Abstract
Stomata open in response to blue light under a background of red light. The plant hormone abscisic acid (ABA) inhibits blue light-dependent stomatal opening, an effect essential for promoting stomatal closure in the daytime to prevent water loss. However, the mechanisms and molecular targets of this inhibition in the blue light signaling pathway remain unknown. Here, we report that phosphatidic acid (PA), a phospholipid second messenger produced by ABA in guard cells, inhibits protein phosphatase 1 (PP1), a positive regulator of blue light signaling, and PA plays a role in stimulating stomatal closure in Vicia faba. Biochemical analysis revealed that PA directly inhibited the phosphatase activity of the catalytic subunit of V. faba PP1 (PP1c) in vitro. PA inhibited blue light-dependent stomatal opening but did not affect red light- or fusicoccin-induced stomatal opening. PA also inhibited blue light-dependent H+ pumping and phosphorylation of the plasma membrane H+-ATPase. However, PA did not inhibit the autophosphorylation of phototropins, blue light receptors for stomatal opening. Furthermore, 1-butanol, a selective inhibitor of phospholipase D, which produces PA via hydrolysis of phospholipids, diminished the ABA-induced inhibition of blue light-dependent stomatal opening and H+ pumping. We also show that hydrogen peroxide and nitric oxide, which are intermediates in ABA signaling, inhibited the blue light responses of stomata and that 1-butanol diminished these inhibitions. From these results, we conclude that PA inhibits blue light signaling in guard cells by PP1c inhibition, accelerating stomatal closure, and that PP1 is a cross talk point between blue light and ABA signaling pathways in guard cells.
Stomatal guard cells in the epidermis of aerial plants regulate gas exchange between leaves and the atmosphere, allowing the uptake of CO2 for photosynthesis and the loss of water by transpiration. Guard cells integrate a wide variety of stimuli such as light, humidity, temperature, CO2, and plant hormones to prevent excessive water loss and optimize plant growth under changing environmental conditions (Vavasseur and Raghavendra, 2005; Shimazaki et al., 2007). Among them, blue light and abscisic acid (ABA) represent key factors that promote stomatal opening and closure, respectively (Assmann and Shimazaki, 1999; Hetherington, 2001; Schroeder et al., 2001; Roelfsema and Hedrich, 2005). Blue light induces H+ pumping by activation of the plasma membrane H+-ATPase, which causes membrane hyperpolarization and drives K+ uptake into guard cells via inward-rectifying K+ channels (Assmann et al., 1985; Shimazaki et al., 1986; Schroeder et al., 1987). By contrast, ABA activates the anion channels, thereby causing membrane depolarization and promoting K+ efflux from guard cells via outward-rectifying K+ channels (Schroeder et al., 1987). There is cross talk between the opening and closure systems, and ABA inhibits blue light-induced activation of the H+-ATPase (Shimazaki et al., 1986; Goh et al., 1996; Roelfsema et al., 1998). Such inhibition of H+-ATPase by ABA is crucial to maintain the plasma membrane depolarization and supports efficient stomatal closure of open stomata. For example, when H+-ATPase is kept in the active state, as was found in the open stomata2 mutants, plants lost the stomatal closure response to ABA, which brought about the wilty phenotype even under well-watered conditions (Merlot et al., 2002, 2007). Although the regulation of the stomatal opening system by ABA is important for plant survival, the mechanism by which ABA inhibits the activation of H+-ATPase by blue light is largely unknown.
Blue light is required for the activation of phototropins, plant-specific Ser/Thr autophosphorylating kinases, and the activated phototropins transmit the signal to the plasma membrane H+-ATPase for its activation (Kinoshita et al., 2001; Christie, 2007). Activation of the H+-ATPase is caused by the phosphorylation of a Thr residue in the C terminus with subsequent binding of a 14-3-3 protein to the Thr residue (Kinoshita and Shimazaki, 1999; Emi et al., 2001). Since phototropins are Ser/Thr protein kinases, it might be possible that phototropins directly phosphorylate the H+-ATPase. However, this has been shown not to be the case. Recently, we demonstrated that protein phosphatase 1 (PP1), a major member of the PPP family of Ser/Thr protein phosphatases, mediates the signaling between phototropins and H+-ATPase in guard cells (Takemiya et al., 2006). Therefore, ABA is likely to inhibit the signaling molecule(s), including phototropins, PP1, H+-ATPase, and other unidentified components.
In guard cells, ABA induces the production of phosphatidic acid (PA), and PA has been implicated in stimulating stomatal closure and inhibiting light-induced stomatal opening (Jacob et al., 1999; Zhang et al., 2004a; Mishra et al., 2006). PA has also been shown to interact with the catalytic subunit of human PP1 (PP1c) and decreases its phosphatase activity (Kishikawa et al., 1999; Jones and Hannun, 2002). It is thus conceivable that PA also functions as an inhibitor of plant PP1c and suppresses the blue light signaling of guard cells.
In this study, we investigated the effect of PA on blue light responses of stomata from Vicia faba. We found that PA inhibited the phosphatase activity of PP1c in vitro, suppressed blue light-dependent H+ pumping and phosphorylation of H+-ATPase, and did not affect the autophosphorylation of phototropins in guard cells.
