Abstract
Acne vulgaris is a common and chronic disorder of the pilosebaceous unit. Standard treatment protocols include topical retinoids, topical and oral antimicrobials, and isotretinoin. Hormonal therapies can be added to the regimen in some patients. This article will review the hormonal pathogenesis of acne, discuss the basics of an endocrine evaluation, and provide an overview of the current hormonal treatment options in women.
Acne vulgaris is a common disorder of the pilosebaceous unit. Its mean prevalence in adolescents is estimated to be 70 to 87 percent.1 Initially miscategorized as an adolescent disorder, acne studies reveal that the mean age of presentation for treatment is 24 years, with 10 percent of visits occurring when patients are between the ages of 35 and 44 years.2,3 Genetics and gender are important factors in the prevalence of acne. One study of 200 patients with postadolescent acne found that 50 percent of patients reported at least one first-degree family relative with acne.4 In regard to gender, acne is significantly higher among women than men in all age groups.3 These findings have led to a new initiative urging dermatologists to take the lead in educating fellow clinicians on the chronicity of acne.5 By recognizing acne as a chronic disease similar to eczema, early and aggressive treatment can be started to avoid the psychological sequela that can result from active disease and scars.5 The direct cost of acne in the United States is estimated to exceed $1 billion per year, with $100 million spent on over-the-counter products.2 Despite this high cost, 81 percent of women report failures with systemic antibiotics, and failures with isotretinoin range from 15 to 30 percent.6 This article will focus on the advanced treatments for acne using hormonal regulation. It will review the hormonal pathogenesis of acne, the basics of an endocrine evaluation, and the current hormonal therapeutic options.
Hormonal Pathogenesis of Acne
The pathogenesis of acne vulgaris is multifactorial and involves four main pathways. These include the following: 1) excess sebum production by androgen-mediated stimulation of sebaceous glands, 2) abnormal keratinization of the follicles leading to plugging and comedone formation, 3) Propionibacterium acnes colonization, and 4) inflammation of the follicle and surrounding dermis.7,8
Sebum production plays a vital role in acne formation. The pilosebaceous unit has four distinct components: the hair follicle, the keratinized follicular infundibulum, the sebaceous gland, and the sebaceous duct that connects the gland to the infundibulum. The number, size, and activity of sebaceous glands may be inherited. While the number of sebaceous glands remains stable throughout life, the size increases with age.8 Human sebum contains unique fatty acids that support the growth of P. acnes, a unique colonizer of human skin.9 Androgens stimulate sebum production and research has demonstrated the intracrine nature of this relationship. Intracrine secretion involves the synthesis of active androgens in peripheral organs, such as the skin, where the androgens exert their action in the same cells where synthesis takes place without release into the general circulation.10 In-vivo studies show that sebaceous glands can act as independent endocrine organs, responding to changes in androgens in a similar manner as the hypothalamus-pituitary-adrenal axis. This intracrine function is regulated by corticotrophin-releasing hormone (CRH), its binding protein, and corticotrophin receptors.5,11 Since CRH levels change during stress and CRH regulates sebaceous gland function, this may explain the relationship between stress and inflammatory skin disorders such as acne.5
A significant portion of the circulating androgens is produced by the adrenal gland and the testes or ovaries. As aforementioned, a large portion of androgens are also synthesized in the skin from inactive adrenal precursors including, dehydroepiandrosterone (DHEA), DHEA-sulfate (DHEA-S), and androstenedione (Figure 1). Besides sebaceous glands, other androgen-sensitive components of skin are hair follicles, sweat glands, epidermis, and dermis. These structures contain enzymes important in converting DHEA, DHEA-S, and androstendione into the potent androgens dihydrotestosterone (DHT) and testosterone.10 DHT and testosterone are the major androgens that interact with the androgen receptors on sebaceous glands with DHT being 5 to 10 times more potent than testosterone.8,19 This conversion of inactive adrenal precursors to potent androgen occurs in sebaceous glands in the presence of several key steroidogenic enzymes: 3Beta-hydroxysteroid dehydrogenase (3B-HSD), 17Beta-hydroxysteroid dehydrogenase (17B-HSD), and 5α-reductase.6,10
Figure 1.
