of solution was 5.6. Se concentration in the shoots (A) and roots (B) was determined. Data are means ± sd (n = 3). Different letters above the columns indicate statistically significant differences at P < 0.05 by Tukey's test.
Transport Activity of OsNIP2;1 for Selenite in Yeast
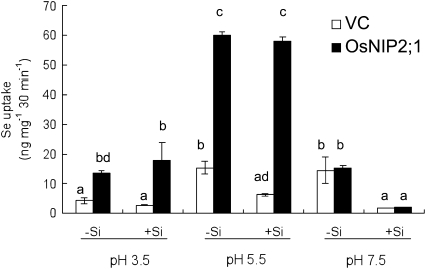
To directly determine the transport activity of OsNIP2;1 for selenite, we expressed OsNIP2;1 gene in yeast. Expression of OsNIP2;1 resulted in increased uptake of selenite at pHs 3.5 and 5.5 (Fig. 5). However, at pH 7.5, there was no difference in the selenite uptake between yeast expressed or not expressed OsNIP2;1 (Fig. 5). Selenite uptake was higher at pH 5.5 than pH 3.5. Addition of silicic acid (100 times of selenite) did not affect the selenite uptake at pH 3.5 and 5.5 (Fig. 5). However, at pH 7.5, silicic acid decreased selenite uptake in both yeast-expressed OsNIP2;1 and vector control (Fig. 5), although the reason for this result is unknown.
Figure 5.
Transport activity of OsNIP2;1 for selenite in yeast. Yeast expressing OsNIP2;1 or not (VC) was exposed to 10 μm selenite in the presence or absence of 1 mm Si as silicic acid at different pHs. After 30-min uptake, the Se in the yeast was determined. Data are means ± sd (n = 3). Different letters above the columns indicate statistically significant differences at P < 0.05 by Tukey's test.
Lsi2 Is Not Involved in the Uptake of Selenite and Selenate
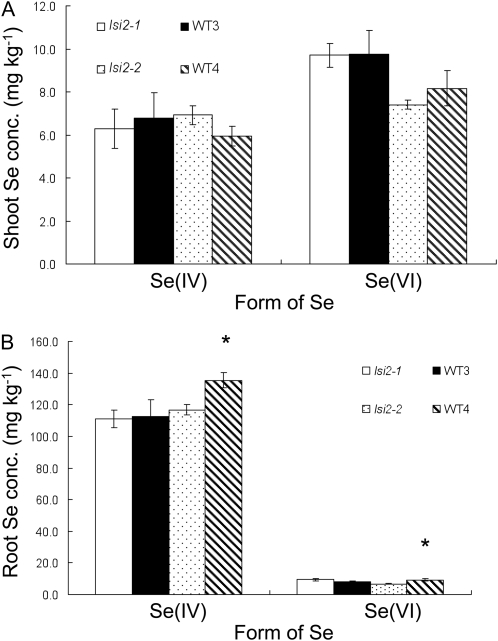
Lsi2 is an efflux transporter of silicic acid (Ma et al., 2007). It also transports arsenite (Ma et al., 2008). To investigate whether Lsi2 is also involved in the uptake of selenite or selenate, we compared the Se uptake between two independent mutants of Lsi2 and their corresponding wild-type rice. lsi2-1 and lsi2-2 have different mutation in the Lsi2 gene (Supplemental Fig. S1). When they were exposed to selenite or selenate, there was no difference in the shoot Se concentration between the wild-type rice and the mutant, irrespectively of mutation (Fig. 6A). There was also no difference in the root Se concentration between the wild-type rice and lsi2-1 (Fig. 6B), and the root Se concentration was slightly higher in the wild-type rice than lsi2-2.
Figure 6.
Concentration of Se in the shoots (A) and roots (B) of rice exposed to selenite or selenate. Seedlings (16-d-old) of WT3 (T-65), lsi2-1 mutant, WT4 (Koshihikari), and lsi2-2 mutant were exposed to a nutrient solution containing 2.5 μm selenate [Se(VI)] or selenite [Se(IV)] for 1 d. The initial pH was 5.6. Data are means ± sd (n = 3). The asterisks above the columns indicate statistically significant differences between wild-type rice and its mutant (P < 0.05 by independent-samples t test).
