Abstract
XBAT32, a member of the RING domain-containing ankyrin repeat subfamily of E3 ligases, was previously identified as a positive regulator of lateral root development. Arabidopsis (Arabidopsis thaliana) plants harboring a mutation in XBAT32 produce fewer lateral roots that wild-type plants. We found that xbat32 mutants produce significantly more ethylene than wild-type plants and that inhibition of ethylene biosynthesis or perception significantly increased xbat32 lateral root production. XBAT32 interacts with the ethylene biosynthesis enzymes AMINOCYCLOPROPANE-1-CARBOXYLIC ACID SYNTHASE4 (ACS4) and ACS7 in yeast-two-hybrid assays. XBAT32 is capable of catalyzing the attachment of ubiquitin to both ACS4 and ACS7 in in vitro ubiquitination assays. These results suggest that XBAT32 negatively regulates ethylene biosynthesis by modulating the abundance of ACS proteins. Loss of XBAT32 may promote the stabilization of ACSs and lead to increased ethylene synthesis and suppression of lateral root formation. XBAT32 may also contribute to the broader hormonal cross talk that influences lateral root development. While auxin treatments only partially rescue the lateral root defect of xbat32, they completely restore wild-type levels of xbat32 lateral root production when coupled with ethylene inhibition. Abscisic acid, an antagonist of ethylene synthesis/signaling, was also found to stimulate rather than inhibit xbat32 lateral root formation, and abscisic acid acts synergistically with auxin to promote xbat32 lateral root production.
Ubiquitin ligases (E3) are central components of the ubiquitination pathway, an essential system that functions to covalently attach ubiquitin molecules to selected proteins. Posttranslational attachment of ubiquitin is accomplished via a conjugation cascade that begins with the ubiquitin-activating enzyme (E1), which activates and transfers ubiquitin to one of many ubiquitin-conjugating enzymes (E2). The substrate-recruiting E3 then facilitates the transfer of ubiquitin from the E2-ubiquitin intermediate to the substrate. One, few, or many ubiquitin moieties can be attached, and the types of ubiquitin-ubiquitin linkages utilized in polyubiquitin chain formation provide functional versatility (Weissman, 2001). The best-characterized outcome of ubiquitination is the degradation of proteins by the 26S proteasome. Proteins targeted for degradation are modified by the attachment of a Lys-48-linked polyubiquitin chain. A number of nonproteolytic functions, including endocytosis, protein activation, and sorting, have also been linked to ubiquitination. These processes utilize alternative ubiquitin linkages, such as Lys-63-linked polyubiquitin chain (Bonifacino and Weissman, 1998; Deng et al., 2000).
The selection of specific targets in the ubiquitination pathway is governed mainly by a very large and diverse family of E3 ligases. The Arabidopsis (Arabidopsis thaliana) genome, for example, is predicted to encode for over 1,300 E3 ligases, which can be classified into different subgroups based on the presence of the U-box, Really Interesting New Gene (RING), or Homologous to E6-AP C-Terminus E2-binding domain (Stone and Callis, 2007). A large complement of the Arabidopsis E3s belongs to the RING class, which is characterized by the presence of a Cys- and His-rich domain (Lovering et al., 1993). The Arabidopsis RING domain-containing E3s can further be classified into 30 different subgroups based on domain architecture (Stone et al., 2005). Members of several subgroups have been linked to important physiological and developmental processes, including XBAT32 (XB3 ortholog 2 in Arabidopsis), which belongs to the RING domain-containing ankyrin repeat subgroup (Nodzon et al., 2004; Stone et al., 2005). Members of this subgroup are referred to as XBAT due to their structural similarity to the rice (Oryza sativa) XB3 protein (Nodzon et al., 2004). XBAT32 has been characterized as a positive regulator of lateral root development (Nodzon et al., 2004). This E3 ligase, which is postulated to be involved in auxin transport, is required for the proper initiation of lateral roots.
Lateral roots are an essential part of the plant root system, which functions as a sensor for abiotic and biotic signals, facilitates the uptake of nutrients and moisture from the soil, and acts as a support for the aerial parts of the plant. The primary root is formed during embryogenesis and emerges from the seed during germination (Dittmer, 1937; Malamy and Benfey, 1997). As the seedling develops further, lateral roots emerge from the primary root to facilitate maximum nutrient absorption from the soil. Following germination, only certain pericycle cells in the primary root that are in contact with the xylem poles acquire the ability to undergo a series of asymmetric cell divisions. This process, referred to as the lateral root initiation stage, leads to the formation of lateral root primordium (Malamy and Benfey, 1997; Dubrovsky et al., 2000; De Smet et al., 2006a). A well-characterized series of cell divisions in the lateral root primordium then forms a meristem, which finally emerges from the primary root by cell elongation (Malamy and Benfey, 1997; Casimiro et al., 2003).
The developmental plasticity of the plant root system, which is affected by external signals and multiple internal hormonal cues, allows the root to adapt to the changing environment. Auxin, a key promoter of lateral root growth, regulates different stages of lateral root formation, including initiation (Casimiro et al., 2003; De Smet et al., 2006a), primordium development (Benková et al., 2003), and emergence (Laskowski et al., 2006). Functional auxin biosynthesis, transport, and signaling pathways are required for lateral root formation (Fukaki et al., 2007). Mutants that overproduce indole-3-acetic acid (IAA), the predominant naturally occurring form of auxin, have an increased number of lateral roots (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995). Altering acropetal and basipetal auxin transport by treatment with auxin transport inhibitors decreases lateral root production (Reed et al., 1998; Casimiro et al., 2001). Other hormones regulate lateral root development either directly or indirectly via their interactions with auxin (Nibau et al., 2008). There is evidence to support cross talk between auxin and abscisic acid (ABA), which is known to inhibit lateral root formation (De Smet et al., 2006b). The ABA signaling mutant abscisic acid insensitive3 shows reduced lateral root formation in response to an auxin stimulus (Brady et al., 2003). Ethylene also inhibits lateral root production (Chilley et al., 2006; Fukaki et al., 2007; Negi et al., 2008). The regulatory effect of ethylene on lateral root production is auxin dependent (Stepanova et al., 2005; Ruzicka et al., 2007; Swarup et al., 2007). High levels of ethylene alter auxin transport, which in turn affects auxin uploading into root cells, to ultimately inhibit lateral root production (Gazzarrini and McCourt, 2001; Negi et al., 2008). Enhanced ethylene response mutants such as polaris display reduced IAA accumulation in roots and are deficient in lateral root development (Chilley et al., 2006). Other ethylene mutants, such as ethylene overproducer1 (eto1) and the constitutive ethylene signaling mutant constitutive triple response1 (ctr1), also display a reduced lateral root phenotype (Negi et al., 2008).
