CELL GROWTH REQUIRES CELL WALL EXTENSION
Plant cell walls are established by different polysaccharides and structural proteins. The exact composition of these complex structures is dependent on the type of cell and the developmental stage and therefore is in a constant flow of remodeling. Cell shape is defined by the balance between turgor pressure from the symplast and the strength of the cell wall. Yet, for cell growth to take place, the controlled loosening of the cell wall is a prerequisite (Cosgrove, 2005). Thus, control mechanisms that closely survey the different steps of cell wall remodeling are necessary, implying that signals from the apoplast to the cell wall and vice versa ascertain the exchange of information. The goal of this article is to give an overview on the understanding of the signaling mechanisms that take place between cell walls and the cytoplasm, with a focus on the recent advances in the field. Plant cell walls are also defense structures against different abiological and biological stresses such as pathogens. The mechanisms of recognition of pathogens and the modification of cell walls upon pathogen encounter are reviewed elsewhere (Hematy et al., 2009).
A number of phenomena demonstrate that a sensing and signaling system must exist in the extracellular matrix that monitors the structure and integrity of cell walls. For example, cellulose synthesis takes place in rosette-forming protein complexes made of cellulose synthases (CesAs). Mutations in Arabidopsis (Arabidopsis thaliana) CesA genes lead to a reduction in cellulose content and induce compensatory mechanisms, including modifications in lignin deposition, pectins, xyloglucan, and AGPs. Thus, a cell wall-sensing process must recognize the cellulose deficiency and induce appropriate responses. These signaling events are clearly not linear but induce many different reactions, including stress-related processes that depend on intact hormone signaling pathways (Ellis et al., 2002; Cano-Delgado et al., 2003; Bosca et al., 2006; Hernandez-Blanco et al., 2007).
TRANSMEMBRANE RECEPTORS
Excellent candidates for a function in sensing the structure of cell walls and transducing this information to the cytoplasm are transmembrane proteins. Plant genomes code for a large number of receptor-like kinases (RLKs; Arabidopsis, more than 600; rice (Oryza sativa), more than 1,100; Shiu and Bleecker, 2001), which can relay a signal to the cytoplasm via the cytoplasmic kinase domain (Fig. 1). Some of these proteins should be able to sense changes in the cell wall structure by, for example, a missing interaction partner. Indeed, a role in cell wall-related signaling has been demonstrated for a number of RLKs.
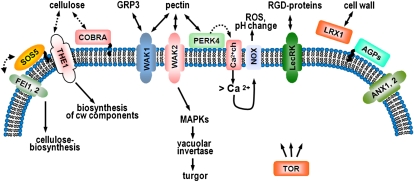
Figure 1.
Functions of proteins implicated in cell wall-related signaling and development. Receptor-like proteins and extracellular proteins are shown that have a function in cell wall sensing and/or signaling. From left to right: FEI1 and FEI2 are LRR-containing RLKs that influence cellulose content and act in a linear pathway with the GPI-anchored SOS5. A direct interaction, however, has so far not been shown. THE1 senses cellulose deficiencies and, under these conditions, induces changes in cell wall structures. COBRA is a GPI-anchored membrane-associated protein important for the orientation of cellulose microfibrils. WAK1 binds GRP3, a Gly-rich protein, and both WAK1 and WAK2 are able to interact with pectin. WAK2 activates MAPK3 and thus possibly other MAPKs and induces a vacuolar invertase to modify turgor pressure. The membrane-associated PERK4 interacts with pectin and activates Ca2+ channels, leading to increased cytosolic Ca2+ content, which can induce changes in intracellular and extracellular pH and the NADPH oxidase (NOX)-dependent production of ROS. The Arg-Gly-Asp (RGD) tripeptide is a cell-adhesion motif and is bound by at least one LecRK. AGPs are GPI-anchored membrane-associated proteins implicated in cell wall-related signaling. LRX1 is an extracellular receptor binding an unknown interactor and is insolubilized in the cell wall. ANX1 and ANX2 are important for pollen tube rupture involving sudden changes in the cell wall. The TOR kinase is central to the TOR pathway that also influences cell wall structure, by a so far unknown mechanism. Solid, double-arrowed lines indicate experimentally shown interactions or signaling outputs; dashed arrows indicate possible, but not experimentally shown, interactions. Wavy lines represent GPI anchors. [See online article for color version of this figure.]
