Abstract
Nodulation of soybean (Glycine max) root hairs by the nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum is a complex process coordinated by the mutual exchange of diffusible signal molecules. A metabolomic study was performed to identify small molecules produced in roots and root hairs during the rhizobial infection process. Metabolites extracted from roots and root hairs mock inoculated or inoculated with B. japonicum were analyzed by gas chromatography-mass spectrometry and ultraperformance liquid chromatography-quadrupole time of flight-mass spectrometry. These combined approaches identified 2,610 metabolites in root hairs. Of these, 166 were significantly regulated in response to B. japonicum inoculation, including various (iso)flavonoids, amino acids, fatty acids, carboxylic acids, and various carbohydrates. Trehalose was among the most strongly induced metabolites produced following inoculation. Subsequent metabolomic analyses of root hairs inoculated with a B. japonicum mutant defective in the trehalose synthase, trehalose 6-phosphate synthase, and maltooligosyltrehalose synthase genes showed that the trehalose detected in the inoculated root hairs was primarily of bacterial origin. Since trehalose is generally considered an osmoprotectant, these data suggest that B. japonicum likely experiences osmotic stress during the infection process, either on the root hair surface or within the infection thread.
The interaction between legumes (Leguminosae) and bacteria in the family Rhizobiaceae leads to the development of a nitrogen-fixing symbiosis. The development of this symbiotic process is complex and is coordinately regulated by the mutual exchange of diffusible signal molecules. Flavonoids, either in root exudates or released from the seed coat during germination, act as specific inducers of the nodulation genes in compatible rhizobia (Subramanian et al., 2007). These bacterial nodulation genes are involved in the production of lipochitooligosaccharide Nodulation (Nod) factors that are essential for inducing root hair deformation and subsequent nodule formation (Oldroyd and Downie, 2008). In addition to these key regulatory molecules, a variety of other compounds, including exopolysaccharides, plant hormones, and vitamins, have been implicated as regulators of the nodulation process (Matamoros et al., 2006; Gibson et al., 2008; Ding and Oldroyd, 2009).
A significant change in the metabolism of both the symbiont and host clearly occurs during the infection process and subsequent nodule development. During the earliest stages of the infection process, the invading rhizobia trigger root hair deformation, depolarization of the cell membrane, cytoskeleton reorientation, calcium spiking, cortical cell division, and the expression of a variety of plant genes (Stacey et al., 2006). Formation of the infection thread, by which the rhizobia gain entry into the plant cell, also clearly requires a drastic change in plant cell metabolism to support the formation of additional cell wall and membrane components. Roth and Stacey (1989) previously estimated that membrane biosynthesis must be elevated 35-fold to support the formation of new membranes during the infection process and nodule development. Plant metabolism must also change to provide carbon sources to support ATP synthesis and to respond to the provision of fixed nitrogen by the symbiont.
Previous studies utilized transcriptomic and proteomic approaches to examine the global changes occurring during legume nodulation. Collectively, these studies identified numerous genes and proteins involved in carbon and nitrogen metabolism, plant defense responses, nutrient exchange, and signal transduction that are significantly regulated in Medicago truncatula, Lotus japonicus, soybean (Glycine max), and Phaseolus vulgaris colonized by their respective rhizobial symbionts (Lohar et al., 2006; Kuster et al., 2007; Benedito et al., 2008; Brechenmacher et al., 2008; Meschini et al., 2008; for review, see Stacey et al., 2006).
Metabolomic approaches have been successfully used to study a variety of plant-pathogen interactions. For example, published studies profiled the metabolomic response of plants infected by pathogenic fungi (Magnaporthe grisea, Sclerotinia sclerotiorum, Penicillium strains, Mycosphaerella graminicola), oomycetes (Pythium sylvaticum), bacteria (Candidatus liberibacter, Pseudomonas syringae, phytoplasma), viruses (Tobacco mosaic virus), and insects (Frankliniella occidentalis; Schmelz et al., 2004; Overy et al., 2006; Jobic et al., 2007; Cevallos-Cevallos et al., 2009; Leiss et al., 2009; Parker et al., 2009; for review, see Allwood et al., 2008). However, few studies have examined the plant metabolome in response to rhizobial infection. Various amino acids (Asn, Gln, Pro), organic acids (threonic acid), sugars (Rib, maltose), and polyols (mannitol) were shown to be more abundant in L. japonicus and M. sativa nodules than in the corresponding roots (Colebatch et al., 2004; Desbrosses et al., 2005; Barsch et al., 2006). However, to our knowledge, no previous studies have sought to characterize metabolites responding during the earliest stages of the rhizobial infection process (e.g. within the first 48 h), when key signaling events are occurring and both host and symbiont are transitioning into the symbiotic state.
In this study, metabolites extracted from soybean roots and root hairs either mock inoculated or inoculated with Bradyrhizobiun japonicum were analyzed by gas chromatography-mass spectrometry (GC-MS) and ultraperformance liquid chromatography-quadrupole time of flight-mass spectrometry (UPLC-QTOF-MS). This analysis resulted in the identification of 166 small molecules whose accumulation was significantly affected by B. japonicum inoculation. Among those compounds showing the most significant response to inoculation was trehalose, which was produced by the invading rhizobium, likely as a response to conditions of osmotic stress.
RESULTS AND DISCUSSION
The aim of this research was to identify metabolites that were significantly regulated in root hairs in response to B. japonicum inoculation. The metabolomic profiles of root hairs and stripped roots, roots from which root hairs were removed, were compared from 0 to 48 h after inoculation with B. japonicum. Mock-inoculated root hairs and stripped root samples served as comparative controls.
Light microscopy was used to estimate the purity and quality of the root hair preparations, as described by Wan et al. (2005) and Brechenmacher et al. (2009). Little or no contamination by epidermal cells was observed. Four independent biological replicates were produced for each time point, and extracted metabolites were analyzed using GC-MS and UPLC-QTOF-MS.
Metabolites Detected in Root Hairs and Stripped Roots
Polar and nonpolar extracts of root hairs and stripped roots were analyzed by GC-MS. A total of 422 and 459 compounds were detected in stripped roots and root hairs from the soluble and lipophilic fractions, respectively (Table I; Supplemental Table S1). However, only 199 of these compounds could be tentatively identified based on available standards and literature data (Supplemental Table S1). Polar metabolites included nucleotides, amino acids, alcohols, organic acids, and sugars, while nonpolar metabolites included fatty acids, sterols, and long-chain alcohols.
Table I. Total number of known and unknown metabolites identified in root hairs and stripped roots by GC-MS (polar and nonpolar) and by UPLC-QTOF-MS (LC-MS).
| Metabolite | GC-MS Polar | GC-MS Polar | GC-MS Nonpolar | GC-MS Nonpolar | LC-MS | LC-MS | Total |
| No. | % | No. | % | No. | % | No. | |
| Known | 123 | 29.1 | 76 | 16.6 | 435 | 25.1 | 634 |
| Unknown | 299 | 70.9 | 383 | 83.4 | 1,294 | 74.9 | 1,976 |
| Total | 422 | 100 | 459 | 100 | 1,729 | 100 | 2,610 |
Due to their relatively low abundance and labile nature, secondary metabolites were examined by UPLC-QTOF-MS, since these compounds are not easily identified by GC-MS. This approach identified 1,729 small molecules in root hairs and stripped roots, of which about 25% could be identified using analytical standards (Table I; Supplemental Table S1). The secondary metabolites identified included flavonoids, isoflavonoids, and triterpenoids.
Collectively, the GC-MS and UPLC-QTOF-MS analyses detected a total of 2,610 small molecules in root hairs and stripped roots, of which 634 could be identified as known compounds. Each compound was characterized by its distinct retention time and mass-to-charge ratio (m/z). No metabolites that were specifically expressed only in root hairs or stripped roots were identified using these analyses.
Metabolites Significantly Regulated in Root Hairs in Response to B. japonicum Inoculation
Statistical analysis was performed to identify metabolites significantly regulated in root hairs colonized by B. japonicum. Bacterial inoculation significantly modified the accumulation of 166 metabolites in root hairs (114 were more abundant after inoculation, 50 were less abundant, and two were more or less abundant at different time points of the time course; Table II; Supplemental Table S2). Out of these 166 small molecules, 48 could be identified using standards (Table III). The analytical methods used could not easily distinguish those metabolites produced by the bacterium from those produced by the plant. However, a bacterial or plant origin was hypothesized for the identified metabolites based on their expression levels, the B. japonicum and soybean genome annotation, and literature mining (Supplemental Table S2). A metabolomic analysis of a bacterial liquid culture cannot identify the origin of the small molecules from this study, since B. japonicum metabolism is greatly affected during the development of the symbiosis. All 48 of the metabolites responding significantly to bacterial inoculation were detected in mock-inoculated root hairs and, therefore, are likely of plant origin. However, the B. japonicum genome encodes for enzymes capable of catalyzing the synthesis of 11 metabolites found significantly regulated in B. japonicum-colonized root hairs (Supplemental Table S2). Except for the trehalose that was analyzed in more detail (see below), none of the bacterial genes possibly involved in the synthesis of the 10 compounds was found regulated in B. japonicum treated with genistein, an isoflavone able to induce nod gene expression, and during nodule development (Pessi et al., 2007; Lang et al., 2008), indicating that these 10 compounds are most likely of plant origin. The fold change for each metabolite after inoculation, the P value, and the se for each regulated metabolite at each time point are indicated in Supplemental Table S2. Principal component analysis clearly segregated metabolites that were significantly regulated in root hairs and stripped roots, providing further evidence for the purity of the root hair preparations analyzed (Supplemental Fig. S1). These data also argue for a distinct set of metabolites that are regulated in root hairs in response to inoculation.
Table II. Distribution of metabolites significantly regulated in roots hairs in response to B. japonicum inoculation identified by GC-MS in polar (p) and nonpolar (np) extracts and by UPLC-QTOF-MS (LC-MS).
The number of known and unknown compounds is indicated. The activation (up), the repression (down), or dual regulation (activation at one time point and repression at another time point; both) are mentioned.
| Method | All Compounds | Total Known | Total Unknown | Up Known | Down Known | Both Known | Up Unknown | Down Unknown |
| LC-MS | 93 | 31 | 62 | 22 | 7 | 2 | 48 | 14 |
| GC-MS np | 33 | 3 | 30 | 3 | 0 | 0 | 27 | 3 |
| GC-MS p | 40 | 14 | 26 | 8 | 6 | 0 | 6 | 20 |
| Total | 166 | 48 | 118 | 33 | 13 | 2 | 81 | 37 |
Table III. Metabolites significantly regulated in root hairs in response to B. japonicum.
