Abstract
The aim of the study was to analyze whether the proliferative effects of insulin in rat liver involve cross-signaling toward the epidermal growth factor receptor (EGFR) and whether this is mediated by insulin-induced hepatocyte swelling. Studies were performed in the perfused rat liver and in primary rat hepatocytes. Insulin (35 nmol/liter) induced phosphorylation of the EGFR at position Tyr845 and Tyr1173, but not at Tyr1045, suggesting that EGF is not involved in insulin-induced EGFR activation. Insulin-induced EGFR phosphorylation and subsequent ERK1/2 phosphorylation were sensitive to bumetanide, indicating an involvement of insulin-induced hepatocyte swelling. In line with this, hypoosmotic (225 mosmol/liter) hepatocyte swelling also induced EGFR and ERK1/2 activation. Insulin- and hypoosmolarity-induced EGFR activation were sensitive to inhibition by an integrin-antagonistic RGD peptide, an integrin β1 subtype-blocking antibody, and the c-Src inhibitor PP-2, indicating the involvement of the recently described integrin-dependent osmosensing/signaling pathway (Schliess, F., Reissmann, R., Reinehr, R., vom Dahl, S., and Häussinger, D. (2004) J. Biol. Chem. 279, 21294–21301). As shown by immunoprecipitation studies, insulin and hypoosmolarity induced a rapid, RGD peptide-, integrin β1-blocking antibody and PP-2-sensitive association of c-Src with the EGFR. As for control, insulin-induced insulin receptor substrate-1 phosphorylation remained unaffected by the RGD peptide, PP-2, or inhibition of the EGFR tyrosine kinase activity by AG1478. Both insulin and hypoosmolarity induced a significant increase in BrdU uptake in primary rat hepatocytes, which was sensitive to RGD peptide-, integrin β1-blocking antibody, PP-2, AG1478, and PD098059. It is concluded that insulin- or hypoosmolarity-induced hepatocyte swelling triggers an integrin- and c-Src kinase-dependent EGFR activation, which may explain the proliferative effects of insulin.
Keywords: Insulin, Integrin, Liver, Signal Transduction, Src, Epidermal Growth Factor Receptor, Osmosensing, Osmosignaling
Introduction
Apart from its metabolic effects, insulin exerts proliferative effects in the liver and other organs (1–5). Much effort has been devoted to the understanding of insulin signaling and its complexity (6–11), which involves tyrosine phosphorylation of the insulin receptor substrate-1 (IRS-1)2 and activation of a variety of protein kinases such as mitogen-activated protein (MAP) kinases ERK1/2 and p38MAPK (3, 12). In rat liver, insulin stimulates Na+/H+ antiport and K+/Na+/2Cl− co-transport, thereby inducing hepatocyte swelling (13). Evidence has been presented that insulin-induced hepatocyte swelling is an integral part of insulin signaling and mediates proteolysis inhibition by the hormone through a swelling-induced p38MAPK activation (14). Like hypoosmotic hepatocyte swelling, insulin-induced hepatocyte swelling is sensed by the integrin system with subsequent activation of c-Src kinase and downstream MAP kinases ERK1/2 and p38MAPK (14–16). In line with this, the insulin- or hypoosmolarity-induced inhibition of autophagic proteolysis is largely abolished in presence of an integrin-antagonistic RGD peptide or the c-Src inhibitor PP-2 (14, 15). Little is known about the contribution of insulin-induced cell swelling to the proliferative effects of the hormone (1–5). Both insulin and EGF increase DNA synthesis in hepatocytes kept under serum-free conditions (17, 18), but it is still unclear whether there is convergence of insulin- and EGF-dependent signaling.
In short term cultured hepatocytes, EGF was reported to induce IRS-1 phosphorylation in an IR-independent manner because no IR-β-subunit phosphorylation occurred (19). IRS-1-phosphorylation was followed by phosphatidylinositol 3-kinase activation (19), indicating that EGF may mimic insulin effects in hepatocytes. On the other hand, insulin (100 nmol/liter) failed to induce EGFR phosphorylation in 3-h cultured rat hepatocytes (19). However, this experimental model may not pick up swelling-dependent components of insulin signaling (20), most likely due to not yet reorganized microtubules or impaired osmosensing. The latter is achieved by hepatocellular integrin/extracellular matrix (ECM) interactions, which require either the intact three-dimensional organ structure, e.g. the intact liver, or long term hepatocyte cultures that allow for endogenous ECM synthesis. A hypoosmolarity-induced EGFR activation was shown by immunofluorescence staining in serum-starved Swiss 3T3 fibroblast (21), but the underlying molecular mechanisms remained unclear. As shown in the present study, insulin-induced cell swelling triggers activation of the EGFR through an integrin- and c-Src kinase-dependent osmosensing/signaling pathway that triggers insulin-induced hepatocyte proliferation.
