Abstract
Breastfed children have lower risk of infectious diseases, post‐neonatal mortality and chronic diseases later in life. Because epidemiologic studies usually rely on reported history of previous breastfeeding, data on the accuracy and precision of recalled histories allow improved interpretation of the epidemiologic findings.
We evaluated the reliability of two reported breastfeeding durations in 567 reproductive‐aged women from Mexico using information obtained from nearly identical sets of questions applied at different times after weaning. We compared differences between reports, and examined the intraclass correlation coefficient (ICC) for any and for exclusive breastfeeding (EBF). Logistic regression was used to evaluate the determinants of poor recall (difference between reports of >20%).
The reliability of duration of any breastfeeding was high (ICC 0.94). Overall, differences between reports of duration were usually <1 month, and for 385/567, the difference was ≤0.5 months. Predictors of poorer recall were having ≥4 children, and time between reports of >2 months. The only predictor of better recall was greater age of the baby at weaning. The reliability of EBF duration was lower (ICC 0.49).
In this population with a relatively long duration of breastfeeding, reliability of any breastfeeding duration was high. Age, education and previous breastfeeding were not important predictors of recall, in contrast to findings in earlier studies. Consistent with previous reports, however, parity and length of recall were associated with poorer recall of duration of any breastfeeding. Future studies that use reported breastfeeding duration may want to consider the effect of these variables on recall.
Keywords: breastfeeding, exclusive breastfeeding, intraclass correlation coefficient, lactation, recall, reliability
Introduction
Breastfed children have lower risk of infectious diseases and post‐neonatal mortality. Breastfeeding may also protect against chronic diseases later in life, for both infant and mother (Leon‐Cava 2002; Gartner et al. 2005). For the infant, breastfeeding may reduce the incidence of asthma (Gdalevich et al. 2001; Oddy et al. 2002; Chulada et al. 2003), hypercholesterolaemia (Owen et al. 2002), overweight and obesity (Armstrong & Reilly 2002; Arenz et al. 2004; Owen et al. 2005), diabetes (types 1 and 2) (Kostraba et al. 1993; Gerstein 1994; Perez‐Bravo et al. 1996; Taylor et al. 2005; Owen et al. 2006), and some cancers (Davis 1998; Smulevich et al. 1999; Martin et al. 2005a). Maternal benefits of breastfeeding include a lower risk of breast (Martin et al. 2005b) and ovarian cancer (Rosenblatt & Thomas 1993), and a possible decrease in the risk of hip fractures and osteoporosis (Cumming & Klineberg 1993).
Epidemiologic studies of breastfeeding usually rely on reported history of previous breastfeeding. Data on the accuracy and precision of recalled histories allow improved interpretation of epidemiologic findings. The reliability of reported breastfeeding duration has been evaluated previously in a diverse group of studies with a wide range of recall time (Kark et al. 1984; Eaton‐Evans & Dugdale 1986; Haaga 1988; Vobecky et al. 1988; Huttly et al. 1990; Launer et al. 1992; Tienboon et al. 1994; Promislow et al. 2005; Gillespie et al. 2006). Overall, the literature suggests that maternal recall of breastfeeding duration is good, but reported correlation coefficients vary between 0.49 and 0.95 (Vobecky et al. 1988; Gillespie et al. 2006). Some of this variation may be due to differing characteristics of the populations studied. However, the role of education and parity in previous studies, for example, is inconsistent (Kark et al. 1984; Eaton‐Evans & Dugdale 1986; Haaga 1988; Huttly et al. 1990). For duration of exclusive breastfeeding (EBF), a previous study showed a poor reliability, with only 28% recalled accurately (Bland et al. 2003).
The main objective of this study was to evaluate reliability of the duration of any breastfeeding based on two reports, and also to assess potential predictors of its reliability in a large sample of reproductive‐aged women from Mexico. Some information on exclusivity of breastfeeding was also obtained and was briefly considered here.
Methods
Participants were recruited from a previous cross‐sectional study of newly delivered male infants and their mothers (n = 872), conducted in 2002–2003 in Tapachula, Chiapas, Mexico (Longnecker et al. 2007; Romano‐Riquer et al. 2007). Because the objective of that study was to evaluate the effects of exposure to an antiandrogen, 1,1‐dichloro‐2,2‐bis(p‐chlorophenyl)ethylene (DDE), only boys were recruited. Subsequently, a follow‐up study was conducted between January 2004 and June 2005 to evaluate the association of the mother's DDE exposure with duration of lactation and other outcomes; 784 healthy mother–son pairs were included in the follow‐up (Cupul‐Uicab et al. 2008). Because follow‐up continued after weaning, a study of the reliability of reported breastfeeding duration was incorporated in the study design. Of the 784 mothers followed, 97 were still breastfeeding at the end of the follow‐up (June 2005) and were therefore ineligible for the present analysis. Of the remaining 687, 120 were excluded because they had only one report of breastfeeding duration (87 reported age of weaning only at the last study visit and 33 were not re‐contacted because they moved from the study area). This left 567 who reported duration of lactation twice; they are the basis of this report. All of these women were known to have attempted breast feeding.
According to the eligibility criteria for the original studies, women younger than 15 or older than 35 years and those with certain medical conditions described previously were excluded, as well as those who used medications that inhibit or increase milk production. Children born at less than 36 weeks of gestational age, under 2500 g of birthweight, or who were to be given up for adoption were also excluded (Longnecker et al. 2007; Romano‐Riquer et al. 2007; Cupul‐Uicab et al. 2008). All mothers gave informed consent. The study was approved by the Institutional Review Boards at the Instituto Nacional de Salud Pública in México and the National Institute of Environmental Health Sciences in the United States.
Measurements
Duration of lactation was obtained from in‐person interviews conducted during each home follow‐up visit. The questions we asked did allow us to evaluate the reliability of duration of any and of EBF. However, we were unable to distinguish the reliability of reporting feeding of liquids from solids with these questions. Interviewers received special training before beginning the study and periodic retraining throughout. Length of any lactation was defined as the last time the child received any breast milk, irrespective of the introduction of liquids or solid foods. In this paper, breastfeeding duration refers to any breastfeeding duration, unless otherwise specified. At each interview, mothers were asked whether they were currently breast feeding. If they had stopped, we asked the number of months and days they had breastfed, and the date on which they completely stopped. Nearly identical questions about breastfeeding duration were asked at the first and last interviews (see Appendix).