Regulated proteolysis also plays an important role in lateral root development. The auxin receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1) is an F-box protein that functions as the substrate-recruiting subunit for the SCFTIR1 E3 ligase complex (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). SCFTIR1 regulates the degradation of AUX/IAA transcriptional repressors that interact with and inhibit the activity of AUXIN RESPONSE FACTOR (ARF) transcription factors. The degradation of AUX/IAA proteins such as SOLITARY ROOT (SLR1)/IAA14 allows ARF7 and ARF19 to activate the transcription of genes required for lateral root initiation (Fukaki et al., 2002; Okushima et al., 2007). Mutations that stabilize SLR1/IAA14 prohibit the formation of lateral roots (Fukaki et al., 2005). Other E3 ligases with a role in lateral root development include the F-box proteins CEGENDUO (CEG), VIER F-BOX PROTEINE (VFB), ARABIDILLO-1 and ARABIDILLO-2, and the RING proteins SINAT5 and XBAT32 (Xie et al., 2002; Nodzon et al., 2004; Coates et al., 2006; Dong et al., 2006; Schwager et al., 2007). Plants that lack VFB and ARABIDILLO produce fewer lateral roots than wild-type plants, and an increase in the number of lateral roots is observed with loss of CEG (Coates et al., 2006; Dong et al., 2006; Schwager et al., 2007). SINAT5 regulates the abundance of the transcription factor NAC1, which promotes lateral root formation (Xie et al., 2000, 2002). Overexpression of SINAT5 reduces NAC1 protein levels in roots and inhibits lateral root growth (Xie et al., 2002).
In this study, we analyzed three of the XBAT RING E3 ligase family members, XBAT32, XBAT34, and XBAT35. The reduced lateral root phenotype is unique to XBAT32. Further analysis of xbat32 demonstrates that the mutant overproduces ethylene, and this contributes to its reduced lateral root phenotype. Ethylene antagonists silver nitrate and ABA stimulate lateral root production in xbat32, and both act synergistically with auxin to fully rescue the lateral root defect. XBAT32 interacts with AMINOCYCLOPROPANE-1-CARBOXYLIC ACID SYNTHASE4 (ACS4) and ACS7 in a yeast two-hybrid assay, and XBAT32 was able to ubiquitinate both ACS4 and ACS7 in in vitro ubiquitination assays. Together, our results indicate that XBAT32 plays an essential role in ethylene biosynthesis as a negative regulator of ACS protein abundance. Loss of XBAT32 E3 activity may lead to an accumulation of ACS4/7 and increased ethylene production, which may account for the xbat32 mutant phenotypes.
RESULTS
ABA Promotes Lateral Root Production in xbat32
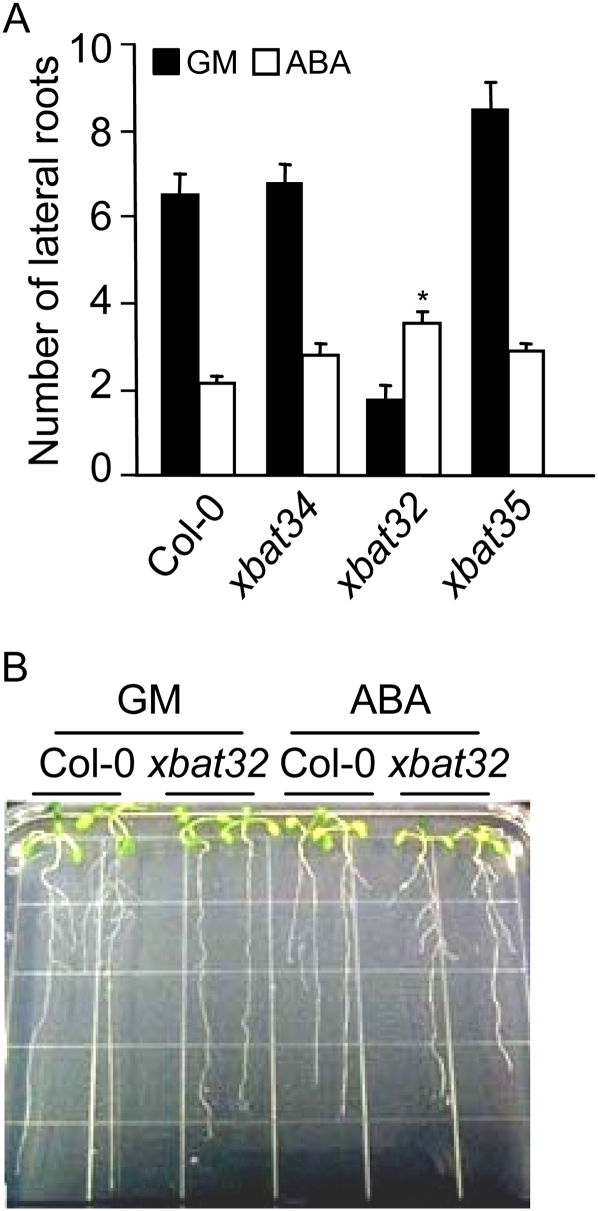
Arabidopsis XBAT and XBAT-related genes from other plant species are involved in development, reproduction, ABA signaling, and biotic and abiotic stress tolerance (Nodzon et al., 2004; Huang et al., 2006; Stone et al., 2006; Wang et al., 2006; Xu et al., 2007; Yang et al., 2008). Loss of Arabidopsis XBAT32, for example, results in a delayed growth phenotype and reduced lateral root production due to a decrease in lateral root initiation (Nodzon et al., 2004). The difference between xbat32 and the wild type was also evident in the larger flowers produced by xbat32 plants (Supplemental Fig. S1). To determine whether other XBAT family members played similar roles in development, we examined the effects of XBAT34 and XBAT35 mutations on plant development. Phenotypic analysis of homozygous XBAT34 and XBAT35 T-DNA insertional lines shows that the xbat32 phenotypes are unique to plants bearing the XBAT32 mutation (Fig. 1A; Supplemental Fig. S1). Given the previous association detected between the XBAT-related genes and ABA signaling (Stone et al., 2006; Yang et al., 2008), xbat32, xbat34, and xbat35 seedlings were treated with ABA and its inhibitory effect on root growth was analyzed. Three-day-old seedlings were transferred to growth medium (GM) with or without ABA, and root development was analyzed after 5 d. Interestingly, from this analysis, we discovered that ABA promoted rather than inhibited xbat32 lateral root production (Fig. 1; Supplemental Fig. S2). In contrast, the response of xbat34 and xbat35 to ABA-mediated inhibition of lateral root production was similar to that of the wild type, because in those three genotypes ABA significantly reduced the number of lateral roots produced (Fig. 1A). Compared with the wild type, xbat32 did not display an altered sensitivity to ABA-mediated inhibition of primary root growth, which suggests that other ABA-mediated responses remain intact (Supplemental Fig. S2).