Wall-Associated Kinases
Wall-associated kinases (WAKs) of Arabidopsis are a family of five transmembrane proteins with a cytoplasmic Ser/Thr kinase domain and an extracellular domain with motifs similar to the vertebrate epidermal growth factor repeats. These proteins are involved in cell expansion but are also induced upon pathogen attack or as a stress response (Wagner and Kohorn, 2001; Sivaguru et al., 2003). The reduction of WAK expression has been shown to lead to a reduction in cell growth (Lally et al., 2001; Wagner and Kohorn, 2001; Kohorn et al., 2006b). Most interestingly with respect to cell wall sensing is the observation that WAK proteins interact strongly, in some cases covalently, with pectin (He et al., 1996; Wagner and Kohorn, 2001). For WAK1 and WAK2, pectin binding could be demonstrated in vivo and in vitro (Decreux and Messiaen, 2005; Kohorn et al., 2009). In addition, in a yeast two-hybrid experiment, WAK1 was shown to interact with the Gly-rich protein GRP3, a structural protein in the cell wall (Park et al., 2001). These results strongly indicate a direct interaction of WAK1 and WAK2 with the cell wall and make them likely candidates for a cell wall-sensing function (Fig. 1). A recent study revealed that WAK1-GFP accumulates in a pectin-containing compartment in the cytoplasm. From there, WAK1-GFP migrates to the plasma membrane, but much slower than RLKs that do not associate with cell walls, such as the brassinosteroid receptor BRI1 (Wang et al., 2001). The migration process is dependent on active cellulose biosynthesis and negatively influenced by Fuc, which is a component of pectin (Kohorn et al., 2006a). The regulation of protein localization by cell wall components adds another level of complexity to the control of RLK signaling activity.
A further insight into the possible function of WAK proteins was provided by a detailed analysis of Arabidopsis WAK2. A wak2 knockout mutant grew normal under standard conditions. However, on medium with low salt and no Suc, it showed a reduced cell elongation rate. The importance of Suc for the wak2 phenotype could be compensated for by expressing a maize (Zea mays) Suc phosphate synthase (Kohorn et al., 2006b), indicating that the wak2 phenotype is linked to Suc-related physiology. Measurements of Suc-related enzymes revealed that vacuolar invertase activity, which converts Suc to Glc and Fru, is reduced in wak2. Most likely, the low level of these sugars in the vacuole reduces turgor pressure in the cell that affects cell elongation (Martin et al., 2001). Next, the authors aimed at making WAK2 expression inducible to follow its effect on cell turgor. The WAK2 extracellular domain was replaced by the one of the brassinosteroid (BR) receptor BRI1 (Wang et al., 2001), thus making WAK2 signaling BR dependent. In protoplast experiments, BR-mediated WAK2 activity induced vacuolar invertase, which resulted in an increased turgor pressure (Kohorn et al., 2006b). Together, these analyses suggest that WAK2 interacts with pectin at the cell surface, upon which the protein induces a signal transduction cascade (see below) that leads to a modification of the turgor pressure in the cell.
Lectin Receptor Kinases
Lectin receptor kinases (LecRKs) contain an extracellular lectin domain and thus represent a second class of RLKs with potential carbohydrate-binding properties. Lectins have been identified as proteins that bind carbohydrates, suggesting that, in the context of LecRKs, the lectin domain establishes a direct link to cell wall polysaccharides (André et al., 2005; Bouwmeester and Govers, 2009). However, at least some LecRKs undergo protein-protein interactions. Upon plasmolysis, during which the plasma membrane detaches from the cell wall, connections remain between these two structures. The addition of peptides containing the RGD (Arg-Gly-Asp) tripeptide motif interferes with the integrity of these linkages (Canut et al., 1998). This is of particular interest since the RGD motif is also found in mammalian extracellular matrix proteins involved in cell adhesion. The search for Arabidopsis proteins that are able to interact with the RGD tripeptide via phage display technology revealed several LecRKs as likely candidates (Gouget et al., 2006). Thus, LecRKs potentially represent a group of receptors that have diverse binding specificities and can have roles in sensing of the cell wall structure/plasma membrane-cell wall connection (Fig. 1).
Leu-Rich Repeat-Containing Receptor Kinases
For the type of RLKs discussed so far, there is evidence that at least some of their members do interact with polysaccharidic components in cell walls. There are, however, a number of RLKs that rather interact with proteins, at least based on the structure of their extracellular domains. A motif particularly well known for undergoing protein-protein interaction is represented by Leu-rich repeats (LRRs), which have been identified in many different organisms such as plants and metazoans, where they are involved in signal transduction activities. In plants, a number of proteins with an LRR domain have been shown to play a role in pathogen recognition or during developmental processes (Kobe and Deisenhofer, 1994; Jones and Jones, 1997). FEI1 and FEI2 are two out of over 200 RLKs in Arabidopsis with an LRR domain (Morillo and Tax, 2006). They are highly homologous, and fei1 fei2 double mutants develop a swollen-root phenotype due to isotropic (instead of longitudinal) cell growth (Xu et al., 2008). This phenotype and the ectopic deposition of lignin are similar to the cellulose synthase (CesA6) mutant procuste1 (prc1; Fagard et al., 2000). Indeed fei1 fei2 double mutants contain reduced levels of crystalline cellulose. Further analyses revealed that FEI1 and FEI2 function in the regulation of cellulose biosynthesis. Interestingly, the kinase activity of the FEI1 and FEI2 kinase domain is not essential for protein function. Corresponding mutations in the kinase domain still allowed for complementation of the fei1 fei2 mutant phenotype, even though the efficiency of complementation was reduced (Xu et al., 2008). Hence, the mode of action of these RLKs appears to be different from the expected and remains to be determined. Genetic evidence suggests that FEI1 and FEI2 function in the same pathway as SALT OVERLY SENSITIVE5 (SOS5), which was identified in a screen for salt-hypersensitive mutants (Fig. 1). The sos5 mutant showed root growth arrest, root swelling, and an increased width in etiolated hypocotyls under high-salt conditions. These sos5 phenotypes are similar to those observed in fei1 fei2 seedlings, but no additive effects were observed in the fei1 fei2 sos5 triple mutant, suggesting that the proteins function in the same pathway. SOS5 codes for a glycosylphosphatidylinositol (GPI)-anchored, and thus extracellular, protein (Shi et al., 2003). Future experiments will have to reveal whether SOS5 does directly interact with FEI1 and/or FEI2 or is involved in perception of the ligand without a direct interaction with the receptor proteins.