RESULTS
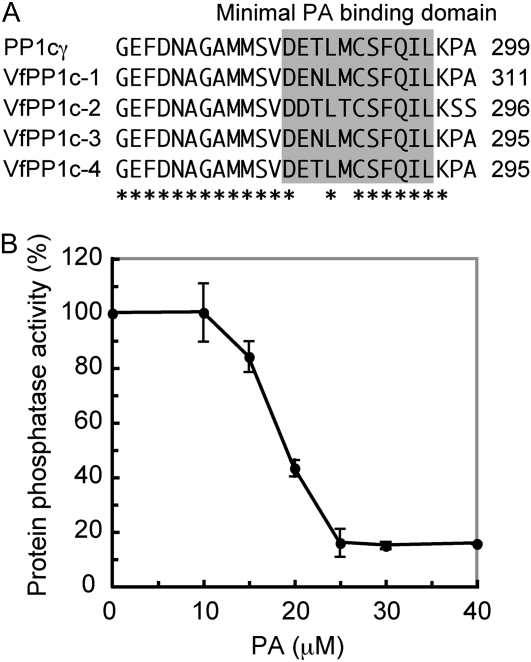
PA Inhibits the Catalytic Activity of V. faba PP1c
Recent study has identified a unique loop-strand fold that acts as a PA-binding domain at the C terminus of human PP1cγ (Jones et al., 2005), and PA inhibits activity via this domain. Alignment of the amino acid sequences of the PA-binding domains of PP1cγ and four V. faba PP1cs (VfPP1c-1 to VfPP1c-4) revealed that the domain is highly conserved in V. faba and human PP1c (Fig. 1A). We thus prepared recombinant VfPP1c-1 protein from Escherichia coli and investigated the effect of PA on the phosphatase activity using 32P-labeled myelin basic protein as a substrate. As shown in Figure 1B, PA inhibited phosphatase activity in a concentration-dependent manner with a half inhibitory concentration of approximately 20 μm. The results indicate that PA acts as an inhibitor of PP1c in plants.
Figure 1.
Inhibition of V. faba PP1c activity by PA. A, Alignment of the amino acid sequences of human PP1cγ and V. faba PP1c (VfPP1c-1 to VfPP1c-4). Asterisks show identical amino acids. Regions corresponding to the minimal PA-binding domain are highlighted in gray. B, Phosphatase activity of VfPP1c-1 was measured using 32P-labeled myelin basic protein as a substrate in the presence or absence of the indicated concentrations of PA. Phosphatase activity is expressed as a percentage of the VfPP1c-1 activity measured without PA. Values shown are means ± se (n = 3). The full activity corresponds to 33,659 ± 2,055 units mg−1 protein (n = 3). One unit represents the release of 1 nmol of phosphate per minute at 30°C.
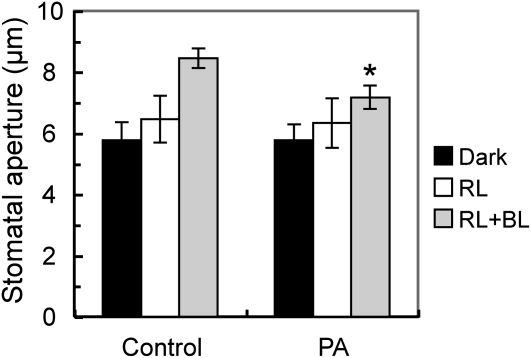
PA Inhibits Blue Light-Specific Stomatal Opening
Light-dependent stomatal opening consists of both photosynthesis-dependent and blue light-specific pathways (Roelfsema and Hedrich, 2005; Shimazaki et al., 2007). We tested the effect of PA on these processes using a dual-beam protocol under a strong red light (150 μmol m–2 s–1) and a weak blue light (10 μmol m–2 s–1) superimposed on the red light. Red light affects photosynthesis-dependent stomatal opening, and blue light affects blue light-specific stomatal opening. When epidermal peels were irradiated with red light alone, stomata opened slightly in both control and PA-treated peels (Fig. 2). Stomatal opening was greater with the superimposed blue light, and this opening was inhibited by PA. This suggests that PA specifically inhibits blue light-dependent stomatal opening.
Figure 2.
Inhibition of blue light-specific stomatal opening by PA. Epidermal peels were preincubated for 2 h in darkness and then illuminated with red light (RL; 150 μmol m–2 s–1) with or without blue light (BL; 10 μmol m–2 s–1) for 2 h. PA (250 μm) was added 1 h before the light illumination. Results are means ± sd (n = 90, pooled from triplicate experiments). Asterisks indicate significant differences between control and PA treatment (P < 0.01).
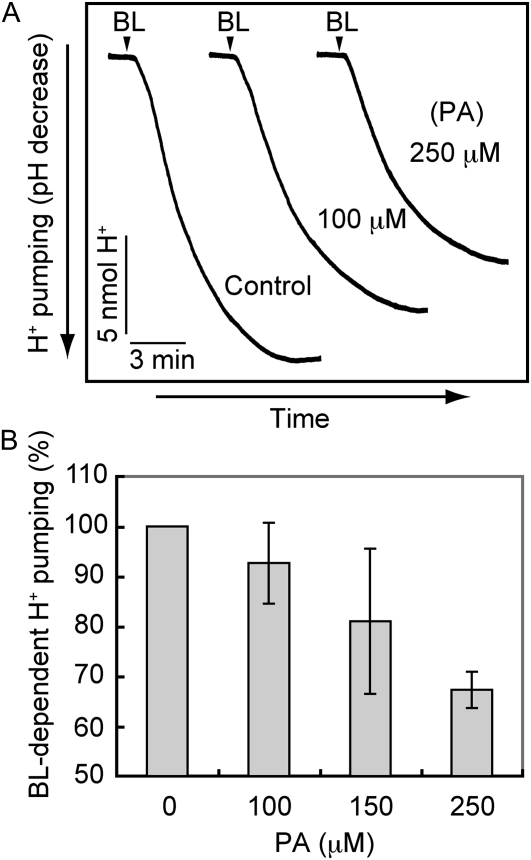
PA Inhibits Blue Light-Dependent H+ Pumping and Phosphorylation of the Plasma Membrane H+-ATPase
Since blue light-dependent stomatal opening is driven by blue light-dependent H+ pumping (Assmann et al., 1985; Shimazaki et al., 1986), we tested the effect of PA on H+ pumping. As shown in Figure 3, H+ pumping in V. faba guard cell protoplasts was inhibited by the application of PA. The degree of inhibition was concentration dependent.