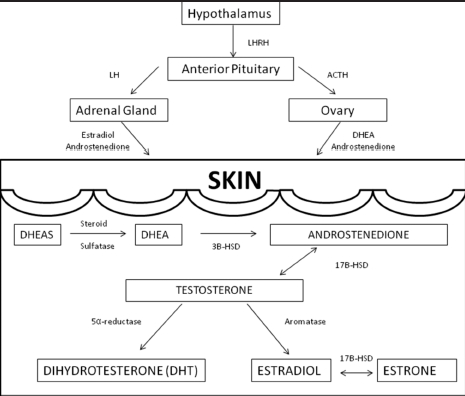
Steroid hormone metabolism
The first step in the synthesis of testosterone and DHT is the conversion of DHEA to androstenedione, which involves 3B-HSD (Figure 1). There are two forms of 3B-HSD. Type I is exclusive to skin and placenta, while in the adrenal and gonads, type II predominates. The next step involves the conversion of androstenedione to testosterone. 17B-HSD is responsible for this reversible conversion. There are multiple forms of 17B-HSD, but the type 2 isozyme12 and the type 5 isozyme10 appear to be the most active in sebaceous glands. Due to its reversible actions, 17B-HSD may function as a gate-keeping enzyme regulating the hormonal environment of the sebaceous gland.12 Finally, testosterone can take two paths: it can be converted to the potent androgen DHT by 5α-reductase activity or the less potent estrogens via aromatase activity. 5α-reductase is an important enzyme in androgen-dependent disorders, such as acne, male pattern baldness, and hirsutism. There are two forms: type 1 and type 2. Type 1 is the predominant form in skin with high concentrations seen in sebaceous glands and face and scalp skin.10,13,14 Finasteride, a type 2 5α-reductase inhibitor, is well known for its use in male pattern baldness. Since type 2 5α-reductase enzymes are not found in the skin, it is unlikely to be a helpful treatment for acne.6
While testosterone and DHT have clear roles in acne pathogenesis, research continues on the role of estrogen. Estrogen is known to suppress sebum production when given in sufficient amounts. Other mechanisms for estrogen’s effect include direct opposition effect on testosterone and inhibition of testosterone secretion.6,15 In addition, through the metabolization of estrogen in the liver, estrogen increases sex hormone-binding globulin (SHBG).16 SHBG has a high affinity for testosterone and will bind to it preferentially over estrogen. Since testosterone and its conversion to DHT are the primary androgens in acne, increased SHBG leads to improvement in acne.17
Endocrine Evaluation
While androgens are essential to the development of acne, routine screening of women with acne or hirsutism usually reveals normal levels of androgens.18 The serum level of DHEAS, testosterone, and DHT in women with acne ranges from high to normal. Several hypotheses exist about why women with acne can have normal androgen levels. One possibility relates to the intracrine relationship of androgens and sebaceous glands, namely, there is increased local production of androgens in patients with acne. Another theory is that the sebaceous glands of patients with acne are more sensitive to androgens’ effects.19 Nonetheless, there is evidence for the successful use of hormonal therapy in women with and without elevated androgen levels.