DISCUSSION
Our results using rice mutants defective in Si influx transporter OsNIP2;1 clearly show that OsNIP2;1 is permeable to selenite (Fig. 1), but not to selenate. This conclusion is also supported by the heterogeneous expression results in yeast (Fig. 5). OsNIP2;1 belongs to the NIP subfamily of aquaporins. Previous studies have shown that NIP proteins are permeable to a wide range of substrates such as silicic acid (Ma et al., 2006), arsenite (Ma et al., 2008), boric acid (Takano et al., 2006), glycerol (Dean et al., 1999), lactic acid (Choi and Roberts, 2007), urea, and formamide (Wallace and Roberts, 2005). To our knowledge, it is the first report that an aquaporin protein is also permeable to selenite.
Although the exact mechanisms regulating substrate specificity of aquaporins are still unknown, it is proposed that the substrate selectivity is mainly controlled by the aromatic/Arg (ar/R) selectivity filter (Wallace and Roberts, 2005; Forrest and Bhave, 2007), which is located in the narrowest region on the extramembrane mouth of the pore. It is formed by four residues, one each from helix 2 and helix 5, as well as two residues from loop E (LE1 and LE2) typically including aromatic residues and an Arg residue (Rouge and Barre, 2008). The properties of the four residues making up the ar/R selectivity filter appear to govern the substrate specificity of the pore. Based on the ar/R regions of aquaporins, NIPs have been newly divided into three groups, NIP I, II, and III (Mitani et al., 2008). OsNIP2;1 belonging to the NIP III subgroup has a unique selectivity filter, which consists of Gly (G), Ser (S), Gly (G), and Arg (R; Mitani et al., 2008). It is predicted that the smaller size of these residues form a larger constriction region compared with other NIP groups (Wang et al., 2005; Forrest and Bhave, 2007). So far NIP III subgroup is also permeable to arsenite (Ma et al., 2008), urea, water, and boric acid (Mitani et al., 2008) in addition to silicic acid (Ma et al., 2006), but not to glycerol (Ma et al., 2006). Since the information on selenite diameter is not available, permeation through OsNIP2;1 suggests that the diameter of selenite is smaller or similar to silicic acid. It will also be interesting to test whether proteins in NIP I and II subgroups are also permeable to selenite in the future.
Different from silicic acid and arsenite, selenite has a pKa of 2.6 in contrast to arsenite (9.2) and silicic acid (9.84). Therefore, at a pH below 8, both silicic acid and arsenite are uncharged, whereas selenite is deprotonated (Fig. 3A). We investigated the effect of pH on selenite uptake at different pHs, at which different species of selenite are present (Fig. 3). At pH 3.5, 90% and 10% of selenite are present in the form of hydrogenselenite ion (HSeO3−) and selenous acid (H2SeO3), respectively (Fig. 3A), and at pH 5.5, about 93% and 7% of selenite are in the form of HSeO3−selenite ion (SeO32−), respectively. However, at pH 7.5, the proportion of HSeO3−SeO32− changes to about 11% and 89%, respectively (Fig. 3A). The Se uptake decreased greatly with increasing pH in the wild-type rice (Fig. 3). These results suggest that there are two possibilities for the Se form of uptake. One is that OsNIP2;1 is also permeable to HSeO3−and the other is that OsNIP2;1 is only permeable to H2SeO3 and the uptake at higher pHs comes from the rapid protonating of HSeO3−SeO32−. In yeast, the difference in Se transport activity between vector control and OsNIP2;1-expressed yeast was also only observed at pHs 3.5 and 5.5, but not at pH 7.5 (Fig. 5). In rice, the selenite uptake was higher in wild-type rice at pH 3.5 than that at pH 5.5 (Fig. 3), however, in yeast expressing OsNIP2;1, the transport activity was higher at pH 5.5 than at pH 3.5 (Fig. 5). The reason for this difference is unknown. At pH 7.5, selenite is also taken up by the yeast (Fig. 5) although there was no difference between vector control and OsNIP2;1-expressed yeast. This indicates that SeO32− is taken up by unidentified transporter rather than OsNIP2;1 at higher pH in yeast. In rice, the uptake of selenite at high pHs is much less compared with that at low pHs (Fig. 3). This means that OsNIP2;1 is the major pathway for selenite in rice. This is supported that when OsNIP2;1 is loss of function, the selenite uptake was only 5% of wild-type rice at pH 3.5 (Fig. 3).