The first report of duration corresponds to the first time the mother reported she had weaned the child. At the time follow‐up began (January 2004), the ages of the children were 3.3 to 25.1 months (median 13.2). For children still being breastfed at the first visit, we continued periodic home visits approximately every 2 months until the child was weaned. The median age of the child at the time the duration of lactation was first reported was 17.7 months (quartiles, 13.3 and 22.7), and the median time from weaning until first report was 9.2 months (quartiles, 3.6 and 14.7).
As noted above, follow‐up visits continued beyond weaning to record other outcomes. Thus, many women who weaned their child during the study period were re‐contacted and, for the reliability study, were asked to report duration again. Because of the design of the study, some women reported duration of lactation more than two times. Therefore, in order to maximize the time between reports for the present analysis, we use the duration recorded at the last study visit as the last report. The interval between the two reports varied; for 91/567 (16%), the last report was recorded ≤2 months after the first report. The median time between the two reports was 4.8 months (quartiles, 2.5 and 7.0).
Duration of EBF was reported twice for 416/567 (73%) women, and its reliability was evaluated in this subset. Identical questions were asked during the first and last interview. EBF was defined as the child's last age in days and months, when he received only breastfeeding, and no other liquids or solid food had yet been introduced.
Covariables
Demographic and breastfeeding characteristics were reported on the questionnaires. Variables considered possibly associated with recall of weaning were hospital of recruitment, rural/urban residence, mother's age at childbirth (Promislow et al. 2005), education (none, 1–6, 7–9, 10–12, 13+ years) (Eaton‐Evans & Dugdale 1986; Haaga 1988; Huttly et al. 1990), poverty status (poorest, somewhat poor, not poor) (Vobecky et al. 1988; Huttly et al. 1990), previous breastfeeding (yes, no) (Gillespie et al. 2006), parity (Kark et al. 1984; Eaton‐Evans & Dugdale 1986; Promislow et al. 2005; Gillespie et al. 2006), difficulty initiating breastfeeding, baby's age at introduction of another kind of milk (months), baby's age at weaning (months) (Eaton‐Evans & Dugdale 1986; Promislow et al. 2005), time between reports (Persson & Carlgren 1984; Launer et al. 1992; Promislow et al. 2005; Gillespie et al. 2006) and time from weaning to the first report.
Poverty status was measured using national standards, based on monthly per capita income in Mexican pesos by residence area. In urban areas, those with an income under 672 Mexican pesos were in the poorest category; they would have difficulty buying adequate food. Of the remainder, those under 1367 pesos lacked adequate income for other human needs, so were still considered poor. In rural areas, the cut points were 495 pesos and 946 pesos, respectively (SEDESOL 2002). If income at recruitment was missing [53/567, (9.3%)], we used income at the first follow‐up visit.
Statistical analysis
Because reported duration of lactation was recorded in days as a continuous variable, we compared the means of both reported durations, and the mean difference between the two reports. Comparisons were done overall and according to selected demographic and breastfeeding characteristics of the mother–son pairs. We calculated intraclass correlation coefficients (ICC) for reliability of breastfeeding duration as the proportion of the total variance explained by the variability between subjects (Deyo et al. 1991). This absolute agreement coefficient was calculated overall and for subgroups defined by variables previously suggested as potentially associated with recall of breastfeeding duration in other populations. To evaluate the possible relationship between the discrepancies in the two reported breastfeeding durations and mean duration, we used Bland–Altman plots (Bland & Altman 1999).
Logistic regression was used to evaluate the determinants of poorer recall, defined as a difference between reports of more than 20%. For epidemiologic purposes, e.g. when estimating a coefficient for breastfeeding duration in a model of a health outcome, a difference between reports greater than 20%, especially among those who breastfed for a short time, would affect estimates more than would differences between reports of >1 month. All variables from 1, 2, plus age at weaning, defined as <6.00, 6.01–12.00, 12.01–18.00, 18.01+ months as reported on the first occasion, were tested as predictors of recall in simple logistic regressions. Those that resulted in a P‐value ≤0.10 were considered for inclusion in the multivariate model (i.e. time between reports, mother's education, parity and baby's age at weaning). To determine the final model, all other variables from Table 1 were added simultaneously and evaluated as potential confounders using the change in estimate method. A variable was considered a confounder if removing it caused a change in at least one of the odds ratios (OR) (from time between reports, mother's education, parity and baby's age at weaning) of 10% or more. Variables that had no such effect on said factors were deleted from the model one by one in a stepwise way (Greenland 1989).
Table 1.