Figure 3.
Inhibition of blue light-dependent H+ pumping from guard cell protoplasts by PA. A, Guard cell protoplasts were preincubated for 30 min with or without (Control) PA under red light (600 μmol m–2 s–1) and then illuminated with a pulse of blue light (BL; 100 μmol m–2 s–1, 30 s) at the times indicated by the arrowheads. B, Concentration-dependent inhibition of blue light-dependent H+ pumping by PA. Values shown are means ± se (n = 3). Experimental conditions were the same as in A.
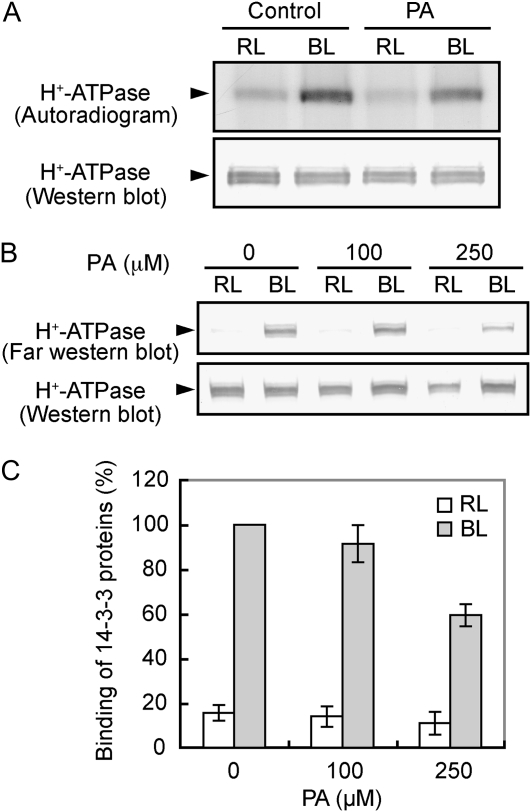
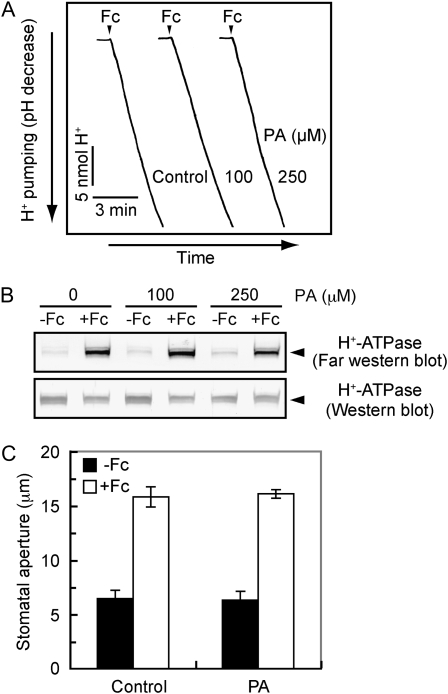
The H+ pump has been identified as the plasma membrane H+-ATPase and is activated by the phosphorylation of the C terminus with subsequent binding of a 14-3-3 protein (Kinoshita and Shimazaki, 1999; Emi et al., 2001). We pretreated 32P-labeled guard cell protoplasts with PA and determined the phosphorylation levels of the H+-ATPase. As shown in Figure 4A, PA inhibited phosphorylation of the H+-ATPase in response to blue light but did not alter the amount of H+-ATPase. We further investigated the effect of PA on the phosphorylation of the H+-ATPase by an indirect method. To test this, we utilized binding of a 14-3-3 protein to the H+-ATPase because the binding depends on phosphorylation levels of the H+-ATPase (Kinoshita and Shimazaki, 1999). PA inhibited the binding in a concentration-dependent manner (Fig. 4, B and C), and the degree of inhibition paralleled that of H+ pumping (Fig. 3). These results indicate that the inhibition of blue light-dependent stomatal opening by PA is due to the impairment of the phosphorylation process of H+-ATPase.
Figure 4.
Inhibition of blue light-dependent phosphorylation of the plasma membrane H+-ATPase and the binding of a 14-3-3 protein to the H+-ATPase by PA. A, Levels of phosphorylation (top) and the amount of the H+-ATPase (bottom) in the presence or absence (Control) of PA. Guard cell protoplasts were preincubated with [32P]orthophosphate for 90 min under red light (RL; 600 μmol m–2 s–1) and then illuminated with a pulse of blue light (BL; 100 μmol m–2 s–1, 30 s). PA at 250 μm was added 30 min before the application of blue light. Reactions were terminated 2.5 min after the blue light. Autoradiography was carried out against H+-ATPase, which was obtained by immunoprecipitation from 100 μg of guard cell proteins. Experiments repeated on three occasions gave similar results. B, Levels of 14-3-3 protein binding to the H+-ATPase in response to blue light. Guard cell protoplasts were treated as described for Figure 3, and the reactions were terminated 2.5 min after the blue light pulse. The binding of a 14-3-3 protein to the H+-ATPase was determined by far western-blot analysis using a recombinant glutathione S-transferase-14-3-3 protein as a probe. C, Quantification of the relative binding of 14-3-3 proteins to the H+-ATPase using ImageJ software. Values shown are means ± se (n = 3).
