Abstract
Exchange dynamics of lipophilic guest molecules, encapsulated in supramolecular nanoassemblies in aqueous solutions, have implications in evaluating the stability of drug delivery vehicle. This is because exchange dynamics is related to the propensity of a nanocarrier to be leaky. We describe a fluorescence resonance energy transfer (FRET) based method to evaluate guest exchange dynamics in the aqueous phase. We have utilized this method to analyze the stability of encapsulation in polymeric nanogels and other related amphiphilic nanoassemblies.
The complications faced in the administration of insoluble and toxic hydrophobic drugs to target sites have spurred tremendous interest in the field of delivery vehicle design.1 Of the many factors considered in such design, the encapsulation stability of the delivery container is critical.2 Encapsulation of a hydrophobic molecule in aqueous media itself is just an indicator of the thermodynamic distribution of the molecules between the container and the bulk solvent. This does not provide indications on the stability of encapsulation in terms of the dynamics of guest exchange with the bulk media. Understanding the dynamics of interchange between the bulk solvent and the nanocontainer is crucial, as this carries clear implications to the potential leakage of encapsulated guest molecules from the vehicles as they pass through a biological system. Thus, an analysis of this process is necessary for optimization of the design and construction of drug delivery carriers. To probe the stability of encapsulation, we describe here the dynamics of guest interchange in nanocarriers using Fluorescence Resonance Energy Transfer (FRET) as a tool. We then utilize this method to investigate the stability of encapsulation in a new class of polymer nanogels and compare it with that observed in classical amphiphilic nanoassemblies.
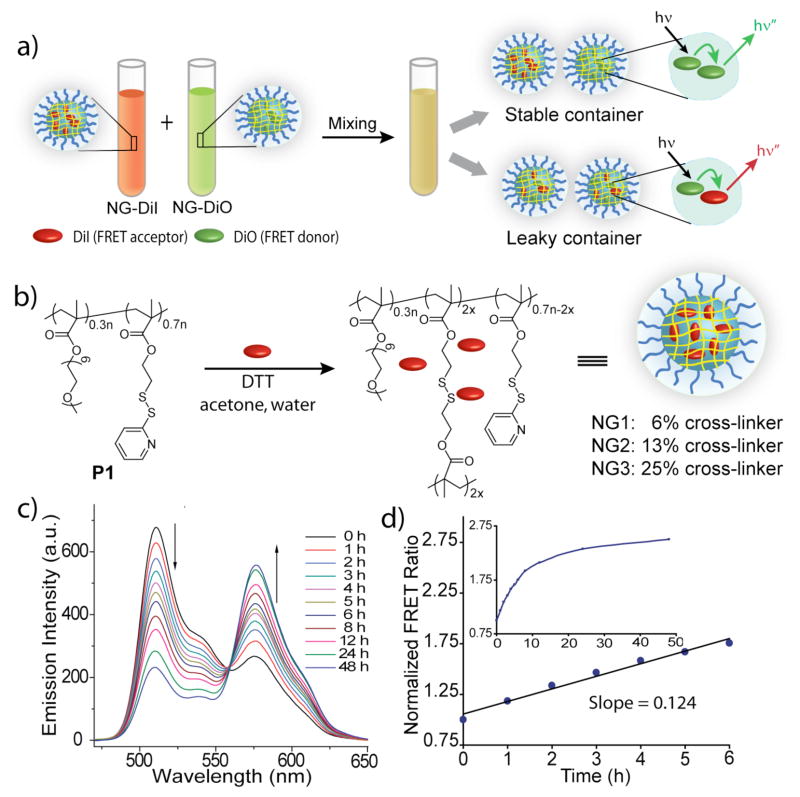
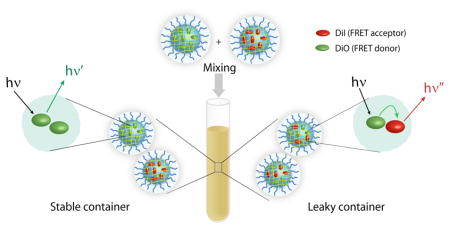
A lipophilic FRET pair, 3,3′-dioctadecyloxacarbocyanine (DiO, donor) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, acceptor), was used for this purpose. These two dye molecules were independently sequestered in nanocarriers. When the solutions containing the dye molecules were mixed, two limiting scenarios are possible (Figure 1a). If the dye molecules are stably encapsulated and do not exchange the guest molecules with the bulk solvent environment, then the two dye molecules will continue to be in two separate nanocontainers. If this were the case, no FRET would be observed since the distance between the two dye molecules is much higher than their Förster radius. However, if there is a significant exchange of the guest molecules between the container interior and the bulk solvent when the two solutions are mixed, it is likely that the dye molecules will equilibrate between the two containers. This is because there would be no registry regarding the nanocontainer from which the dye molecule leaked. The resulting equilibration will cause DiI and DiO molecules to occupy the same container, leading to increased FRET. Thus, tracing the evolution of FRET in such systems provides insight into the dynamics of guest interchange and potential carrier leakage.