Distribution of the demographic characteristics and reported duration of lactation among women from Tapachula, Chiapas*
| n | Mean (SD) of breastfeeding duration † (month) | Mean (SD) of the difference ‡ (month) | ICC § | With >20% difference ¶ (%) | |
|---|---|---|---|---|---|
| All women | 567 | 8.2 (5.8) | 0.07 (2.0) | 0.94 | 18.0 |
| Mother's age (y) | |||||
| 15–<20 | 121 | 8.3 (5.6) | −0.20 (2.7) | 0.89 | 20.7 |
| 20–<25 | 212 | 8.4 (5.8) | 0.05 (1.6) | 0.96 | 17.5 |
| 25–<30 | 162 | 7.7 (5.5) | 0.20 (1.9) | 0.94 | 13.0 |
| ≥30 | 72 | 8.7 (6.5) | 0.28 (1.9) | 0.96 | 26.4 |
| Mother's educational background (y) | |||||
| None | 20 | 12.0 (7.3) | 0.54 (2.0) | 0.96 | 20.0 |
| 1–6 | 161 | 8.7 (5.8) | −0.13 (3.0) | 0.87 | 19.3 |
| 7–9 | 173 | 8.0 (6.1) | 0.15 (1.2) | 0.98 | 22.0 |
| 10–12 | 147 | 8.2 (5.2) | 0.17 (1.8) | 0.94 | 14.3 |
| >12 | 66 | 6.4 (4.6) | −0.02 (0.9) | 0.98 | 12.1 |
| Hospital of recruitment | |||||
| Social Security | 292 | 7.3 (5.3) | 0.05 (1.6) | 0.96 | 15.8 |
| Ministry of health | 275 | 9.1 (6.1) | 0.09 (2.4) | 0.92 | 20.4 |
| Residence area | |||||
| Urban | 350 | 7.7 (5.6) | 0.10 (1.3) | 0.97 | 17.4 |
| Rural | 217 | 8.9 (5.9) | 0.02 (2.7) | 0.89 | 18.9 |
| Poverty index | |||||
| Poorest | 387 | 9.0 (6.0) | 0.10 (2.4) | 0.92 | 18.6 |
| Less poor | 112 | 6.3 (4.7) | 0.01 (0.7) | 0.99 | 17.9 |
| Not poor | 68 | 6.5 (4.9) | −0.01 (0.7) | 0.99 | 14.7 |
| Birthweight (g) | |||||
| 2500–<3000 | 121 | 7.4 (5.4) | 0.06 (1.7) | 0.95 | 18.2 |
| 3000–<3500 | 275 | 8.5 (5.8) | 0.16 (2.1) | 0.93 | 17.8 |
| 3500–<4000 | 148 | 8.4 (5.8) | −0.12 (2.0) | 0.94 | 16.9 |
| ≥4000 | 23 | 7.9 (6.4) | 0.22 (0.8) | 0.99 | 26.1 |
| Primiparous | |||||
| Yes | 243 | 7.6 (5.6) | −0.12 (1.9) | 0.94 | 16.5 |
| No | 324 | 8.7 (5.9) | 0.21 (2.0) | 0.94 | 19.1 |
| Previous breastfeeding | |||||
| Yes | 289 | 8.9 (5.9) | 0.25 (2.1) | 0.94 | 18.3 |
| No | 278 | 7.5 (5.5) | −0.12 (1.9) | 0.94 | 17.6 |
| Number of living children | |||||
| 1 | 257 | 7.7 (5.6) | −0.08 (1.9) | 0.94 | 16.7 |
| 2 | 197 | 8.4 (5.6) | 0.08 (1.7) | 0.95 | 16.8 |
| 3 | 90 | 8.9 (6.0) | 0.28 (2.0) | 0.94 | 18.9 |
| ≥4 | 23 | 9.0 (7.7) | 0.76 (4.0) | 0.85** | 39.1 |
| Mother had difficulty initiating breasfeeding | |||||
| Yes | 94 | 6.3 (5.4) | −0.07 (0.9) | 0.99 | 20.2 |
| No | 473 | 8.6 (5.8) | 0.10 (2.1) | 0.93 | 17.5 |
| Nipple anatomy | |||||
| Inverted | 12 | 4.7 (4.0) | 0.14 (0.7) | 0.99 | 0.0 |
| Flat | 57 | 4.1 (4.7) | 0.04 (0.6) | 0.99 | 26.3 |
| Neither | 498 | 8.8 (5.7) | 0.07 (2.1) | 0.93 | 17.5 |
| Baby's age at introduction of other kind of milk (month) | |||||
| ≤1 | 174 | 5.8 (5.2) | 0.13 (1.5) | 0.96 | 21.5 |
| >1–6 | 172 | 7.4 (5.1) | 0.11 (1.9) | 0.92 | 19.4 |
| >6 | 92 | 12.6 (5.0) | −0.04 (2.7) | 0.85** | 11.4 |
| No other milk | 20 | 12.9 (4.2) | −0.17 (2.4) | 0.85** | 10.3 |
SD, standard deviation. *The table is based on the 567 women who reported breastfeeding duration twice. Information on those who were still breastfeeding (n = 97) at the end of the study or who had had only one report (n = 120) was not included. †Duration of breastfeeding based on first report. ‡First minus last breastfeeding duration (months). Significant differences in the means of the differences between reports were observed only for Primiparous (P = 0.05) and Previous breastfeeding (P = 0.02). §ICC, Intraclass correlation coefficient. ¶Proportion of women with >20% difference between reports. P‐values from Pearson's chi‐squared or Fisher's exact tests were >0.10 for all variables included in the table, except for Mother's age (P = 0.08), Number of living children (P = 0.08) and Baby's age at introduction of other kind of milk (P = 0.08). **95% confidence intervals are: Number of living children (≥4), 0.73–0.96; Baby's age at introduction of other kind of milk (>6 months), 0.80–0.90; and No other milk, 0.74–0.95. For the ICC that were larger the 95% CI were more narrow.
Table 2.