PA Does Not Affect Fusicoccin-Dependent Stomatal Responses
Since PA inhibited the phosphorylation of H+-ATPase, it is possible that PA inhibits unidentified protein kinases that phosphorylate the H+-ATPase. To test this, we utilized the fungal toxin fusicoccin, which enhances the phosphorylation levels of the H+-ATPase by inhibiting the dephosphorylation process via stabilization of the complex between phosphorylated H+-ATPase and a 14-3-3 protein (Kinoshita and Shimazaki, 2001). If PA inhibits the protein kinases, PA would inhibit phosphorylation of the H+-ATPase, thereby decreasing the 14-3-3 protein binding. As shown in Figure 5B, PA did not inhibit fusicoccin-induced binding of a 14-3-3 protein to the H+-ATPase. Consistent with this result, PA neither affected the fusicoccin-induced H+ pumping in guard cell protoplasts nor stomatal opening in the epidermal peels (Fig. 5, A and C). These results suggest that PA does not affect the protein kinases that are responsible for H+-ATPase phosphorylation.
Figure 5.
Effects of PA on fusicoccin-dependent stomatal opening, H+ pumping, and the binding of a 14-3-3 protein to the H+-ATPase. A, Fusicoccin (Fc)-dependent H+ pumping in the presence or absence (Control) of PA. Guard cell protoplasts were preincubated for 30 min with the indicated concentrations of PA under red light, and then fusicoccin (10 μm) was applied at the times indicated by the arrowheads. B, Levels of 14-3-3 protein binding to the H+-ATPase in response to fusicoccin. The reactions were terminated 5 min after application of fusicoccin (10 μm). Other reaction conditions were the same as in Figure 4B. C, Fusicoccin-dependent stomatal opening in the presence of PA. Epidermal peels were preincubated for 2 h in darkness and further incubated with 10 μm fusicoccin for 2 h. PA (250 μm) was added 1 h before the application of fusicoccin. Results are means ± sd (n = 90, pooled from triplicate experiments).
PA Does Not Affect the Blue Light-Dependent Phosphorylation of Phototropins
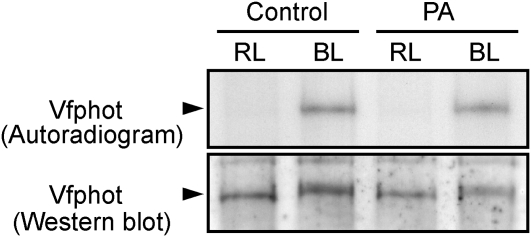
To test the effect of PA on phototropins, we determined the phosphorylation levels of V. faba phototropins (Vfphots) in response to blue light using guard cell protoplasts labeled with 32P. Autoradiograms revealed that PA had no effect on the autophosphorylation of phototropins (Fig. 6). In accordance with this, phototropins underwent a mobility shift with blue light treatment, which is indicative of autophosphorylation, in the presence of PA. These results indicate that PA inhibits the signaling components that transmit the blue light signal from phototropins to H+-ATPase in guard cells and support the idea that PP1 could be a molecular target of PA.
Figure 6.
Effects of PA on the blue light-dependent phosphorylation of phototropins. Levels of phosphorylation (top) and the amounts (bottom) of V. faba phototropin (Vfphot) in response to blue light (BL) in the presence or absence (Control) of PA are shown. Vfphot was obtained by immunoprecipitation from 200 μg of 32P-labeled guard cell protoplasts 1 min after the blue light pulse. Other experimental conditions were the same as in Figure 4A. Experiments repeated on three occasions gave similar results. RL, Red light.
1-Butanol Partially Decreases the Inhibitions of Blue Light-Dependent Stomatal Responses by ABA, Hydrogen Peroxide, and Nitric Oxide
ABA induces the production of hydrogen peroxide (H2O2) and nitric oxide (NO) in guard cells (Pei et al., 2000; Zhang et al., 2001; Garcia-Mata and Lamattina, 2002; Neill et al., 2002). Previously, we have demonstrated that ABA inhibits the blue light-dependent activation of H+-ATPase through the actions of H2O2 and NO (Zhang et al., 2004b, 2007). Recent studies have indicated that both ABA and NO induce PA production through phospholipase D (PLD) in V. faba guard cells (Jacob et al., 1999; Distéfano et al., 2008). We thus suspected that ABA, H2O2, and NO inhibit the activation of H+-ATPase via PA produced by PLD. To test this, we obtained an Arabidopsis (Arabidopsis thaliana) T-DNA insertion mutant of PLDα1, since PLDα1 has been reported to be required for ABA-induced stomatal closure (Sang et al., 2001; Zhang et al., 2004a). We expected a decrease in the ABA-induced inhibition of blue light-dependent H+ pumping in the pldα1 mutant. However, there was no difference in ABA-induced inhibition of H+ pumping between the wild type and the mutant (data not shown).