When should an endocrine disorder be suspected? Hyperandrogenism should be considered if a woman presents with acne that is severe, associated with hirsutism, or irregular menstrual periods. Other signs include cushingoid features, increased libido, presence of acanthosis nigricans, and androgenetic alopecia. Further tests and referrals are appropriate since these women may have insulin resistance with resultant development of diabetes and cardiovascular disease. Screening tests include serum DHEAS, total and free testosterone, and luteinizing hormone/follicle stimulating hormone (LH/FSH) ratio. These tests should be obtained during the two weeks prior to the onset of menses to avoid the LH surge associated with ovulation. In addition, if the patient is on oral contraceptives, these should be stopped 4 to 6 weeks before the endocrine evaluation.19–21
When interpreting the results of an endocrine evaluation, remember there are three sources of androgen production in women: the ovary, the adrenal gland, and within the skin itself. The first parameter to evaluate is DHEAS. An elevation indicates an adrenal source of the androgens. DHEAS levels >8000ng/mL (normal: <3500ng/mL) indicates an adrenal tumor. If the level is 4,000 to 8,000ng/mL, consider congenital adrenal hyperplasia. The next parameters to interpret are total and free testosterone. Elevation in testosterone, while not precluding an adrenal abnormality, commonly indicates an ovarian source of the androgens. Free testosterone is elevated in all forms of hyperandrogenemia, but can be useful if there is a SHBG dysfunction. Total testosterone >150 to 200ng/dL (normal range: 20 to 80ng/dL) indicates an ovarian tumor. Mild elevation suggests polycystic ovary syndrome (PCOS). Further findings in PCOS include an elevated LH/FSH ratio greater than 2 to 3, in addition to irregular menstrual periods, reduced fertility, obesity, hirsutism, and insulin resistance. If PCOS is diagnosed, ultrasound is indicated and the patient should be referred to a gynecologist for further evaluation.18–21
Hormonal Therapy
As previously noted, acne is a chronic condition for many women, and 81 percent of women report failures with systemic antibiotics. Recurrence rates after isotretinoin treatment range from 15 to 30 percent.6 In addition, women with signs of hyperandrogenism usually do not respond to conventional topical therapy. These women, along with those who report a premenstrual flare of facial acne22 or have deep-seated nodules of the lower face and neck2 are excellent candidates for hormonal therapy. Drugs used in the hormonal treatment of acne fall into four categories: 1) androgen receptor blockers (spironolactone, flutamide, cyproterone acetate), which block the effect of androgens on the sebaceous gland; 2) oral contraceptives, which suppress ovarian androgen production; 3) glucocorticoids, which cause adrenal suppression of androgen production; and 4) enzyme inhibitors (5α-reductase inhibitors).18–21 This article focuses on the agents in the first two treatment categories. Glucocorticoids are used in patients with late-onset congenital adrenal hyperplasia and its use is beyond the scope of this article. Finasteride, the only drug marketed as a 5α-reductase inhibitor, blocks the type 2 isozyme of 5α-reductase. Since the type 1 isozyme is found in skin, there are limited studies evaluating finasteride for acne. Furthermore, concerns about the potential for a fetal feminizing effect have limited its use in hyperandrogenic women. Inhibitors of the type 1 isozyme of 5α-reductase are being investigated.18
Androgen Receptor Blockers
The available androgen receptor blockers are spironolactone, cyproterone acetate, and flutamide. These three agents are not US Food and Drug Administration (FDA)-approved for the treatment of acne and cyproterone acetate is not available in the United States.
Spironolactone. Spironolactone is a synthetic steroidal androgen receptor blocker, which has been used for more than 30 years for the treatment of acne and hirsutism. It is also used to treat noncutaneous disorders, such as hypertension and congestive heart failure. In these disorders, it acts as an aldosterone antagonist and competes with aldosterone receptors in the kidney to produce diuresis, reduction in blood pressure, and potassium retention.23 The anti-androgenic effects are achieved through several mechanisms: 1) competition with testosterone and DHT for androgen receptors, thereby decreasing androgen-stimulated sebum production; 2) inhibition of androgen synthesis decreasing type 2 17B-HSD, thereby halting the conversion of androstenedione to testosterone; 3) inhibition of 5α-reductase, thus halting the conversion of testosterone to DHT; and 4) increasing the level of SHBG.6,8,24 After oral administration, spironolactone metabolism occurs in the liver where it is converted to its primary metabolite, canrenone, which has a serum half-life of 4 to 8 hours.23
