Abstract
Acne vulgaris is the most common disorder encountered in ambulatory clinical practice comprising 11.3 percent of office visits to dermatologists in 2005.1 By comparison, eczematous dermatoses, psoriasis, and skin cancer accounted for 6.2, 3.5, and 10 percent of office visits, respectively.1 A variety of topical therapeutic options are available for treatment of acne vulgaris, including benzoyl peroxide, antibiotics, retinoids, azelaic acid, and sodium sulfacetamide-sulfur.2,3 Sodium sulfacetamide 10%-sulfur 5% has been used for the topical treatment of seborrheic dermatitis, acne vulgaris, and rosacea since the mid-1950s and is available in a variety of formulations, including lotions, creams, cleansers, and emollient foams.4 Recently, an emollient foam sodium sulfacetamide 10%-sulfur 5% formulation indicated for topical therapy of acne vulgaris, rosacea, and seborrheic dermatitis has become available.5 This article provides an overview of the sodium sulfacetamide 10%-sulfur 5% emollient foam and reports the results of a case report series of patients with acne vulgaris treated with sodium sulfacetamide 10%-sulfur 5% emollient foam as monotherapy or in combination with other topical acne products.
The sodium sulfacetamide 10%-sulfur 5% (SSS) emollient foam is formulated as an alcohol-free and fragrance-free topical aerosol foam that has been shown to exhibit moisturization properties.5,6 An evaluation of the rate of release of the active ingredients from the SSS emollient foam formulation demonstrated active release characteristics conducive to both wash-off and leave-on treatment regimens in clinical practice.7 In addition, SSS emollient foam has been shown to markedly reduce colony counts of Propionibacterium acnes in vitro.8 Lastly, SSS emollient foam has been shown to exhibit reduced sulfur odor intensity as compared to other conventional SSS formulations,9 without the use of potentially sensitizing, odor-masking additives, such as fragrance.
Case Series
The following case series discusses the use of SSS emollient foam in the treatment of mild-to-moderate acne vulgaris either as monotherapy or in combination with other topical acne medications. In all cases, SSS emollient foam was used as a leave-on formulation according to directions outlined in the product prescribing information. The frequency of application and use of any concomitant therapies were determined by the author who evaluated and treated all patients included in the case series.
Case 1
A 35-year-old Asian woman presented with facial acne vulgaris “on and off” for a duration of several years. She reported that her acne had not responded to treatment with benzoyl peroxide (BPO) 5.5% cream over the past few months. On examination, she exhibited 15 inflammatory papules and 26 closed comedones. She was prescribed SSS emollient foam in the morning and tretinoin microsphere gel 0.04% at bedtime.
At the follow-up visit four weeks later, the number of facial papules and comedones decreased to seven and 15, respectively. She reported mild pruritus of the temple regions over the first few days of medication use, which resolved without discontinuation of therapy. There was no visible evidence of cutaneous side effects on examination. At the eight-week follow-up visit, the number of facial papules and comedones decreased to three and three, respectively. Intermittent mild dryness of the temple regions, which was not evident on clinical examination, was reported by the patient; however, this subjective finding did not result in discontinuation of treatment. With regard to use of the SSS emollient foam, the patient stated it was very easy to apply and that she was satisfied with the results of her treatment program.
Case 2
A 29-year-old Asian woman presented with a 10-year history of intermittent facial acne vulgaris involving predominantly the forehead region and occasionally the cheeks. She describes her acne as mild, but frustrating, stating, “there are always a few, especially on the forehead.” She has separately used tretinoin cream 0.025% daily and BPO cream 3.5% with modest success. On examination, 12 inflammatory papules and 10 closed comedones were noted. The patient was prescribed SSS emollient foam once daily.
At follow up six weeks later, the number of inflammatory lesions decreased to three and no comedones were observed. Two weeks later (Week 8), no inflammatory lesions were noted and only one closed comedone was observed. No adverse reactions were reported or observed. With regard to use of SSS emollient foam, the patient reported that it was very easy to use and aesthetically pleasing. She was very satisfied with the results of treatment, and the current regimen was continued.
Case 3
A 13-year-old Asian boy presented with a one-year history of facial acne vulgaris that was only modestly responsive to previous treatment with BPO 5%-clindamycin 1% gel. Clinical examination demonstrated 23 inflammatory lesions (predominantly papules with no nodules) and 31 noninflammatory lesions (predominantly open comedones) (Figure 1). The predominant areas of involvement were the forehead and cheeks. The patient was prescribed SSS emollient foam in the morning and tretinoin microsphere gel 0.04% in the evening.
Figure 1.