EXPERIMENTAL PROCEDURES
Materials
Collagenases were from Roche Applied Science. William's E medium, collagen, insulin, and bumetanide were from Sigma-Aldrich. Penicillin and streptomycin were from Biochrom (Berlin, Germany). Fetal calf serum was from Invitrogen. The integrin antagonistic GRGDSP peptide (RGD peptide) was from Bachem (Heidelberg, Germany). PP-2 and AG1478 were from Calbiochem.
Rabbit anti-EGFR, rabbit anti-p38MAPK, and rabbit anti-FAK were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse anti-phospho-ERK1/2, rabbit anti-phospho-EGFR (EGFR Tyr1045-P) antibodies, and rabbit anti-phospho-Src family-Tyr418 were from Cell Signaling (Beverly, MA). Mouse anti-phospho-c-Src-Tyr418 was from Calbiochem. Rabbit anti-phospho-p38MAPK, rabbit anti-phospho-EGFR antibodies (EGFR Tyr845-P and Tyr1173-P), rabbit anti-phospho-FAK-Tyr397, and rabbit anti-c-Src were from BioSource (Camarillo, CA). Rabbit anti-phospho-IRS-Tyr612 was from Invitrogen. Rabbit anti-IRS-1, rabbit anti-ERK1/2, rabbit anti-Yes, and rabbit anti-Fyn were from Upstate Biotechnology (Lake Placid, NY). Integrin-blocking antibodies anti-integrin β1 (clone 6S6) (22), anti-integrin β3 (clone B3A) (23), and anti-integrin αVβ5 (clone P1F6) (24) were from Millipore.
Protein A/G-agarose was obtained from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated anti-mouse IgG and protein assay were from Bio-Rad. The enhanced chemiluminescence detection kit was from Amersham Biosciences. All other chemicals were from Merck at the highest quality available.
Primary Hepatocyte Preparation and Culture
As described previously (25), hepatocytes were isolated from livers of male Wistar rats fed ad libitum with a standard diet by a collagenase perfusion technique. Aliquots of 1.5 × 106 hepatocytes were plated on collagen-coated 6-well culture plates (Falcon) and cultured as published recently (25) for 48 h, unless indicated otherwise, before the respective experiments were started. Osmolarity changes were performed by the appropriate addition or removal of NaCl from the medium. The viability of the hepatocytes was >95% as assessed by trypan blue exclusion.
Liver Perfusion
The experiments were approved by the responsible local authorities. Livers from male Wistar rats (120–150 g body mass), fed a standard chow, were perfused as described previously (26) in a nonrecirculating manner. The perfusion medium was the bicarbonate-buffered Krebs-Henseleit saline plus l-lactate (2.1 mm) and pyruvate (0.3 mm) gassed with O2/CO2 (95/5 v/v). The temperature was 37 °C. In normoosmotic perfusions, the osmolarity was 305 mosmol/liter. Hypoosmotic exposure (225 mosmol/liter) was performed by lowering the NaCl concentration in the perfusion medium. The addition of inhibitors to influent perfusate was made either by use of precision micropumps or by dissolution into the Krebs-Henseleit buffer. Viability of the perfused livers was assessed by measuring lactate dehydrogenase leakage from livers, which did not exceed 20 milliunits min−1 g liver−1. The portal pressure was routinely monitored with a pressure transducer (Hugo Sachs Electronics, Hugstetten, Germany) (14–16). If not stated otherwise, the compounds used in this study did not affect portal perfusion pressure.