Figure 1.
(a) Mixed nanogels encapsulated DiI/DiO and FRET behavior; (b) Synthesis of nanogels containing hydrophobic guest molecule; (c) Fluorescence emission spectra of mixed NG1 encapsulated DiI/DiO; (d) Plot of FRET ratio vs. time.
We sought to investigate the versatility of such an approach using crosslinked polymeric nanogels. The reason for this choice is the assertion that variations in crosslinking densities provide a method for tuning encapsulation stabilities and thus an opportunity to test our FRET-based strategy. Understandably, this feature also renders nanogels to be of great interest for controlled release applications in the area of pharmaceutical and biomedical research.3 Generating water-soluble polymeric nanogels that encapsulate lipophilic molecules can be cumbersome. However, we have recently introduced a new emulsion-free method for nanogel preparation in aqueous solution that also provides for facile hydrophobic guest encapsulation (Figure 1b).4
To probe the guest exchange dynamics, aqueous solutions of NG1 (6% crosslinked; ~200 nm in size) containing 1 wt% DiO or 1 wt% DiI were prepared by in situ loading (Figure 1b).4,6 The two solutions containing the separate dyes, referred as NG1-DiO and NG1-DiI, were then mixed in water. Fluorescence from the DiO excitation (450 nm) was monitored over time. The evolution of FRET was obtained by tracing the decrease in the donor (DiO) emission and concurrent increase in acceptor’s (DiI). The results show that there is a gradual equilibration of the dye molecules over a 48 h period (Figure 1c). Interestingly, the thermodynamic distribution of the dye molecule significantly favors the nanogel interior, as discerned by the overall concentration of the otherwise insoluble dyes in the aqueous phase.6 Thus, the continuous interchange of the dyes among the nanocarriers is responsible for observed enhancement in FRET. The FRET ratio Ia/(Id+Ia), where Ia and Id are the fluorescence intensities of the acceptor (DiI) and the donor (DiO) respectively, was plotted against time (Figure 1d). The slope of the linear fit is related to the dynamics of the guest exchange and we define this as the leakage coefficient (Λ), which was found to be about 0.124 h−1 for the first six hours in NG1 (Figure 1d).