Mean (SD) of reported duration of breastfeeding, mean difference (SD) between reports, and intraclass correlation coefficient according to time between weaning and first report and time between reports
| n | All women | Time between first and last report (month) | |||
|---|---|---|---|---|---|
| <2.00 | 2.00–5.99 | 6.00–13.99 | |||
| 567 | 87 | 270 | 210 | ||
| All women | |||||
| First duration* | 8.2 (5.8) | 10.8 (6.4) | 8.7 (5.8) | 6.5 (4.9) | |
| Last duration* | 8.1 (5.7) | 10.7 (6.3) | 8.7 (5.8) | 6.3 (4.6) | |
| Difference † | 0.1 (2.0) | 0.1 (2.1) | 0.0 (2.0) | 0.2 (1.9) | |
| ICC ‡ | 0.94 | 0.95 | 0.94 | 0.92 | |
| 95% CI | 0.93–0.95 | 0.92–0.97 | 0.92–0.95 | 0.90–0.94 | |
| Time between weaning and first report (month) | |||||
| ≤4.0 | 156 | ||||
| First duration* | 12.6 (5.2) | 18.0 (3.7) | 15.1 (3.7) | 9.4 (4.4) | |
| Last duration* | 12.1 (4.9) | 17.7 (3.5) | 14.5 (3.2) | 9.0 (4.0) | |
| Difference † | 0.5 (2.1) | 0.2 (1.0) | 0.6 (1.9) | 0.4 (2.4) | |
| ICC ‡ | 0.91 | 0.96 | 0.83 | 0.83 | |
| 95% CI | 0.88–0.94 | 0.93–1.00 | 0.75–0.91 | 0.76–0.90 | |
| 4.1–12.0 | 200 | ||||
| First duration* | 8.1 (5.5) | 14.6 (4.4) | 9.8 (4.6) | 5.2 (4.6) | |
| Last duration* | 7.9 (5.4) | 13.8 (4.3) | 9.6 (4.8) | 5.0 (4.5) | |
| Difference † | 0.2 (1.8) | 0.8 (3.3) | 0.1 (1.7) | 0.2 (1.5) | |
| ICC ‡ | 0.94 | 0.71 | 0.94 | 0.95 | |
| 95% CI | 0.93–0.96 | 0.49–0.94 | 0.91–0.96 | 0.92–0.97 | |
| 12.1–38.0 | 211 | ||||
| First duration* | 5.0 (4.0) | 6.2 (3.7) | 4.9 (4.2) | 3.8 (3.4) | |
| Last duration* | 5.4 (4.6) | 6.5 (4.2) | 5.3 (5.0) | 4.2 (3.7) | |
| Difference † | −0.4 (2.0) | −0.3 (1.8) | −0.4 (2.3) | −0.3 (1.2) | |
| ICC ‡ | 0.89 | 0.89 | 0.88 | 0.94 | |
| 95% CI | 0.86–0.92 | 0.84–0.95 | 0.84–0.92 | 0.91–0.98 | |
SD, standard deviation; CI, confidence interval. *Mean (Standard Deviation). †First minus last breastfeeding duration (months).P‐values for the means of the differences between reports were 0.61 for Time between first and last report, and <0.001 for Time between weaning and first report. ‡ICC, intraclass correlation coefficient.
Because previous studies suggest that the reliability of breastfeeding duration decreases over time, ICCs were calculated according to categories of time between reports. As mentioned earlier, in the present study for 91/567 (16%), the last report was recorded ≤2 months after the first report, and we repeated the analyses after excluding the 91 to evaluate whether the reliability changed. All analyses were conducted using Stata (Stata Statistical Software, release 9.0; StataCorp, College Station, TX, USA).
Results
The participants were young (mean 24 years), few attended college, and the majority lived in the city and its surrounding areas (Table 1). Because Tapachula is located in one of the poorest states in the country, the fact that 387/567 (68%) of the participants were in the poorest category and only 68/567 (12%) were not poor was expected. Compared with the subjects who were included, those who were excluded [had only one report or were still breastfeeding at the end of the study (n = 217)] were more likely to be: less educated, come from rural areas, recruited in the Ministry of Health hospital (i.e. without health insurance coverage), and in the poorest category (data not shown). Previous studies of breastfeeding duration among women in Mexico have reported that the poorest breastfeed the longest (Long‐Dunlap et al. 1995, Consejo Nacional de Población 2000; Gonzalez‐Cossio et al. 2003), and this finding was apparent in the present data (Table 1).
Differences between reports of breastfeeding duration
The mean duration of lactation in both reports was similar (8.2 and 8.1 months). The range for the first report (0–29 months) was comparable to that for the last report (0–27 months), and the overall mean of the difference was small (∼2 days), although large variability was observed in the standard deviation (SD, 2 months). Across categories of the variables listed in Table 1, the mean of the differences between the two reports were small (range, −0.20 to 0.76), although, again some large discrepancies were reflected in the SD (range, 0.6 to 4.0). A somewhat larger mean difference was observed among women with no education (0.54) compared with those who were more educated (>12 year, −0.02), and those with four or more children (0.76) versus those with fewer (−0.08) (Table 1).
At the last report, 311/567 (55%) women agreed exactly with their first report, while essentially the same proportion reported longer [125/567, (22%)] or shorter durations [131/567, (23%)]. For 177/567 (31%), the reports differed by 1 month or less; for 59/567 (10.4%), the difference was more than 1 but less than 3 months, and for 20/567 (3.5%), the difference exceeded 3 months. Only 102/567 (18%) of the participants had a difference of more than 20% between the two reports, and across the variables shown in Table 1, the difference varied from 0/12 (0%) to 9/23 (39%) (Table 1). Of the 10 women who initially reported never breastfeeding, two reported a duration of 5 days in the second interview.
In the cross‐tabulation of time from weaning to the first report and time between the two reports (Table 2), the mean of the differences were again small (range, −0.4 to 0.8), with a somewhat large SD (range, 1.0 to 3.3). For those whose first report was >12 months after weaning, the mean of the differences were consistently negative, indicating a tendency to over‐report breastfeeding duration in the last interview. Those whose first report was closer to weaning had the opposite tendency. However, as noted above, all means of the difference were <1 month.
Reliability of breastfeeding duration
The intraclass correlation of reported breastfeeding duration was high, and varied only slightly by selected characteristics of the mothers and children (range, 0.85–0.99) (Table 1); and the 95% confidence intervals (CI) were generally from 0.73 to 1.00 (not shown). In particular, women with four or more children had lower reliability compared with those with fewer children (Table 1).
We also observed a good agreement between the two reports, and no systematic differences were apparent in the Bland–Altman plot (Fig. 1). The Spearman correlation of the differences between reports and their mean (r = 0.04, P = 0.34) showed no obvious relation; however, the plot suggested that the disagreement between reports increased slightly with duration of breastfeeding, especially for durations longer than 10 months.
Figure 1.
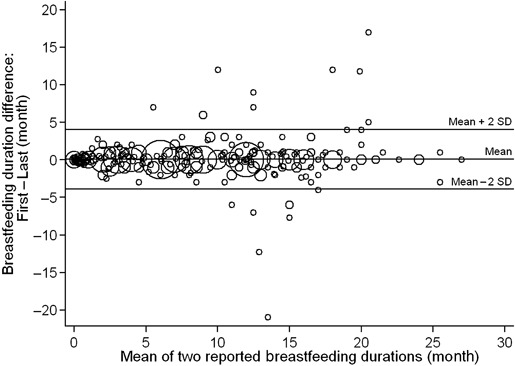
Bland–Altman plot of the differences between two breastfeeding durations versus the mean of the two durations, reported by reproductive‐aged women from Tapachula, Chiapas, Mexico (n = 567). Size of circle is proportional to the number of women. Limits of agreement: Mean ± 2SD, 0.069 ± 2*1.991.