Western Blot Analysis
At the end of the incubations, the medium was removed, and the cells were washed briefly with phosphate-buffered saline (PBS) and immediately lysed. Samples were transferred to SDS/PAGE, and proteins were then blotted to nitrocellulose membranes using a semidry transfer apparatus (GE Healthcare) as recently described (25, 27). Blots were blocked for 2 h in 5% (w/v) BSA-containing 20 mmol/liter Tris, pH 7.5, 150 mmol/liter NaCl, and 0.1% Tween 20 (TBS-T) and then incubated at 4 °C overnight with the respective first antibody (antibodies used: anti-phospho-EGFR Tyr845, Tyr1045, Tyr1173 and anti-phospho-c-Src-Tyr418 (1:2,500); anti-c-Src, anti-phospho-IRS-1-Tyr612, anti-IRS-1, anti-phospho-Src family-Tyr418, and anti-phospho-FAK (1:5,000); anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38MAPK, anti-p38MAPK, anti-EGFR, anti-FAK, anti-Yes, and anti-Fyn (1:10,000)). Following washing with TBS-T and incubation with horseradish peroxidase-coupled anti-mouse, anti-sheep, or anti-rabbit IgG antibody (all diluted 1:10,000) at room temperature for 2 h, respectively, the blots were washed extensively and developed using enhanced chemiluminescent detection (Amersham Biosciences). Blots were exposed to Kodak X-OMAT AR-5 film (Eastman Kodak Co., Rochester, NY).
Immunoprecipitation
Hepatocytes were harvested in lysis buffer as recently published (27). Equal protein amounts (200 μg) of each sample were incubated for 2 h at 4 °C with polyclonal rabbit anti-EGFR, anti-Yes, or anti-Fyn antibodies (dilution 1:100; Santa Cruz Biotechnology) to immunoprecipitate EGFR, Yes, or Fyn, respectively (28, 29). Then, 10 μl of protein A-agarose and 10 μl of protein G-agarose (Santa Cruz Biotechnology) were added and incubated at 4 °C overnight. Immunoprecipitates were washed three times as published recently (27) and then transferred to Western blot analysis as described above. c-Src association of the immunoprecipitated EGFR samples was detected by Western blot analysis using rabbit anti-c-Src antibody from BioSource. Activating phosphorylation of residue Tyr418 of either Yes or Fyn in the respective immunoprecipitated samples was detected using a rabbit anti-phospho-Src family-Tyr418 antibody (28, 29).
EGFR Translocation in Primary Rat Hepatocytes
To detect EGFR translocation, isolated hepatocytes were cultured for 24 h on collagen-coated glass coverslips (℘ 30 mm) in 6-well culture plates. After treatment for the indicated time periods with EGF (10 ng/ml), insulin (100 nmnol/liter), or hypoosmotic medium (205 mosmol/liter), cells were fixed for 15 min using paraformaldehyde (4% v/v) and then permeabilized using Triton X-100 (0.1% v/v in PBS, 10 min, room temperature). Cells were washed briefly with PBS (4 °C) and then exposed to a rabbit anti-EGFR antibody (1 h, 4 °C, 1:100 in PBS), washed off, and then stained with an anti-rabbit Cy3-conjugated antibody (1 h, 4 °C, 1:500 in PBS). Coverslips were mounted with diazabicyclo[2.2.2]octane 0.1% in glycine:PBS (9:1). Confocal pictures were taken using the LSM 510 META (Zeiss, Oberkochen, Germany).
Detection of Primary Rat Hepatocyte Proliferation
Hepatocyte proliferation was measured using a colorimetric BrdU cell proliferation assay ((+)-5-bromo-2′-deoxyuridine-ELISA; Roche Applied Science). Therefore, primary rat hepatocytes were cultured on collagen-coated flat-bottomed 96-well microtiter plates, respectively, for up to 48 h. The culture medium was removed and replaced by culture medium containing BrdU. For the respective samples, BrdU incorporation was determined according to the manufacturer's recommendations.
Statistics
Results from at least three independent experiments are expressed as means ± S.E. of the mean. n refers to the number of independent experiments. Results were analyzed using Student's t test: p < 0.05 was considered statistically significant.