Catharanthus roseus Protein Kinase1-Like Receptor Kinases
THESEUS1 (THE1) is a member of the subfamily of Catharanthus roseus Protein Kinase1-Like receptor kinases, for which a function in cell wall integrity sensing has been demonstrated (Hematy et al., 2007). The the1 mutant was identified as a suppressor of prc1-1, a mutant affected in CesA6 (Fagard et al., 2000). The prc1-1 mutant is characterized by reduced levels of cellulose and develops short hypocotyls when grown in the dark. While the short-hypocotyl phenotype is suppressed by the1, the cellulose deficiency is not, indicating that THE1 is involved in sensing structural defects in the cell wall. Cellulose-deficient mutants tend to accumulate ectopic lignin, and this effect is dependent on THE1, as prc1 the1 mutants failed to accumulate lignin ectopically. Overexpression of THE1 led to an overaccumulation of ectopic lignin, but only in the prc1 mutant background. In addition, the1 was able to partially suppress other cesa mutants, but as a single mutant it did not develop a mutant phenotype. This again indicates that THE1 is involved in sensing cellulose deficiency and adapts cell wall development upon changes or irregularities in the cell wall structure (Fig. 1). THE1 exhibits in vitro phosphorylation activity and was localized to the plasma membrane, further supporting the hypothesis that THE1 indeed acts as an active RLK. It is not clear at present whether THE1 directly interacts with the cell wall or rather acts as a signaling intermediate.
Recently, two RLKs related to THE1, FERONIA (FER) and HERKULES1 (HERK1), were characterized. These three homologous genes are influenced by BRs, as they were down-regulated in the BR receptor mutant bri1 (Tang et al., 2008) and up-regulated in the constitutive BR-response mutant bes1-D (Yin et al., 2002). FER was previously shown to mediate male-female interaction during fertilization by enabling pollen tube reception at the synergid cell (Escobar-Restrepo et al., 2007), and RNA interference plants show reduced cell elongation (Guo et al., 2009). HERK1 and THE1 revealed genetic interaction, as a herk1 the1 double mutant develops a dwarf phenotype. Together with microarray data, Guo and coworkers (2009) showed that THE1, HERK1, and FER are likely to function in a pathway to regulate cell elongation that is influenced by, but still largely independent of, BR-induced signaling. ANX1 and ANX2 represent the two closest homologs of FER in Arabidopsis. These two RLKs are expressed in pollen and localize to the tip of the growing pollen tube, where they are thought to prevent rupture of the tube prior to arrival at the female gametophyte. The anx1 anx2 double mutant was characterized by premature pollen tube rupture (Boisson-Dernier et al., 2009; Miyazaki et al., 2009). The interaction partner(s) of ANX1 and ANX2 and the mechanism of pollen tube rupture remain to be shown, but it is safe to assume that this also involves sudden changes in the cell wall of the tip region and, hence, that ANX1 and ANX2 are involved in cell wall-related signaling (Fig. 1).
PROTEINS AT THE MEMBRANE
Transmembrane receptor proteins are obvious candidates for transducing signals from the extracellular matrix to the cytoplasm. In addition, proteins can be attached to the outer surface of the membrane via the membrane anchor GPI that is posttranslationally attached to proteins (Borner et al., 2003) and integrated into the membrane. Several GPI-containing proteins have been identified that play a role in the transfer of cell wall-related information across the membrane. The Arabidopsis peanut (pnt) mutants show a defect in the biosynthesis of GPI and develop a severe phenotype. The pnt1 mutant displays a strongly retarded morphology, swollen shoots and roots, and can only be maintained as calli. Compared with the wild type, pnt1 cell walls show a strongly reduced cellulose content and ectopic accumulation of pectin, xyloglucan, and callose (Gillmor et al., 2005). Hence, GPI-containing proteins play an important role during cell wall development. Indeed, several of these proteins have been identified as being important for the establishment and maintenance of the cell wall.