The usual dosage for the treatment of acne is 50 to 200mg daily. However, lower daily doses may be effective in controlling acne and have a reduced side-effect profile. Three placebo-controlled, randomized, controlled trials of spironolactone in acne have been performed. One 12-week study of 21 women taking 200mg daily showed significant improvement in acne. Another 12-week trial of 36 men and women at doses from 50 to 200mg daily showed a dose-dependent improvement, with maximum benefit at doses of 100 to 200mg daily. The final 12-week trial of spironolactone 50mg daily showed acne clearance in 24 of the 34 participants. Despite this, based on the limited number and small sample sizes of the trials, the efficacy of spironolactone for the treatment of acne is considered indeterminate by the Cochrane review group.23,24
Spironolactone is a generally well-tolerated medication by women. In men, dose-related side effects, such as decreased libido, impotence, and gynecomastia, have limited its use in this population.25 The incidence of side effects is high, ranging from 75 to 91 percent, but fortunately, these effects are usually mild and most patients choose to continue the medication.23,25 The most common side effects are menstrual irregularities and breast tenderness/enlargement. The most common metabolic adverse effect and most-feared is hyperkalemia. In a study of 28 patients taking 50mg twice daily (BID) of spironolactone for three months, potassium levels before and after treatment were found to be within normal limits and there were no significant changes in blood pressure.25 Another study of 85 patients found a five-percent reduction in blood pressure in most patients and clinically insignificant hyperkalemia in 10 percent of the study population.18 It is generally recommended to check potassium levels in older patients with other medical problems or one month into therapy when high doses are utilized. Neurological side effects include headache, dizziness, drowsiness, and confusion. Gastrointestinal side effects are nausea, vomiting, anorexia, and diarrhea. At one time, there were concerns about the potential for developing breast cancer after five women developed it while taking spironolactone and other medications. A follow-up study proved a lack of association of spironolactone and breast cancer.23 Long-term studies in rats showed that spironolactone can lead to adenomas on endocrine organs and the liver. This led to a black box warning by the FDA.26 Spironolactone is contraindicated in pregnancy due to the potential feminizing effect of spironolactone on a developing male fetus. Due to the risk of birth defects and the reduction of side effects, spironolactone should be used in conjunction with oral contraceptives.6
Topical spironolactone has been investigated for its ability to produce localized anti-androgenic effects. One study found a lack of effect of topical spironolactone on sebum excretion.27 Later studies have shown that when compared to vehicle gel in a split-face trial, topical 5% spironolactone gel led to a significant reduction in the rate of sebum secretion at 12 weeks (4 weeks after termination of application), but not at eight weeks (end of treatment).8 Overall, the efficacy of topical spironolactone in the treatment of facial acne requires further studies.
Cyproterone acetate. Cyproterone is used in Canada, Europe, and Asia and is one of the first androgen receptor-blocking agents to be studied. It has dual activity of directly inhibiting androgen receptors and can serve as the progesterone in combination oral contraceptives. It works by inhibiting the conversion of DHEA to androstenedione by blocking 3B-HSD activity (Figure 1). This leads to an overall decrease in testosterone with subsequent decrease in sebum production. Single-agent use of cyproterone 50 to 100mg/day has shown acne improvement rates as high as 75 to 90 percent.6,19–21 It is usually combined in doses of 2mg with 35µg of ethinyl estradiol (Diane-35, Bayer Schering Pharma, Berlin, Germany). The most common side effects are breast tenderness, headache, nausea, and breakthrough bleeding, which resolve by the second cycle. Serious side effects include fatal hepatotoxicity, which is dose dependent, and in women of childbearing age, there is a risk of feminization of the male fetus.6,8,18–21