RESULTS
Insulin-induced EGFR Activation in Perfused Rat Liver
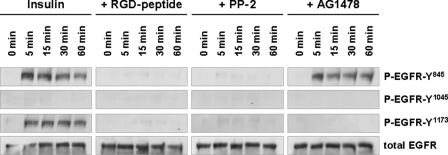
Insulin (35 nmol/liter) induced within 5-min phosphorylation of the EGFR tyrosine residue Tyr845 (Fig. 1) a known Src-inducible EGFR trans-activation site (30), which is known to induce EGFR tyrosine kinase activity (31). In line with this also, phosphorylation of the EGFR residue Tyr1173, a known EGFR autophosphorylation target (32), became detectable within 5 min (Fig. 1). No phosphorylation of EGFR residue Tyr1045, which represents an EGFR internalization site (33, 34), was observed, suggestive of ligand (EGF)-independent EGFR activation. As for control, EGF was shown to induce EGFR Tyr1045 phosphorylation (supplemental Fig. 1). AG1478, an inhibitor of EGFR tyrosine kinase activity, largely abolished insulin-induced EGFR autophosphorylation at Tyr1173, but not phosphorylation at Tyr845, indicating that insulin induces an EGFR trans-activation by another kinase.
FIGURE 1.
Insulin-induced EGFR phosphorylation in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), or AG1478 (1 μmol/liter) was added 30 min prior to the insulin institution (35 nmol/liter) to the perfusate to inhibit integrins, c-Src or EGFR tyrosine kinase activity, respectively. Liver samples were taken at the time points indicated, and phosphorylation of EGFR Tyr845, Tyr1045, and Tyr1173 was analyzed by use of phospho-specific antibodies. Total EGFR served as loading control. Representative Western blots of three independent perfusion experiments are shown. Within 5 min insulin induced an RGD peptide- and PP-2-sensitive phosphorylation of EGFR residues Tyr845 and Tyr1173, whereas no phosphorylation at position Tyr1045 is detectable within 60 min. AG1478 blunted insulin-induced Tyr1173 phosphorylation, whereas Tyr845 phosphorylation remained unchanged, suggestive of an EGFR Tyr845 transphosphorylation leading to EGFR Tyr1173 autophosphorylation.
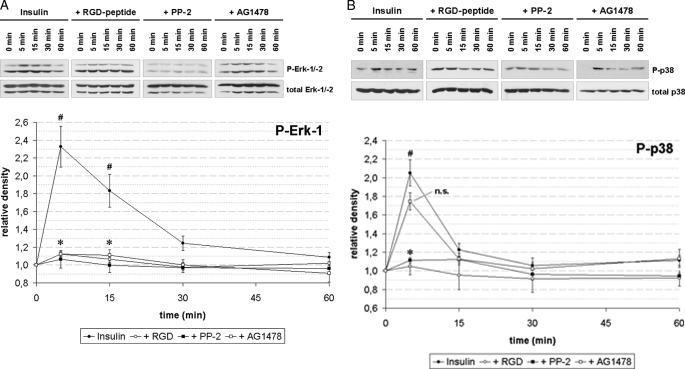
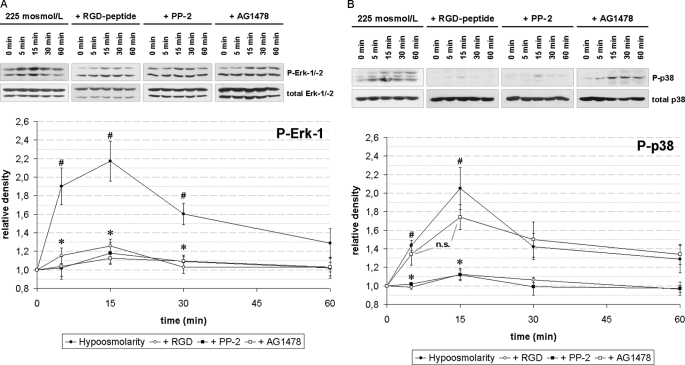
Insulin (35 nmol/liter) induced within 5 min an activation of ERKs (Fig. 2A) and p38MAPK (Fig. 2B), which was recently shown to be mediated by insulin-induced hepatocyte swelling (15, 35). In line with this, inhibition of the integrin system using the integrin antagonistic RGD peptide or inhibition of c-Src kinase activity by PP-2 inhibited both insulin-induced ERK and p38MAPK activation (Fig. 2). Because EGFR has been reported to activate ERKs (for reviews, see 36, 37), it was tested whether inhibition of EGFR tyrosine kinase activity might influence insulin-induced ERK activation. AG1478 significantly inhibited insulin-induced ERK activation (Fig. 2A, p < 0.05), but not insulin-induced p38MAPK activation (Fig. 2B, p > 0.05).
FIGURE 2.