We envisaged that crosslinking density could be used to tune the rate of exchange/leakage. The preparation method indeed allows for control over the degree of crosslinking.4,6 Accordingly, nanogels NG1, NG2, and NG3, with crosslinking densities of 6%, 13%, and 25% respectively, with encapsulated DiI and DiO were prepared. Dynamics of guest interchange were monitored and the FRET ratios were plotted against time. NG2 and NG3 exhibited minimal exchange over 6 hours at a Λ of 0.002 h−1 or below, compared to 0.124 h−1 for NG1 (Figures 1c, d, and 2a).6 These results suggest that degree of crosslinking is effective in tuning guest exchange dynamics. Since the precursor polymer P1 itself is capable of encapsulating DiO and DiI, we were interested in evaluating the exchange dynamics before and after crosslinking. P1 exhibited a Λ of 0.739 after mixing, indicating rapid guest interchange as compared to the crosslinked nanogels.6
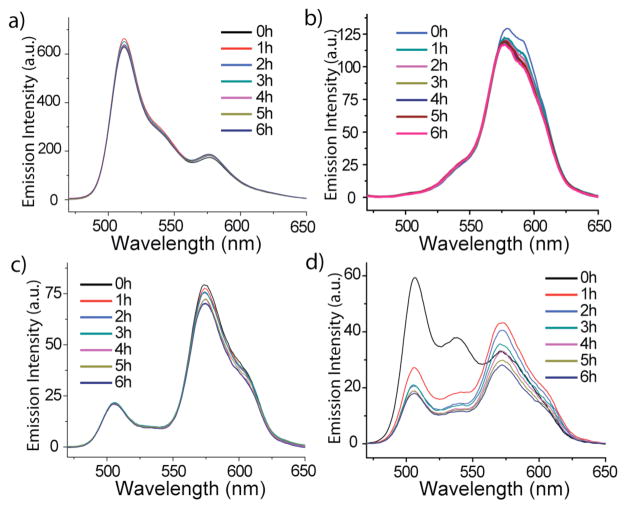
Figure 2.
FRET behavior of mixed nanocontainers encapsulated DiI/DiO: (a) NG2; (b) CTAB; (c) Amphiphilic random copolymers (d) Pluronic block copolymer.
The key motivation in developing these experiments is to develop a screening method for probing exchange dynamics, and thus the potential, of drug delivery vehicles. Therefore, we were interested in investigating the guest exchange characteristics of other supramolecular assemblies that are known to encapsulate lipophilic guest molecules. The simplest supramolecular assembly that encapsulates lipophilic guest molecules involves small molecule surfactant-based micelles. Accordingly, we encapsulated DiO and DiI in CTAB (cetyl trimethylammonium bromide) and Tween80 micelles and carried out the FRET experiments. Complete equilibration of the dye molecules was observed instantaneously upon mixing the DiO and DiI based micelles with the Λ of 0.850 and 0.501 respectively (Figure 2b).6 Since the nanogels are based on polymers, we also investigated other polymer-based nanoassemblies. We measured the guest exchange dynamics of an amphiphilic random copolymer (structure shown in Supporting Information).7 The Λ in these assemblies was found to be 0.788 (Figure 2c). Similarly, the encapsulation stability of pluronic block copolymer micellar assemblies,8 which are widely used in biomedical applications, exhibited a Λ of 0.358 (Figure 2d). This is slower than assemblies based on small molecule surfactants and random copolymers, but faster than the nanogels.
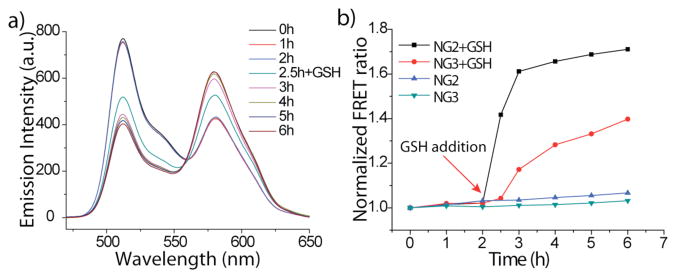
The results above suggest that the crosslinked polymer nanogels exhibit high encapsulation stability and the leakage dynamics can be tuned by varying the crosslinking density. While encapsulation stability is important, a practical delivery vehicle must also be able to release its contents in response to a biologically-relevant stimulus. As the nanogels consist of biodegradable disulfide crosslinkers, the release of encapsulated molecules can be potentially triggered upon exposure to reducing agents. To test this, we utilized NG2 that exhibited very stable encapsulation. Glutathione (GSH, 10 mM), a disulfide reducing agent present at higher concentrations inside cells, was added to the solution containing NG2-DiO and NG2-DiI after 2.5 h. Significant increase of the FRET ratio was observed at this point (Figure 3a and 3b). This is presumably caused by dye leakage upon cleavage of the disulfide crosslinkers due to the presence of GSH, which loosens the gel and reduces encapsulation stability. Moreover, significant decreases in the emission intensities of the individual dyes indicate that the GSH reaction reduces the distribution coefficient of these dyes as well.6 These results, combined with experiments with NG3,6 confirm that the dynamics of guest interchange can be controlled by crosslinking density and that release can be externally triggered (Figure 3b).