Figure 1.
ABA treatment increases xbat32 lateral root production. A and B, Three-day-old xbat34, xbat32, xbat35, and wild-type Arabidopsis (Col-0) seedlings were transferred to GM alone or GM with 5 μm ABA and grown vertically for 5 d, after which the number of visible lateral roots was quantified (A) and photographs were taken (B). Each bar represents the average number of lateral roots ± se. Statistical analysis was performed using Student's t test, with significant differences relative to untreated xbat32 and ABA-treated Col-0 (* P < 0.05).
Inhibition of Ethylene Perception Drastically Increases xbat32 Lateral Root Production
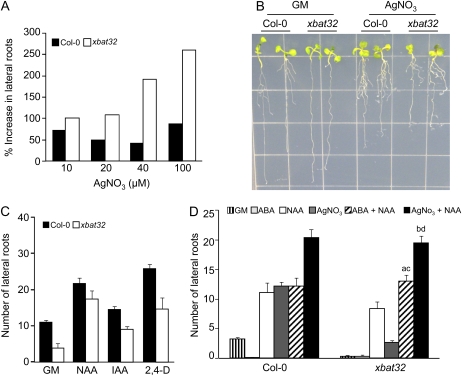
ABA stimulation of xbat32 lateral root production could be due to ABA relieving the inhibitory effects of ethylene on root growth. During lateral root development, high levels of ethylene inhibit lateral root formation (Ivanchenko et al., 2008; Negi et al., 2008). If ABA is indeed alleviating the inhibitory effects of ethylene on xbat32 lateral root development, then blocking ethylene synthesis or perception should increase xbat32 lateral root numbers. To determine if this is the case, 3-d-old xbat32 seedlings were treated for 5 d with increasing concentrations of silver nitrate (AgNO3), an antagonist of the ethylene receptor (Beyer, 1976). Blocking ethylene perception greatly increased the number of lateral roots produced by xbat32 (Fig. 2, A and B; Supplemental Fig. S3). An increase in lateral root production was also observed for wild-type seedlings. However, the effect of AgNO3 on xbat32 lateral root production was considerably greater than that observed for the wild type (Fig. 2A). The fact that xbat32 mutants are more sensitive than wild-type plants to the effects of the ethylene receptor antagonist suggests hyperactivity of ethylene production or response in the mutant. Overall, these results indicate that ethylene signaling is a target for the xbat32 mutation.
Figure 2.
Response of xbat32 to inhibition of ethylene signaling and/or increase in auxin levels. A and B, Three-day-old Col-0 and xbat32 seedlings were treated with increasing concentrations of AgNO3 for 5 d, after which the number of visible lateral roots was scored and seedlings were photographed. A shows percentage increase in lateral root numbers on AgNO3 compared with the control (without AgNO3). C, Comparison of lateral root growth of xbat32 and Col-0 seedlings grown on GM and GM supplemented with 2 μm NAA, 2 μm IAA, or 2 μm 2,4-D. The average number of lateral roots ± se is shown. D, Col-0 and xbat32 seedlings were grown for 3 d on GM and then transferred to GM supplemented with 2 μm NAA, 0.5 μm ABA, or 100 μm AgNO3 alone or in combination, 0.5 μm ABA plus 2 μm NAA or 100 μm AgNO3 plus 2 μm NAA. Lateral root growth was assessed 5 d after transfer. Each bar represents the average number of lateral roots ± se. Significant differences (P < 0.05) between xbat32 on NAA alone and xbat32 on NAA with ABA (a) or NAA with AgNO3 (b) is indicated. No significant difference (P > 0.05) was found between Col-0 and xbat32 treated with NAA and ABA (c) or NAA and AgNO3 (d). Statistical analysis was performed using Student's t test.
Inhibition of Ethylene Perception or Signaling Enhances Auxin's Ability to Rescue xbat32 Lateral Root Production
Recent studies have suggested an interaction between ethylene and auxin in regulating lateral root development (Ivanchenko et al., 2008; Negi et al., 2008). Auxin can suppress the inhibitory effects of ethylene on lateral root formation, and auxin mutants are insensitive to ethylene-induced inhibition of lateral root initiation (Ivanchenko et al., 2008;Negi et al., 2008). Treatment of xbat32 seedlings with IAA and the synthetic auxin analogs naphthaleneacetic acid (NAA) or 2,4-dichlorophenoxy acetic acid (2,4-D) only partially rescued the reduced lateral root phenotype (Fig. 2C; Nodzon et al., 2004). Therefore, we analyzed the combined effect of blocking ethylene perception and/or signaling and increasing auxin levels. Three-day-old xbat32 and wild-type seedlings were treated with NAA or AgNO3 alone or NAA plus AgNO3 for 5 d, after which lateral root production was quantified. The combined effects of blocking ethylene perception and increasing auxin levels brought about a greater rescue of the lateral root defect of xbat32 compared with auxin or AgNO3 alone (Fig. 2D). Since ABA promotes xbat32 lateral root formation, we also examined the combined effect of ABA and auxin on lateral root production. As observed with AgNO3, ABA acted synergistically with auxin to fully rescue xbat32 lateral root production compared with auxin or ABA treatment alone (Fig. 2D). The fact that AgNO3 and ABA treatment enhances the actions of auxin on xbat32 lateral root production suggests that increased ethylene synthesis and/or signaling contributes to the xbat32 lateral root phenotype.