The predicted compound origin, the P value (pVal; comparison of inoculated versus uninoculated condition), and the fold change ratio (FC; inoculated/uninoculated) for each time point (t0, t12, t18, t24, t36, and t48 hours after inoculation with the symbiotic bacterium) are indicated. Metabolite abundance was also statistically analyzed by combining all the time points and comparing the metabolite abundance in all inoculated conditions versus all uninoculated conditions (pVal Comb). Significant changes are indicated in boldface.
| Compound | Metabolite Origin | pVal t0 | FC t0 | pVal t12 | FC t12 | pVal t18 | FC t18 | pVal t24 | FC t24 | pVal t36 | FC t36 | pVal t48 | FC t48 | pVal Comb |
| (Iso)flavonoid | ||||||||||||||
| Amino-flavonoid | Plant | 5.2E-02 | 2.5 | 2.4E-02 | 4.0 | 4.4E-04 | 4.8 | 5.2E-05 | 3.6 | 1.5E-03 | 2.9 | 1.6E-04 | 3.7 | 2.0E-13 |
| Aureusidin | Plant | 9.3E-01 | 1.2 | 3.5E-01 | 2.8 | 7.1E-01 | 1.3 | 7.7E-01 | 1.5 | 6.4E-01 | 1.3 | 2.8E-03 | 0.5 | 9.1E-01 |
| Isoliquiritigenin | Plant | 8.9E-01 | 1.0 | 2.8E-01 | 1.1 | 3.2E-03 | 1.7 | 3.3E-03 | 1.6 | 1.4E-03 | 1.4 | 1.1E-02 | 1.4 | 1.2E-08 |
| Apigenin 7-glucoronide | Plant | 3.8E-01 | 2.6 | 9.1E-01 | 1.4 | 1.3E-02 | 1.4 | 5.3E-01 | 1.0 | 7.4E-01 | 1.2 | 1.9E-02 | 0.7 | 3.8E-01 |
| Dihydrokaempferol b | Plant | 2.2E-01 | 0.8 | 1.7E-01 | 2.3 | 3.1E-01 | 1.4 | 7.8E-01 | 1.1 | 3.3E-01 | 1.7 | 1.3E-01 | 1.9 | 7.9E-03 |
| Afrormosin | Plant | 3.5E-01 | 0.9 | 3.0E-01 | 1.3 | 1.2E-02 | 1.5 | 5.5E-01 | 1.3 | 7.7E-02 | 1.4 | 1.4E-01 | 1.4 | 5.5E-03 |
| Genkwanin a | Plant | 9.0E-01 | 1.2 | 7.8E-02 | 1.5 | 1.4E-03 | 2.9 | 5.9E-01 | 1.6 | 1.7E-01 | 1.4 | 1.5E-01 | 1.3 | 5.2E-04 |
| Prunetin a | Plant | 3.0E-01 | 1.9 | 8.4E-02 | 1.5 | 5.6E-04 | 2.0 | 5.0E-01 | 1.6 | 1.8E-01 | 1.3 | 1.7E-01 | 1.3 | 9.2E-04 |
| Liquiritigenin | Plant | 8.2E-01 | 1.0 | 1.6E-01 | 1.1 | 8.7E-03 | 1.3 | 2.9E-01 | 1.2 | 4.0E-01 | 1.1 | 1.7E-01 | 1.2 | 2.7E-03 |
| Liquiritin | Plant | 8.1E-01 | 1.0 | 1.4E-01 | 1.1 | 7.5E-03 | 1.3 | 2.8E-01 | 1.2 | 3.9E-01 | 1.1 | 1.8E-01 | 1.2 | 2.4E-03 |
| Sissotrin | Plant | 1.1E-01 | 0.9 | 4.8E-01 | 1.0 | 1.4E-01 | 1.2 | 1.5E-01 | 1.3 | 2.3E-01 | 1.2 | 1.9E-01 | 1.5 | 4.1E-03 |
| Isorhamnetin 7-α-d-glucosamine | Plant | 6.7E-01 | 1.3 | 5.5E-01 | 1.0 | 2.5E-01 | 1.6 | 7.2E-03 | 0.5 | 5.5E-01 | 1.6 | 2.3E-01 | 2.1 | 7.8E-01 |
| Apigenin | Plant | 7.7E-01 | 1.0 | 2.0E-07 | 5.0 | 4.1E-02 | 4.4 | 8.8E-01 | 1.1 | 9.2E-01 | 1.0 | 3.1E-01 | 0.9 | 1.8E-01 |
| Prunetin b | Plant | 4.4E-02 | 0.6 | 8.4E-01 | 1.1 | 5.9E-05 | 4.7 | 5.4E-01 | 1.5 | 5.4E-01 | 0.8 | 4.0E-01 | 1.9 | 3.2E-02 |
| Delphinidin 3-rhamnoside-5-glucoside | Plant | 5.6E-01 | 0.9 | 2.0E-01 | 0.7 | 2.0E-01 | 0.5 | 1.7E-02 | 0.3 | 2.6E-01 | 0.7 | 6.0E-01 | 1.2 | 6.5E-03 |
| Naringenin 4′-O-glucoside | Plant | 2.0E-01 | 9.6 | 1.5E-02 | 1.4 | 3.4E-02 | 1.6 | 4.1E-01 | 1.1 | 4.0E-02 | 1.4 | 6.9E-01 | 1.1 | 6.1E-02 |
| Fatty acid | ||||||||||||||
| Lignoceric acid | Plant | 2.8E-02 | 0.8 | 3.2E-01 | 1.1 | 8.7E-02 | 1.4 | 3.2E-01 | 1.1 | 3.8E-01 | 1.1 | 3.7E-03 | 1.3 | 1.1E-02 |
| α-Eleosteric acid | Plant | 3.7E-01 | 1.2 | 6.1E-01 | 1.0 | 5.6E-01 | 1.1 | 6.7E-01 | 1.1 | 8.3E-01 | 0.9 | 6.0E-03 | 1.5 | 4.5E-01 |
| α-Linolenic acid | Plant | 3.5E-01 | 1.1 | 8.0E-01 | 1.0 | 3.7E-01 | 1.2 | 4.0E-01 | 0.9 | 6.6E-01 | 0.9 | 8.4E-03 | 1.5 | 5.7E-01 |
| Octadecenoic fatty acid | Plant | 6.2E-02 | 15.1 | 3.2E-02 | 2.6 | 5.2E-04 | 4.3 | 1.1E-02 | 2.6 | 3.5E-03 | 3.2 | 8.5E-03 | 3.3 | 1.0E-10 |
| Lauric acid | Plant/bacteria | 2.5E-01 | 0.8 | 5.9E-02 | 0.8 | 7.2E-01 | 1.0 | 2.8E-01 | 0.9 | 1.8E-01 | 0.9 | 3.3E-01 | 0.9 | 6.7E-03 |
| Myristic acid | Plant/bacteria | 1.8E-01 | 0.9 | 1.8E-02 | 0.9 | 3.5E-01 | 0.9 | 4.2E-01 | 0.9 | 2.2E-01 | 0.9 | 4.0E-01 | 0.9 | 6.2E-03 |
| Amino acid | ||||||||||||||
| l-Aspartic acid, N, O, O-TMS | Plant/bacteria | 7.1E-02 | 15.5 | 2.9E-05 | 5.0 | 6.4E-02 | 15.4 | 1.6E-03 | 7.4 | 3.8E-03 | 7.0 | 2.3E-03 | 6.4 | 1.0E-07 |
| l-Aspartic acid, O, O-TMS | Plant/bacteria | 7.2E-02 | 3.6 | 6.8E-05 | 5.4 | 5.9E-02 | 7.3 | 1.3E-02 | 9.7 | 1.7E-05 | 6.3 | 2.2E-02 | 7.9 | 5.7E-09 |
| l-Aspartic acid, 4TMS | Plant/bacteria | 4.3E-01 | 1.3 | 1.7E-01 | 1.6 | 7.8E-02 | 2.8 | 2.0E-02 | 3.5 | 9.9E-04 | 2.3 | 6.9E-02 | 3.6 | 8.2E-05 |
| 4-Aminobutyric acid, N, O, O-TMS | Plant | 1.3E-01 | 1.6 | 2.9E-01 | 0.8 | 7.6E-01 | 1.0 | 2.6E-03 | 0.7 | 2.9E-01 | 0.9 | 1.2E-01 | 0.8 | 2.1E-02 |
| l-Glutamic acid, O, O-TMS | Plant/bacteria | 1.3E-01 | 1.6 | 9.7E-01 | 1.2 | 6.8E-01 | 1.4 | 2.6E-03 | 0.7 | 5.5E-01 | 1.0 | 1.2E-01 | 0.8 | 1.2E-01 |
| Proline, N, O-TMS | Plant/bacteria | 7.8E-01 | 1.2 | 7.0E-01 | 1.0 | 1.5E-01 | 0.8 | 4.7E-01 | 1.1 | 4.8E-03 | 0.7 | 1.4E-01 | 0.8 | 1.0E-01 |
| Carboxylic acid | ||||||||||||||
| Lactic acid, O, O-TMS | Plant/bacteria | 6.6E-01 | 5.0 | 6.3E-02 | 70.1 | 5.0E-03 | 54.9 | 1.2E-02 | 19.0 | 1.2E-01 | 10.8 | 7.6E-03 | 64.9 | 3.2E-08 |
| Benzoic acid, O-TMS | Plant | 9.8E-01 | 1.1 | 2.4E-01 | 1.5 | 4.9E-01 | 1.2 | 7.7E-03 | 1.4 | 7.3E-01 | 1.0 | 2.4E-01 | 0.9 | 2.2E-01 |
| Sydnic acid | Plant | 1.4E-01 | 0.8 | 5.6E-01 | 1.3 | 4.1E-02 | 0.6 | 7.6E-01 | 2.0 | 3.4E-02 | 2.4 | 5.5E-01 | 1.3 | 6.6E-01 |
| Threonic acid, O, O, O, O-TMS | Plant | 2.4E-01 | 1.7 | 2.3E-02 | 1.6 | 2.5E-02 | 1.6 | 6.0E-01 | 1.1 | 7.7E-01 | 1.2 | 9.4E-01 | 1.0 | 1.6E-01 |
| Disaccharide | ||||||||||||||
| Trehalose, 8TMS | Plant/bacteria | 1.2E-01 | 1.4 | 5.2E-05 | 15.6 | 4.9E-05 | 17.6 | 3.8E-03 | 7.8 | 3.0E-03 | 32.7 | 9.9E-04 | 27.4 | 6.1E-11 |
| Maltose monohydrate MEOX1, TMS | Plant | 5.7E-03 | 1.8 | 2.1E-01 | 2.9 | 1.1E-01 | 7.0 | 4.6E-01 | 1.7 | 2.8E-03 | 32.6 | 4.9E-02 | 19.3 | 1.9E-04 |
| Maltose methoxyamine | Plant | 2.1E-03 | 1.6 | 3.6E-03 | 1.6 | 6.2E-03 | 1.8 | 6.0E-01 | 1.0 | 9.4E-03 | 1.8 | 2.3E-01 | 1.3 | 4.2E-03 |
| Glucosinolate | ||||||||||||||
| 4-Hydroyxindol-3-yl-methyl-glucosinolate | Plant | 3.2E-01 | 1.8 | 2.3E-02 | 0.6 | 7.9E-01 | 1.2 | 2.2E-01 | 2.5 | 6.0E-01 | 1.1 | 3.0E-02 | 2.1 | 2.7E-01 |
| 7-Methylsulfinyl-n-heptyl-glucosinolate | Plant | 7.7E-01 | 1.6 | 7.7E-01 | 1.3 | 9.1E-03 | 5.5 | 2.0E-02 | 0.5 | 8.4E-01 | 1.2 | 9.1E-02 | 0.6 | 4.4E-01 |
| Other | ||||||||||||||
| Beiwutine | Plant | 6.1E-01 | 0.9 | 1.0E-01 | 0.6 | 6.1E-01 | 1.4 | 1.9E-01 | 0.7 | 1.8E-01 | 0.8 | 2.1E-04 | 2.7 | 2.3E-01 |
| O-Methyl inositol, 5TMS | Plant | 6.0E-02 | 0.6 | 9.2E-01 | 4.8 | 5.8E-03 | 0.2 | 8.9E-01 | 6.0 | 2.6E-01 | 0.9 | 2.0E-01 | 0.4 | 3.0E-02 |
| Ascochalasin | Plant | 8.4E-01 | 1.0 | 3.8E-01 | 0.7 | 9.1E-01 | 1.2 | 3.6E-02 | 1.6 | 6.0E-01 | 1.1 | 2.8E-02 | 0.7 | 7.5E-01 |
| 3,3′,4,4′5-Pentahydroxystilbene | Plant | 7.5E-01 | 1.4 | 8.8E-01 | 1.1 | 3.9E-04 | 2.4 | 4.8E-01 | 1.4 | 4.2E-01 | 1.3 | 2.9E-01 | 1.6 | 9.7E-03 |
| 4’,5′-Trihydroxy-3′,6′′,6′′-trimetylpyranol (2′′,3′′:6′′,5′′)dihydrochalcone | Plant | 1.2E-01 | 0.8 | 1.7E-01 | 3.0 | 6.2E-03 | 2.3 | 2.7E-01 | 0.8 | 3.5E-01 | 0.8 | 7.8E-01 | 0.9 | 8.9E-01 |
| Neoxanthin | Plant | 4.7E-01 | 1.3 | 5.2E-01 | 2.3 | 3.8E-01 | 0.8 | 1.7E-01 | 0.6 | 3.2E-03 | 0.6 | 1.1E-01 | 0.6 | 1.5E-02 |
| Medicagenic acid 3-O-triglucoside | Plant | 4.4E-01 | 2.6 | 1.2E-01 | 7.2 | 4.1E-01 | 1.8 | 3.6E-01 | 0.8 | 8.1E-03 | 2.3 | 4.9E-01 | 1.5 | 3.6E-02 |
| α-Glycerophosphate ester, TMS | Plant | 8.1E-01 | 1.2 | 2.4E-02 | 1.7 | 3.0E-01 | 1.6 | 5.4E-01 | 1.7 | 3.7E-02 | 3.1 | 5.9E-01 | 1.2 | 1.9E-02 |
| Cytosine, N, N-TMS | Plant/bacteria | 2.2E-01 | 0.6 | 2.2E-01 | 0.8 | 1.6E-02 | 0.5 | 3.3E-01 | 1.0 | 9.0E-02 | 0.7 | 5.8E-01 | 0.9 | 2.5E-03 |
| Xylose methoxyamine | Plant/bacteria | 4.5E-02 | 2.0 | 4.4E-01 | 0.9 | 3.4E-01 | 1.2 | 5.4E-01 | 1.0 | 6.7E-01 | 1.0 | 8.6E-03 | 0.8 | 3.3E-01 |
| Rha-Hex-Hex-Hex-gypsogenic acid | Plant | 2.5E-01 | 1.3 | 2.7E-01 | 1.1 | 7.0E-03 | 1.3 | 9.4E-01 | 1.0 | 1.6E-02 | 1.3 | 2.1E-01 | 1.1 | 5.9E-02 |
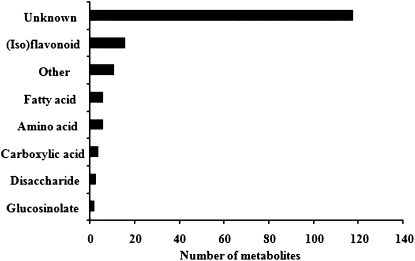
Metabolites were classified into various functional categories. Many compounds, including various flavonoids and isoflavonoids, amino acids, carboxylic acids, fatty acids, and carbohydrates, were found to be significantly regulated in root hairs colonized by B. japonicum (Fig. 1). Their possible functions during the nodulation process are discussed below. The fold change ratios and statistical values of all these compounds are summarized in Table III.