Flutamide. Flutamide is a nonsteroidal androgen receptor blocker approved by the FDA for the treatment of prostate cancer. It has been effective in treating acne, androgenetic alopecia, and hirsutism. After oral administration, it is converted to the potent metabolite 2-hydroxyflutamide, which selectively inhibits the binding of DHT to the androgen receptor. It may also enhance androgen metabolism to inactive metabolites.28 Doses range from as low as 62.5 to 500mg/day. One study found an 80-percent improvement in acne with flutamide 250mg/day.6,18 Side effects include breast tenderness, gastrointestinal upset, hot flashes, and decreased libido. Serious side effects include fatal hepatitis, which is dose and age related; therefore, regular liver function tests are necessary. Since it is an antiandrogen, pregnancy risks are another concern.6,8,18–21
Ovarian Androgen Blockade
Oral contraceptives. Another option in the arsenal of hormonal treatments for acne is oral contraceptives (OCs), which work largely by suppressing ovarian androgen production. They are particularly useful for women affected by acne who are also interested in contraceptive benefits. Pills combining both an estrogen [usually ethinyl estradiol (EE)] and progestin are used for acne as progestin-only containing pills may exacerbate the condition. Oral contraceptives exert their therapeutic effect by decreasing androgens and therefore sebum production. One important mechanism by which this is achieved is the suppression of luteinizing hormone production by the pituitary gland, which in turn decreases androgen synthesis by the ovaries. Androgen production by the adrenal glands and peripheral androgen production are also reduced by the use of oral contraceptives. The estrogen component of hormonal contraceptives works to increase levels of sex hormone-binding globulin (SHBG), thereby decreasing levels of free testosterone. Lastly, oral contraceptives inhibit 5α-reductase in hair follicles and skin. This enzyme is responsible for the conversion of testosterone to dihydrotestosterone, which is the most potent and active androgen in skin. The progestin component is responsible for this enzymatic inhibition.29
Several OCs have been used to treat acne, but only three have been approved by the FDA for this purpose (Table 1). All three approved OCs have low doses of the estrogen, ethinyl estradiol (EE), combined with differing progestin components: EE 20/30/35µg plus norethindrone 1mg (Estrostep, Warner Chilcott Company, Inc., Fajardo, Puerto Rico), EE 35µg plus norgestimate 180/215/250µg (Ortho-Tri-Cyclen, Ortho-McNeil Pharmaceutical, Inc., Raritan, New Jersey), and EE 20µg plus drospirenone 3mg (Yaz, Bayer HealthCare Pharmaceuticals Inc., Wayne, New Jersey). Drospirenone is a novel progestin, which is a 17α-spironolactone derivative that has both antimineralo-corticoid and antiandrogenic properties, which may improve estrogen-related weight gain and bloating.30
Table 1.
Oral contraceptives used in treatment of acne
| TRADE NAME | ETHINYL ESTRADIOL (µg) | PROGESTERONE (mg) |
|---|---|---|
| Estrostep* (Warner Chilcott Company, Inc., Fajardo, Puerto Rico) |
20/30/35 | Norethindrone 1 |
| Ortho Tri-Cyclen* (Ortho-McNeil Pharmaceutical, Inc., Raritan, New Jersey) |
35 | Norgestimate 0.18/0.215/0.25 |
| Yaz* (Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ) |
20 | Drospirenone 3 |
| Yasmin (Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ) |
30 | Drospirenone 3 |
| Alesse (Wyeth Pharmaceuticals Inc., A Wyeth-Ayerst Company, Philadelphia, PA) |
20 | Levonorgestrel 0.1 |
| Nordette/Microgynon/Levlen (Duramed Pharmaceuticals, Inc., subsidiary of Barr Pharmaceuticals, Inc., Pomona, NY) |
30 | Levonorgestrel 0.15 |
| Triphasil (Wyeth Laboratories, A Wyeth-Ayerst Company, Philadelphia, PA) |
30/40/30 | Levonogestrel 0.5/0.75/0.125 |
| Mircette (Duramed Pharmaceuticals, Inc., subsidiary of Barr Pharmaceuticals, Inc., Pomona, NY) |
20/10 | Desogestrel 0.15 |
| Desogen (N.V. Organon, Oss, Holland or Organon (Ireland) Ltd, Swords, Co. Dublin, Ireland) |
30 | Desogestrel 0.15 |
| Ortho Cyclen (Ortho-McNeil Pharmaceutical, Inc., Raritan, New Jersey) |
35 | Norgestimate 0.25 |
| Femodene/Femovan (Bayer HealthCare Pharmaceuticals Inc., Wayne,NJ) |
30 | Gestodene .75 |
| Diane-35 (Bayer Schering Pharma, Berlin, Germany) |
35 | Cyproterone 2 |
Approved by the FDA for use in acne treatment.