COBRA is a GPI-anchored protein that was identified based on aberrant cell growth in roots and reduced cellulose content in a corresponding mutant (Schindelman et al., 2001). A detailed analysis revealed that cellulose is not properly deposited in the cell wall due to the lack of this membrane-anchored protein (Fig. 1). Cellulose is a major determinant of the direction of cell growth, as the microfibrils align transversely to the axis of cell elongation (Taiz, 1984). The orientation of cellulose microfibrils is critical, as they are thought to be the load-bearing structure of the cell wall, in combination with hemicelluloses, and resist the turgor-driven cell enlargement (Carpita and Gibeaut, 1993). Thus, the disoriented cellulose deposition in cobra mutants may explain the misshaped cells in elongating root tissue. The orientation of the microtubule cytoskeleton and the movement of the rosette complexes synthesizing cellulose in the membrane have been shown to be strikingly similar, and it was assumed that cortical microtubules define the movement of the rosettes (Giddings and Staehelin, 1988). Interfering with the microtubule cytoskeleton by mutations or application of cytotoxic drugs also led to aberrant cellulose deposition (Burk and Ye, 2002; Baskin et al., 2004). Together, these data suggest that COBRA relays the positional information on the microtubule cytoskeleton to the cellulose synthase complexes and therefore is involved in establishing a continuum between the cytoskeleton and the cell wall (Roudier et al., 2005).
Pro-Rich Extensin-Like Receptor Kinases
A direct interaction with cell wall polysaccharides appears also to be established by the membrane-associated Pro-rich extensin-like receptor kinases (PERKs). Pro-rich proteins and extensins (Hyp-rich glycoproteins) are structural cell wall proteins known to insolubilize in the cell wall (Cassab, 1998; Held et al., 2004), and the extracellular domains of PERKs resemble these proteins (Nakhamchik et al., 2004). The best-studied PERK4 of Arabidopsis is effectively extracted by pectinase treatment, indicating a possible interaction with pectin (Bai et al., 2009). On the functional level, the Arabidopsis perk4 mutation induces a long-root phenotype caused by an increased cell length, indicating that PERK4 negatively influences cell elongation. The perk4 mutant also shows a decreased sensitivity to abscisic acid (but not other hormones) with respect to root growth, Ca2+ channel currents, and cytosolic free Ca2+ levels (Bai et al., 2009). Ca2+ is an important signaling component, and mechanostimulation leads to changes in Ca2+ fluxes (Nakagawa et al., 2007). Increased Ca2+ influx induces the production of reactive oxygen species, which induce again Ca2+ influx and pH changes on both sites of the plasma membrane that affect cell wall extension (Fig. 1; Foreman et al., 2003; Takeda et al., 2008; Monshausen et al., 2009). Together, these data allow for the (admittedly speculative) model in which PERKs, covalently linked to the cell wall, sense mechanical stresses in the cell wall and influence Ca2+ fluxes across the membrane that modulate a number of cell wall-related processes, resulting in the alteration of cell (wall) growth.
ARABINOGALACTAN-PROTEINS
Arabinogalactan-proteins (AGPs) belong to the family of Hyp-rich glycoproteins and consist of a rather small protein moiety that is highly glycosylated. AGPs can be classified according to the composition of the peptide backbone. Classical AGPs contain Hyp, Ser, Thr, and Gly. Nonclassical AGPs deviate considerably from classical ones in their sequence. AGPs usually have an N-terminal GPI anchor (Fig. 1; Showalter, 2001). As described above, different proteins that are attached to the plasma membrane via a GPI anchor have been shown to be involved in linking the intracellular and extracellular space and thus influence cell wall development (Gillmor et al., 2005; Roudier et al., 2005). Analysis of AGP function revealed important roles in cell expansion, proliferation, and differentiation (Showalter, 2001; Yang et al., 2007). AGPs have been shown to bind components of the cell wall. This interaction can be covalent and suggests that AGPs are able to physically link the plasma membrane and the cell wall (Kjellbom et al., 1997; Nothnagel, 1997; Kohorn, 2000). Recently, the analysis of tomato (Solanum lycopersicum) LeAGP1 revealed a mutual dependence of the distribution of LeAGP1 and the microtubule and F-actin cytoskeleton. Precipitating AGPs with the β-Yariv reagent affects microtubules and F-actin, and interfering with these cytoskeleton components changes the distribution of LeAGP1 in tobacco (Nicotiana tabacum) protoplasts (Sardar et al., 2006). These data suggest a link between the cytoskeleton and LeAGP1. Hence, GPI-anchored AGPs are likely not only involved in establishing a connection between the cell wall and the plasma membrane but appear to extend this to the cytoplasm, establishing a continuum that might serve as a means to relay information between the intracellular and extracellular compartments.