Figure 1.
Distribution of root hair metabolites regulated in response to B. japonicum and identified by GC-MS and UPLC-QTOF-MS into eight functional categories.
(Iso)flavonoids
Sixteen metabolites belonging to the (iso)flavonoid family were clearly responsive to inoculation by B. japonicum. Bacterial inoculation triggered the induction in root hairs of afrormosin (18 h), amino-flavonoid (12, 18, 24, 36, 48 h), apigenin (12, 18 h), dihydrokaempferol b, genkwanin a (18 h), naringenin 4′-O-glucoside b (12, 18, 36 h), liquiritin (18 h), liquiritigenin (18 h), isoliquiritigenin (18, 24, 36, 48 h), sissotrin, and prunetins (18 h; Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). In contrast, the accumulation of apigenin 7-glucoronide (48 h), aureusidin (48 h), isorhamnetin 7-α-d-glucosamine (24 h), and delphinidin 3-rhamnoside-5-glucoside (24 h) was reduced in root hairs upon B. japonicum inoculation. Moreover, none of these compounds was significantly regulated in stripped roots in response to bacterial inoculation.
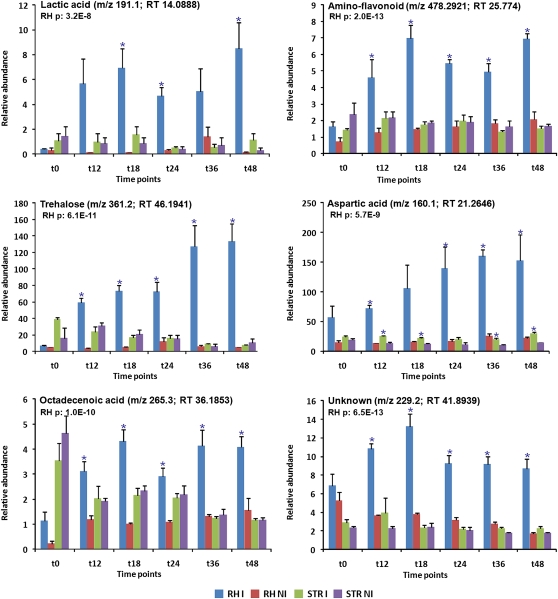
Figure 2.
Relative abundance of lactic acid, amino-flavonoid, trehalose, Asp, octadecenoic acid, and an unknown compound in root hairs (RH) and stripped roots (STR) 0, 12, 18, 24, 36, and 48 h after inoculation with the symbiotic bacterium B. japonicum (I) or mock inoculated (NI). Asterisks indicate significantly regulated compounds colonized by B. japonicum. se values are also indicated.
The (iso)flavonoid class of compounds is of particular interest since they are essential for the infection process and are known to induce the nodulation genes in Bradyrhizobium (Subramanian et al., 2007). Daidzein and genistein are soybean isoflavones required for the establishment of the nitrogen-fixing symbiosis (Subramanian et al., 2006). Although these two isoflavones were detected in root hairs and stripped roots, their levels were not significantly elevated in response to B. japonicum inoculation. In contrast, several other (iso)flavonoids (listed above) were significantly increased in root hairs inoculated by B. japonicum (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). Liquiritigenin, isoliquiritigenin, apigenin, and prunetin were reported to induce nod gene expression in Sinorhizobium meliloti, Sinorhizobium medicae, Rhizobium leguminosarum, or B. japonicum (Kape et al., 1992; Zuanazzi et al., 1998; Broughton et al., 2000; Zhang and Cheng, 2006; Yokoyama, 2008; Watkin et al., 2009). The abundance of liquiritigenin and isoliquiritigenin was increased in P. vulgaris root exudates when common bean was inoculated with R. leguminosarum in comparison with the mock-inoculated control (Bolanos-Vasquez and Werner, 1997). The two latter flavonoids also possess antifungal and antibacterial activities (Machado et al., 2005; Kusuma and Tachibana, 2007; Cui et al., 2008) and might be compounds involved in controlling B. japonicum development. Apigenin levels were also elevated in alfalfa (Medicago sativa) and soybean grown under conditions of elevated atmospheric CO2 (Agrell et al., 2004; Kretzschmar et al., 2009) and increased in response to herbivore damage in alfalfa (Agrell et al., 2003). Conjugation of apigenin with glycosides inactivates the molecule (Scervino et al., 2006). This may explain the observation that apigenin 7-glucuronide was down-regulated in B. japonicum-inoculated root hairs 48 h after inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Conjugates of delphinidin and isorhamnetin were also repressed in root hairs 24 h after bacterial inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2).
The naringenin 4′-O-glucoside conjugate accumulated to higher levels in root hairs 12, 18, and 36 h after inoculation with B. japonicum compared with the mock-inoculated control (Table III; Supplemental Fig. S2; Supplemental Table S2). Naringenin is known to be an inducer of nod genes in Rhizobium, to stimulate nodulation, and to directly interact with NodD, the key regulator of nod gene expression (Begum et al., 2001; Novak et al., 2002; Li et al., 2008). However, further study is required to determine the role of naringenin 4′-O-glucoside in the early events of soybean nodulation.
Afrormosin levels were also up-regulated in root hairs inoculated with B. japonicum (Table III; Supplemental Fig. S2; Supplemental Table S2), and this compound was also found in M. truncatula cell cultures treated with a yeast elicitor and methyl jasmonate (Farag et al., 2008). Afrormosin was also implicated in soybean insect resistance (Caballero et al., 1986).
Taken together, the metabolomic data indicated that only a subset of (iso)flavonoid compounds were induced in root hairs in response to B. japonicum inoculation. While these compounds play a key role in the symbiotic process by inducing the bacterial nodulation genes, they likely have a variety of other biological functions. For example, isoflavonoids are also strong inhibitors of polar auxin transport (Subramanian et al., 2007). However, this function does not appear to be essential for soybean nodulation (Zhang et al., 2009).
Flavonoid compounds are precursors of dihydrochalcones (Nazareno et al., 2000; Kamara et al., 2005). The 2′,4′,5′-trihydroxy-3′,6′′,6′′-trimetylpyranol(2′′,3′′:6′′,5′′) dihydrochalcone was up-regulated in root hairs 18 h after inoculation with B. japonicum (Table III; Supplemental Fig. S2; Supplemental Table S2). Dihydrochalcone derivatives are known to possess antifungal and antibacterial properties (Awouafack et al., 2008; Pontais et al., 2008; Poumale et al., 2008). Therefore, an interesting question is whether the production of this compound adversely affects B. japonicum growth during the infection process.
Amino Acids
The amino acids Pro, Glu, Asp, and 4-aminobutyric acid (GABA) were significantly regulated in root hairs colonized by B. japonicum (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). Asp was significantly more abundant in root hairs 12, 24, 36, and 48 h after inoculation, as well as in stripped roots at similar time points. In contrast, Pro, Glu, and GABA were less abundant in root hairs 24 and 36 h after inoculation. Pro is known to have an osmoprotective function during the plant response to drought, cold, salt, and heavy metal treatment (Cook et al., 2004; Singh et al., 2004; Gong et al., 2005; Charlton et al., 2008; Shulaev et al., 2008; Urano et al., 2009). Furthermore, Pro was also shown to accumulate in nodules of Cicer arietinum and Vicia faba during salt stress (Trinchant et al., 1998; Nandwal et al., 2007). Nitrogen fixation was less sensitive to osmotic stress in M. truncatula plants overaccumulating Pro (Verdoy et al., 2006). As discussed below, the production of trehalose by B. japonicum during infection of root hairs suggests that the bacteria may be responding to osmotic stress. In contrast, the reduction of Pro in root hairs after inoculation suggests that the plant cell may not experience osmotic stress or that B. japonicum colonization triggers a different regulation of compounds involved in osmotic stress tolerance.
Glu is a precursor of GABA. Accumulation of the latter compound was reduced in root hairs 24 h after inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Interestingly, a Glu dehydrogenase gene involved in Glu synthesis was also found down-regulated in root hairs inoculated by B. japonicum (Libault et al., 2010). GABA accumulated very quickly in rice (Oryza sativa) during the germination process and was suggested to function as a signaling molecule in plants, as it does in animal systems (Bouche and Fromm, 2004; Howell et al., 2009). GABA was also implicated in regulating pollen tube development (Yang, 2003; Yu and Sun, 2007). GABA is known to accumulate in response to abiotic stresses, including drought, cold, heat, salt, ozone exposure, mechanical damage, and anoxia (Kinnersley and Turano, 2000; Cho et al., 2008). GABA is also involved in the plant defense reaction against insects (Shelp et al., 1999; Bouche and Fromm, 2004) and was induced in rice cells in response to fungal elicitor (Takahashi et al., 2008). The reduction of Glu and GABA in response to B. japonicum inoculation could be interpreted as the result of an active suppression of a plant innate immunity response. This conclusion is supported by our recent transcriptomic results showing that transcripts known to be associated with the plant innate immunity system were initially elevated but then repressed within 48 h after B. japonicum inoculation (Libault et al., 2010).