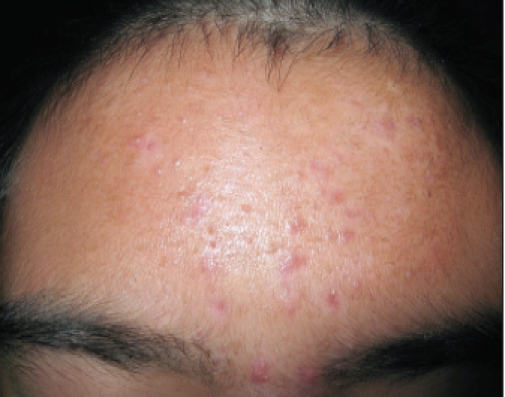
Multiple inflammatory and comedonal acne lesions noted on the forehead at baseline.
Follow up at eight weeks revealed 16 inflammatory lesions and five closed comedones (Figure 2). No adverse reactions were reported or observed. The patient stated that SSS emollient foam was easy to apply, and he was satisfied with the results of therapy. The current regimen was continued.
Figure 2.
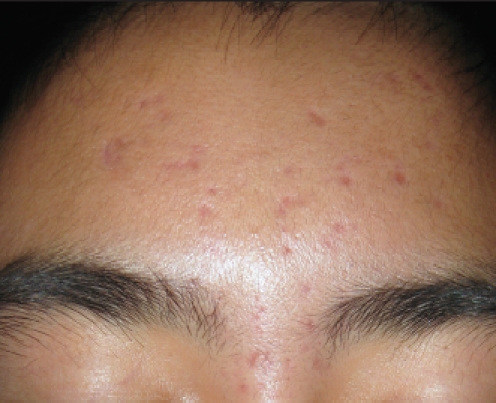
Marked reduction in inflammatory and comedonal acne lesions on the forehead observed after eight weeks of treatment. Note significant improvement with only residual post-inflammatory erythema and hyperpigmentation.
Case 4
A 30-year-old Asian woman presented with a 15-year history of facial acne vulgaris treated previously with multiple medications including topical tretinoin, clindamycin, and adapalene for durations of approximately three months. The patient had discontinued therapy with topical adapalene two weeks prior to her visit. On examination, eight inflammatory papules and 30 noninflammatory lesions (predominantly closed comedones) were noted, involving the forehead, temple, cheeks, and chin. Mild postinflammatory hyperpigmentation was also noted. The patient was prescribed SSS emollient foam to be applied twice daily.
At her four-week follow-up visit, seven inflammatory papules and 27 noninflammatory lesions were noted. She reported mild facial dryness, which was ameliorated by use of a facial moisturizer cream without interruption of therapy. Examination at eight weeks revealed six inflammatory papules and 19 comedonal lesions. No adverse reactions were reported or observed. The patient reported that SSS emollient foam was easy to apply, and satisfaction with response to treatment was high. The current regimen was continued.
Case 5
A 14-year-old Caucasian girl presented with truncal acne vulgaris involving the back over the past few years. Despite the use of BPO creamy wash 8% daily there was some persistence of acne lesions that was bothersome to the patient. On examination, 17 inflammatory papules and 40 closed comedones were noted on the back. BPO creamy wash 8% once daily was continued and SSS emollient foam twice daily was added to the regimen.
After four weeks of treatment, the patient reported marked improvement. On examination, six inflammatory papules and 12 closed comedones were observed. Improvement progressed through Week 8 with one inflammatory papule and three closed comedones noted. No adverse reactions were reported or observed at either visit. The patient and her mother stated they were very satisfied with the treatment results and reported that SSS emollient foam was very easy to apply and was aesthetically pleasing to use. The current treatment regimen was continued.
Case 6
A 19-year-old Caucasian woman presented with facial acne vulgaris present for several months. Previous treatment had included a BPO-containing skin care system, topical adapalene, and oral minocycline. The patient has been off of all treatment for several months and admits to inconsistent compliance with previous therapies. On examination, 22 inflammatory lesions (predominantly papules with no nodules) and 36 noninflammatory lesions (predominantly closed comedones) were observed on the forehead, cheeks, and chin. The patient was instructed to apply SSS emollient foam in the morning and tazarotene cream 0.1% in the evening. Additionally, the patient was administered a ceramide-based moisturizer cream to be applied in the evening prior to application of topical tazarotene.
At the four-week follow-up visit, 27 inflammatory lesions and 24 noninflammatory lesions were noted. The patient reported mild facial redness occurring after application of topical tazarotene; however, this dissipated by the following morning and did not interfere with continuation of treatment. By Week 8, the numbers of inflammatory and noninflammatory lesions were 12 and 26, respectively. Regarding the use of SSS emollient foam, the patient reported it was easy to use and exhibited good aesthetic qualities. Additionally, she was satisfied with the results of the treatment regimen, which was continued with the addition of oral doxycycline.