Figure 3.
Release of encapsulated DiI/DiO: (a) NG2 added GSH at 2.5h (b) Dynamics of interchange of NG2 and NG3 with/without adding GSH.
In summary, we report on a FRET-based method to monitor the guest exchange dynamics in water-soluble nanoassemblies, which provides insight into the leakage characteristics of these nanocontainers. We have thus defined a leakage coefficient parameter, Λ, from the FRET experiments and analyzed this value for a variety of nanoassemblies. We find that the guest exchange is slower in crosslinked polymer nanogels and that this can be conveniently tuned by altering the degree of crosslinking. We have also investigated the relative guest exchange rates in other amphiphilic nanoassemblies. We find that while pluronic block copolymers exhibit higher encapsulation stabilities compared to small molecules and random copolymers, they do not compare to those offered by crosslinked polymer nanogels. In addition, we show that the stably encapsulated guests in the nanogels can be released in response to an external trigger. The dyes employed model the hydrophobic nature of efficacious drug molecules that commonly face solubility issues during administration. Thus, understanding the dynamics of encapsulated guest exchange between nanocarriers, using this method, provides a useful starting point for evaluating viable drug delivery vehicles. Moreover, the exchange dynamics provide information on the possibility of utilizing a vehicle to deliver multiple drugs in separate containers with timed release. These FRET-based studies also highlight the advantages of the newly developed polymeric nanogels in drug delivery applications.
Supplementary Material
Acknowledgments
We thank DARPA and NIH-NIGMS for support and BASF (NJ) for a gift of Pluronic-P85.
Footnotes
Supporting Information Available: Experimental details and fluorescence data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]; (b) Allen TM, Culis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 2.Savić R, Eisenberg A, Maysinger D. J Drug Target. 2006;14:343–355. doi: 10.1080/10611860600874538. [DOI] [PubMed] [Google Scholar]
- 3.(a) Byrne ME, Park K, Peppas NA. Adv Drug Deliv Rev. 2002;54:149–161. doi: 10.1016/s0169-409x(01)00246-0. [DOI] [PubMed] [Google Scholar]; (b) Kabanov AV, Vinogradov S. Angew Chem Int Ed. 2009;48:5418–5429. doi: 10.1002/anie.200900441. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kopeček J. Nature. 2002;417:388–391. doi: 10.1038/417388a. [DOI] [PubMed] [Google Scholar]; (d) Hamidi M, Azadi A, Rafiei P. Adv Drug Deliv Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Ryu JH, Jiwpanich S, Chacko R, Bickerton S, Thayumanavan S. J Am Chem Soc. 2010;132:8246–8247. doi: 10.1021/ja102316a. [DOI] [PubMed] [Google Scholar]
- 5.(a) Harth E, Horn BV, Lee VY, Germack DS, Gonzales CP, Miller RD, Hawker CJ. J Am Chem Soc. 2002;124:8653–8660. doi: 10.1021/ja026208x. [DOI] [PubMed] [Google Scholar]; (b) Jiang J, Thayumanavan S. Macromolecules. 2005;38:5886–5891. [Google Scholar]; (c) Cherian AE, Sun FC, Sheike SS, Coates GW. J Am Chem Soc. 2007;129:11350–11351. doi: 10.1021/ja074301l. [DOI] [PubMed] [Google Scholar]
- 6.See Supporting Information for details.
- 7.Ryu JH, Roy R, Ventura J, Thayumanavan S. Langmuir. 2010;26:7086–7092. doi: 10.1021/la904437u. [DOI] [PubMed] [Google Scholar]
- 8.(a) Batrakova EV, Kabanov AV. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kabanov AV, Batrakova EV, Alakhov VY. Adv Drug Deliv Rev. 2002;54:759–779. doi: 10.1016/s0169-409x(02)00047-9. [DOI] [PubMed] [Google Scholar]; (c) Sharma PK, Bhatia SR. Int J Pharm. 2004;278:361–377. doi: 10.1016/j.ijpharm.2004.03.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.