In contrast to Glu, GABA, and Pro, which were repressed in root hairs colonized by B. japonicum, Asp levels were significantly elevated in root hairs and stripped roots after inoculation (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). Prell and Poole (2006) suggested that Asp is produced by bacteroids in nodules and plays a critical part in the nutrient exchange process between symbiont and host. The current data, however, do not allow firm conclusions as to whether the Asp seen in root hairs is of bacterial or plant origin.
Fatty Acids
Six fatty acids were significantly regulated only in root hairs in response to bacterial inoculation. Octadecenoic acid was more abundant in root hairs from 12 to 48 h after inoculation with B. japonicum (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). α-Eleosteric, α-linolenic, and lignoceric acids accumulated in root hairs 48 h after B. japonicum inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). The release of fatty acids from the membrane affects its fluidity and is involved in the plant tolerance to biotic and abiotic stresses (Upchurch, 2008). Linolenic acid is the most abundant fatty acid in plant membranes, and its accumulation in B. japonicum-colonized root hairs may be involved in modifying membrane fluidity necessary for the bacteria to colonize the cell. Another possibility is that elevation of this fatty acid, as well as the others, may simply be a reflection of an up-regulation of metabolic pathways necessary for the synthesis of new membranes required during the infection process.
However, fatty acids also have other biological activities. For example, free α-linolenic acid possesses antifungal activity and was found to accumulate in potato (Solanum tuberosum) leaves infected with Pseudomonas syringae, in parsley (Petroselinum crispum) cells treated with a fungal elicitor, and in Silybum marianum cell cultures exposed to yeast extract (Kirsch et al., 1997; Gobel et al., 2002; Walters et al., 2004; Sanchez-Sampedro et al., 2007). These data suggest that the accumulation of free α-linolenic acid may be an indicator of an active plant defense response. Trienoic fatty acids also play a role in the plant response to abiotic stresses, including cold and heat, as well as wounding (Conconi et al., 1996; Matsuda and Iba, 2005). Linolenic acid is a precursor of jasmonic acid, which plays a critical role in plant responses to biotic and abiotic stresses (Wasternack, 2007). Incubation of B. japonicum with either jasmonate or methyl jasmonate was reported to induce expression of the nod genes and enhance nodulation and nitrogen fixation (Mabood and Smith, 2005).
Root hairs colonized by B. japonicum accumulated more octadecenoic acid (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). Accumulation of oleic acid, which is an octadecenoic acid, was detected in mycorrhizal M. truncatula roots (Stumpe et al., 2005; Schliemann et al., 2008). Mycorrhizal and nitrogen-fixing symbioses require the synthesis of plant membranes surrounding the fungi and bacteria, respectively. Given the projected need for a 35-fold increase in membrane biogenesis for infection thread formation and subsequent symbiosome membrane production in nodules (Roth and Stacey, 1989), it is more likely that the noted increases in the presence of free fatty acids may reflect a cell in the process of up-regulating membrane biosynthetic pathways.
In contrast to those free fatty acids that accumulated to higher levels after inoculation, lauric and myristic acids were repressed in root hairs 12 h after inoculation with B. japonicum (Table III; Supplemental Fig. S2; Supplemental Table S2). Lauric acid exhibits antifungal properties, and its repression in root hairs colonized by B. japonicum may reflect a down-regulation of the soybean defense reaction (Walters et al., 2003). To our knowledge, changes in myristic acid levels in response to plant-microbe interaction have not been previously reported. N-Myristoylation regulates weak interactions of proteins with the cell membrane as well as protein-protein interactions. Many proteins involved in signal transduction are N-myristoylated (Farazi et al., 2001), which suggests that the repression of myristic acid, leading to a presumed decrease of protein N-myristoylation, could have an effect on the cell signaling processes at work during nodulation.
Carboxylic Acids
Lactic acid accumulated specifically in root hairs 18, 24, and 48 h after B. japonicum inoculation, but not in stripped roots (Fig. 2; Table III; Supplemental Fig. S2; Supplemental Table S2). Lactic acid was previously reported to be 4.8 times more abundant in alfalfa nodules than in roots and accumulated in nodules during salt stress (Swaraj and Bishnoi, 1999; Barsch et al., 2006). Polymers of lactic acid were shown to stimulate plant growth in corn (Zea mays) and soybean (Kinnersley et al., 1990; Chang et al., 1996). However, further studies are required to determine a possible role for lactic acid in root hairs colonized by B. japonicum.
Likewise, benzoic acid was significantly up-regulated only in B. japonicum-infected root hairs 24 h after inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Benzoic acid itself exhibits antifungal activity, and pretreatment of plants with this compound increases plant resistance to fungi (Khan et al., 2001; Williams et al., 2003; Shabana et al., 2008). Similarly, benzoic acid levels increase in response to both bacterial and viral infection (Shapiro and Gutsche, 2003; Matsuura et al., 2009). The level of benzoic acid was also shown to increase in plants treated with manganese and cadmium and was correlated with improved plant resistance to cold, drought, and heat (Senaratna et al., 2003; Pal et al., 2005; Fuhrs et al., 2009). Benzoic acid was shown to be metabolized to salicylic acid in tobacco (Nicotiana tabacum) during ozone stress (Ogawa et al., 2005). However, salicylic acid abundance was not modified in root hairs after B. japonicum inoculation (Supplemental Table S1).
Threonic acid is another carboxylic acid that was significantly up-regulated in root hairs 12 and 18 h after inoculation with B. japonicum (Table III; Supplemental Fig. S2; Supplemental Table S2). Threonic acid is enriched in L. japonicus nodules in comparison with other plant organs (Desbrosses et al., 2005) and arises from ascorbic acid, which is a strong antioxidant (Loewus, 1999). Increased activities of antioxidant enzymes as well as accumulation of hydrogen peroxide occur during the early colonization of M. sativa by S. meliloti (Bueno et al., 2001). Hydrogen peroxide was also shown to be critical for the development of the infection thread within the root hair (Jamet et al., 2007). The levels of reactive oxygen species increase very rapidly in root hairs treated with Nod factor (Cardenas et al., 2008). Altogether, these data suggest that a tight regulation of the oxidative burst is required for soybean root hair colonization by the symbiotic bacteria. The accumulation in B. japonicum-colonized root hairs of threonic acid, which is degradation product of ascorbic acid, might be part of this regulation.
Carbohydrates
The pentose Xyl was repressed in root hairs in response to B. japonicum 48 h after inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Xyl can be used as a carbon source by B. japonicum and other rhizobia under both normal and salt stress conditions (Wagner et al., 1995; Ghosh et al., 2005; El Idrissi and Abdelmoumen, 2008). The level of Xyl was also shown to decrease in wheat (Triticum aestivum) roots treated with calcium (Hossain et al., 2006). Interestingly, Xyl exudation was reduced from wheat roots infected by Helminthosporium sativum, the agent responsible for root-rot disease (Jalali and Suryanarayana, 1971). Further experiments are required to determine whether the repression of Xyl in B. japonicum-colonized root hairs is due to its use as a carbon source by the symbiotic bacterium, to a plant stress response, or to an elevation in intracellular calcium levels, which is known to occur in root hairs during the infection process (Talukdar et al., 2009).
Trehalose: A Disaccharide Required for Root Hair Colonization
The inoculation of root hairs by B. japonicum triggered the accumulation of the disaccharides trehalose (12, 18, 24, 36, 48 h) and maltose (36, 48 h; Table III; Fig. 2; Supplemental Fig. S2; Supplemental Table S2). Trehalose is a nonreducing disaccharide of Glc whose metabolic pathway is implicated in regulating embryo, leaf, and flower development. In many cases, an accumulation of trehalose is correlated with a stress response, especially in response to osmotic stress (Iordachescu and Imai, 2008).
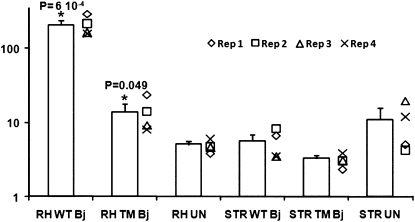
During the early events of the nitrogen-fixing symbiosis, trehalose can be synthesized by both plant and bacterial cells. In order to determine if the trehalose accumulation seen in root hairs was of plant or bacterial origin, root hairs were inoculated with a B. japonicum mutant strain defective in trehalose synthase (treS), trehalose 6-phosphate synthase (otsA), and maltooligosyltrehalose synthase (treY) activities. This mutant produced little or no trehalose in culture (Sugawara et al., 2010). Root hairs were collected 48 h after inoculation, since this coincided with the highest accumulation of trehalose when plants were inoculated with wild-type B. japonicum (Fig. 2). The amount of trehalose in root hairs inoculated with the wild type was 16 times greater than those inoculated with the triple mutant, clearly indicating that the accumulation of trehalose in root hairs after inoculation is most likely due to bacterial synthesis (Fig. 3). However, we cannot rule out some plant synthesis of trehalose, since a higher accumulation of this compound was observed in root hairs inoculated with the B. japonicum triple mutant than in mock-inoculated root hairs (Fig. 3). Furthermore, a soybean trehalose phosphatase gene was found up-regulated in root hairs colonized by B. japonicum (Libault et al., 2010), suggesting that a small amount of the accumulated trehalose in root hairs might be of plant origin. It is also possible that the bacterial triple mutant synthesizes a limited amount of trehalose.
Figure 3.
Average trehalose abundance (bars) determined by GC-MS in root hairs (RH) and stripped roots (STR) inoculated with B. japonicum wild type (WT) or the triple mutant for trehalose synthesis (TM) and in the uninoculated control (UN). The abundance of trehalose for each of the four replicates and each condition is also included (diamonds, squares, triangles, crosses). Asterisks indicate significant differences in trehalose accumulation. se values are also indicated.
Trehalose is known to accumulate and/or confer resistance to plants in response to drought, cold, and salt stress (Garcia et al., 1997; Garg et al., 2002; Pramanik and Imai, 2005; Iordachescu and Imai, 2008) and is induced in Arabidopsis (Arabidopsis thaliana) and cucumber (Cucumis sativus) infected by fungal pathogens (Brodmann et al., 2002; Abood and Losel, 2003). Trehalose also accumulated in ectomycorrhized and endomycorrhized roots, particularly during drought stress (Schellenbaum et al., 1999; Pfeffer et al., 2004; Lopez et al., 2007). Genetic engineering of tobacco, potato, and tomato (Solanum lycopersicum) transformed with trehalose biosynthetic genes conferred higher resistance to drought, low and high temperature, salt, and oxidative stresses (Yeo et al., 2000; Almeida et al., 2005; Cortina and Culianez-Macia, 2005). Trehalose was detected in nodules of M. truncatula, P. vulgaris, and soybean, where it accumulated in response to water stress (Muller et al., 1996; Farias-Rodriguez et al., 1998; Streeter and Gomez, 2006; Lopez et al., 2008; Suarez et al., 2008). This disaccharide is also produced by bacteroids in soybean nodules (Muller et al., 1996). Trehalose synthesis by soybean during the early events of the nitrogen-fixing symbiosis might be part of a stress response triggered by bacterial colonization or infection, perhaps indicating conditions that confer osmotic stress. Unfortunately, the methods used cannot distinguish between trehalose produced by B. japonicum cells attached to the surface of the root hair and those cells contained within the infection threads.
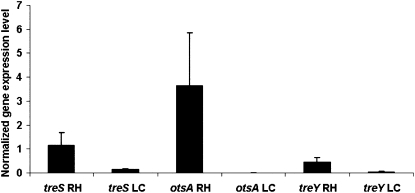
It was previously described that three different pathways, showing different regulation in in vitro culture and in nodules, exist in B. japonicum for the synthesis of trehalose and involved three genes: treS (encoding trehalose synthase), otsA (encoding trehalose 6-phosphate synthase), and treY (encoding maltooligosyltrehalose synthase; Streeter and Gomez, 2006). However, it was recently suggested that treS is involved in the catabolism of trehalose to maltose (Sugawara et al., 2010). The expression levels of treS, otsA, and treY were quantified by real-time PCR in sprayed root hairs 12 h after inoculation with B. japonicum and in liquid culture. All three genes were strongly expressed in B. japonicum sprayed root hairs. The elevated expression of these genes is also consistent with the conclusion that trehalose accumulation in sprayed root hairs is primarily of bacterial origin. Little or no expression of treS, otsA, and treY was found in B. japonicum liquid cultures (Fig. 4).