LRR-EXTENSINS
LRR-extensins (LRXs) are chimeric extracellular proteins containing an N-terminal LRR domain and a C-terminal extensin domain, a typical Hyp-rich glycoprotein-like structural protein domain (Rubinstein et al., 1995; Baumberger et al., 2003a). Considering the function of LRR domains in protein-protein interaction, these proteins are candidates for a signaling function in cell wall development (Ringli, 2005). The best characterized LRX protein is LRX1 of Arabidopsis, which is expressed in root hairs. LRX1 is insolubilized in the cell wall, a function that is probably provided by the extensin moiety, since extensins are known to cross-link in the cell wall (Cassab, 1998). For LRX1, it could be shown that the LRR-containing N terminus of the protein undergoes an interaction in the cell wall, since expressing this extensin-less LRX1 protein in the wild type results in a dominant root hair phenotype similar to the lrx1 mutant (Fig. 1). This suggests that this protein titrates out the binding partner of the endogenous LRX1 (Baumberger et al., 2001). Mutations in LRX1 and its paralog LRX2 result in the formation of aberrant cell wall structures (Baumberger et al., 2003b), confirming a function of LRX proteins in cell wall development, possibly as a signaling intermediate. So far, the LRX1 interaction partner remains elusive, leaving open the question of the exact function of LRX1 and possibly other LRX-like proteins during cell wall development. Genetic evidence points at the possibility of LRX1 being involved in a pectin-related process. This is deduced from the finding that the pectin-modifying rol1 mutants were identified as suppressors of the lrx1 root hair phenotype (Diet et al., 2006).
SIGNAL TRANSDUCTION BEYOND RECEPTOR PROTEINS
The identification of potential receptor proteins, particularly those localized in the plasma membrane, is relatively straightforward based on bioinformatics approaches. A number of domains involved in protein-protein interaction were identified in plants and (predominantly) metazoans, and they can serve as a selection parameter in the identification of potential candidate proteins for a signaling function in cell wall-related processes. In the past, forward and reverse genetic approaches have allowed for considerable progress in this field. However, information on how the signals are relayed to other components in the signal transduction cascade is still scarce. Yet, recent work has shed light on signal transduction pathways that are involved in cell wall sensing.
MITOGEN-ACTIVATED PROTEIN KINASE PATHWAY
As mentioned above, WAK2 activates a vacuolar invertase (Inv), leading to the production of Glc and Fru, which modulates turgor pressure and hence cell expansion (Kohorn et al., 2006b). But how is this activation taking place? To identify the trigger of WAK2 activity, protoplasts were transiently transformed with a reporter gene construct consisting of the Inv promoter fused to the red fluorescent protein gene RFP. This construct led to a strong induction of red fluorescence (i.e. activity of the Inv promoter) upon addition of pectin to the protoplasts. Since this induction was not observed in protoplasts derived from a wak2 knockout mutant, the pectin-induced gene expression appears to be WAK2 dependent, providing in vivo evidence for WAK2 being a pectin receptor. The question remained of how the signal is transferred from the WAK2 kinase domain. The mitogen-activated protein kinase (MAPK) pathway is an important signaling pathway, and some MAPKs are activated upon pectin treatment (Colcombet and Hirt, 2008). Indeed, the kinase activity of MAPK3 was induced in wild-type protoplasts upon addition of pectin to the medium, while this induction was not observed in wak2 protoplasts. Further evidence for a connection between MAPK3 and WAK2 was obtained by a genetic analysis. A mapk3 mutant develops no visible mutant phenotype, while a dominant negative allele of wak2 shows a slight growth phenotype. However, a mapk3 wak2 double mutant develops a strong growth phenotype that is by far more pronounced that just a sum of both single mutant phenotypes (Kohorn et al., 2009). This observed synergistic interaction between the two mutants also points toward a function in a related process (i.e. that WAK2 and MAPK3 are part of the same signal transduction process; Fig. 1).
Evidence for a MAPK pathway playing a role in cell wall sensing has previously been obtained from yeast (Heinisch et al., 1999), where cell wall-related sensing mechanisms have been extensively studied (for review, see Levin, 2005). Cell walls of the yeast Saccharomyces cerevisiae differ structurally from plant cell walls but serve the same purpose. In yeast, the inner cell wall is composed of different polysaccharides, mainly Glc (β1-3 glucan, β1-6 glucan linkages) or a Glc derivative (chitin: β1-4 acetylglucosamine linkages), whereas the outer layer is rich in proteins glycosylated with Man. Since this cell wall is a rigid structure, a well-controlled mechanism of wall enlargement is required to allow for proper cell growth to take place. Under particular circumstances, yeast has to undergo dramatic changes in cell and cell wall development, such as during the transition to pseudohyphal growth under nutrient-limited conditions. Hence, the cell wall integrity (CWI) pathway system is vitally important, ensuring a controlled adaptation of cell wall development to the prevailing conditions and thus necessary developmental transitions. The protein kinase C-MAPK pathway has been referred to as the CWI pathway, since it controls the expression of cell wall-related genes and is induced in response to changes in growth or when cell wall damage occurs. A number of different sensory proteins localize to the yeast plasma membrane, where they monitor changes in the cell wall or mechanical stresses. These are described by others (Levin, 2005; Humphrey et al., 2007) and shall not be discussed here. Instead, I prefer to pick out one regulatory pathway that is important for cell (wall) development in yeast and that turns out to have a similar function in plants.