Two multicenter, randomized, double-blind, placebo-controlled trials compared EE 20/30/35µg plus norethindrone 1mg (Estrostep) with placebo in 593 women over six cycles. Subjects treated with Estrostep showed statistically significant reductions from baseline in inflammatory and total lesion count, improvements in acne quality-of-life ratings, and global assessments versus placebo.31
Similarly, the efficacy of EE 35µg plus norgestimate 180/215/250µg (Ortho-Tri-Cyclen) has been proven in two multicenter, randomized, double-blind, placebo-controlled trials. Statistically significant reductions in inflammatory and total lesion counts and improvement in global assessments were achieved after six months in the OC group as compared to placebo.32,33 In addition, significant reductions in free testosterone and increases in SHBG were noted in one treatment group.33
The newest available oral contraceptive, which is approved for the treatment of acne, is EE 20µg plus drospirenone 3mg (Yaz). Drospirenone is a novel progestin that has antiandrogenic properties. There have been two multicenter, double-blind, randomized, placebo-controlled trials evaluating its efficacy in the management of acne in women over six cycles. Both demonstrated significant reductions in total lesion counts and greater improvement in investigator global assessment in the treatment group.34,35 Treatment with Yaz also led to significantly lower serum free testosterone and androstenedione and increased SHBG levels.35
Another drospirenone-containing oral contraceptive, EE 30µg plus drospirenone 3mg (Yasmin, Bayer HealthCare Pharmaceuticals Inc., Wayne, New Jersey), has also been studied, but is not approved by the FDA for treatment of acne. In a multicenter, double-blind, randomized study, the efficacy of Yasmin was compared with that of EE 35µg plus 2mg cyproterone acetate (Diane-35) in 128 women over nine cycles. Total acne lesion counts were decreased by 62.5 percent in the Yasmin group and 58.8 percent in the Diane-35 group. Both treatments resulted in reduction of sebum production and hair growth on the upper lip and chin as well as a three-fold increase in SHBG with concomitant decrease in levels of androgens and luteinizing hormone.36 Another double-blind study compared the efficacy of Yasmin with the triphasic preparation EE 35µg plus norgestimate 180/215/250µg (Ortho Tri-Cyclen) in 1,154 women over six cycles. While Yasmin was found to be superior to Ortho Tri-Cyclen in reduction of total lesion count and investigator and subject assessments of effect, the two treatments were comparable in reduction of inflammatory lesion count. Increased levels of SHBG and decreased levels of androgens were noted in each treatment group.37 Other oral contraceptives are available and have shown to be beneficial in acne, but are not yet FDA approved (Table 1). EE 35µg plus cyproterone 2mg (Diane-35) is extensively used for this purpose in other countries as it is not currently available in the United States.
While the use of oral contraceptives is generally considered quite safe, there are some important safety considerations when prescribing these medications. The most serious potential side effects of oral contraceptives are venous and arterial complications. The earliest forms of oral contraceptives contained increased concentrations of estrogen and progestin compared to currently used formulations, imparting a significantly increased risk of thromboembolic events and myocardial infarction. More recent oral contraceptives have lessened these concerns, though there still exists a small elevation in risk of vascular complications. However, in healthy nonsmokers who are 35 years old or younger, the risk is quite low.38 Of note, the risk of venous thromboembolism is greatest during the first year of oral contraceptive use.39 While oral contraceptives have been linked to decreased risk of ovarian cancer after five years of use, there has been concern about the possible association between oral contraceptive use and the risk of breast cancer. Several epidemiological studies have not validated this association.38 For prescribing purposes, there exist several conditions that are considered contra-indications to the use of oral contraceptives. These include history of venous thromboembolic disease, heart disease or genetic clotting disorder (such as Factor V Leiden); hypertension; smoking in women older than 35; severe obesity; liver disease; diabetes mellitus; migraine headaches; current pregnancy or breastfeeding; prolonged immobilization; hypersensitivity to any component of the oral contraceptive pills; and history of malignancy, such as breast, endometrial, or liver. It is important to determine existence of any of these risk factors in patients when considering starting an oral contraceptive. In addition to obtaining a thorough past medical history, a blood pressure should be documented prior to prescribing oral contraceptives. Oral contraceptives have demonstrated significant clinical efficacy in reduction of acne lesions as well as concurrent reductions in levels of androgens and increases in SHBG. Oral contraceptives are a valuable tool either solely or in combination with other modalities for the treatment of acne in women.