Although selenite and silicic acid share the same transporter, the antagonistic interaction between silicic acid and selenite was not observed in both rice and yeast (Figs. 4 and 5). The antagonistic interaction between selenite and arsenite was also not observed in rice (data not shown). This is different from arsenite, which uptake is significantly inhibited by silicic acid in rice (Ma et al., 2008). This difference might be attributed to different metabolism processes of these metalloids. Selenite taken up by the roots is readily converted to other organic forms such as selenomethionine and selenomethionine Se-oxide hydrate in the roots (Li et al., 2008) and little selenite was transported into xylem. By contrast, silicic acid and arsenite are the main form in the xylem sap in rice (Mitani et al., 2005; Ma et al., 2008). OsNIP2;1 is an influx transporter that is localized at the distal side of both exodermis and endodermis of rice roots (Ma et al., 2006). It is responsible for the transport of silicic acid, arsenite, and selenite from the external solution to the root cells. To reach the xylem, an efflux transporter is required. For silicic acid and arsenite, Lsi2, an efflux transporter localized at the proximal side of root exodermis and endodermis, has been demonstrated to be responsible for the efflux toward the xylem (Ma et al., 2007, 2008). Therefore, the antagonistic interaction observed between silicic acid and arsenite might occur at the efflux transporter Lsi2. This is supported by the fact that Lsi2 is not involved in the selenite uptake in rice roots (Fig. 6) because of Se species change from selenite to organic forms as described above. In fact, in yeast expressing plant NIP genes including OsNIP2;1, As(III) uptake was not inhibited by Si (Bienert et al., 2008). Li et al. (2009) reported that silicic acid did not inhibit methylated As uptake by rice roots although OsNIP2;1 is also permeable to methylated As in rice. Silicic acid also did not inhibit As(III) influx into Xenopus oocytes expressing OsNIP2;1 (J.F. Ma and N. Mitani, unpublished data). One possible explanation is that different from carrier-type transporters like Lsi2, aquaporins such as OsNIP2;1 permit extremely fast flux of solutes (Maurel et al., 2008), and therefore competition between analogous substrates are less apparent (Li et al., 2009).
In conclusion, to our knowledge, OsNIP2;1 is the first transporter of selenite identified in plants, animals, and microorganism. After selenite is taken up by OsNIP2;1, it will be converted to organic forms. Therefore, other transporters are required to efflux organic Se into the xylem, which remain to be identified in the future.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Rice (Oryza sativa) mutants (lsi1-1, lsi1-2, lsi2-1, and lsi2-2) and their corresponding wild-type rice (cultivars Oochikara, Nipponbare, T-65, and Koshihikari) were used in this study. These mutants were isolated previously (Ma et al., 2002, 2006, 2007; Chiba et al., 2009), their mutation points are shown in Supplemental Figure S1. Seedlings were cultured in a half-strength Kimura B nutrient solution (pH 5.6) in a controlled-environment growth chamber as described previously (Ma et al., 2002). The solution was renewed every 3 d. Each experiment was conducted with three replicates.
In all treatment experiments, selenite and selenate were supplied as Na2SeO3 and Na2SeO4, respectively. Silicic acid was prepared by passing potassium silicate through a cation-exchange resin (Amberlite IR-120B, H+ form; Ma et al., 2002).
Se Uptake Experiment
For the comparison of Se uptake from selenate and selenite, seedlings (20-d-old) of two lsi1 mutants and their wild-type rice were exposed to a nutrient solution (pH 5.4) containing 0, 0.05, 0.25, or 2.0 μm of either selenite or selenate for 1 d. The solution was buffered with 5 mm MES.