Increased Ethylene Signaling Inhibits Lateral Root Production on Preexisting xbat32 Primary Roots
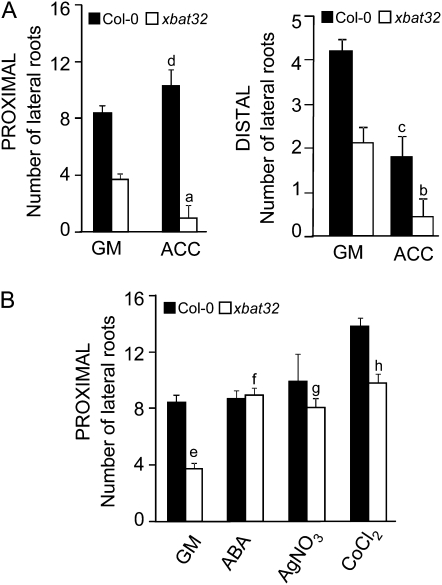
Applications of exogenous ethylene have been shown to inhibit lateral root initiation only on roots that have formed in the presence of a treatment with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC; Ivanchenko et al., 2008). The ethylene-induced inhibitory effect is not observed on roots formed prior to ACC treatment; in fact, in this instance, ethylene is observed to moderately promote the emergence of lateral roots. To determine if xbat32 response to ethylene was similar to that of the wild type, we observed the effects of ACC treatment on roots formed prior to the ACC treatment (designated as proximal) and roots formed during the treatment (designated as distal; Fig. 3A). For wild-type seedlings, ACC inhibited lateral root formation on the distal portion of primary roots. A slight increase in lateral root numbers was observed for ACC-treated proximal primary roots; however, the change was not significantly different from untreated roots (Fig. 3A). For xbat32 seedlings, both proximal and distal root portions showed reduced lateral root numbers in response to ACC (Fig. 3A). These results suggest that the xbat32 mutation enhanced ethylene synthesis or signaling, which led to increased sensitivity to ethylene and inhibition of lateral root formation in the proximal portion of the primary root. Therefore, treatment with ACC only serves to enhance the effects of the xbat32 mutation. To further explore the role of ethylene in the xbat32 mutant phenotype, the effect of the ethylene antagonists ABA and AgNO3 on the production of lateral roots on the proximal portion of primary roots was analyzed. Five-day-old seedlings were treated with AgNO3 or ABA for 5 d, after which the number of lateral roots produced on the proximal portion of the primary root was quantified (Fig. 3B). Application of either ABA or AgNO3 restored xbat32 lateral root numbers to wild-type levels. The fact that treatment with ABA and AgNO3 had similar effects on lateral root formation indicates that the xbat32 mutation alters ethylene biosynthesis. To determine if increased ethylene synthesis is involved in producing the xbat32 phenotype, seedlings were treated as described above with cobalt chloride (CoCl2), which blocks the ACC oxidase activity required for the conversion of ACC to ethylene. Exposure to CoCl2 increased xbat32 lateral root numbers on proximal roots to wild-type levels (Fig. 3B). An increase in wild-type lateral root numbers was also observed. These results further support the hypothesis that ethylene overproduction contributes to the xbat32 lateral root defect.
Figure 3.
Effects of modulating ethylene signaling on lateral root production on preexisting and new primary root growth. A, Five-day-old Col-0 and xbat32 seedlings were grown on GM or GM supplemented with 50 μm ACC for 5 d, after which lateral roots produced were quantified. Lateral roots produced on preexisting primary roots at the time of transfer (proximal) and those produced on primary roots that developed during treatment (distal) were quantified separately. Differences between ACC-treated xbat32 (a and b) or Col-0 (c) and untreated are statistically significant (P < 0.05). ACC-treated Col-0 (d) is not significantly different from untreated Col-0 (P > 0.05). B, Col-0 and xbat32 seedlings were grown on GM for 5 d and then transferred to GM containing 5 μm ABA, 100 μm AgNO3, or 100 μm CoCl2 for another 5 d. The number of lateral roots that developed on preexisting primary roots at the time of transfer (proximal) was quantified. Each bar represents the average number of lateral roots ± se. Significant difference (P < 0.05) between Col-0 and xbat32 on GM (e) is indicated. No significant difference (P > 0.05) between xbat32 treated with ABA (f), AgNO3 (g), or CoCl2 (h) and untreated Col-0 was found. Statistical analysis was performed using Student's t test.
XBAT32 Mutant Seedlings Overproduce Ethylene
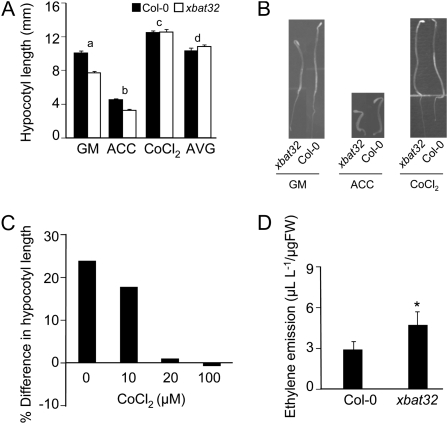
The results obtained from growth assays using ethylene inhibitors are consistent with XBAT32’s involvement in regulating ethylene biosynthesis. Based on these observations, we predicted that the xbat32 mutation would enhance ethylene production. To test this hypothesis, we observed the xbat32 dark-grown phenotype, studied the response of xbat32 etiolated seedlings to ethylene, and measured ethylene production. Dark-grown wild-type seedlings exposed to ethylene and ethylene-overproducing mutants display a characteristic triple response that includes shorter hypocotyls, shorter roots, and exaggerated apical hooks (Guzman and Ecker, 1990). Dark-grown xbat32 seedlings had shorter hypocotyls than wild-type seedlings when grown in air (Fig. 4, A and B). The xbat32 dark-grown phenotype is consistent with ethylene overproduction; however, this phenotype could result from alterations in ethylene signaling. To demonstrate that ethylene synthesis and not signaling is affected by the mutation, we examined the response of xbat32 etiolated seedlings to ACC, CoCl2, and aminoethoxyvinylglycine (AVG), an inhibitor of ACS activity that is required for the conversion of S-adenosyl-l-methionine (SAM) to the ethylene precursor ACC. Similar to the wild type, xbat32 hypocotyl growth was inhibited upon exposure to ACC (Fig. 4, A and B). Treatment with the ethylene biosynthesis inhibitors AVG and CoCl2 restored xbat32 hypocotyl length to wild-type levels (Fig. 4, A and B). The difference between wild-type and xbat32 hypocotyl length became progressively smaller as the concentration of cobalt was increased (Fig. 4C). Consistent with the results from the growth assays, xbat32 seedlings were repeatedly found to produce significantly more ethylene than the wild type (Fig. 4D). These findings indicate that xbat32’s phenotype is due to an increase in ethylene synthesis.
Figure 4.
Response of etiolated xbat32 seedlings to ethylene, cobalt, and AVG and measurement of ethylene emission. A, Hypocotyl lengths of Col-0 and xbat32 etiolated seedlings grown on GM or GM supplemented with 50 μm ACC, 100 μm CoCl2, or 2 μm AVG. Each bar represents the average number of lateral roots ± se. Significant differences (P < 0.05) between xbat32 and Col-0 on GM (a; P = 0.000) and with ACC treatment (b; P = 0.000) are indicated. No significant difference (P > 0.05) was found between CoCl2-treated (c) and AVG-treated (d) Col-0 and xbat32. Statistical analysis was performed using Student's t test. B, Representative 3-d-old etiolated Col-0 and xbat32 seedlings grown on GM or GM supplemented with 50 μm ACC or 100 μm CoCl2. C, Hypocotyl elongation of etiolated xbat32 seedlings in response to increasing concentrations of CoCl2. Percentage difference in hypocotyl length between Col-0 and xbat32 is shown. D, Ethylene emission from 10-d-old Col-0 and xbat32 seedlings. Col-0 and xbat32 seedlings were grown in sealed vials, and ethylene emitted was quantified by gas chromatography. Each bar represents the average measurement ± se from two separate trials each with duplicate vials. Each vial was measured in triplicate. Difference between xbat32 and Col-0 is statistically significant (* P < 0.05) using Student's t test. FW, Fresh weight.