Figure 4.
B. japonicum treS, otsA, and treY gene expression levels determined by real-time PCR in root hairs (RH) 12 h after inoculation and in liquid culture (LC). se values are also indicated.
Maltose levels were elevated in root hairs in response to B. japonicum inoculation. The enzyme trehalose synthase (treS) was suggested to convert trehalose to maltose (Sugawara et al., 2010). Therefore, it is likely that the accumulation of maltose seen in the inoculated root hairs reflects the catabolism of trehalose.
Trehalose accumulation in B. japonicum was shown to be crucial for its survival during desiccation (Streeter, 2003). Free-living rhizobia were shown to synthesize trehalose in response to phenanthrene, hyperosmolarity, high temperature, salt stress, very low oxygen levels, and desiccation (Hoelzle and Streeter, 1990; Ghittoni and Bueno, 1996; Dardanelli et al., 1997, 2000; Cytryn et al., 2007; Essendoubi et al., 2007; McIntyre et al., 2007; Keum et al., 2008). Furthermore, the resistance of Rhizobium etli to salt stress was improved in a strain overexpressing a trehalose 6-phosphate synthase gene and decreased in a strain mutated in the same gene (Suarez et al., 2008). Trehalose accumulation in rhizobia appears to be a general bacterial response to stress, suggesting that trehalose induction in B. japonicum-sprayed root hairs during the early events of the nitrogen-fixing symbiosis is due to a perceived stress during the infection process. Consistent with this hypothesis, nodule formation by the B. japonicum ΔtreS, ΔotsA, ΔtreY mutant is significantly reduced in comparison with the wild type (Sugawara et al., 2010). These findings are also consistent with the report by Ampomah et al. (2008), who demonstrated that S. meliloti strains mutated in genes involved in trehalose catabolism (presumably allowing trehalose accumulation) were more competitive than the wild type, particularly at the stage of infection thread development.
Other Compounds
O-Methyl inositol, a polyol, was significantly less abundant in root hairs 18 h after inoculation with B. japonicum (Table III; Supplemental Fig. S2; Supplemental Table S2). Methyl inositol was implicated in drought or salt stress tolerance through the stabilization of membranes and proteins in soybean, alfalfa, and the ice plant (Mesembryanthemum crystallinum; Fougere et al., 1991; Muller et al., 1996; Guo and Oosterhuis, 1997; Streeter et al., 2001; Agarie et al., 2009). O-Methyl inositol is also secreted from soybean roots (Timotiwu and Sakurai, 2002) and exhibits antibacterial and antifungal activities (Tan et al., 1999; Agnese et al., 2001). Therefore, its repression in root hairs inoculated with B. japonicum may prevent the inhibition of bacterial growth.
Ascochalasin accumulated in root hairs 24 h after inoculation with B. japonicum, but levels decreased at 48 h (Table III; Supplemental Fig. S2; Supplemental Table S2). Ascochalasin is a member of the cytochalasin family, which includes molecules with antibiotic and antifungal properties (Bottalico et al., 1990), and therefore would also have the potential of affecting bacterial growth during the infection process.
The levels of 4-hydroxy-indole-3-yl-methyl-glucosinolate were reduced in root hairs 12 h after inoculation with B. japonicum but increased at 48 h (Table III; Supplemental Fig. S2; Supplemental Table S2). 7-Methylsulfinyl-n-heptyl-glucosinolate abundance increased in root hairs 18 h after B. japonicum inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Glucosinolates are secondary metabolites that function in plant defense against insects and pathogens (Hopkins et al., 2009; van Dam et al., 2009). Indole glucosinolate was also suggested to be a precursor of auxin (Ludwig-Muller, 2009), a hormone implicated in nodule development (Mathesius, 2008).
Medicagenic acid 3-O-triglucoside levels also increased in root hairs 36 h after B. japonicum inoculation (Table III; Supplemental Fig. S2; Supplemental Table S2). Medicagenic acid and its glycoside derivatives exhibit antifungal and nemacidal activities (Zehavi and Polacheck, 1996; Argentieri et al., 2008). However, to our knowledge, they have never been tested for activity against B. japonicum.
CONCLUSION
Metabolomic analysis of soybean roots and root hairs following B. japonicum inoculation identified a variety of compounds, most of which were previously implicated in various plant-microbe interactions. The regulation of several metabolites (e.g. GABA, O-methyl inositol, Glu, lauric acid) suggests the B. japonicum may actively suppress plant defense pathways during infection. This conclusion is strongly supported by transcriptomic studies of root hair mRNA taken at similar time points and under identical treatment conditions (Libault et al., 2010). The regulation of other compounds, such as isoflavonoids and free fatty acids, may be due to their signaling role during the symbiosis or may reflect the need for increased cellular biosynthesis (e.g. membrane lipids) to create the structural components (e.g. infection thread) needed to support symbiotic development.
The methods used in this study did not allow a distinction between metabolites produced by the invading symbiont and those produced by the plant host. This is both a limitation and an advantage: the latter since it allows conclusions to be drawn about the interaction between host and symbiont. For example, the noted accumulation of trehalose in the inoculated root hairs likely reflects bacterial synthesis but strongly suggests that the symbiont is perceiving a stressful environment during the earliest stages of the infection process. Previous studies suggested that the response of bacteroids within the infected nodule cells may reflect a perception of osmotic stress or a related stress (Boscari et al., 2006). For example, a variety of protein chaperone proteins are induced in bacteroids (Djordjevic, 2004). However, our data suggest that the symbiont likely responds to the stress of a plant intracellular lifestyle very early during the infection process.
The soybean root hair is a single, differentiated cell type. The methods used in this work, as well as similar publications from our laboratory (Wan et al., 2005; Brechenmacher et al., 2009; Libault et al. 2010) seek to provide a thorough functional genomic description of this single-cell model system by using B. japonicum inoculation as a probe for physiological changes. It is hoped that the final integration of these data sets will provide a foundation for single-cell, systems biology studies of plant function.
MATERIALS AND METHODS
Bradyrhizobium japonicum Growth, Root Hair, and Stripped Root Isolation
Sterilization of the soybean seeds (Glycine max ‘Williams 82’) was performed by soaking seeds twice in 20% bleach for 10 min each. Seeds were then rinsed five times in sterile water, neutralized for 10 min in 0.1 n HCl, and rinsed five more times in sterile water. Sterilized seeds were germinated in a dark growth chamber (25°C, 80% humidity) for 3 d on nitrogen-free B&D agar medium (Broughton and Dilworth, 1971).
B. japonicum strain USDA110 was grown at 30°C for 3 d in HM medium (HEPES, 1.3 g L−1; MES, 1.1 g L−1; Na2HPO4, 0.125 g L−1; Na2SO4, 0.25 g L−1; NH4Cl, 0.32 g L−1; MgSO4 7H2O, 0.18 g L−1; FeCl3, 0.004 g L−1; CaCl2 2H2O, 0.013 g L−1; yeast extract, 0.25 g L−1; d-Ara, 1 g L−1; sodium gluconate, 1 g L−1, pH 6.6) containing 20 μg mL−1 chloramphenicol. Cells were centrifuged, washed twice with sterile water, diluted to an optical density at 600 nm of 0.8 in sterile water, and used to inoculate the 3-d-old seedlings using a perfume sprayer in order to consistently deliver a thorough inoculant dose. Control seedlings were mock inoculated with sterile water. The B. japonicum ΔotsAΔtreSΔtreY triple mutant defective in trehalose synthesis was grown as described above with the following additions: 100 μg mL−1 streptomycin and 100 μg mL−1 spectinomycin (Sugawara et al., 2010).
Soybean roots (about 1,500 per experiment) were collected 0, 12, 18, 24, 36, and 48 h after inoculation by cutting and allowing the roots to fall directly into liquid nitrogen. The roots were gently stirred for 20 min to break off root hairs from the roots. The liquid nitrogen slurry was filtered through a wire mesh to separate root hairs from stripped roots (roots with no root hairs). Root hairs and stripped roots were stored at −80°C until metabolite extraction. Four biological replicates were produced for each time point. This procedure was previously used to successfully isolate root hairs largely free of any other root cell contamination. (Wan et al., 2005; Brechenmacher et al., 2009).
GC-MS
GC-MS analyses of root metabolites were carried out as described previously (Broeckling et al., 2005). Root hairs and stripped roots were lyophilized until dry. The dried root tissues were homogenized using mortar and pestle, and 6 mg was transferred into a 4-mL glass vial. Metabolites were extracted by adding 1 mL of chloroform containing 10 μg mL−1 docosanol (the standard for nonpolar metabolites), vortexed, and incubated for 45 min at 50°C. HPLC-grade water (1.5 mL) containing 25 μg mL−1 ribitol (the standard for polar metabolites) was added and incubated a second time at 50°C for 45 min. The two phases were separated by centrifugation at 2,900g for 30 min at 4°C. One milliliter of each layer was collected into a vial and dried in a rotary evaporator for the polar layer or under nitrogen for the nonpolar layer. Methoxyamine hydrochloride (50 μL at 15 mg mL−1) in pyridine was added to the polar metabolites. Compounds were sonicated until they were completely dissolved and incubated at 50°C for at least 1 h for methoximation. Silylation was performed by incubating the samples for 1 h at 50°C with 50 μL of N-methyl-N-trimethylsilyltrifluoroacetamide and 1% trimethylchlorosilane (Pierce Biotechnology). The sample was equilibrated to room temperature, transferred to a 200-μL glass insert, and analyzed using an Agilent 6890 GC apparatus coupled to a 5973 MSD scanning from m/z 50 to 650. One microliter of the sample solution was injected at a 15:1 split ratio, and the inlet and transfer line were held at 280°C. Separation was achieved on a 60-m DB-5MS column (J&W Scientific; 0.25 mm i.d., 0.25 μm film thickness) at a constant flow of 1.0 mL min−1 with a temperature program of 80°C for 2 min, ramped at 5°C min−1 to 315°C, and held for 12 min.
The nonpolar compounds were resuspended in 0.8 mL of chloroform and hydrolyzed for 4 h at 50°C by adding 0.5 mL of HCl (1.25 m) in methanol. HCl and solvent were removed by evaporation under nitrogen. Solubilization was achieved by sonication in 70 μL of pyridine and incubation at 50°C. Derivatization was performed, as described above, by using 30 μL of N-methyl-N-trimethylsilyltrifluoroacetamide and 1% trimethylchlorosilane. Metabolites were analyzed by GC-MS as described above for the polar compounds using a 1:1 split ratio.
UPLC-QTOF-MS
Root hairs and stripped roots were lyophilized until dry and ground to a fine powder. Ten milligrams of the powder was extracted for 2 h with 1 mL of 80% methanol containing 0.018 mg mL−1 umbelliferone as an internal standard. Samples were centrifuged at 2,900g for 30 min at 4°C, and the supernatants were collected. Five microliters of the supernatant was injected into the Waters UPLC system coupled to a quadrupole-time of flight mass spectrometer (QTOF Premier). Separation of metabolites was achieved on a 2.1- × 150-mm i.d., 1.7-μm UPLC BEH C18 column (Waters Acquity) using the following gradient: mobile phase B (acetonitrile) increased from 5% to 70% over 30 min, then to 95% in 3 min, held at 95% for 3 min, and returned to 95% mobile phase A (0.1% acetic acid in water) for equilibration for 3 min. The flow rate of the mobile phases was 0.56 mL min−1, and the column temperature and the autosampler temperature were maintained at 60°C and 4°C, respectively. Mass spectral data were acquired from m/z 50 to 2,000 in the negative ion electrospray mode, with the nebulization gas at 850 L h−1 (350°C) and the cone gas at 50 L h−1 (120°C). Raffinose (m/z 503.1612) was used as the reference compound in the independent lock-mass mode, with the lock mass scan (1 s) collected every 10 s for the accurate mass measurement. The concentration of raffinose was 50 fmol mL−1, and the flow rate 0.2 mL h−1.