Conclusion
For women with hormonal flares of acne, such as increased breakouts related to the menstrual cycle, hormonal therapy can be a helpful addition to the treatment armamentarium. Although it may help decrease outbreaks, it will not be as effective for existing lesions and is used as an adjunct rather than a stand-alone therapy. In healthy women who do not smoke, OCs can first be introduced. It can take up to three months to see improvement. At that time, spironolactone can be added if the response is inadequate. Dosing can range from 25 to 100mg per day. Laboratory follow up is usually not necessary in healthy women. As the acne clears, other treatments, such as oral antibiotics, can be discontinued. OCs, usually in combination with topical retinoids, can be continued as part of a maintenance regimen.
References
- 1.Dreno B, Poli F. Epidemiology of acne. Dermatology. 2003;206:7–10. doi: 10.1159/000067817. [DOI] [PubMed] [Google Scholar]
- 2.James W. Acne. N Engl J Med. 2005;352(14):1463–1472. doi: 10.1056/NEJMcp033487. [DOI] [PubMed] [Google Scholar]
- 3.Collier C, Harper J, Cantrell W, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Goulden V, Clark S, Cunliffe W. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66–70. [PubMed] [Google Scholar]
- 5.Thiboutot D, Gollnick H, Betolli V, et al. New insights into the management of acne: An update from the Global Alliance to Improve Outcomes in Acne Group. J Am Acad Dermatol. 2009;60:S1–S50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 6.George R, Clarke S, Thiboutot D. Hormonal therapy for acne. Semin Cutan Med Surg. 2008;27:188–196. doi: 10.1016/j.sder.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Haider A, Shaw JC. Treatment of acne vulgaris. JAMA. 2004;292(6):726–735. doi: 10.1001/jama.292.6.726. [DOI] [PubMed] [Google Scholar]
- 8.Clarke S, Nelson A, George R, et al. Pharmacologic modulation of sebaceous gland activity: Mechanisms and clinical applications. Dermatol Clin. 2007;25:137–146. doi: 10.1016/j.det.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Thiboutot D. Overview of acne and its treatment. Cutis. 2008;81(S1):3–7. [PubMed] [Google Scholar]
- 10.Labrie F, Luu-The V, Labrie C, et al. Intracrinology of the skin. Horm Res. 2000;54:218–229. doi: 10.1159/000053264. [DOI] [PubMed] [Google Scholar]
- 11.Zoubolis C, Baron J, Bohm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 12.Thiboutot D, Martin P, Volikos L, et al. Oxidative activity of the type 2 isozyme of 17B-Hydroxysteroid dehydrogenase (17B-HSD) predominates in human sebaceous glands. J Invest Dermatol. 1998;111:390–395. doi: 10.1046/j.1523-1747.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 13.Thiboutot D, Harris G, Iles V, et al. Activity of the type 1 5α–reductase exhibits regional differences in isolated sebaceous glands and whole skin. J Invest Dermatol. 1995;105:209–214. doi: 10.1111/1523-1747.ep12317162. [DOI] [PubMed] [Google Scholar]
- 14.Thiboutot D, Knaggs H, Gilliland K, et al. Activity of type 1 5. -reductase is greater in the follicular infrainfundibulum compared with the epidermis. Br J Dermatol. 1997;136:166–171. [PubMed] [Google Scholar]
- 15.Bhambri S, Del Ross J, Bhambri A. Pathogenesis of acne vulgaris: recent advances. J Drugs Dermatol. 2009;8:615–618. [PubMed] [Google Scholar]
- 16.Rich P. Hormonal contraceptives for acne management. Cutis. 2008;81(1):13–18. [PubMed] [Google Scholar]
- 17.O’Connell K, Westhoff C. Pharmocology of hormonal contraceptives and acne. Cutis. 2008;81(1):8–12. [PubMed] [Google Scholar]
- 18.Shaw J. Acne: Effects on hormones on pathogenesis and management. Am J Clin Dermatol. 2002;3:571–578. doi: 10.2165/00128071-200203080-00007. [DOI] [PubMed] [Google Scholar]