XBAT32 Interacts with and Ubiquitinates ACS4 and ACS7
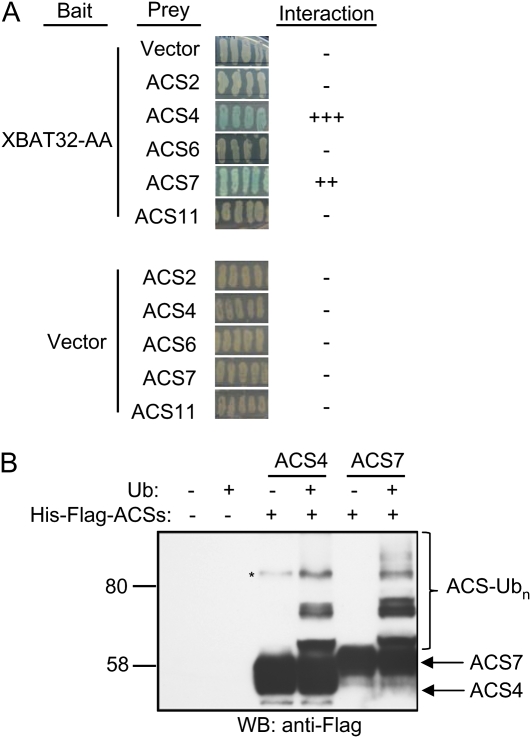
The increased production of ethylene observed for xbat32 is most likely due to an increase in the activity or levels of one or more of the ethylene biosynthesis enzymes. The rate-limiting conversion of SAM to ACC by ACS is regulated by ubiquitin-dependent proteasomal degradation (Vogel et al., 1998; Chae et al., 2003; Wang et al., 2004; Yoshida et al., 2005; Christians et al., 2009). XBAT32, a functional RING-type E3 ligase capable of E2-dependent protein ubiquitination (Nodzon et al., 2004), may regulate the abundance of one or more of the ACS enzymes. The ACSs are grouped into three classes, type 1 (ACS1, ACS2, and ACS6), type 2 (ACS4, ACS5, ACS8, and ACS9), and type 3 (ACS7 and ACS11; Yoshida et al., 2005). We first used a yeast two-hybrid screen to determine if XBAT32 can interact with representative type 1 (ACS2 and ACS6), type 2 (ACS4), or type 3 (ACS7 and ACS11) ACSs. To prevent ubiquitination and subsequent degradation of any interacting proteins, the RING domain of XBAT32 was made nonfunctional by mutating two essential zinc coordinating residues to Ala (XBAT32-AA). XBAT32 was found to interact with ACS4 and ACS7 (Fig. 5A). XBAT32-AA repeatedly induced higher β-galactosidase activity with ACS4 and ACS7 relative to controls and other ACSs tested. This interaction was also observed with XBAT32 containing a functional RING domain; however, evidence for this interaction (blue color) took longer to appear (data not shown). This observation can be explained by the existence of a conserved ubiquitination pathway in yeast; ACS4 and ACS7 protein levels may have taken longer to accumulate and turn on expression of the reporter gene if XBAT32 ubiquitinated and targeted some of the ACS4 and ACS7 for degradation by the yeast 26S proteasome. Recombinant His-glutathione S-transferase (GST)-tagged XBAT32 was capable of attaching ubiquitin to both His-Flag-tagged ACS4 and ACS7 in in vitro ubiquitination assays (Fig. 5B). This is evident by the higher Mr forms of His-Flag-ACS4 and -ACS7 detected by Flag antibodies following assays carried out in the presence of ubiquitin. The higher Mr forms of His-Flag-ACS4 or -ACS7 were not observed when ubiquitin was omitted from the assay (Fig. 5B). These results suggest that XBAT32 negatively regulates ethylene biosynthesis via ubiquitin-dependent degradation of ACS enzymes, in particular ACS4 and ACS7.
Figure 5.
XBAT32 interacts with and ubiquitinates ACS4 and ACS7 in vitro. A, Full-length XBAT32 with a mutated RING domain (XBAT32-AA) fused to a LexA DNA binding domain (bait) in pNLexAattR was tested by yeast two-hybrid screening for interactions with various ACSs fused to a Gal4 activation domain (prey) in pJZ4attR. Empty pNLexAattR and pJZ4attR vectors were used as controls. Interaction between XBAT32-AA and ACS is indicated by β-galactosidase activity (blue color). −, No interaction; ++, moderate interaction; +++, strong interaction. B, His-GST-XBAT32 (E3) promotes the ubiquitination of Flag-His-ACS4 and -ACS7 in in vitro ubiquitination assays containing recombinant yeast E1, His-AtUBC8 (E2), and ubiquitin (Ub). Omission of ubiquitin from the assay resulted in a loss of ACS ubiquitination. ACS and ubiquitinated ACSs were visualized by western-blot analysis using Flag antibodies. The asterisk indicates a nonspecific protein detected by Flag antibodies. WB, Western blot.
DISCUSSION
Ethylene is known to play a major role in regulating root development (Hussain and Roberts, 2002). Older studies established that in Arabidopsis high levels of ethylene inhibit primary root growth (Le et al., 2001; Swarup et al., 2007), and recent experiments have indicated that high levels of ethylene also negatively regulate lateral root formation (Ivanchenko et al., 2008; Negi et al., 2008). Previous studies have shown that the xbat32 mutation causes a number of aberrant phenotypes, including a striking and pronounced reduction in the number of lateral roots (Nodzon et al., 2004). Here, we show that XBAT32, a RING domain-containing ankyrin repeat E3 ligase, is a negative regulator of ethylene biosynthesis and that through this activity XBAT32 modulates lateral root development. Homozygous xbat32 plants display an ethylene overproduction phenotype when grown in the dark and produce significantly more ethylene than the wild type. The dark-grown ethylene overproduction phenotype and reduced lateral root numbers were rescued by treating xbat32 seedlings with inhibitors of ethylene biosynthesis and perception. XBAT32 interacts with the ethylene biosynthesis enzymes ACS4 and ACS7, which convert SAM to the ethylene precursor ACC. Furthermore, XBAT32 is capable of catalyzing the attachment of ubiquitin to ACS4 and ACS7 in vitro. Altogether, these data suggest that XBAT32 regulates ethylene biosynthesis by modulating the stability of one or more ACS enzymes.