Metabolomic Data Analysis
For the GC-MS data, metabolites were identified through spectral and retention time matching against a custom library compiled with authentic standards prepared in an identical manner and then against the National Institutes of Standards and Technology library for confirmation and/or identification of compounds not included in the custom database. For the UPLC-MS data, tentative identifications were made by matching the retention time and the accurate mass (m/z) of ions to those in a custom library compiled with authentic standards and/or plant metabolites previously reported in the literature. Relative metabolite abundances were calculated using custom MET-IDEA software to extract peak areas of individual ions characteristic of each component (Broeckling et al., 2006). Peak areas were normalized to the internal standard, and the normalized peak areas were used in statistical analyses. Principal component analysis was performed on normalized data sets using Pirouette (InfoMetrix). A t test was performed to identify metabolites significantly regulated in root hairs and stripped roots in response to B. japonicum inoculation. All metabolites having P < 0.01 for at least one time point or P < 0.05 for more than one time point were considered significantly regulated.
Gene Expression Quantification
Trizol reagent was used to extract RNA from soybean root hairs 12 h after inoculation with B. japonicum and from B. japonicum grown in liquid culture according to the manufacturer's protocol (Invitrogen). RNAs were subsequently treated with DNase (Ambion) to remove genomic DNA contamination according to the manufacturer's instructions. One microgram of total RNA was employed as template for cDNA synthesis as described by Brechenmacher et al. (2008), modified by the use of a mixture of random primers. Primers used to amplify treS (blr6767), otsA (bll0322), and treY (blr6771) genes are described by Cytryn et al. (2007). PCR conditions, control of the absence of genomic DNA, and the method to quantify the transcripts are identical to those in Brechenmacher et al. (2008) except for the use of a penicillin-binding protein (bll0910) to normalize the data (Chang et al., 2007).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Principal component analysis of metabolites significantly regulated in root hairs and stripped roots.
Supplemental Figure S2. Relative abundance of known compounds in root hairs and stripped roots after inoculation with the symbiotic bacterium B. japonicum or mock inoculated.
Supplemental Table S1. Normalized data of metabolites detected in root hairs and stripped roots.
Supplemental Table S2. Metabolites significantly regulated in root hairs in response to B. japonicum inoculation.
Supplementary Material
References
- Abood JK, Losel DM. (2003) Changes in carbohydrate composition of cucumber leaves during the development of powdery mildew infection. Plant Pathol 52: 256–265 [Google Scholar]
- Agarie S, Kawaguchi A, Kodera A, Sunagawa H, Kojima H, Nose A, Nakahara T. (2009) Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Prod Sci 12: 37–46 [Google Scholar]
- Agnese AM, Perez C, Cabrera JL. (2001) Adesmia aegiceras: antimicrobial activity and chemical study. Phytomedicine 8: 389–394 [DOI] [PubMed] [Google Scholar]
- Agrell J, Anderson P, Oleszek W, Stochmal A, Agrell C. (2004) Combined effects of elevated CO2 and herbivore damage on alfalfa and cotton. J Chem Ecol 30: 2309–2324 [DOI] [PubMed] [Google Scholar]
- Agrell J, Oleszek W, Stochmal A, Olsen M, Anderson P. (2003) Herbivore-induced responses in alfalfa (Medicago sativa). J Chem Ecol 29: 303–320 [DOI] [PubMed] [Google Scholar]
- Allwood JW, Ellis DI, Goodacre R. (2008) Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiol Plant 132: 117–135 [DOI] [PubMed] [Google Scholar]
- Almeida AM, Villalobos E, Araujo SS, Leyman B, Van Dijck P, Alfaro-Cardoso L, Fevereiro PS, Torne JM, Santos DM. (2005) Transformation of tobacco with an Arabidopsis thaliana gene involved in trehalose biosynthesis increases tolerance to several abiotic stresses. Euphytica 146: 165–176 [Google Scholar]
- Ampomah OY, Jensen JB, Bhuvaneswari TV. (2008) Lack of trehalose catabolism in Sinorhizobium species increases their nodulation competitiveness on certain host genotypes. New Phytol 179: 495–504 [DOI] [PubMed] [Google Scholar]
- Argentieri MP, D'Addabbo T, Tava A, Agostinelli A, Jurzysta M, Avato P. (2008) Evaluation of nematicidal properties of saponins from Medicago spp. Eur J Plant Pathol 120: 189–197 [Google Scholar]
- Awouafack MD, Kouam SF, Hussain H, Ngamga D, Tane P, Schulz B, Green IR, Krohn K. (2008) Antimicrobial prenylated dihydrochalcones from Eriosema glomerata. Planta Med 74: 50–54 [DOI] [PubMed] [Google Scholar]
- Barsch A, Tellstrom V, Patschkowski T, Küster H, Niehaus K. (2006) Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol Plant Microbe Interact 19: 998–1013 [DOI] [PubMed] [Google Scholar]
- Begum AA, Leibovitch S, Migner P, Zhang F. (2001) Specific flavonoids induced nod gene expression and pre-activated nod genes of Rhizobium leguminosarum increased pea (Pisum sativum L.) and lentil (Lens culinaris L.) nodulation in controlled growth chamber environments. J Exp Bot 52: 1537–1543 [DOI] [PubMed] [Google Scholar]
- Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T, et al. (2008) A gene expression atlas of the model legume Medicago truncatula. Plant J 55: 504–513 [DOI] [PubMed] [Google Scholar]
- Bolanos-Vasquez MC, Werner D. (1997) Effects of Rhizobium tropici, R. etli, and R. leguminosarum bv. phaseoli on nod gene-inducing flavonoids in root exudates of Phaseolus vulgaris. Mol Plant Microbe Interact 10: 339–346 [Google Scholar]
- Boscari A, Van De Sype G, Le Rudulier D, Mandon K. (2006) Overexpression of BetS, a Sinorhizobium meliloti high-affinity betaine transporter, in bacteroids from Medicago sativa nodules sustains nitrogen fixation during early salt stress adaptation. Mol Plant Microbe Interact 19: 896–903 [DOI] [PubMed] [Google Scholar]
- Bottalico A, Capasso R, Evidente A, Randazzo G, Vurro M. (1990) Cytochalasins: structure-activity relationships. Phytochemistry 29: 93–96 [Google Scholar]
- Bouche N, Fromm H. (2004) GABA in plants: just a metabolite? Trends Plant Sci 9: 110–115 [DOI] [PubMed] [Google Scholar]
- Brechenmacher L, Kim MY, Benitez M, Li M, Joshi T, Calla B, Lee MP, Libault M, Vodkin LO, Xu D, et al. (2008) Transcription profiling of soybean nodulation by Bradyrhizobium japonicum. Mol Plant Microbe Interact 21: 631–645 [DOI] [PubMed] [Google Scholar]
- Brechenmacher L, Lee J, Sachdev S, Song Z, Nguyen THN, Joshi T, Oehrle N, Libault M, Mooney B, Xu D, et al. (2009) Establishment of a protein reference map for soybean root hair cells. Plant Physiol 149: 670–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann D, Schuller A, Ludwig-Muller J, Aeschbacher RA, Wiemken A, Boller T, Wingler A. (2002) Induction of trehalase in Arabidopsis plants infected with the trehalose-producing pathogen Plasmodiophora brassicae. Mol Plant Microbe Interact 15: 693–700 [DOI] [PubMed] [Google Scholar]
- Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW. (2005) Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56: 323–336 [DOI] [PubMed] [Google Scholar]
- Broeckling CD, Reddy IR, Duran AL, Zhao X, Sumner LW. (2006) MET-IDEA: data extraction tool for mass spectrometry-based metabolomics. Anal Chem 78: 4334–4341 [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Dilworth MJ. (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton WJ, Jabbouri S, Perret X. (2000) Keys to symbiotic harmony. J Bacteriol 182: 5641–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno P, Soto MJ, Rodriguez-Rosales MP, Sanjuan J, Olivares J, Donaire JP. (2001) Time-course of lipoxygenase, antioxidant enzyme activities and H2O2 accumulation during the early stages of Rhizobium-legume symbiosis. New Phytol 152: 91–96 [DOI] [PubMed] [Google Scholar]
- Caballero P, Smith CM, Fronczek FR, Fischer NH. (1986) Isoflavones from an insect-resistant variety of soybean and the molecular structure of afrormosin. J Nat Prod 49: 1126–1129 [Google Scholar]
- Cardenas L, Martinez A, Sanchez F, Quinto C. (2008) Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J 56: 802–813 [DOI] [PubMed] [Google Scholar]
- Cevallos-Cevallos JM, Rouseff R, Reyes-De-Corcuera JI. (2009) Untargeted metabolite analysis of healthy and Huanglongbing-infected orange leaves by CE-DAD. Electrophoresis 30: 1240–1247 [DOI] [PubMed] [Google Scholar]
- Chang WS, Franck WL, Cytryn E, Jeong S, Joshi T, Emerich DW, Sadowsky MJ, Xu D, Stacey G. (2007) An oligonucleotide microarray resource for transcriptional profiling of Bradyrhizobium japonicum. Mol Plant Microbe Interact 20: 1298–1307 [DOI] [PubMed] [Google Scholar]
- Chang YN, Mueller RE, Iannotti EL. (1996) Use of low MW polylactic acid and lactide to stimulate growth and yield of soybeans. Plant Growth Regul 19: 223–232 [Google Scholar]
- Charlton AJ, Donarski JA, Harrison M, Jones SA, Godward J, Oehlschlager S, Arques JL, Ambrose M, Chinoy C, Mullineaux PM, et al. (2008) Responses of the pea (Pisum sativum L.) leaf metabolome to drought stress assessed by nuclear magnetic resonance spectroscopy. Metabolomics 4: 312–327 [Google Scholar]
- Cho K, Shibato J, Agrawal GK, Jung YH, Kubo A, Jwa NS, Tamogami S, Satoh K, Kikuchi S, Higashi T, et al. (2008) Integrated transcriptomics, proteomics, and metabolomics analyses to survey ozone responses in the leaves of rice seedling. J Proteome Res 7: 2980–2998 [DOI] [PubMed] [Google Scholar]
- Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, Kopka J, Udvardi MK. (2004) Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J 39: 487–512 [DOI] [PubMed] [Google Scholar]
- Conconi A, Miquel M, Browse JA, Ryan CA. (1996) Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant Physiol 111: 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Fowler S, Fiehn O, Thomashow MF. (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA 101: 15243–15248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortina C, Culianez-Macia FA. (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169: 75–82 [Google Scholar]
- Cui HB, Mei WL, Miao CD, Lin HP, Hong K, Dai HF. (2008) Antibacterial constituents from the endophytic fungus Penicillium sp. 0935030 of mangrove plant Acrostichum aureurm. Chin J Antibiot 33: 407–410 [Google Scholar]
- Cytryn EJ, Sangurdekar DP, Streeter JG, Franck WL, Chang WS, Stacey G, Emerich DW, Joshi T, Xu D, Sadowsky MJ. (2007) Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation-induced stress. J Bacteriol 189: 6751–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardanelli MS, Gonzalez PS, Bueno MA, Ghittoni NE. (2000) Synthesis, accumulation and hydrolysis of trehalose during growth of peanut rhizobia in hyperosmotic media. J Basic Microbiol 40: 149–156 [DOI] [PubMed] [Google Scholar]
- Dardanelli MS, Woelke MR, Gonzalez PS, Bueno MA, Ghittoni NE. (1997) The effects of nonionic hyperosmolarity and of high temperature on cell-associated low molecular weight saccharides from two peanut rhizobia strains. Symbiosis 23: 73–84 [Google Scholar]