For lsi2 mutants and their wild-type rice, the seedlings (16-d-old) were exposed to a nutrient solution containing 2.5 μm selenate [Se(VI)] or selenite [Se(IV)] for 1 d. The pH of treatment solution was 5.6 and 4.0 at initial and end of the experiment. After the exposure, the roots were washed three times with an ice-old solution containing 1 mm K2HPO4, 0.5 mm Ca(NO3)2, and 5 mm MES (pH 5.6) to remove apoplastic Se, and then separated from the shoots. The samples were dried at 75°C in an oven for 2 d and ground for analysis.
Xylem Sap Collection
For the collection of xylem sap, 21-d-old seedlings were exposed to a nutrient solution (pH 5.6) containing 2.5 μm selenite or selenate. After 1 d, the solution pH was reduced to 4.0 and the shoots were cut at about 2 cm above the roots, and xylem sap was then collected for 1 h with a micropipette. At the same time, xylem saps of seedlings without selenite and selenate exposure were collected for the determination of background Se concentration in the present experimental system, which was found to be negligible. Total Se in xylem sap was analyzed as described below.
Effect of pH on Selenite Uptake in Rice
To investigate the effect of pH on selenite uptake by rice, a short-term (30 min) uptake experiment was performed. Seedlings (14-d-old) were exposed to a nutrient solution containing 2.5 μm selenite at different pHs (3.5, 4.5, 5.5, 6.5, or 7.5) buffered with 5 mm MES. After 30 min, roots were desorbed, separated from the shoots, and blotted as described above. The solution pHs before and after the uptake were measured and no significant changes were found during the uptake experiments. The Se concentration in the roots was determined as described below.
Effect of Silicic Acid on Selenite Uptake in Rice
To investigate the effects of silicic acid on selenite uptake, seedlings (14-d-old) were exposed to a nutrient solution (pH 5.6) containing 2.5 μm selenite in the presence of 0, 0.1, 0.5, or 1 mm silicic acid. After 1 d, the plants were harvested and analyzed as described above.
Se Transport Activity Assay in Yeast
For transport assay of Se in yeast (Saccharomyces cerevisiae), the OsNIP2;1 cDNA was cloned into yeast expression vector pYES2 (Invitrogen). Transformation reactions were performed according to the manufacturer's protocols using yeast strain INVSc-1 (S.c. easy comp transformation kit; Invitrogen). OsNIP2;1 transformed yeast and vector control line were precultured in synthetic complete (−Ura) medium with 2% Gal until OD600 of 1.5 or 2.5. The precultured yeast were collected by centrifugation and resuspended in the synthetic complete (−Ura) medium containing 10 μm Se as Na2SeO3, at pH 3.5, 5.5, or 7.5, in the presence or absence of 1.0 mm Si. After 30 min treatment at 30°C with gently shaking, yeast was collected by centrifugation and washed twice by ice-cold water. The yeast pellet was freeze dried and digested with HNO3. The Se concentration was determined by inductively coupled plasma-mass spectrometry. Each treatment was replicated three times.
Se Determination
The ground plant samples were first digested with a mixture of HNO3/HClO4 (80/20, v/v), and then 6 m HCl was added to reduce selenate to selenite. Total Se in the diluted digestion solution and xylem sap was analyzed by hydride generation flame atomic fluorescence spectrometry (AF-610A, Beijing Ruili Analytical Instrument Co.). To control the quality of the analysis procedure, a reagent blank and a reference material BGW07605 (GSV-4) were applied during the experiment of total Se determination.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Mutation point in lsi1-1, lsi1-2, lsi2-1, and lsi2-2 mutants.
Supplementary Material
Acknowledgments
We thank Shiwei Guo (Institute of Food Crops, Jiangsu Academy of Agricultural Sciences) for increasing rice seeds in the field.