The reliability of reports for all women varied little across time between reports or time from weaning to first report. For the cross‐tabulation of the time between weaning and first report with recall time, correlations did not show any pattern and reliability remained high. The lowest coefficient (0.71) was based on a relatively small group of subjects (n = 19) with the largest SD for the mean difference between reports (Table 2).
Because not all women were interviewed by the same interviewer on both occasions, we evaluated whether the reliability differed by interviewer. For those whose two reports were obtained by the same interviewer (n = 46), the ICC was 0.99 (95% CI, 0.98, 0.99); the time between reports was >2 months for 40/46 (87%). For women whose two reports were obtained from different interviewers (n = 521), the ICC was 0.94 (95% CI, 0.92, 0.95); for 440/521 (84%), the time between reports was >2 months. The results were similar using random effects model that included a term for interviewer (not shown).
To facilitate comparison of our data with a previous study where the reliability of breastfeeding duration was evaluated in women who weaned their infants in the first 3 months after birth (Gillespie et al. 2006), we re‐calculated the ICC for the subset of women whose breastfeeding duration at first report was ≤3 months (n = 145). The overall ICC fell to 0.45 (95% CI, 0.32, 0.58) and the Spearman correlation of the difference between reports and their mean was −0.17 (P = 0.07). After removing an outlier observation (first report, 3 months; last report, 24 months), the ICC increased to 0.83 (95% CI, 0.78, 0.88), but still remained lower than when all 567 subjects were included. The Bland–Altman plot showed that there was a small systematic difference between the two reports of breastfeeding (i.e. the mean of the differences is slightly below zero in Fig. 2). The Spearman correlation between the differences of reports and their means (−0.13) was not statistically significant (P = 0.11) even after excluding the outlier observation. The disagreement between reports, however, increased as the duration of breastfeeding increased (Fig. 2).
Figure 2.
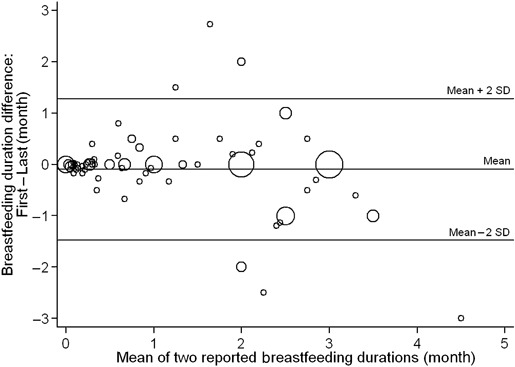
Bland–Altman plot of the differences between two reported breastfeeding durations versus their mean, for the subset of women whose breastfeed duration was ≤3 months (n = 144a). Size of circle is proportional to the number of women. Limits of agreement: Mean ± 2SD, −0.095 ± 2*0.688. aFor a better visualization of the distribution, one outlier observation was removed from the plot. Its values were: difference between reports, −21 mo; mean of reports, 13.5 mo.
Reliability of EBF duration
For EBF duration, the overall ICC, 0.49 (95% CI, 0.42, 0.56), was lower than for any breastfeeding (Table 1). The smallest ICC (0.01) was for women with no education (n = 18), and the largest (ICC, 0.78) was for those who introduced another kind of milk within the first month of life (n = 146). In contrast to what we observed for any breastfeeding, the reliability of EBF for women with one child was lower, ICC (0.40), than for women with two or more children (ICC: 0.54 for two children; 0.64 for three; and 0.51 for ≥4). Restricting the calculation to those with time between reports of >2 months showed a slight increase in the overall reliability of EBF (ICC, 0.52).
Determinants of poorer recall of any breastfeeding
We also examined determinants of poorer recall, defined as a difference between reports of more than 20% (data not shown because of similarity of results to those shown in 1, 2). The results showed that women with four or more children were more likely to have a poorer recall compared with those with two or fewer children (adjusted OR, 2.9; 95% CI 1.12, 7.44). Time between reports longer than 2 months was also associated with poorer recall (adjusted OR, 3.7; 95% CI 1.43, 9.71). Comparing women who weaned in the first 6 months, those who weaned after 6.01–12.00 months (adjusted OR, 0.32, 95% CI 0.19, 0.54) and after 12.01–18.00 months (adjusted OR, 0.13, 95% CI 0.05, 0.34) were less likely to have poorer recall. No other variables were found to be statistically significant independent determinants of poorer recall. Excluding women whose length of recall was ≤2 months gave similar results.
Discussion
In this population of reproductive‐aged women who breastfed for a relatively long time, reported duration of any breastfeeding showed high reliability. Overall, reports of the duration of any breastfeeding usually differed by less than a month, with SDs of about 2 months; however, for 385/567 (68%), the mean of the differences was ≤0.5 months. Predictors of poorer recall, defined as a difference between reports of more than 20%, were having four or more children and time between reports (recall period) longer than 2 months, while the only predictor of better recall was greater age of baby at weaning.
Recall of breastfeeding duration (as measured with an ICC) in this study was similar to that reported previously among Malaysians (recall period, 4 months) and among a small group of Canadians (recall period, 8 years) (Haaga 1988; Vobecky et al. 1988). However, it is greater than that reported previously for college‐educated US women with a recall period of 50 years, among whom the overall weighted kappa was 0.55 (Promislow et al. 2005). The reliability we observed is also greater than in another report based on US women in which the recall period was similar to ours. In that study, the breastfeeding duration was shorter (limited to those who weaned within the first 3 months), and the correlation reported was just 0.49 (Gillespie et al. 2006). When we similarly restricted our calculation to those whose breastfeeding duration was ≤3 months, the overall ICC was low (0.45). But as noted before, after removing one observation whose two reports were very different (3 and 24 months, respectively), the ICC increased (0.83), showing that this observation was influential. We suspect the outlier was due to a recording error on the questionnaire for the first report of breastfeeding duration, but a memory error was also a possibility. Nonetheless, it is possible that the low correlation reported for the US population was largely the result of the narrow range of time considered. Our data also suggested a tendency to slightly overestimate the duration of breastfeeding in the second report for this group, as shown in the Bland–Altman plot (Fig. 2); and there was evidence that the error in reporting duration of breastfeeding increased as the duration increased. These errors could mask associations between breastfeeding duration and health outcomes, particularly in populations whose average duration of breastfeeding is relatively short.