TARGET OF RAPAMYCIN PATHWAY
The Target of Rapamycin (TOR) pathway is a major controller of eukaryotic growth, where it adapts growth properties to the presence of growth factors and nutrient availability (Wullschleger et al., 2006). The TOR protein, a Ser/Thr kinase central to this pathway, is sensitive to rapamycin, making rapamycin a drug with anticancer activities (Mao et al., 2008). It was shown for yeast that some proteins involved in the transition to pseudohyphal growth (including rearrangements of the cell wall) also have a function in TOR signaling (Goehring et al., 2003, and refs. therein). Thus, it is conceivable that TOR is a modifier of cell wall structures. Recent work in the yeast Candida albicans identified Rhb1, a small G-protein of the Ras superfamily, to be required for proper filamentation of C. albicans under nitrogen-limited conditions. A mutation in Rhb1 causes rapamycin hypersensitivity, indicating that Rhb1 also has a function in TOR signaling. In addition, the rhb1 mutant shows an enhanced sensitivity toward and a reduced induction of the CWI-MAPK pathway upon treatment with cell wall-disrupting agents (Tsao et al., 2009). In plants, several components of the TOR pathway, including the TOR protein, have been identified. Even though there is evidence that the plant TOR pathway is somewhat different from the TOR pathway in yeast (Bögre et al., 2003), it is likely that it has CWI-sensing functions as well (Fig. 1). In Arabidopsis, the TOR kinase is essential for plant development. A knockout mutant is embryo lethal, but RNA interference plants with reduced TOR expression show retardation in growth (Menand et al., 2002; Deprost et al., 2007). Mutations in other TOR pathway components also have an effect on plant development (Mahfouz et al., 2006; Berkowitz et al., 2008). There is evidence that the Arabidopsis TOR pathway is indeed involved in regulating cell wall development. A suppressor screen was performed on the lrx1 mutant that shows aberrant cell wall development in root hairs (Baumberger et al., 2001, 2003b). A suppressor (rol5) was identified that induces changes in cell wall structures and leads to the formation of wild-type-like root hairs in the lrx1 mutant background. Comparison with the ROL5 homolog of yeast revealed a function of ROL5 in the TOR pathway. Inhibition of the TOR pathway in Arabidopsis by treatment with rapamycin not only leads to suppression of the lrx1 root hair phenotype but also induces changes in cell wall structures that are comparable to those of the rol5 mutant (Leiber et al., 2010). Future analyses will be necessary to get a better insight into the extent and the mechanism by which the TOR pathway affects cell wall development in plants.
OUTLOOK
The last few years have led to the accumulation of a wealth of information on receptor proteins that perceive signals from the apoplast and transduce them to the cytoplasm. Particular progress was obtained on WAK2, where the binding partner, one step of the signal transduction, and a signaling output could be elucidated. Proteins have been identified that do not act as receptors but are involved in transducing positional information from the cytoplasm to the extracellular matrix (e.g. COBRA, which functions in relaying the orientation of the cortical microtubules to the movement of cellulose-synthesizing rosette complexes). For many receptors, the interaction partner(s) remains to be determined. There is also a lack of understanding about the steps between signal perception and signaling output. The latter was assessed in some cases by microarray experiments, yet the signal-transducing network remains to be elucidated. In some instances, the comparison of plants with other systems such as yeast will certainly help to identify possible candidate proteins. For plant-specific functions, it will be exciting to follow the progress that will be made in the years to come, providing us with a much better insight into complex processes of cell wall-sensing mechanisms.
Acknowledgments
I thank James Breen for critical reading of the manuscript.