Exogenous auxin treatment partially rescued the lateral root phenotype in xbat32 (Nodzon et al., 2004; this study). Based on our findings, a feasible explanation for only a partial rescue for xbat32 could be the presence of high levels of ethylene in the mutant plants. Several studies point to an interaction between ethylene and auxin during lateral root production (Stepanova et al., 2007; Swarup et al., 2007; Ivanchenko et al., 2008; Negi et al., 2008). High ethylene levels promote both acropetal and basipetal auxin transport (Negi et al., 2008), which affects auxin uploading into root cells of the elongation zone, thus resulting in reduced lateral root production (Negi et al., 2008). In enhanced ethylene signaling mutants, such as the polaris mutant, which displays reduced lateral root production, auxin transport in roots is also defective (Chilley et al., 2006). The overproduction of ethylene in xbat32 could be the factor responsible for preventing a complete rescue of the lateral root defect by auxin. Since high ethylene enhances auxin transport (Negi et al., 2008), which alters its availability to root cells, simply adding exogenous auxin would not be sufficient to fully rescue the lateral root defect. Blocking ethylene signaling would restore proper auxin transport, allowing the exogenously supplied auxin to be made more available for lateral root production. ABA, another developmentally essential plant hormone known to inhibit Arabidopsis lateral root development (De Smet et al., 2006b), was found to increase the number of lateral roots in xbat32 seedlings. In contrast to Arabidopsis, ABA stimulates lateral root development in legumes. ABA has been shown to rescue the meristem defect and promote lateral root formation in the Medicago truncatula lateral root organ defective mutant (Liang et al., 2007). A possible explanation for stimulatory effects of ABA on xbat32 lateral root production may be that ABA inhibits ethylene synthesis and/or signaling. ABA and ethylene are antagonistic during many stages of plant development. For example, ABA inhibits early seedling establishment, whereas ethylene has the opposite effect. Interactions between ABA and ethylene may occur during hormone synthesis and/or between signaling pathways (Beaudoin et al., 2000; Ghassemian et al., 2000; Spollen et al., 2000; LeNoble et al., 2004; Cheng et al., 2009). Several lines of evidence support the hypothesis that ABA negatively regulates ethylene synthesis and vice versa (Spollen et al., 2000; LeNoble et al., 2004; Cheng et al., 2009). Inhibition of ABA synthesis chemically via fluridone treatment or genetically using ABA-deficient mutants such as abscisic acid deficient2 increases ethylene production, and application of ABA inhibits ethylene production (Spollen et al., 2000; LeNoble et al., 2004; Cheng et al., 2009). Exogenous ABA, therefore, may reduce xbat32 ethylene levels, thus alleviating the negative effects of ethylene on auxin transport, allowing for lateral root initiation and primordia development.
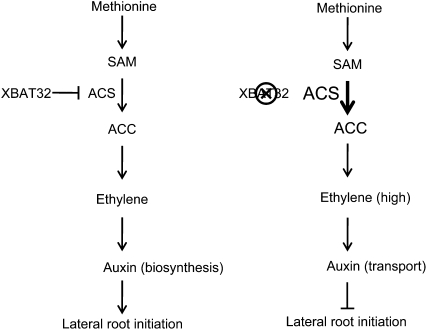
The role of XBAT32 as a negative regulator of ethylene biosynthesis is further strengthened by the display of an ethylene-associated phenotype of a shortened hypocotyl in xbat32 etiolated seedlings. Wild-type Arabidopsis seedlings exposed to ethylene and ethylene-overproducing mutants such as eto1 and ctr1-1 that activate the ethylene response pathway (Kieber et al., 1993) display a dark-grown constitutive triple response phenotype, which is characterized by a shortened hypocotyl as well as an exaggerated apical hook and a short seedling root (Guzman and Ecker, 1990). Gas chromatography results indicated that xbat32 produced significantly more ethylene than the wild type. XBAT32 mutant seedlings do not seem to have an exaggerated apical hook or shorter roots; however, the shorter hypocotyls compared with wild-type seedlings indicate that the level of ethylene overproduction in xbat32 does alter some aspects of growth. Blocking ethylene synthesis via cobalt or AVG application rescues the shortened hypocotyl phenotype and restores it to wild-type levels, as for ethylene-overproducing mutants like eto1 (Guzman and Ecker, 1990) and the triple mutant eto1 eol1 eol2 (Christians et al., 2009). Similar to xbat32, the ethylene overproducer eto1 also shows reduced lateral root formation, whereas ethylene-insensitive mutants such as ein2 and ert1 produce more lateral roots than the wild type (Negi et al., 2008). The fact that XBAT32 can interact with and ubiquitinate ACS4 and ACS7 provides further evidence for a role for XBAT32 in ethylene biosynthesis. The abundance of the type 2 ACSs, ACS5 and ACS9, is regulated by ETO1 and ETO1-like (EOL1) broad complex/tramtrack/bric-a-brac proteins, which are substrate-recruiting components of cullin-based RING E3 ligases (Vogel et al., 1998; Chae et al., 2003; Wang et al., 2004; Yoshida et al., 2005; Christians et al., 2009). Similarly, XBAT32 may negatively regulate the abundance of ACSs to modulate ethylene production. Our results suggest a model where XBAT32 regulates lateral root production by maintaining low levels of specific ACSs and reducing ethylene synthesis (Fig. 6). The low levels of ethylene may support lateral root formation by up-regulating auxin biosynthesis, which promotes lateral root primordia initiation (Swarup et al., 2007; Ivanchenko et al., 2008). Loss of XBAT32 would result in a stabilization of ACSs and increased ethylene synthesis (Fig. 6). Increased ethylene levels may enhance auxin transport, which negatively affects auxin's ability to promote lateral root formation. The role of XBAT32 in regulating ethylene synthesis may also be responsible for the delayed growth phenotype of xbat32 plants. Similar to ethylene overproducers or constitutively active ethylene signaling mutants (Ogawara et al., 2003; Christians et al., 2009), xbat32 plants are shorter in stature than wild-type plants. However, unlike these ethylene mutants, the short stature phenotype of xbat32 is only observed early in development (Nodzon et al., 2004; this study). XBAT32 may be required to regulate ACS abundance and ethylene synthesis during early seedling and plant growth and development. Thus, the XBAT32 mutation may only affect early plant development. Other E3 ligases may regulate ACS abundance at later stages of development, allowing xbat32 plants to achieve wild-type levels of growth later in development.
Figure 6.