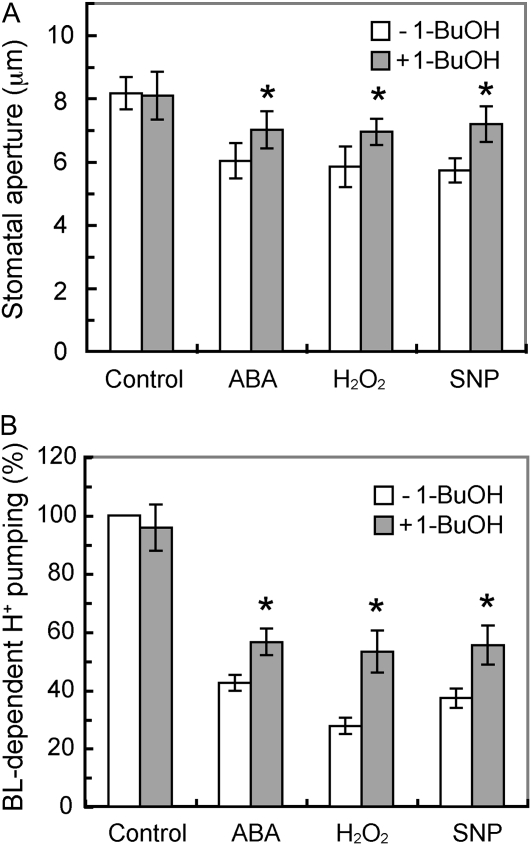
We thus utilized 1-butanol (1-BuOH), which inhibits PA production by PLD in guard cells through the transphosphatidylation of 1-BuOH (Munnik et al., 1995). We first confirmed the inhibitions of blue light-dependent stomatal opening and H+ pumping by ABA, H2O2, and a NO donor, sodium nitroprusside (SNP), in epidermal peels and guard cell protoplasts (Fig. 7). Treatment of these tissues with 1-BuOH partially decreased ABA-, H2O2-, and SNP-induced inhibitions of blue light-dependent stomatal opening and H+ pumping. Addition of 1-BuOH alone had no effect on blue light responses. These results suggest that inhibitions of the activation of H+-ATPase by ABA, H2O2, and NO are at least partially mediated via PA produced by PLD.
Figure 7.
Restoration of ABA-, H2O2-, or NO-induced inhibition of blue light-dependent stomatal opening and H+ pumping by 1-BuOH. A, Epidermal peels were preincubated for 2 h in darkness and then illuminated with red light (RL; 150 μmol m–2 s–1) with or without blue light (BL; 10 μmol m–2 s–1) for 2 h. ABA (10 μm), H2O2 (1 mm), or SNP (100 μm) was added 30 min before and 1-BuOH (0.2%, v/v) was added 1 h before the light illumination. Results are means ± sd (n = 90, pooled from triplicate experiments). B, Guard cell protoplasts were preincubated for 40 min under red light (600 μmol m–2 s–1) and then illuminated with a pulse of blue light (100 μmol m–2 s–1, 30 s). ABA (0.1 μm), H2O2 (1 mm), or SNP (100 μm) was added 30 min before and 1-BuOH (0.2%, v/v) was added 40 min before the blue light pulse. Values shown are means ± se (n = 3). Asterisks indicate significant differences between treatments with and without 1-BuOH (P < 0.01).
In addition to the PLD-dependent pathway, PA can also be produced via the sequential action of phospholipase C and diacylglycerol kinase. Inhibitions of the blue light responses by ABA, H2O2, and NO might be due to PA production through this pathway. We tried to utilize U73122, an inhibitor of phospholipase C, since U73122 inhibits NO-induced PA production (Distéfano et al., 2008). Unfortunately, application of U73122 to guard cell protoplasts inhibited both blue light- and fusicoccin-dependent H+ pumping (data not shown); therefore, we did not use the reagent for our purposes.
DISCUSSION
In this study, we demonstrate that ABA inhibits blue light-dependent stomatal opening at PP1 in the guard cell signaling pathway and that ABA causes this inhibition via PA production. PA inhibited the phosphatase activity of V. faba PP1c in vitro (Fig. 1B). We also show that PA inhibited stomatal opening, H+ pumping, and the phosphorylation of the plasma membrane H+-ATPase but did not affect the autophosphorylation of phototropins (Figs. 2–4 and 6). These inhibitory effects of PA were essentially the same as for tautomycin, an inhibitor of PP1c, with respect to blue light responses in stomata (Takemiya et al., 2006). These results suggest that PA affects PP1c, thereby suppressing the signaling between phototropins and the H+-ATPase. However, some components other than PP1c in blue light signaling pathways could be a target of PA, and it is possible that PA affects the protein kinase that phosphorylates the H+-ATPase. This is not the case, however, because PA did not affect the fusicoccin-induced phosphorylation of H+-ATPase (Fig. 5). From these results, we conclude that PP1c is a target of PA and is a cross talk point between blue light and ABA signaling in guard cells. This is the first evidence, to our knowledge, that identifies a protein molecule involved in cross talk between blue light and ABA signaling in guard cells.
We previously demonstrated that blue light activates the H+-ATPase via phosphorylation (Kinoshita and Shimazaki, 1999). We initially suspected that phototropins directly phosphorylate the H+-ATPase, which was inconsistent with PP1 intervening between phototropins and the H+-ATPase (Takemiya et al., 2006). In this study, we have provided evidence that blue light and ABA signaling converge at PP1. This situation would allow stomatal guard cells to integrate closure and opening signals in order to adjust stomatal aperture. For example, drought conditions would be able to influence light-induced stomatal opening through the regulation of PP1 activity via ABA and efficiently stimulate stomatal closure in the daytime. Therefore, PP1 may be an entry point to blue light signaling from the ABA signaling pathways and thereby play a role in finely regulating stomatal aperture. By contrast, we note here that blue light is reported to inhibit the plasma membrane S-type anion channels that drive stomatal closure and accelerate stomatal opening (Marten et al., 2007). It will be intriguing to elucidate how blue light affects these anion channels.