References
- André S, Siebert HC, Nishiguchi M, Tazaki K, Gabius HJ. (2005) Evidence for lectin activity of a plant receptor-like protein kinase by application of neoglycoproteins and bioinformatic algorithms. Biochim Biophys Acta 1725: 222–232 [DOI] [PubMed] [Google Scholar]
- Bai L, Zhang GZ, Zhou Y, Zhang ZP, Wang W, Du YY, Wu ZY, Song CP. (2009) Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca2+ signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J 60: 314–327 [DOI] [PubMed] [Google Scholar]
- Baskin TI, Beemster GTS, Judy-March JE, Marga F. (2004) Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol 135: 2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, Simmons MP, Bedinger P, Goff SA, Ringli C, et al. (2003a) Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice: a conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131: 1313–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Ringli C, Keller B. (2001) The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev 15: 1128–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumberger N, Steiner M, Ryser U, Keller B, Ringli C. (2003b) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35: 71–81 [DOI] [PubMed] [Google Scholar]
- Berkowitz O, Jost R, Pollmann S, Maslea J. (2008) Characterization of TCTP, the translationally controlled tumor protein, from Arabidopsis thaliana. Plant Cell 20: 3430–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Okresz L, Henriques R, Anthony RG. (2003) Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci 8: 424–431 [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A, Roy S, Kritsas K, Grobei MA, Jaciubek M, Schroeder JI, Grossniklaus U. (2009) Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136: 3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Lilley KS, Stevens TJ, Dupree P. (2003) Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis: a proteomic and genomic analysis. Plant Physiol 132: 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosca S, Barton CJ, Taylor NG, Ryden P, Neumetzler L, Pauly M, Roberts K, Seifert GJ. (2006) Interactions between MUR10/CesA7-dependent secondary cellulose biosynthesis and primary cell wall structure. Plant Physiol 142: 1353–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester K, Govers F. (2009) Arabidopsis L-type lectin receptor kinases: phylogeny, classification, and expression profiles. J Exp Bot 60: 4383–4396 [DOI] [PubMed] [Google Scholar]
- Burk DH, Ye ZH. (2002) Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14: 2145–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Delgado A, Penfield S, Smith C, Catley M, Bevan M. (2003) Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J 34: 351–362 [DOI] [PubMed] [Google Scholar]
- Canut H, Carrasco A, Galaud JP, Cassan C, Bouyssou H, Vita N, Ferrara P, Pont-Lezica R. (1998) High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J 16: 63–71 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Cassab GI. (1998) Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 49: 281–309 [DOI] [PubMed] [Google Scholar]
- Colcombet J, Hirt H. (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413: 217–226 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Decreux A, Messiaen J. (2005) Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol 46: 268–278 [DOI] [PubMed] [Google Scholar]
- Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolai M, Bedu M, Robaglia C, Meyer C. (2007) The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep 8: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diet A, Link B, Seifert GJ, Schellenberg B, Wagner U, Pauly M, Reiter WD, Ringli C. (2006) The Arabidopsis root hair cell wall formation mutant lrx1 is suppressed by mutations in the RHM1 gene encoding a UDP-l-rhamnose synthase. Plant Cell 18: 1630–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Karafyllidis I, Wasternack C, Turner JG. (2002) The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 14: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. (2007) The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660 [DOI] [PubMed] [Google Scholar]
- Fagard M, Desnos T, Desprez T, Goubet F, Refregier G, Mouille G, McCann M, Rayon C, Vernhettes S, Hofte H. (2000) PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell 12: 2409–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Giddings TH, Staehelin LA. (1988) Spatial relationship between microtubules and plasma-membrane rosettes during the deposition of primary wall microfibrils in Closterium sp. Planta 173: 22–30 [DOI] [PubMed] [Google Scholar]
- Gillmor CS, Lukowitz W, Brininstool G, Sedbrook JC, Hamann T, Poindexter P, Somerville C. (2005) Glycosylphosphatidyl inositol-anchored proteins are required for cell wall synthesis and morphogenesis in Arabidopsis. Plant Cell 17: 1128–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring AS, Rivers DM, Sprague GF. (2003) Urmylation: a ubiquitin-like pathway that functions during invasive growth and budding in yeast. Mol Biol Cell 14: 4329–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, Pont-Lezica RP, Canut H. (2006) Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis. Plant Physiol 140: 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HQ, Li L, Ye HX, Yu XF, Algreen A, Yin YH. (2009) Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA 106: 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Fujiki M, Kohorn BD. (1996) A cell wall-associated, receptor-like protein kinase. J Biol Chem 271: 19789–19793 [DOI] [PubMed] [Google Scholar]
- Heinisch JJ, Lorberg A, Schmitz HP, Jacoby JJ. (1999) The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol Microbiol 32: 671–680 [DOI] [PubMed] [Google Scholar]
- Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewski MJ. (2004) Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. J Biol Chem 279: 55474–55482 [DOI] [PubMed] [Google Scholar]
- Hematy K, Cherk C, Somerville S. (2009) Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol 12: 406–413 [DOI] [PubMed] [Google Scholar]
- Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Hofte H. (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, Berrocal-Lobo M, Keller H, Barlet X, Sanchez-Rodriguez C, et al. (2007) Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19: 890–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR. (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176: 7–21 [DOI] [PubMed] [Google Scholar]