The proportion of those who recalled breastfeeding duration within a month (i.e. accuracy of recall) in the present study [488/567, (86%)] was similar to that reported previously for US women (88%) (Quandt 1987), but higher than reported for Australians (37% and 79%) (Eaton‐Evans & Dugdale 1986; Tienboon et al. 1994).
Reliability of EBF in this population was lower than for duration of any breastfeeding. The proportion of women who accurately recalled EBF duration was low [99/416 (24%)] and was comparable with a previous study [23/81 (28%)] conducted in a rural area of South Africa (Bland et al. 2003). The proportion who over‐reported EBF duration was lower in this sample [181/416 (43.5%)] compared with Bland et al. (2003) where 46/81 (57%) did so; however, this difference might be accounted by the fact that our main focus was on any breastfeeding rather than EBF duration as in the previous study.
Whether the duration of breastfeeding is related to the accuracy of recall (as measured by the difference between reports) was evaluated in two previous studies, in populations that were more educated than in the present study. In those studies, less accuracy of recall was observed among those who reported shorter (<2 months) and longer (>6 and >9 months) breastfeeding durations (Eaton‐Evans & Dugdale 1986; Promislow et al. 2005). Our study, with poorer recall defined as the percentage of the difference between reports (>20%), showed that women who breastfed for ≤6 months were more likely to have poorer recall than those who breastfed longer. However, with poorer recall defined as the absolute difference between reports >1 month, women who breastfed ≤6 months were less likely to have poorer recall than those who breastfed longer. As mentioned before, for epidemiologic purposes, (e.g. when estimating a coefficient for breastfeeding duration in a model related to a health outcome), a difference between reports greater than 20%, especially among those who breastfed for a short time, would affect estimates more than would differences between reports of >1 month.
Other predictors of poorer recall in these data were having four or more children and time between reports longer than 2 months. Parity has been related to both poorer and better recall in previous studies (Kark et al. 1984; Eaton‐Evans & Dugdale 1986; Promislow et al. 2005; Gillespie et al. 2006). We observed that high parity was related to poorer recall even after adjusting for time between reports, baby's age at weaning and mother's educational background. However, our results were opposite to those reported for US women with a longer recall period where a greater parity was related with better recall (Promislow et al. 2005). Longer length of recall has been consistently associated previously with poor recall (Launer et al. 1992; Promislow et al. 2005; Gillespie et al. 2006), and we also observed that a recall period longer than 2 months increased almost four times the likelihood of having poorer recall in the last report of breastfeeding duration. Other variables reported previously as predictors, such as previous breastfeeding, age and education, were not significantly related to recall in the present study. Moreover, reliability was consistently high across categories of these variables (Table 1).
A preference in reporting certain numbers is always a possibility when evaluating reported outcomes, and can lead to some degree of bias. In this study, we observed such a preference in reported breastfeeding durations. For example, the numbers of subjects reporting the following months of duration the first time were: 5 months, n = 18; 6 months, n = 49; 7 months, n = 25; 11 months, n = 12; 12 months, n = 54; and 13 months, n = 17. However, we cannot determine whether this was a real preference or whether the high proportions around these times were produced because women in this population weaned preferentially at 6 and 12 months.
A limitation of the present study was that we only included boys because the original study was limited to that sex. Previous studies suggest that reliability is slightly better for girls (Kark et al. 1984; Promislow et al. 2005), which means that this exclusion could cause an underestimation in the reliability of breastfeeding duration in this population. The fact that recall time was short (<2 years) could limit the generalizability of our results to settings with a very long recall time; however, reliability in this study was similar to that in a previous study with a much longer recall (8 years) (Vobecky et al. 1988). Women who were excluded from the present analysis [gave one report or were still breastfeeding at the last study visit (n = 217)] had characteristics similar to women who breastfed longer. Because longer breastfeeding duration was associated with better recall in this population, we probably have underestimated the reliability of breastfeeding due to this exclusion. Also, because all participants attempted to breastfeed in this population, we could not evaluate the reliability of reports of ever breastfeeding (i.e. as a yes/no event), which has been evaluated in previous studies.
Because we asked subjects about breastfeeding status many times during follow‐up, this could have increased the observed reliability because it might have increased their awareness of such information. Therefore, the reliability of reported breastfeeding duration may be somewhat lower than that reported here. Although some information about introduction of liquids and solids was obtained, the questions we used did not allow us to distinguish the reliability of liquids from solids. One of the questions used to determine the duration of any breastfeeding was slightly different the first and last time it was asked (see Appendix); however, we consider that the difference was too small to account for any of the discrepancies that we observed in the agreement.
To our knowledge, this is the first study of the reliability of breastfeeding duration conducted in a Mexican population; the sample includes mostly poor women, with low education and long breastfeeding duration. Those characteristics have been previously related to poor recall (Eaton‐Evans & Dugdale 1986; Haaga 1988; Huttly et al. 1990; Promislow et al. 2005; Gillespie et al. 2006), but we nonetheless observed high reliability. We were also able to evaluate other potential determinants of recall reported by others, such as parity and education. However, only greater parity was an important determinant of poorer recall in this population. We were also able to evaluate the effect of different interviewers on the reliability of breastfeeding duration and found that the ICCs remained high whether both reports were obtained from the same interviewer or different interviewers.
The reliability of any breastfeeding duration in this population with a relatively long duration of breastfeeding was high and mean differences between reports were small. Length of recall longer than 2 months was strongly related to poorer recall; however, the ICC was still high across categories of this variable. Education was not an important predictor of recall, probably because this association has been observed mostly for college‐educated women and our power was limited for that group. The other determinants of recall for this population were greater parity and younger age of the baby at weaning. Future studies that use reported breastfeeding duration may want to consider the effect of these variables on recall in their populations.