- Desbrosses GG, Kopka J, Udvardi MK. (2005) Lotus japonicus metabolic profiling: development of gas chromatography-mass spectrometry resources for the study of plant-microbe interactions. Plant Physiol 137: 1302–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Oldroyd GED. (2009) Positioning the nodule, the hormone dictum. Plant Signal Behav 4: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MA. (2004) Sinorhizobium meliloti metabolism in the root nodule: a proteomic perspective. Proteomics 4: 1859–1872 [DOI] [PubMed] [Google Scholar]
- El Idrissi MM, Abdelmoumen H. (2008) Carbohydrates as carbon sources in rhizobia under salt stress. Symbiosis 46: 33–44 [Google Scholar]
- Essendoubi M, Brhada F, Eljamali JE, Filali-Maltouf A, Bonnassie S, Georgeault S, Blanco C, Jebbar M. (2007) Osmoadaptative responses in the rhizobia nodulating Acacia isolated from south-eastern Moroccan Sahara. Environ Microbiol 9: 603–611 [DOI] [PubMed] [Google Scholar]
- Farag MA, Huhman DV, Dixon RA, Sumner LW. (2008) Metabolomics reveals novel pathways and differential mechanistic and elicitor-specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiol 146: 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Waksman G, Gordon JI. (2001) The biology and enzymology of protein N-myristoylation. J Biol Chem 276: 39501–39504 [DOI] [PubMed] [Google Scholar]
- Farias-Rodriguez R, Mellor RB, Arias C, Pena-Cabriales JJ. (1998) The accumulation of trehalose in nodules of several cultivars of common bean (Phaseolus vulgaris) and its correlation with resistance to drought stress. Physiol Plant 102: 353–359 [Google Scholar]
- Fougere F, Rudulier DL, Streeter JG. (1991) Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol 96: 1228–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrs H, Gotze S, Specht A, Erban A, Gallien S, Heintz D, Van Dorsselaer A, Kopka J, Braun HP, Horst WJ. (2009) Characterization of leaf apoplastic peroxidases and metabolites in Vigna unguiculata in response to toxic manganese supply and silicon. J Exp Bot 60: 1663–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AB, De Almeida Engler J, Iyer S, Gerats T, Van Montagu M, Caplan AB. (1997) Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol 115: 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg AK, Kim JK, Owens TG, Ranwala AP, Do Choi Y, Kochian LV, Wu RJ. (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99: 15898–15903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghittoni NE, Bueno MA. (1996) Changes in the cellular content of trehalose in four peanut rhizobia strains cultured under hypersalinity. Symbiosis 20: 117–127 [Google Scholar]
- Ghosh AC, Ghosh S, Basu PS. (2005) Production of extracellular polysaccharide by a Rhizobium species from root nodules of the leguminous tree Dalbergia lanceolaria. Eng Life Sci 5: 378–382 [Google Scholar]
- Gibson KE, Kobayashi H, Walker GC. (2008) Molecular determinants of a symbiotic chronic infection. Annu Rev Genet 42: 413–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel C, Feussner I, Hamberg M, Rosahl S. (2002) Oxylipin profiling in pathogen-infected potato leaves. Biochim Biophys Acta 1584: 55–64 [DOI] [PubMed] [Google Scholar]
- Gong Q, Li P, Ma S, Indu Rupassara S, Bohnert HJ. (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J 44: 826–839 [DOI] [PubMed] [Google Scholar]
- Guo C, Oosterhuis DM. (1997) Effect of water-deficit stress and genotypes on pinitol occurrence in soybean plants. Environ Exp Bot 37: 147–152 [Google Scholar]
- Hoelzle I, Streeter JG. (1990) Increased accumulation of trehalose in rhizobia cultured under 1% oxygen. Appl Environ Microbiol 56: 3213–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RJ, van Dam NM, van Loon JJ. (2009) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54: 57–83 [DOI] [PubMed] [Google Scholar]
- Hossain A, Hossain M, Asgar M, Tosaki T, Koyama H, Hara T. (2006) Changes in cell wall polysaccharides and hydroxycinnamates in wheat roots by aluminum stress at higher calcium supply. J Plant Nutr 29: 601–613 [Google Scholar]
- Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J. (2009) Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol 149: 961–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordachescu M, Imai R. (2008) Trehalose biosynthesis in response to abiotic stresses. J Integr Plant Biol 50: 1223–1229 [DOI] [PubMed] [Google Scholar]
- Jalali BL, Suryanarayana D. (1971) Shift in the carbohydrate spectrum of root exudates of wheat in relation to its root-rot disease. Plant Soil 34: 261–267 [Google Scholar]
- Jamet A, Mandon K, Puppo A, Hérouart D. (2007) H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J Bacteriol 189: 8741–8745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobic C, Boisson AM, Gout E, Rascle C, Fevre M, Cotton P, Bligny R. (2007) Metabolic processes and carbon nutrient exchanges between host and pathogen sustain the disease development during sunflower infection by Sclerotinia sclerotiorum. Planta 226: 251–265 [DOI] [PubMed] [Google Scholar]
- Kamara BI, Manong DTL, Brandt EV. (2005) Isolation and synthesis of a dimeric dihydrochalcone from Agapanthus africanus. Phytochemistry 66: 1126–1132 [DOI] [PubMed] [Google Scholar]
- Kape R, Parniske M, Brandt S, Werner D. (1992) Isoliquiritigenin, a strong nod gene- and glyceollin resistance-inducing flavonoid from soybean root exudates. Appl Environ Microbiol 58: 1705–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum YS, Seo JS, Li QX, Kim JH. (2008) Comparative metabolomic analysis of Sinorhizobium sp. C4 during the degradation of phenanthrene. Appl Microbiol Biotechnol 80: 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SH, Aked J, Magan N. (2001) Control of the anthracnose pathogen of banana (Colletotrichum musae) using antioxidants alone and in combination with thiabendazole or imazalil. Plant Pathol 50: 601–608 [Google Scholar]
- Kinnersley AM, Scott TC, III, Yopp JH, Whitten GH. (1990) Promotion of plant growth by polymers of lactic acid. Plant Growth Regul 9: 137–146 [Google Scholar]
- Kinnersley AM, Turano FJ. (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. CRC Rev Plant Sci 19: 479–509 [Google Scholar]
- Kirsch C, Takamiya-Wik M, Reinold S, Hahlbrock K, Somssich IE. (1997) Rapid, transient, and highly localized induction of plastidial ω-3 fatty acid desaturase mRNA at fungal infection sites in Petroselinum crispum. Proc Natl Acad Sci USA 94: 2079–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar Fd S, Aidar MPM, Salgado I, Braga MR. (2009) Elevated CO2 atmosphere enhances production of defense-related flavonoids in soybean elicited by NO and a fungal elicitor. Environ Exp Bot 65: 319–329 [Google Scholar]
- Kuster H, Vieweg MF, Manthey K, Baier MC, Hohnjec N, Perlick AM. (2007) Identification and expression regulation of symbiotically activated legume genes. Phytochemistry 68: 8–18 [DOI] [PubMed] [Google Scholar]
- Kusuma IW, Tachibana S. (2007) Antifungal compounds isolated from tropical and temperate woods. ACS Symp Ser 954: 377–390 [Google Scholar]
- Lang K, Lindemann A, Hauser F, Gottfert M. (2008) The genistein stimulon of Bradyrhizobium japonicum. Mol Genet Genomics 279: 203–211 [DOI] [PubMed] [Google Scholar]
- Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PGL. (2009) Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol 150: 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Hou B, Chen L, Yao Z, Hong G. (2008) In vitro observation of the molecular interaction between NodD and its inducer naringenin as monitored by fluorescence resonance energy transfer. Acta Biochim Biophys Sin (Shanghai) 40: 783–789 [PubMed] [Google Scholar]
- Libault M, Farmer A, Brechenmacher L, Franck WL, Drnevich J, Langley RJ, Bilgin DD, Radman O, Clough SJ, May G, et al. (2010) Complete transcriptome of the soybean root hair cell, a single cell model, and its alteration in response to Bradyrhizobium japonicum infection. Plant Physiol 52: 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus FA. (1999) Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52: 193–210 [Google Scholar]
- Lohar DP, Sharopova N, Endre G, Penuela S, Samac D, Town C, Silverstein KAT, VandenBosch KA. (2006) Transcript analysis of early nodulation events in Medicago truncatula. Plant Physiol 140: 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Tejera NA, Iribarne C, Lluch C, Herrera-Cervera JA. (2008) Trehalose and trehalase in root nodules of Medicago truncatula and Phaseolus vulgaris in response to salt stress. Physiol Plant 134: 575–582 [DOI] [PubMed] [Google Scholar]
- Lopez MF, Manner P, Willmann A, Hampp R, Nehls U. (2007) Increased trehalose biosynthesis in Hartig net hyphae of ectomycorrhizas. New Phytol 174: 389–398 [DOI] [PubMed] [Google Scholar]
- Ludwig-Muller J. (2009) Glucosinolates and the clubroot disease: defense compounds or auxin precursors? Phytochem Rev 8: 135–148 [Google Scholar]
- Mabood F, Smith DL. (2005) Pre-incubation of Bradyrhizobium japonicum with jasmonates accelerates nodulation and nitrogen fixation in soybean (Glycine max) at optimal and suboptimal root zone temperatures. Physiol Plant 125: 311–323 [Google Scholar]
- Machado TDB, Leal ICR, Kuster RM, Amaral ACF, Kokis V, De Silva MG, Dos Santos KRN. (2005) Brazilian phytopharmaceuticals: evaluation against hospital bacteria. Phytother Res 19: 519–525 [DOI] [PubMed] [Google Scholar]
- Matamoros MA, Loscos J, Coronado MJ, Ramos J, Sato S, Testillano PS, Tabata S, Becana M. (2006) Biosynthesis of ascorbic acid in legume root nodules. Plant Physiol 141: 1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U. (2008) Goldacre Paper. Auxin: at the root of nodule development? Funct Plant Biol 35: 651–668 [DOI] [PubMed] [Google Scholar]
- Matsuda O, Iba K. (2005) Trienoic fatty acids and stress responses in higher plants. Plant Biotechnol 22: 423–430 [Google Scholar]
- Matsuura H, Aoi A, Satou C, Nakaya M, Masuta C, Nabeta K. (2009) Simultaneous UPLC MS/MS analysis of endogenous jasmonic acid, salicylic acid, and their related compounds. Plant Growth Regul 57: 293–301 [Google Scholar]
- McIntyre HJ, Davies H, Hore TA, Miller SH, Dufour JP, Ronson CW. (2007) Trehalose biosynthesis in Rhizobium leguminosarum bv. trifolii and its role in desiccation tolerance. Appl Environ Microbiol 73: 3984–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschini EP, Blanco FA, Zanetti ME, Beker MP, Kuster H, Puhler A, Aguilar OM. (2008) Host genes involved in nodulation preference in common bean (Phaseolus vulgaris)-Rhizobium etli symbiosis revealed by suppressive subtractive hybridization. Mol Plant Microbe Interact 21: 459–468 [DOI] [PubMed] [Google Scholar]
- Muller J, Boller T, Wiemken A. (1996) Pools of non-structural carbohydrates in soybean root nodules during water stress. Physiol Plant 98: 723–730 [Google Scholar]
- Nandwal AS, Kukreja S, Kumar N, Sharma PK, Jain M, Mann A, Singh S. (2007) Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J Plant Physiol 164: 1161–1169 [DOI] [PubMed] [Google Scholar]
- Nazareno MA, Giannuzzo AN, Mishima HT, Lopez De Mishima BA. (2000) Catalytic hydrogenation reaction of naringin-chalcone: study of the electrochemical reaction. Molecules 5: 589–590 [Google Scholar]