- Jones DA, Jones JDG. (1997) The role of leucine-rich repeat proteins in plant defences. Adv Bot Res 24: 89–167 [Google Scholar]
- Kjellbom P, Snogerup L, Stohr C, Reuzeau C, McCabe PF, Pennell RI. (1997) Oxidative cross-linking of plasma membrane arabinogalactan proteins. Plant J 12: 1189–1196 [DOI] [PubMed] [Google Scholar]
- Kobe B, Deisenhofer J. (1994) The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 19: 415–421 [DOI] [PubMed] [Google Scholar]
- Kohorn BD. (2000) Plasma membrane-cell wall contacts. Plant Physiol 124: 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Johansen S, Shishido A, Todorova T, Martinez R, Defeo E, Obregon P. (2009) Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J 60: 974–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, Friedman HP, Fischer A, Byers N. (2006a) Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. J Cell Sci 119: 2282–2290 [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, Riese J, Huang LF, Koch K, Fu S, Dotson A, Byers N. (2006b) An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J 46: 307–316 [DOI] [PubMed] [Google Scholar]
- Lally D, Ingmire P, Tong HY, He ZH. (2001) Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 13: 1317–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber RM, John F, Verhertbruggen Y, Diet A, Knox JP, Ringli C. (2010) The TOR pathway modulates the structure of cell walls in Arabidopsis. Plant Cell (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE. (2005) Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz MM, Kim S, Delauney AJ, Verma DPS. (2006) Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell 18: 477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. (2008) FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 321: 1499–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Bhatt K, Baumann K. (2001) Shaping in plant cells. Curr Opin Plant Biol 4: 540–549 [DOI] [PubMed] [Google Scholar]
- Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, Robaglia C. (2002) Expression and disruption of the Arabidopsis TOR (Target Of Rapamycin) gene. Proc Natl Acad Sci USA 99: 6422–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Murata T, Sakurai-Ozato N, Kubo M, Demura T, Fukuda H, Hasebe M. (2009) ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr Biol 19: 1327–1331 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21: 2341–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo SA, Tax FE. (2006) Functional analysis of receptor-like kinases in monocots and dicots. Curr Opin Plant Biol 9: 460–469 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104: 3639–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhamchik A, Zhao ZY, Provart NJ, Shiu SH, Keatley SK, Cameron RK, Goring DR. (2004) A comprehensive expression analysis of the Arabidopsis proline-rich extensin-like receptor kinase gene family using bioinformatic and experimental approaches. Plant Cell Physiol 45: 1875–1881 [DOI] [PubMed] [Google Scholar]
- Nothnagel EA. (1997) Proteoglycans and related components in plant cells. Int Rev Cytol 174: 195–291 [DOI] [PubMed] [Google Scholar]
- Park AR, Cho SK, Yun UJ, Jin MY, Lee SH, Sachetto-Martins G, Park OK. (2001) Interaction of the Arabidopsis receptor protein kinase WAK1 with a glycine-rich protein, AtGRP3. J Biol Chem 276: 26688–26693 [DOI] [PubMed] [Google Scholar]
- Ringli C. (2005) The role of extracellular LRR-extensin (LRX) proteins in cell wall formation. Plant Biosyst 139: 32–35 [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al. (2005) COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17: 1749–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein AL, Broadwater AH, Lowrey KB, Bedinger PA. (1995) PEX1, a pollen-specific gene with an extensin-like domain. Proc Natl Acad Sci USA 92: 3086–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar HS, Yang J, Showalter AM. (2006) Molecular interactions of arabinogalactan proteins with cortical microtubules and F-actin in Bright Yellow-2 tobacco cultured cells. Plant Physiol 142: 1469–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN. (2001) COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev 15: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Kim Y, Guo Y, Stevenson B, Zhu JK. (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15: 19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter AM. (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58: 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Ezaki B, He ZH, Tong HY, Osawa H, Baluska F, Volkmann D, Matsumoto H. (2003) Aluminum-induced gene expression and protein localization of a cell wall-associated receptor kinase in Arabidopsis. Plant Physiol 132: 2256–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L. (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol Plant Mol Biol 35: 585–657 [Google Scholar]
- Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. (2008) Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Tang WQ, Kim TW, Oses-Prieto JA, Sun Y, Deng ZP, Zhu SW, Wang RJ, Burlingame AL, Wang ZY. (2008) BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CC, Chen YT, Lan CY. (2009) A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet Biol 46: 126–136 [DOI] [PubMed] [Google Scholar]
- Wagner TA, Kohorn BD. (2001) Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13: 303–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383 [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. (2006) TOR signaling in growth and metabolism. Cell 127: 5–19 [DOI] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. (2008) Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20: 3065–3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sardar HS, McGovern KR, Zhang YZ, Showalter AM. (2007) A lysine-rich arabinogalactan protein in Arabidopsis is essential for plant growth and development, including cell division and expansion. Plant J 49: 629–640 [DOI] [PubMed] [Google Scholar]
- Yin YH, Wang ZY, Mora-Garcia S, Li JM, Yoshida S, Asami T, Chory J. (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191 [DOI] [PubMed] [Google Scholar]