Key messages
-
•
In general, available literature suggests that maternal recall of breastfeeding duration is fairly good. Reported correlation coefficients vary between 0.49 and 0.95.
-
•
The reliability of the reported duration of any breastfeeding in this population, with a relatively long duration of breastfeeding, was high (ICC, 0.94) and differences between reports were small; however, the reliability of exclusive breastfeeding duration was low (ICC, 0.49).
-
•
Predictors of poorer recall were having four or more children and a recall period longer than 2 months. Education was not an important predictor of recall in this population.
-
•
Future studies that use reported breastfeeding duration should consider the effect of these variables on recall in their populations.
Conflicts of interest
None declared.
Source of funding
This study was supported in part by a contract from the National Institute of Environmental Health Sciences, National Institutes of Health (NIH) (N01‐ES‐15467), in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA, and in part by the Instituto Nacional de Salud Pública, Cuernavaca, Morelos, México.
Acknowledgements
We wish to thank the field work team in Tapachula, Chiapas, México, for their valuable support during data collection.
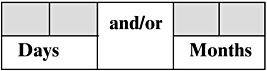
The original Spanish questions from the questionnaires, used to determine duration of any breastfeeding for first and last interview, and its corresponding English translations were:
First interview (Spanish)
4. En la actualidad, ¿Continúa dando pecho a su bebé?

5. ¿Durante cuánto tiempo amamantó a su bebé? (Si la señora conoce el tiempo exacto, anote dias y meses)

English translation:
4. Currently, do you continue breastfeeding your baby?

5. How long did you breastfed your baby? (If the women know the exact duration, write days and months)
Last interview (Spanish):
4. En la actualidad, ¿Continúa dando pecho a su bebé?

5. ¿Durante cuánto tiempo ha amamantado a su bebé? (Si la señora conoce el tiempo exacto, anote dias y meses)

English translation:
4. Currently, do you continue breastfeeding your baby?

5. How long have you breastfed your baby? (If the women know the exact duration, write days and months)
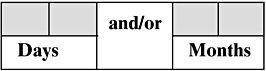
Note that the difference is one word having to do with a subtle difference in verb tense.
References
- Arenz S., Ruckerl R., Koletzko B. & Von Kries R. (2004) Breast‐feeding and childhood obesity – a systematic review. International Journal of Obesity and Related Metabolic Disorders 28, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Armstrong J. & Reilly J.J. (2002) Breastfeeding and lowering the risk of childhood obesity. Lancet 359, 2003–2004. [DOI] [PubMed] [Google Scholar]
- Bland J.M. & Altman D.G. (1999) Measuring agreement in method comparison studies. Statistical Methods in Medical Research 8, 135–160. [DOI] [PubMed] [Google Scholar]
- Bland R.M., Rollins N.C., Solarsh G., Van den Broeck J. & Coovadia H.M. (2003) Maternal recall of exclusive breast feeding duration. Archives of Disease in Childhood 88, 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulada P.C., Arbes S.J. Jr, Dunson D. & Zeldin D.C. (2003) Breast‐feeding and the prevalence of asthma and wheeze in children: analyses from the Third National Health and Nutrition Examination Survey, 1988–1994. Journal of Allergy and Clinical Immunology 111, 328–336. [DOI] [PubMed] [Google Scholar]
- Consejo Nacional de Población (2000) Lactancia Materna In: Cuadernos de Salud Reproductiva. República Mexicana (ed. Zúñiga E., Zubieta B. & Araya C.). p 152 CONAPO: México D.F. [Google Scholar]
- Cumming R.G. & Klineberg R.J. (1993) Breastfeeding and other reproductive factors and the risk of hip fractures in elderly women. International Journal of Epidemiology 22, 684–691. [DOI] [PubMed] [Google Scholar]
- Cupul‐Uicab L.A., Gladen B.C., Hernández‐Ávila M., Weber J.‐P. & Longnecker M.P. (2008) DDE, a degradation product of DDT, and duration of lactation in a highly exposed area of Mexico. Environmental Health Perspectives 116, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.K. (1998) Review of the evidence for an association between infant feeding and childhood cancer. International Journal of Cancer. Supplement 11, 29–33. [PubMed] [Google Scholar]
- Deyo R.A., Diehr P. & Patrick D.L. (1991) Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Controlled Clinical Trials 12, 142S–158S. [DOI] [PubMed] [Google Scholar]
- Eaton‐Evans J. & Dugdale A.E. (1986) Recall by mothers of the birth weights and feeding of their children. Human Nutrition. Applied Nutrition 40, 171–175. [PubMed] [Google Scholar]
- Gartner L.M., Morton J., Lawrence R.A., Naylor A.J., O'Hare D., Schanler R.J. et al (2005) Breastfeeding and the use of human milk. Pediatrics 115, 496–506. [DOI] [PubMed] [Google Scholar]
- Gdalevich M., Mimouni D. & Mimouni M. (2001) Breast‐feeding and the risk of bronchial asthma in childhood: a systematic review with meta‐analysis of prospective studies. The Journal of Pediatrics 139, 261–266. [DOI] [PubMed] [Google Scholar]
- Gerstein H.C. (1994) Cow's milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care 17, 13–19. [DOI] [PubMed] [Google Scholar]
- Gillespie B., D’Arcy H., Schwartz K., Bobo J.K. & Foxman B. (2006) Recall of age of weaning and other breastfeeding variables. International Breastfeeding Journal 1, 4. Doi: 10.1186/1746‐4358‐1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Cossio T., Moreno‐Macias H., Rivera J.A., Villalpando S., Shamah‐Levy T., Monterrubio E.A. et al (2003) Breast‐feeding practices in Mexico: results from the Second National Nutrition Survey 1999. Salud Publica de Mexico 45 (Suppl. 4), S477–S489. [DOI] [PubMed] [Google Scholar]