- Novak K, Chovanec P, Skrdleta V, Kropacova M, Lisa L, Nemcova M. (2002) Effect of exogenous flavonoids on nodulation of pea (Pisum sativum L.). J Exp Bot 53: 1735–1745 [DOI] [PubMed] [Google Scholar]
- Ogawa D, Nakajima N, Sano T, Tamaoki M, Aono M, Kubo A, Kanna M, Ioki M, Kamada H, Saji H. (2005) Salicylic acid accumulation under O3 exposure is regulated by ethylene in tobacco plants. Plant Cell Physiol 46: 1062–1072 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA. (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59: 519–546 [DOI] [PubMed] [Google Scholar]
- Overy DP, Smedsgaard J, Frisvad JC, Phipps RK, Thrane U. (2006) Host-derived media used as a predictor for low abundant, in planta metabolite production from necrotrophic fungi. J Appl Microbiol 101: 1292–1300 [DOI] [PubMed] [Google Scholar]
- Pal M, Horvath E, Janda T, Paldi E, Szalai G. (2005) Cadmium stimulates the accumulation of salicylic acid and its putative precursors in maize (Zea mays) plants. Physiol Plant 125: 356–364 [Google Scholar]
- Parker D, Beckmann M, Zubair H, Enot DP, Caracuel-Rios Z, Overy DP, Snowdon S, Talbot NJ, Draper J. (2009) Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J 59: 723–737 [DOI] [PubMed] [Google Scholar]
- Pessi G, Ahrens CH, Rehrauer H, Lindemann A, Hauser F, Fischer HM, Hennecke H. (2007) Genome-wide transcript analysis of Bradyrhizobium japonicum bacteroids in soybean root nodules. Mol Plant Microbe Interact 20: 1353–1363 [DOI] [PubMed] [Google Scholar]
- Pfeffer PE, Douds DD, Jr, Bucking H, Schwartz DP, Shachar-Hill Y. (2004) The fungus does not transfer carbon to or between roots in an arbuscular mycorrhizal symbiosis. New Phytol 163: 617–627 [DOI] [PubMed] [Google Scholar]
- Pontais I, Treutter D, Paulin JP, Brisset MN. (2008) Erwinia amylovora modifies phenolic profiles of susceptible and resistant apple through its type III secretion system. Physiol Plant 132: 262–271 [DOI] [PubMed] [Google Scholar]
- Poumale HMP, Randrianasolo R, Rakotoarimanga JV, Raharisololalao A, Krebs HC, Tchouankeu JC, Ngadjui BT. (2008) Flavonoid glycosides and other constituents of Psorospermum androsaemifolium Baker (Clusiaceae). Chem Pharm Bull (Tokyo) 56: 1428–1430 [DOI] [PubMed] [Google Scholar]
- Pramanik MHR, Imai R. (2005) Functional identification of a trehalose 6-phosphate phosphatase gene that is involved in transient induction of trehalose biosynthesis during chilling stress in rice. Plant Mol Biol 58: 751–762 [DOI] [PubMed] [Google Scholar]
- Prell J, Poole P. (2006) Metabolic changes of rhizobia in legume nodules. Trends Microbiol 14: 161–168 [DOI] [PubMed] [Google Scholar]
- Roth LE, Stacey G. (1989) Bacterium release into host cells of nitrogen-fixing soybean nodules: the symbiosome membrane comes from three sources. Eur J Cell Biol 49: 13–23 [PubMed] [Google Scholar]
- Sanchez-Sampedro A, Kim HK, Choi YH, Verpoorte R, Corchete P. (2007) Metabolomic alterations in elicitor treated Silybum marianum suspension cultures monitored by nuclear magnetic resonance spectroscopy. J Biotechnol 130: 133–142 [DOI] [PubMed] [Google Scholar]
- Scervino JM, Ponce MA, Erra-Bassells R, Bompadre MJ, Vierheilig H, Ocampo JA, Godeas A. (2006) Glycosidation of apigenin results in a loss of its activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biol Biochem 38: 2919–2922 [Google Scholar]
- Schellenbaum L, Sprenger N, Schuepp H, Wiemken A, Boller T. (1999) Effects of drought, transgenic expression of a fructan synthesizing enzyme and of mycorrhizal symbiosis on growth and soluble carbohydrate pools in tobacco plants. New Phytol 142: 67–77 [Google Scholar]
- Schliemann W, Ammer C, Strack D. (2008) Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 69: 112–146 [DOI] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. (2004) The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. Plant J 39: 790–808 [DOI] [PubMed] [Google Scholar]
- Senaratna T, Merritt D, Dixon K, Bunn E, Touchell D, Sivasithamparam K. (2003) Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul 39: 77–81 [Google Scholar]
- Shabana YM, Abdel-Fattah GM, Ismail AE, Rashad YM. (2008) Control of brown spot pathogen of rice (Bipolaris oryzae) using some phenolic antioxidants. Braz J Microbiol 39: 438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD, Gutsche AT. (2003) Capillary electrophoresis-based profiling and quantitation of total salicylic acid and related phenolics for analysis of early signaling in Arabidopsis disease resistance. Anal Biochem 320: 223–233 [DOI] [PubMed] [Google Scholar]
- Shelp BJ, Bown AW, McLean MD. (1999) Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci 4: 446–452 [DOI] [PubMed] [Google Scholar]
- Shulaev V, Cortes D, Miller G, Mittler R. (2008) Metabolomics for plant stress response. Physiol Plant 132: 199–208 [DOI] [PubMed] [Google Scholar]
- Singh S, Kayastha AM, Asthana RK, Singh SP. (2004) Response of garden pea to nickel toxicity. J Plant Nutr 27: 1543–1560 [Google Scholar]
- Stacey G, Libault M, Brechenmacher L, Wan J, May GD. (2006) Genetics and functional genomics of legume nodulation. Curr Opin Plant Biol 9: 110–121 [DOI] [PubMed] [Google Scholar]
- Streeter JG. (2003) Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J Appl Microbiol 95: 484–491 [DOI] [PubMed] [Google Scholar]
- Streeter JG, Gomez ML. (2006) Three enzymes for trehalose synthesis in Bradyrhizobium cultured bacteria and in bacteroids from soybean nodules. Appl Environ Microbiol 72: 4250–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter JG, Lohnes DG, Fioritto RJ. (2001) Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant Cell Environ 24: 429–438 [Google Scholar]
- Stumpe M, Carsjens JG, Stenzel I, Gobel C, Lang I, Pawlowski K, Hause B, Feussner I. (2005) Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry 66: 781–791 [DOI] [PubMed] [Google Scholar]
- Suarez R, Wong A, Ramírez M, Barraza A, Orozco MDC, Cevallos MA, Lara M, Hernandez G, Iturriaga G. (2008) Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol Plant Microbe Interact 21: 958–966 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O. (2006) Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J 48: 261–273 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O. (2007) Distinct, crucial roles of flavonoids during legume nodulation. Trends Plant Sci 12: 282–285 [DOI] [PubMed] [Google Scholar]
- Sugawara M, Cytryn EJ, Sadowsky MJ. (2010) Functional role of Bradyrhizobium japonicum trehalose biosynthetic and metabolic genes during physiological stress and nodulation. Appl Environ Microbiol 76: 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaraj K, Bishnoi NR. (1999) Effect of salt stress on nodulation and nitrogen fixation in legumes. Indian J Exp Biol 37: 843–848 [PubMed] [Google Scholar]
- Takahashi H, Matsumura H, Kawai-Yamada M, Uchimiya H. (2008) The cell death factor, cell wall elicitor of rice blast fungus (Magnaporthe grisea) causes metabolic alterations including GABA shunt in rice cultured cells. Plant Signal Behav 3: 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar T, Gorecka KM, de Carvalho-Niebel F, Downie JA, Cullimore J, Pikula S. (2009) Annexins: calcium- and membrane-binding proteins in the plant kingdom. Potential role in nodulation and mycorrhization in Medicago truncatula. Acta Biochim Pol 56: 199–210 [PubMed] [Google Scholar]
- Tan RX, Lu H, Wolfender JL, Yu TT, Zheng WF, Yang L, Gafner S, Hostettmann K. (1999) Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med 65: 64–67 [DOI] [PubMed] [Google Scholar]
- Timotiwu PB, Sakurai N. (2002) Identification of mono-, oligo-, and polysaccharides secreted from soybean roots. J Plant Res 115: 77–85 [DOI] [PubMed] [Google Scholar]
- Trinchant JC, Yang YS, Rigaud J. (1998) Proline accumulation inside symbiosomes of faba bean nodules under salt stress. Physiol Plant 104: 38–49 [Google Scholar]
- Upchurch RG. (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30: 967–977 [DOI] [PubMed] [Google Scholar]
- Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, et al. (2009) Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 57: 1065–1078 [DOI] [PubMed] [Google Scholar]
- Van Dam NM, Tytgat TOG, Kirkegaard JA. (2009) Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem Rev 8: 171–186 [Google Scholar]
- Verdoy D, Coba De La Pena T, Redondo FJ, Lucas MM, Pueyo JJ. (2006) Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ 29: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Wagner SC, Skipper HD, Hartel PG. (1995) Medium to study carbon utilization by Bradyrhizobium strains. Can J Microbiol 41: 633–636 [Google Scholar]
- Walters D, Raynor L, Mitchell A, Walker R, Walker K. (2004) Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 157: 87–90 [DOI] [PubMed] [Google Scholar]
- Walters DR, Walker RL, Walker KC. (2003) Lauric acid exhibits antifungal activity against plant pathogenic fungi. J Phytopathol 151: 228–230 [Google Scholar]
- Wan J, Torres M, Ganapathy A, Thelen J, DaGue BB, Mooney B, Xu D, Stacey G. (2005) Proteomic analysis of soybean root hairs after infection by Bradyrhizobium japonicum. Mol Plant Microbe Interact 18: 458–467 [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot (Lond) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkin ELJ, Mutch LA, Rome S, Reeve WG, Castelli JM, Gruchlik Y, Best WM, O'Hara GW, Howieson JG. (2009) The effect of acidity on the production of signal molecules by Medicago roots and their recognition by Sinorhizobium. Soil Biol Biochem 41: 163–169 [Google Scholar]
- Williams M, Senaratna T, Dixon K, Sivasithamparam K. (2003) Benzoic acid induces tolerance to biotic stress caused by Phytophthora cinnamomi in Banksia attenuate. Plant Growth Regul 41: 89–91 [Google Scholar]
- Yang Z. (2003) GABA, a new player in the plant mating game. Dev Cell 5: 185–186 [DOI] [PubMed] [Google Scholar]
- Yeo ET, Kwon HB, Han SE, Lee JT, Ryu JC, Byun MO. (2000) Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol Cells 10: 263–268 [PubMed] [Google Scholar]
- Yokoyama T. (2008) Flavonoid-responsive nodY-lacZ expression in three phylogenetically different Bradyrhizobium groups. Can J Microbiol 54: 401–410 [DOI] [PubMed] [Google Scholar]
- Yu GH, Sun MX. (2007) Deciphering the possible mechanism of GABA in tobacco pollen tube growth and guidance. Plant Signal Behav 2: 393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehavi U, Polacheck I. (1996) Saponins as antimycotic agents: glycosides of medicagenic acid. Adv Exp Med Biol 404: 535–546 [DOI] [PubMed] [Google Scholar]
- Zhang J, Subramanian S, Stacey G, Yu O. (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 57: 171–183 [DOI] [PubMed] [Google Scholar]
- Zhang XS, Cheng HP. (2006) Identification of Sinorhizobium meliloti early symbiotic genes by use of a positive functional screen. Appl Environ Microbiol 72: 2738–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuanazzi JAS, Clergeot PH, Quirion JC, Husson HP, Kondorosi A, Ratet P. (1998) Production of Sinorhizobium meliloti nod gene activator and repressor flavonoids from Medicago sativa roots. Mol Plant Microbe Interact 11: 784–794 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.