Model for XBAT32's role during ethylene/auxin regulation of lateral root development. XBAT32 negatively regulates ethylene biosynthesis, possibly by modulating the stability of specific ACS through ubiquitin-dependent proteasomal degradation. Loss of XBAT32 leads to stabilization of these ACSs and increased ethylene synthesis, which results in inhibition of lateral root initiation. Ethylene's role in regulating lateral root development may occur through modulation of auxin biosynthesis and transport.
Collectively, our results indicate that an overproduction of ethylene in xbat32 seedlings contributes to the lateral root defect. The mechanism by which high ethylene inhibits lateral roots in xbat32 is not yet clear; however, this study provides evidence to support the recent line of studies that suggest an interaction between ethylene and auxin in lateral root development, and it also emphasizes the essential role of E3 ligases in regulating hormonal levels during root development.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds from Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) or mutant plant lines surface sterilized with 30% (v/v) bleach and 0.1% (v/v) Triton X-100 were grown on solid GM (pH 5.7) containing 0.8% agar with half-strength Murashige and Skoog medium (Sigma-Aldrich) supplemented with 1% Suc. Sterilized seeds were stratified for 2 to 3 d in the dark at 4°C and then transferred to room temperature under continuous light. Seven-day-old seedlings were transferred to soil and grown under photoperiodic cycles of 16 h of light and 8 h of dark at 22°C.
Identification of T-DNA Insertional Plants
All T-DNA insertional lines were obtained from the Salk Institute Genomic Analysis Laboratory via the Arabidopsis Biological Resource Center (ABRC; Alonso et al., 2003). xbat34 (SAIL_395_E02), xbat32 (Salk_015002), and xbat35 (Salk_104813) are T-DNA insertion lines in At4g14365, At5g57740, and At3g23280, respectively. T-DNA homozygous plants were confirmed by PCR analysis of genomic DNA using the Extract N′ Amp kit (Sigma-Aldrich) as per the manufacturer's instructions. Genotyping was done using gene-specific primers for the wild-type allele in combination with T-DNA-specific primers for the mutant allele.
Growth Assays
For root growth assays, surface-sterilized and stratified seeds were germinated on GM and grown vertically for 3 or 5 d before transfer to GM with or without the indicated concentrations of ABA, ACC, AVG, AgNO3, CoCl2, NAA, or 2,4-D. All chemicals used were obtained from Sigma-Aldrich. Seedlings were then grown vertically for 5 d, after which time the number of lateral roots and/or primary root length was quantified. The number of lateral roots was scored with a compound light microscope. For hypocotyl growth assays, surface-sterilized and stratified seeds were germinated on GM with or without the indicated quantities of ACC, CoCl2, or AVG and grown for 3 d in the dark at room temperature. Seedlings were then photographed, and measurements of root and hypocotyl length were performed using ImageJ Image Processing and Analysis Software (Abramoff et al., 2004). All assays were repeated a minimum of four times with two replicates for each trial and at least 10 seedlings per replicate.
Ethylene Production Measurements
Surface-sterilized and stratified seeds were germinated and grown on GM for 3 d before transfer to vials containing liquid GM. Each vial contained 50 seedlings. After 3 d, vials were sealed and seedlings were grown for an additional 3 d. The accumulated ethylene in each vial was measured by gas chromatography (3900 gas chromatograph; Varian) on the 4th d after sealing. An injection volume of 1 mL was used. An ethylene standard (μL L−1; Praxair) was used as a positive control. Assays were carried out at two separate times with two replicates per sample. Measurements were done in triplicate for each vial. Results from each vial were represented as means of the three readings. Ethylene concentration was calculated per milligram of seedling weight.
Yeast Two-Hybrid Analysis
XBAT32, ACS2, ACS4, ACS6, ACS7, and ACS11 cDNAs were obtained from the ABRC. To create XBAT32-AA, Phusion site-directed mutagenesis (New England Biolabs) was used to mutate RING domain metal ligand residues Cys-336 and His-338 to Ala. cDNAs were introduced into the Gateway entry vector pDONR201 (Invitrogen). XBAT32 and XBAT32-AA were then introduced into the bait Gateway destination vector pNLexAattR containing the LexA DNA binding domain as well as a nuclear localization signal. ACS2, ACS4, ACS6, ACS7, and ACS11 cDNAs were introduced into the prey Gateway destination vector pJZ4attR, which contains the activation domain and a Gal-inducible promoter. Sequence-verified yeast two-hybrid Gateway destination vectors were a gift from the Finley laboratory (Wayne State University; Serebriiskii et al., 2001). The reporter plasmid pSH18-34 was first transformed into yeast stain EGY48 using the TRAFO Protocol (Agatep et al., 1998), followed sequentially by the bait and prey vectors. Transformed yeast were selected and maintained on dropout medium (−His/uracil/Trp; BioShop Canada). To detect an interaction, yeast colonies containing all three plasmids were streaked onto medium containing Gal/raffinose and 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside (BioShop Canada). Binding of activation domain fusion proteins to binding domain fusion proteins was detected by the presence of a blue color produced by the metabolism of 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside by β-galactosidase, the protein product of the reporter gene.
In Vitro Ubiquitination Assays
XBAT32, ACS4, and ACS7 cDNAs were cloned via Gateway into the pDEST565 or modified pDEST527 (a FLAG tag was inserted into the vector to create a Flag-His tag) vectors to obtain His-GST- or Flag-His-tagged fusion proteins, respectively. pDEST565 and pDEST527 were obtained from Addgene, plasmids 11520 and 11518, respectively (plasmids donated by Dominic Esposito, National Cancer Institute). Fusion proteins were expressed in Escherichia coli strain Rosetta (DE3) and purified using nickel-charged resin (Bio-Rad) according to the manufacturer's protocols. Ubiquitination assays were performed as described previously (Stone et al., 2006). Briefly, reactions (30 μL) containing 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 0.05 mm ZnCl2, 1 mm ATP (Sigma-Aldrich), 0.2 mm dithiothreitol, 10 mm phosphocreatine, 0.1 unit of creatine kinase (Sigma-Aldrich), 50 ng of yeast E1 (BostonBiochem), 250 ng of purified 6× His-AtUBC8 (E2), 2 μg of ubiquitin (BostonBiochem), 500 ng of His-GST-XBAT32, and 500 ng of Flag-His-ACSs were incubated at 30°C for 2 h. Reactions were stopped by adding sample buffer (125 mm Tris-HCl, pH 6.8, 20% [v/v] glycerin, 4% [w/v] SDS, and 10% [v/v] β-mercaptoethanol) and analyzed by SDS-PAGE followed by protein gel blotting using anti-Flag antibodies.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: At4g14365, DQ086842; At5g57740, NM_125157.3; At3g23280, DQ086844.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Phenotypic analysis of xbat mutants.
Supplemental Figure S2. Comparison of wild-type Col-0 and xbat32 response to ABA.