- Greenland S. (1989) Modeling and variable selection in epidemiologic analysis. American Journal of Public Health 79, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaga J.G. (1988) Reliability of retrospective survey data on infant feeding. Demography 25, 307–314. [PubMed] [Google Scholar]
- Huttly S.R., Barros F.C., Victora C.G., Beria J.U. & Vaughan J.P. (1990) Do mothers overestimate breast feeding duration? An example of recall bias from a study in southern Brazil. American Journal of Epidemiology 132, 572–575. [DOI] [PubMed] [Google Scholar]
- Kark J.D., Troya G., Friedlander Y., Slater P.E. & Stein Y. (1984) Validity of maternal reporting of breast feeding history and the association with blood lipids in 17‐year‐olds in Jerusalem. Journal of Epidemiology Community Health 38, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostraba J.N., Cruickshanks K.J., Lawler‐Heavner J., Jobim L.F., Rewers M.J., Gay E.C. et al (1993) Early exposure to cow's milk and solid foods in infancy, genetic predisposition, and risk of IDDM. Diabetes 42, 288–295. [PubMed] [Google Scholar]
- Launer L.J., Forman M.R., Hundt G.L., Sarov B., Chang D., Berendes H.W. et al (1992) Maternal recall of infant feeding events is accurate. Journal of Epidemiology Community Health 46, 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon‐Cava N. (2002) Cuantificacion de los Beneficios de la Lactancia Materna: Reseña de la Evidencia. Organizacion Panamericana de la Salud: Washington, DC. [Google Scholar]
- Long‐Dunlap K., Rivera‐Dommarco J., Rivera‐Pasquel M., Hernandez‐Avila M. & Lezana M.A. (1995) Feeding patterns of Mexican infants recorded in the 1988 National Nutrition Survey. Salud Publica de Mexico 37, 120–129. [PubMed] [Google Scholar]
- Longnecker M.P., Gladen B.C., Cupul‐Uicab L.A., Romano‐Riquer S.P., Weber J.P., Chapin R.E. et al (2007) In utero exposure to the antiandrogen 1,1‐Dichloro‐2,2‐bis(p‐chlorophenyl)ethylene (DDE) in relation to anogenital distance in male newborns from Chiapas, Mexico. American Journal of Epidemiology 165, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.M., Gunnell D., Owen C.G. & Smith G.D. (2005a) Breast‐feeding and childhood cancer: a systematic review with metaanalysis. International Journal of Cancer 117, 1020–1031. [DOI] [PubMed] [Google Scholar]
- Martin R.M., Middleton N., Gunnell D., Owen C.G. & Smith G.D. (2005b) Breast‐feeding and cancer: the Boyd Orr cohort and a systematic review with meta‐analysis. Journal of the National Cancer Institute 97, 1446–1457. [DOI] [PubMed] [Google Scholar]
- Oddy W.H., Peat J.K. & De Klerk N.H. (2002) Maternal asthma, infant feeding, and the risk of asthma in childhood. Journal of Allergy and Clinical Immunology 110, 65–67. [DOI] [PubMed] [Google Scholar]
- Owen C.G., Whincup P.H., Odoki K., Gilg J.A. & Cook D.G. (2002) Infant feeding and blood cholesterol: a study in adolescents and a systematic review. Pediatrics 110, 597–608. [DOI] [PubMed] [Google Scholar]
- Owen C.G., Martin R.M., Whincup P.H., Smith G.D. & Cook D.G. (2005) Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 115, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Owen C.G., Martin R.M., Whincup P.H., Smith G.D. & Cook D.G. (2006) Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. American Journal of Clinical Nutrition 84, 1043–1054. [DOI] [PubMed] [Google Scholar]
- Perez‐Bravo F., Carrasco E., Gutierrez‐Lopez M.D., Martinez M.T., Lopez G. & De Los Rios M.G. (1996) Genetic predisposition and environmental factors leading to the development of insulin‐dependent diabetes mellitus in Chilean children. Journal of the Molecular Medicine 74, 105–109. [DOI] [PubMed] [Google Scholar]
- Persson L.A. & Carlgren G. (1984) Measuring children's diets: evaluation of dietary assessment techniques in infancy and childhood. International Journal of Epidemiology 13, 506–517. [DOI] [PubMed] [Google Scholar]
- Promislow J.H., Gladen B.C. & Sandler D.P. (2005) Maternal recall of breastfeeding duration by elderly women. American Journal of Epidemiology 161, 289–296. [DOI] [PubMed] [Google Scholar]
- Quandt S.A. (1987) Maternal recall accuracy for dates of infant feeding transitions. Human Organization 46, 152–160. [Google Scholar]
- Romano‐Riquer S.P., Hernandez‐Avila M., Gladen B.C., Cupul‐Uicab L.A. & Longnecker M.P. (2007) Reliability and determinants of anogenital distance and penis dimensions in male newborns from Chiapas, Mexico. Paediatric and Perinatal Epidemiology 21, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt K.A. & Thomas D.B. (1993) Lactation and the risk of epithelial ovarian cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. International Journal of Epidemiology 22, 192–197. [DOI] [PubMed] [Google Scholar]
- SEDESOL (2002) Technical Note on the Measurement of Poverty Based on the Results of the National Survey of Household Income and Expenditures [In Spanish]. SEDESOL: México D.F. Available at: http://sedesol2006.sedesol.gob.mx/subsecretarias/prospectiva/main_medicionpobreza.htm (last accessed 29 March 2007). [Google Scholar]
- Smulevich V.B., Solionova L.G. & Belyakova S.V. (1999) Parental occupation and other factors and cancer risk in children: I. Study methodology and non‐occupational factors. International Journal of Cancer 83, 712–717. [DOI] [PubMed] [Google Scholar]
- Taylor J.S., Kacmar J.E., Nothnagle M. & Lawrence R.A. (2005) A systematic review of the literature associating breastfeeding with type 2 diabetes and gestational diabetes. Journal of the American College of Nutrition 24, 320–326. [DOI] [PubMed] [Google Scholar]
- Tienboon P., Rutishauser I. & Walhqvist M. (1994) Maternal recall of infant feeding practices after an interval of 14 to 15 years. The Australian Journal of Nutrition and Dietetics 51, 25–27. [Google Scholar]
- Vobecky J.S., Vobecky J. & Froda S. (1988) The reliability of the maternal memory in a retrospective assessment of nutritional status. Journal of Clinical Epidemiology 41, 261–265. [DOI] [PubMed] [Google Scholar]