In plants as well as in animal cells, the function of PP1c is determined by the multiple regulatory subunits that specify the catalytic activity, substrate specificity, and subcellular localization of PP1c (Bollen, 2001; Cohen, 2002; Takemiya et al., 2009). Interestingly, it has been suggested that the binding sites of PA and regulatory subunits to PP1c share common sequences (Jones et al., 2005). Although the specific regulatory subunit responsible for stomatal opening has yet to be identified, it is conceivable that PA competes with the regulatory subunit and binds to PP1c, resulting in the decreases in phosphatase activity. This binding could result in suppression of the blue light response of stomata.
The half inhibitory concentration value of PA for V. faba PP1c phosphatase activity shown here is much higher than those reported for mammalian PP1c (Kishikawa et al., 1999; Jones and Hannun, 2002). The reason for this difference is not clear at present. It is probably due to isoform specificity in the reactivity to PA, as reported by Jones and Hannun (2002). As shown in Figure 1A, although the minimal PA binding domain of V. faba PP1cs resembles that of human PP1cγ, a few amino acids within this domain are different. For example, Thr-288 of human PP1cγ is substituted by Asn in VfPP1c-1. Such a difference of local amino acid sequence in the domain may affect the reactivity of PP1c with PA. Furthermore, there may be a difference between the recombinant PP1c protein prepared using E. coli and the endogenous protein. The activity of recombinant PP1c requires divalent cations such as Mn2+ or Co2+ (Chu et al., 1996), and the mammalian PP1c used in previous experiments required Mn2+ (Kishikawa et al., 1999; Jones and Hannun, 2002). In this study, we prepared PP1c with a requirement for Co2+, because the Co2+-dependent PP1c resembled the native PP1c more than the Mn2+-dependent form (Zhang et al., 1998; Watanabe et al., 2003). Further studies will be needed to clarify this point.
Recent genetic and pharmacological studies have demonstrated that ABA induces NO synthesis through H2O2 (Lü et al., 2005; Bright et al., 2006) and that NO in turn causes PA production in guard cells (Distéfano et al., 2008). Thus, PA is likely a molecule that is directly responsible for the inhibition of stomatal blue light response by ABA. In accordance with this, PA inhibited stomatal responses to blue light, and the inhibitory effect mimicked those of ABA, H2O2, and NO (Zhang et al., 2004b, 2007). It is unclear, however, how PA is produced in guard cells in response to ABA. PA is produced through the activity of PLD in guard cells (Jacob et al., 1999; Distéfano et al., 2008). Consistent with this, we showed that 1-BuOH, an inhibitor of PLD, diminished the ABA-, H2O2-, and NO-induced inhibitions of stomatal responses (Fig. 7). This suggests that PA is produced at least partly through PLD activity in guard cells.
Although PA inhibits blue light-dependent H+ pumping, the maximum inhibition by PA was only half that caused by ABA (Fig. 3; Goh et al., 1996). Such a low degree of inhibition might be attributed to the low solubility of PA in the reaction buffer and/or the low permeability of PA across the plasma membrane. We cannot exclude the existence of parallel inhibitory pathways other than the PLD-dependent one that participates in the inhibition, as suggested previously (Jacob et al., 1999).
It has been demonstrated that PA inhibits the inward-rectifying K+ channels in V. faba guard cell protoplasts (Jacob et al., 1999). Our results here show that PA inhibited H+ pumping (Fig. 3), which would favor membrane depolarization and inhibit the inward K+ channels. These results suggest that PA may cause stomatal closure through simultaneous inhibition of the H+-ATPase and the inward K+ channels. Consistent with this, the light-dependent stomatal opening was more sensitive to PA than the H+ pumping (Fig. 3; Jacob et al., 1999).
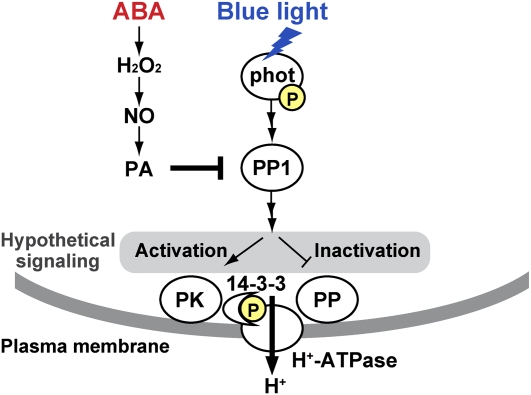
We summarize our results and illustrate a model in Figure 8. The activation of the H+-ATPase requires an increase in phosphorylation level of Thr residues in the H+-ATPase. Activation of the protein kinases or inactivation of the phosphatases, or both, can achieve this situation. The inhibition of PP1 by PA would suppress the kinase activation or the phosphatase inactivation, thereby inhibiting the H+-ATPase activation. We also mention another possibility here, although this case is not included in the model: PKS5/CIPK11, a Ser/Thr protein kinase, phosphorylates Ser-931 of the H+-ATPase (AHA2) and prevents the binding of a 14-3-3 protein to the H+-ATPase (Fuglsang et al., 2007). Thus, the inhibition of PP1 may increase the PKS5/CIPK11 activity and inactivate the H+-ATPase.
Figure 8.