- 19.Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57–67. doi: 10.1159/000067823. [DOI] [PubMed] [Google Scholar]
- 20.Thiboutot D. Acne: Hormonal concepts and therapy. Clin Dermatol. 2004;22:419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Thiboutot D. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(3):57–61. doi: 10.1046/j.0926-9959.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 22.Lucky A. Quantitative documentation of a premenstrual flare of facial acne in adult women. Arch Dermatol. 2004;140:423–424. doi: 10.1001/archderm.140.4.423. [DOI] [PubMed] [Google Scholar]
- 23.Shaw J. Spironolactone in dermatologic therapy. J Am Acad Dermatol. 1991;24:236–243. doi: 10.1016/0190-9622(91)70034-y. [DOI] [PubMed] [Google Scholar]
- 24.Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. 2009;(2) doi: 10.1002/14651858.CD000194.pub2. Cochrane Database of Systemic Reviews. [DOI] [PubMed] [Google Scholar]
- 25.Yemisci A, Gorgulu A, Piskin S. Effects and side effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163–166. doi: 10.1111/j.1468-3083.2005.01072.x. [DOI] [PubMed] [Google Scholar]
- 26.Walton S, Cunliffe W, Lookingbill P, et al. Lack of effect of topical spironolactone on sebum excretion. Br J Dermatol. 1986;114:261–264. doi: 10.1111/j.1365-2133.1986.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 27.Tan J. Hormonal treatment of acne: Review of current best evidence. J Cutan Med Surg. 2005;(8) 4:11–15. doi: 10.1007/s10227-004-0754-8. [DOI] [PubMed] [Google Scholar]
- 28.Spironolactone. [October 8, 2009.]. http://www.drugs.com/pro/aldactone.html
- 29.Cassidenti DL, Paulson RJ, Serafini P, et al. Effects of sex steroids on skin 5α-reductase activity in vitro . Obstet Gynecol. 1991;78:103–107. [PubMed] [Google Scholar]
- 30.Thorneycroft IH. Evolution of progestins. Focus on the novel progestin drospirenone. J Reprod Med. 2002;47:975–980. [PubMed] [Google Scholar]
- 31.Maloney M, Arbit D, Flack M, et al. Use of a low-dose oral contraceptive containing norethindrone acetate and ethinyl estradiol in the treatment of moderate acne vulgaris. Clin J Women’s Health. 2001;1:123–131. [Google Scholar]
- 32.Lucky AW, Henderson TA, Olson WH, et al. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997;37:746–754. doi: 10.1016/s0190-9622(97)70112-9. [DOI] [PubMed] [Google Scholar]
- 33.Redmond GP, Olson WH, Lippman JS, et al. Norgestimate and ethinyl stradiol in the treatment of acne vulgaris: a randomized, placebo-controlled trial. Obstet Gynecol. 1997;89:615–622. doi: 10.1016/S0029-7844(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 34.Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3mg drospirenone/20µg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143–150. [PubMed] [Google Scholar]
- 35.Maloney M, Dietze P, Watson D, et al. Treatment of acne using a 3mg drospirenone/20µg ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773–781. doi: 10.1097/AOG.0b013e318187e1c5. [DOI] [PubMed] [Google Scholar]
- 36.van Vloten WA, van Haselen CW, van Zuuren EJ, et al. The effect of two combined oral contraceptives containing either drospirenone or cyproterone acetate on acne and seborrhea. Cutis. 2002;69(4):2–15. [PubMed] [Google Scholar]
- 37.Thorneycroft H, Gollnick H, Schellschmidt I. Superiority of a combined contraceptive containing drospirenone to a triphasic preparation containing norgestimate in acne treatment. Cutis. 2004;74:123–130. [PubMed] [Google Scholar]
- 38.Frangos JE, Alavian CN, Kimball AB. Acne and oral contraceptives: update on women’s health screening guidelines. J Am Acad Dermatol. 2008;58:781–786. doi: 10.1016/j.jaad.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro S. Oral contraceptives, hormone therapy and cardiovascular risk. Climacteric. 2008;11:355–363. doi: 10.1080/13697130802322848. [DOI] [PubMed] [Google Scholar]