Case 7
A 32–year-old Caucasian man presented with facial acne vulgaris present for several months involving the forehead and cheeks. He had previously utilized BPO 5%-clindamycin 1% gel with modest success, with cutaneous dryness leading to inconsistent use. Clinical evaluation revealed 19 inflammatory lesions (predominantly papules) and 14 noninflammatory lesions (predominantly closed comedones). The patient was instructed to use SSS emollient foam in the morning and tretinoin 0.05% aqueous gel in the evening.
After four weeks, the inflammatory and noninflammatory lesion counts decreased to five and nine, respectively. At Week 6, eight inflammatory papules and one closed comedone were observed. No adverse reactions were reported or observed over the course of treatment. With regard to SSS emollient foam, the patient stated it was very easy to apply with very good aesthetic characteristics. He was satisfied with the response to treatment.
Case 8
A 23-year-old Caucasian woman presented with a recent flare of facial acne vulgaris involving primarily the forehead and cheeks over the past two months. She was previously treated with oral minocycline for only a short duration. A total of 18 inflammatory papules and 19 closed comedones were noted on examination. SSS emollient foam twice daily was prescribed.
At the four-week follow-up visit, inflammatory and noninflammatory lesions decreased to eight and seven, respectively. Follow up at Week 8 demonstrated seven inflammatory papules and seven closed comedones. Facial dryness was reported by the patient, but did not interfere with treatment. No other adverse reactions were reported or observed. The patient stated that SSS emollient foam was easy to apply and aesthetically pleasing. She was moderately satisfied with the results of treatment, but requested a switch in her therapy to an alternative approach that would lead to further clearance of acne lesions.
Discussion
The above cases demonstrate the use of SSS emollient foam in the treatment of mild-to-moderate acne vulgaris as either monotherapy or in combination with a topical retinoid. Use of a topical retinoid is a mainstay of both initial and maintenance treatment of acne vulgaris.2 Application of a topical retinoid often causes some degree of cutaneous irritation (“retinoid dermatitis”) within the first few weeks of use. Therefore, the availability of other topical agents that are inherently not irritating and that do not compound the potential for or the intensity of “retinoid dermatitis” is an important factor when selecting topical agents for use in combination to treat acne vulgaris. In the cases where SSS emollient foam was used in combination with a topical retinoid, the cutaneous tolerability was favorable and all patients continued treatment without interruption. This may relate at least partially to the moisturization properties of the SSS emollient foam vehicle.6
Recognized advantages of a foam vehicle include rapid penetration into the skin without a messy residue, cosmetic acceptability, and ease of use, especially for widespread application.10 The case involving truncal acne on the back was effectively treated with SSS emollient foam and a BPO cleanser.
In summary, SSS emollient foam is indicated for the treatment of acne vulgaris, as well as seborrheic dermatitis and rosacea. SSS emollient foam appears to be a welcome addition to the therapeutic armamentarium based on formulation characteristics, active ingredient release properties, moisturization capacity, and clinical use.
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics, 2005 National Ambulatory Medical Care Survey. Mar, 2005.
- 2.Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a global alliance to improve outcomes in acne. J Am Acad Dermatol. 2003;49(Suppl):S1–S37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- 3.Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427–431. [PubMed] [Google Scholar]
- 4.Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1):29–33. [PubMed] [Google Scholar]
- 5.Cumberland, RI: Onset Therapeutics; 2008. Clarifoam-EF Emollient Foam [package insert] [Google Scholar]
- 6.Trumbore M. The moisturization ability of a sodium sulfacetamide (10%)/sulfur (5%) emollient foam. J Am Acad Dermatol. 2007;56 AB21 (Abstract P134) [Google Scholar]
- 7.Trumbore MW, Macey JM, Schilling W. San Antonio, TX: Feb 1-5, 2008. The rate of release of active ingredients from a novel wash-off and leave-on sodium sulfacetamide 10%/sulfur 5% emollient foam. Poster presented at: 66th Annual Meeting of the American Academy of Dermatology. [Google Scholar]
- 8.Trumbore MW. New York, NY: Aug 1-5, 2007. The development of an in-vitro assay for determining the antimicrobial efficacy of leave-on and wash-off topical acne medications. Poster presented at: American Academy of Dermatology Summer Academy Meeting. [Google Scholar]
- 9.Gurge R, Trumbore MW, Varanasi R. Chicago, IL: 2008. The evaluation of a novel pharmaceutical foam vehicle technology optimized to improve patient compliance. Poster presented at: American Academy of Dermatology Summer Academy Meeting; July 30–August 3. [Google Scholar]
- 10.Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327–332. [PubMed] [Google Scholar]