A model of ABA-induced inhibition of blue light-dependent activation of the plasma membrane H+-ATPase in guard cells. Blue light activates phototropins (phot) by autophosphorylation, and the activated phototropins increase the PP1 activity. The PP1 finally results in the activation of the H+-ATPase by phosphorylation with a subsequent binding of a 14-3-3 protein. The increase in phosphorylation levels of the H+-ATPase is brought about by activation of the protein kinases (PK) or inactivation of the protein phosphatases (PP) or both. Neither PK nor PP had been identified. ABA causes the production of PA via H2O2 and NO, and the resulting PA inhibits PP1, thereby suppressing the activation of the H+-ATPase. Arrows and T-bars represent signaling direction and inhibition, respectively. P in the yellow discs indicates phosphorylation.
In conclusion, this study has revealed a novel mechanism by which ABA inhibits blue light signaling. Further genetic studies will provide direct evidence for the physiological function of PA in the regulation of PP1 in guard cells.
MATERIALS AND METHODS
Plant Materials and Isolation of Guard Cell Protoplasts
Vicia faba ‘Ryosai Issun’ was cultured hydroponically in a greenhouse as described previously (Shimazaki et al., 1992). Guard cell protoplasts were obtained enzymatically from the lower epidermis of 5- to 6-week-old leaves according to previous methods with slight modifications (Shimazaki et al., 1992; Kinoshita and Shimazaki, 1999). The first digestion medium (pH 5.4) contained 1% (w/v) cellulase R-10, 0.1% (w/v) polyvinylpyrrolidone K-30, 0.25 m mannitol, 1 mm CaCl2, and 10 mm MES. The second digestion medium (pH 5.4) contained 0.5% (w/v) cellulase RS, 0.125% (w/v) macerozyme R-10, 0.4 m mannitol, 1 mm CaCl2, 5 mm ascorbic acid, 0.002% (w/v) chloramphenicol, and 10 mm MES. Isolated protoplasts were stored in 0.4 m mannitol and 1 mm CaCl2 on ice in the dark until use. Protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Preparation of Recombinant PP1c and Determination of PP1c Activity
Full-length VfPP1c-1 was subcloned into pGEX-2T (GE Healthcare), and the recombinant protein was prepared as described previously (Takemiya et al., 2006). Ser/Thr phosphatase activity was measured using 32P-labeled myelin basic protein as a substrate according to previous methods (Takemiya et al., 2009). Briefly, recombinant VfPP1c-1 (0.25 ng) and various concentrations of PA were preincubated for 30 min on ice, followed by incubation for 5 min at 30°C, and the reaction was started by adding 32P-labeled myelin basic protein. The reaction was terminated by adding cold trichloroacetic acid after 10 min, and the amount of [32P]phosphate released was measured in the supernatant obtained by centrifugation. All assays were performed within the linear ranges of activities (less than 20% substrate dephosphorylation).
Measurement of Stomatal Aperture
Epidermal strips prepared from the abaxial side of leaves were preincubated in a petri dish containing 10 mm MES-NaOH (pH 6.1), 5 mm KCl, and 0.1 mm CaCl2 for 1 h in the dark and then transferred to the same buffer supplemented with PA and further incubated for 1 h. Afterward, epidermal strips were illuminated with red light (150 μmol m–2 s–1) with or without blue light (10 μmol m–2 s–1) for 2 h. To determine the effect of 1-BuOH on ABA-, H2O2-, or NO-induced inhibition of blue light-dependent stomatal opening, 1-BuOH was added to epidermal strips 1 h before, and ABA, H2O2, or SNP was added 30 min before, light irradiation, respectively. To determine fusicoccin-dependent stomatal opening, 10 μm fusicoccin was added to the epidermal peels instead of light irradiation. After the incubation, stomatal apertures were measured with a bright-field microscope (Eclipse TS100; Nikon).
Measurements of Blue Light- or Fusicoccin-Dependent H+ Pumping
Blue light- or fusicoccin-dependent H+ pumping from guard cell protoplasts was measured as described previously (Shimazaki et al., 1992). To examine the effect of PA on blue light- or fusicoccin-dependent H+ pumping, guard cell protoplasts were preincubated with PA for 30 min under the background red light (600 μmol m–2 s–1), and a pulse of blue light (100 μmol m–2 s–1, 30 s) or fusicoccin at 10 μm was applied. To determine the effect of 1-BuOH on ABA-, H2O2-, or NO-induced inhibition of blue light-dependent H+ pumping, 1-BuOH was added to guard cell protoplasts 40 min before, and ABA, H2O2, or SNP was added 30 min before, the blue light pulse.
Far Western-Blot Analysis and Immunodetection
The binding of 14-3-3 proteins to H+-ATPase was determined by far western-blot analysis using glutathione S-transferase-14-3-3 according to methods described previously (Kinoshita et al., 2003). The plasma membrane H+-ATPase and Vfphots were detected immunologically as described previously (Zhang et al., 2004b; Takemiya et al., 2006).
Determination of Phosphorylation Levels of Vfphots and Plasma Membrane H+-ATPase
Phosphorylation levels of Vfphots and the plasma membrane H+-ATPase were determined using 32P-labeled guard cell protoplasts as described previously (Kinoshita and Shimazaki, 1999; Kinoshita et al., 2003). Briefly, guard cell protoplasts were preincubated with [32P]orthophosphate for 90 min under a background red light (600 μmol m–2 s–1), and a pulse of blue light (100 μmol m–2 s–1, 30 s) was applied. PA was added 30 min before the blue light pulse. Vfphots and the H+-ATPase were obtained by immunoprecipitation with individual antibodies and separated by SDS-PAGE. Autoradiography was carried out by exposing the dried gel to x-ray films (Fuji Film) for 2 d at room temperature.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BAA92244 (VfPP1c-1), BAF31130 (VfPP1c-2), BAF31131 (VfPP1c-3), and BAF31132 (VfPP1c-4).