Supplemental Figure S3. Inhibition of ethylene signaling in xbat32.
Supplementary Material
Acknowledgments
We thank Drs. D. Goring and K. Dreher for invaluable comments and suggestion on the manuscript, Dr. A. Gunawardena for use of the Reichert-Jung Polyvar compound microscope, Dr. R. Prange and P. Harrison for use of the gas chromatograph, and the ABRC for Arabidopsis cDNAs and seeds.
References
- Abramoff MD, Magalhaes P, Ram SJ. (2004) Image processing with ImageJ. Biophoton Int 11: 36–43 [Google Scholar]
- Agatep R, Kirkpatrick RD, Parchaliuk DL, Woods RA, Gietz RD. (1998) Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Technical Tips Online. http://tto.trends.com (August 15, 2009)
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Beyer EM. (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58: 268–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Cervera M, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Onckelen H, Montagu M, Inzé D. (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Weissman AM. (1998) Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu Rev Cell Dev Biol 14: 19–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P. (2003) The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ, et al. (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL Jr, Grisafi PL, Fink GR. (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. (2003) The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Chiang MH, Hwang SG, Lin PC. (2009) Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Mol Biol 71: 61–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilley PM, Casson SA, Tarkowski P, Hawkins N, Wang KLC, Hussey PJ, Beale M, Ecker JR, Sandberg GK, Lindsey K. (2006) The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell 18: 3058–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians MJ, Gingerich DJ, Hansen M, Binder BM, Kieber JJ, Vierstra RD. (2009) The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J 57: 332–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates JC, Laplaze L, Haseloff J. (2006) Armadillo-related proteins promote lateral root development in Arabidopsis. Proc Natl Acad Sci USA 103: 1621–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361 [DOI] [PubMed] [Google Scholar]
- De Smet I, Vanneste S, Inzé D, Beeckman T. (2006a) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60: 871–887 [DOI] [PubMed] [Google Scholar]
- De Smet I, Zhang H, Inzé D, Beeckman T. (2006b) A novel role for abscisic acid emerges from underground. Trends Plant Sci 11: 434–439 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. (2005) The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dittmer HJ. (1937) A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale). Am J Bot 24: 417–420 [Google Scholar]
- Dong L, Wang L, Zhang Y, Zhang Y, Deng X, Xue Y. (2006) An auxin-inducible F-box protein CEGENDUO negatively regulates auxin-mediated lateral root formation in Arabidopsis. Plant Mol Biol 60: 599–615 [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124: 1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M. (2005) Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J 44: 382–395 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Okushima Y, Tasaka M. (2007) Auxin-mediated lateral root formation in higher plants. Int Rev Cytol 256: 111–137 [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tameda S, Masuda H, Tasaka M. (2002) Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 29: 153–168 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P. (2001) Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr Opin Plant Biol 4: 387–391 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chen F, Del Casino C, Autino A, Shen M, Yuan S, Peng J, Shi H, Wang C, Cresti M, et al. (2006) An ankyrin repeat-containing protein, characterized as a ubiquitin ligase, is closely associated with membrane-enclosed organelles and required for pollen germination and pollen tube growth in lily. Plant Physiol 140: 1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Roberts JA. (2002) Role of ethylene in coordinating root growth and development. Waisel Y, Eshel A, Kafkafi U, , Plant Roots: The Hidden Half; Marcel Dekker, New York, pp 449–459 [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG. (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55: 335–347 [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. (1995) A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7: 2023–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R. (2006) Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol 47: 788–792 [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. (2001) In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol 125: 519–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNoble ME, Spollen WG, Sharp RE. (2004) Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot 55: 237–245 [DOI] [PubMed] [Google Scholar]
- Liang Y, Mitchell DM, Harris JM. (2007) Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Dev Biol 304: 297–307 [DOI] [PubMed] [Google Scholar]
- Lovering R, Hanson IM, Borden KLB, Martin S, O'Reilly NJ, Evan GI, Rahman D, Pappin DJC, Trowsdale J, Freemont PS. (1993) Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci USA 90: 2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55: 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Gibbs DJ, Coates JC. (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179: 595–614 [DOI] [PubMed] [Google Scholar]
- Nodzon LA, Xu WH, Wang Y, Pi LY, Chakrabarty PK, Song WY. (2004) The ubiquitin ligase XBAT32 regulates lateral root development in Arabidopsis. Plant J 40: 996–1006 [DOI] [PubMed] [Google Scholar]
- Ogawara T, Higashi K, Kamada H, Ezura H. (2003) Ethylene advances the transition from vegetative growth to flowering in Arabidopsis thaliana. J Plant Physiol 160: 1335–1340 [DOI] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. (2007) ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118: 1369–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager KM, Calderon-Villalobos LI, Dohmann EM, Willige BC, Knierer S, Nill C, Schwechheimer C. (2007) Characterization of the VIER F-BOX PROTEINE genes from Arabidopsis reveals their importance for plant growth and development. Plant Cell 19: 1163–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebriiskii IG, Toby GG, Finley RL, Golemis EA. (2001) Genomic analysis utilizing the yeast two-hybrid system. Methods Mol Biol 175: 415–454 [DOI] [PubMed] [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol 122: 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Callis J. (2007) Ubiquitin ligases mediate growth and development by promoting protein death. Curr Opin Plant Biol 10: 624–632 [DOI] [PubMed] [Google Scholar]
- Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J. (2005) Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 137: 13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Williams LA, Farmer LM, Vierstra RD, Callis J. (2006) KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 18: 3415–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. (1998) Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KLC, Yoshida H, Lurin C, Ecker JR. (2004) Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428: 945–950 [DOI] [PubMed] [Google Scholar]
- Wang YS, Pi LY, Chen X, Chakrabarty PK, Jiang J, De Leon AL, Liu GZ, Li L, Benny U, Oard J, et al. (2006) Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. Plant Cell 18: 3635–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM. (2001) Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2: 169–178 [DOI] [PubMed] [Google Scholar]
- Xie Q, Frugis G, Colgan D, Chua NH. (2000) Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev 14: 3024–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Guo HS, Dallman G, Fang S, Weissman AM, Chua NH. (2002) SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature 419: 167–170 [DOI] [PubMed] [Google Scholar]
- Xu X, Jiang CZ, Donnelly L, Reid MS. (2007) Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J Exp Bot 58: 3623–3630 [DOI] [PubMed] [Google Scholar]
- Yang X, Sun C, Hu Y, Lin Z. (2008) Molecular cloning and characterization of a gene encoding RING zinc finger ankyrin protein from drought-tolerant Artemisia desertorum. J Biosci 33: 103–112 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nagata M, Saito K, Wang KL, Ecker JR. (2005) Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.