References
- Arvy MP. (1993) Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris). J Exp Bot 44: 1083–1087 [Google Scholar]
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP. (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyer TC, Johnson CM, Huston RP. (1972) Selenium and nutrition of Astragalus II: ionic sorption interactions among selenium, phosphate, and the macro- and micronutrient cations. Plant Soil 36: 651–669 [Google Scholar]
- Chiba Y, Mitani N, Yamaji N, Ma JF. (2009) HvLsi1 is a silicon influx transporter in barley. Plant J 57: 810–818 [DOI] [PubMed] [Google Scholar]
- Choi WG, Roberts DM. (2007) Arabidopsis NIP2;1, a major intrinsic protein transporter of lactic acid induced by anoxic stress. J Biol Chem 282: 24209–24218 [DOI] [PubMed] [Google Scholar]
- Combs GF., Jr (2001) Selenium in global food systems. Br J Nutr 85: 517–547 [DOI] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM. (1999) Purification and functional reconstitution of soybean nodulin 26: an aquaporin with water and glycerol transport properties. Biochemistry 38: 347–353 [DOI] [PubMed] [Google Scholar]
- Forrest KL, Bhave M. (2007) Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Funct Integr Genomics 7: 263–289 [DOI] [PubMed] [Google Scholar]
- Hopper JL, Parker DR. (1999) Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil 210: 199–207 [Google Scholar]
- Kassis EE, Cathala N, Rouached H, Fourcroy P, Berthomieu P, Terry N, Davidian JC. (2007) Characterization of a selenate-resistant Arabidopsis mutant: root growth as a potential target for selenate toxicity. Plant Physiol 143: 1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett JE, Epstein E. (1956) Kinetics of sulfate absorption by barley roots. Plant Physiol 31: 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HF, McGrath SP, Zhao FJ. (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178: 92–102 [DOI] [PubMed] [Google Scholar]
- Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ. (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150: 2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Ichii M, Wu GF. (2002) A rice mutant defective in Si uptake. Plant Physiol 130: 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. (2006) A silicon transporter in rice. Nature 440: 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M. (2007) An efflux transporter of silicon in rice. Nature 448: 209–212 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ. (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105: 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V. (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59: 595–624 [DOI] [PubMed] [Google Scholar]
- Mitani N, Ma JF, Iwashita T. (2005) Identification of the silicon form in xylem sap of rice (Oryza sativa L.). Plant Cell Physiol 46: 279–283 [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF. (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch 456: 679–686 [DOI] [PubMed] [Google Scholar]
- Rayman MP. (2000) The importance of selenium to human health. Lancet 356: 233–241 [DOI] [PubMed] [Google Scholar]
- Rayman MP. (2002) The argument for increasing selenium intake. Proc Nutr Soc 61: 203–215 [DOI] [PubMed] [Google Scholar]
- Rayman MP. (2008) Food-chain selenium and human health: emphasis on intake. Br J Nutr 100: 254–268 [DOI] [PubMed] [Google Scholar]
- Rouge P, Barre A. (2008) A molecular modeling approach defines a new group of Nodulin 26-like aquaporins in plants. Biochem Biophys Res Commun 367: 60–66 [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP. (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Shrift A, Ulrich JM. (1969) Transport of selenate and selenite into Astragalus roots. Plant Physiol 44: 893–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sors TG, Ellis DR, Salt DE. (2005) Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res 86: 373–389 [DOI] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N, Zayed AM, de Souza MP, Tarun AS. (2000) Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol 51: 401–432 [DOI] [PubMed] [Google Scholar]
- Wallace IS, Roberts DM. (2005) Distinct transport selectivity of two structural subclasses of the nodulin-like intrinsic protein family of plant aquaglyceroporin channels. Biochemistry 44: 16826–16834 [DOI] [PubMed] [Google Scholar]
- Wang Y, Schulten K, Tajkhorshid E. (2005) What makes an aquaporin a glycerol channel? A comparative study of AqpZ and GlpF. Structure 13: 1107–1118 [DOI] [PubMed] [Google Scholar]
- Whanger PD. (2004) Selenium and its relationship to cancer: an update. Br J Nutr 91: 11–28 [DOI] [PubMed] [Google Scholar]
- Zhang LH, Shi WM, Wang XC. (2006) Difference in selenite absorption between high- and low-selenium rice cultivars and its mechanism. Plant Soil 282: 183–193 [Google Scholar]
- Zhu YG, Pilon-Smits EAH, Zhao FJ, Williams PN, Meharg AA. (2009) Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci 14: 436–442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.