Acknowledgments
We are grateful to Dr. H. Wada (University of Tokyo) and Dr. M. Shibata (Nagaoka National College of Technology) for providing technical information for the preparation of PA suspension.
References
- Assmann SM, Shimazaki K. (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI. (1985) Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318: 285–287 [Google Scholar]
- Bollen M. (2001) Combinational control of protein phosphatase-1. Trends Biochem Sci 26: 426–431 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir I, Neill SJ. (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Christie JM. (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Chu Y, Lee EYC, Schlender KK. (1996) Activation of protein phosphatase 1: formation of a metalloenzyme. J Biol Chem 271: 2574–2577 [DOI] [PubMed] [Google Scholar]
- Cohen PTW. (2002) Protein phosphatase 1: targeted in many directions. J Cell Sci 115: 241–256 [DOI] [PubMed] [Google Scholar]
- Distéfano AM, García-Mata C, Lamattina L, Laxalt AM. (2008) Nitric oxide-induced phosphatidic acid accumulation: a role for phospholipases C and D in stomatal closure. Plant Cell Environ 31: 187–194 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinishita T, Shimazaki K. (2001) Specific binding of a vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125: 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, et al. (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128: 790–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CH, Kinoshita T, Oku T, Shimazaki K. (1996) Inhibition of blue light-dependent H+ pumping by abscisic acid in Vicia guard-cell protoplasts. Plant Physiol 111: 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM. (2001) Guard cell signaling. Cell 107: 711–714 [DOI] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S. (1999) Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA 96: 12192–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JA, Hannun YA. (2002) Tight binding inhibition of protein phosphatase-1 by phosphatidic acid: specificity of inhibition by the phospholipid. J Biol Chem 277: 15530–15538 [DOI] [PubMed] [Google Scholar]
- Jones JA, Rawles R, Hannun YA. (2005) Identification of a novel phosphatidic acid binding domain in protein phosphatase-1. Biochemistry 44: 13235–13245 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Emi T, Tominaga M, Sakamoto K, Shigenaga A, Doi M, Shimazaki K. (2003) Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol 133: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. (2001) Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol 42: 424–432 [DOI] [PubMed] [Google Scholar]
- Kishikawa K, Chalfant CE, Perry DK, Bielawska A, Hannun YA. (1999) Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J Biol Chem 274: 21335–21341 [DOI] [PubMed] [Google Scholar]
- Lü D, Zhang X, Jiang J, An GY, Zhang LR, Song CP. (2005) NO may function in the downstream of H2O2 in ABA-induced stomatal closure in Vicia faba L. J Plant Physiol Mol Biol 31: 62–70 [PubMed] [Google Scholar]
- Marten H, Hedrich R, Roelfsema MRG. (2007) Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J 50: 29–39 [DOI] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, et al. (2007) Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J. (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30: 601–609 [DOI] [PubMed] [Google Scholar]
- Mishra G, Zhang W, Deng F, Zhao J, Wang X. (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science 312: 264–266 [DOI] [PubMed] [Google Scholar]
- Munnik T, Arisz SA, De Vrije T, Musgrave A. (1995) G-protein activation stimulates phospholipase D signaling in plants. Plant Cell 7: 2197–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT. (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cell. Plant Physiol 128: 13–16 [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JL. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. (2005) In the light of stomatal opening: new insights into ‘the Watergate’. New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Staal M, Prins HBA. (1998) Blue light-induced apoplastic acidification of Arabidopsis thaliana guard cells: inhibition by ABA is mediated through protein phosphatases. Physiol Plant 103: 466–474 [Google Scholar]
- Sang Y, Zheng S, Li W, Huang B, Wang X. (2001) Regulation of plant water loss by manipulating the expression of phospholipase Dα. Plant J 28: 135–144 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E. (1987) Voltage dependence of K+ channels in guard cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E. (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319: 324–326 [Google Scholar]
- Shimazaki K, Kinoshita T, Nishimura M. (1992) Involvement of calmodulin and calmodulin-dependent myosin light chain kinase in blue light-dependent H+ pumping by guard cell protoplasts from Vicia faba L. Plant Physiol 99: 1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Ariyoshi C, Shimazaki K. (2009) Identification and functional characterization of inhibitor-3, a regulatory subunit of protein phosphatase 1 in plants. Plant Physiol 150: 144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Kinoshita T, Asanuma M, Shimazaki K. (2006) Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba. Proc Natl Acad Sci USA 103: 13549–13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS. (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Watanabe T, da Cruz e Silva EF, Huang HB, Starkova N, Kwon YG, Horiuchi A, Greengard P, Nairn AC. (2003) Preparation and characterization of recombinant protein phosphatase 1. Methods Enzymol 366: 321–338 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Zhao S, Lee EYC. (1998) Identification and characterization of human HCG V gene product as a novel inhibitor of protein phosphatase-1. Biochemistry 37: 16728–16734 [DOI] [PubMed] [Google Scholar]
- Zhang W, Qin C, Zhao J, Wang X. (2004a) Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101: 9508–9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Takemiya A, Kinoshita T, Shimazaki K. (2007) Nitric oxide inhibits blue light-specific stomatal opening via abscisic acid signaling pathways in Vicia guard cells. Plant Cell Physiol 48: 715–723 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang H, Takemiya A, Song CP, Kinoshita T, Shimazaki K. (2004b) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136: 4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang L, Dong FC, Gao JF, Galbraith DW, Song CP. (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126: 1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]