Insulin-induced MAP kinase activation in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), or AG1478 (1 μmol/liter) was added 30 min prior to insulin (35 nmol/liter) to the perfusate. Liver samples were taken at the time points indicated and phosphorylation of ERK-1/-2 (A) and p38MAPK (B) was analyzed by use of phospho-specific antibodies. Total ERK-1/-2 or p38MAPK, respectively, served as loading controls. Western blots were analyzed densitometrically. Phosphorylation level at t = 0 min (i.e. immediately before insulin addition) was arbitrarily set to 1. Representative blots and statistics of at least three independent perfusion experiments are shown. A, insulin induced a significant increase in ERK phosphorylation at t = 5 and t = 15 min (#, p < 0.05), which was significantly inhibited by RGD peptide, PP-2, and AG1478 (*, p < 0.05). B, insulin induced a significant increase (#, p < 0.05) in p38MAPK phosphorylation at t = 5 min, which was significantly inhibited by RGD peptide and PP-2 (*, p < 0.05), but not by AG1478 (p > 0.05; n.s., not significant). Error bars, S.E. of the mean.
Hepatocyte Swelling Is Required for Insulin-induced EGFR and MAP Kinase Activation
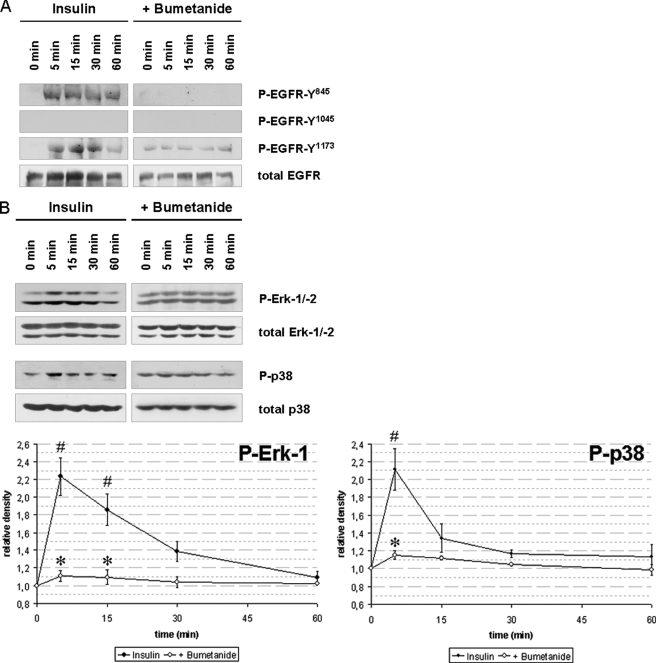
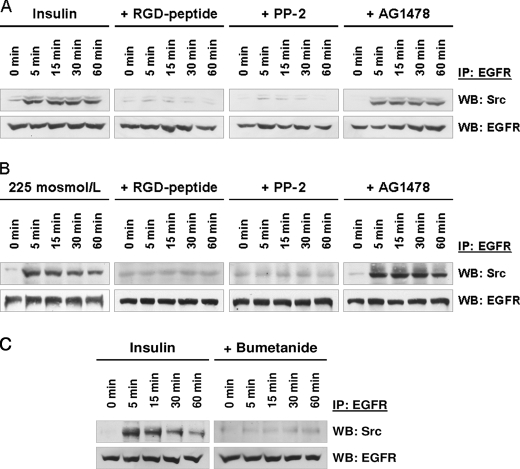
To test whether insulin-induced hepatocyte swelling mediates EGFR activation, experiments with bumetanide (5 μmol/liter), a known inhibitor of the Na+/K+/2Cl− co-transporter (38) and of insulin-induced hepatocyte swelling (13, 35), were performed. As shown in Fig. 3, both insulin-induced EGFR phosphorylation (Fig. 3A) as well as ERK and p38MAPK activation were largely abolished in presence of bumetanide (Fig. 3B, p < 0.05). These data suggest that not only insulin-dependent MAP kinase activation, but also EGFR phosphorylation depend on insulin-induced hepatocyte swelling. This is further supported by the findings that inhibition of the integrin system using an RGD motif-containing hexapeptide (10 μmol/liter) or inhibition of c-Src by PP-2 (250 nmol/liter) abolished the otherwise observed insulin-induced EGFR phosphorylation at positions Tyr845 and Tyr1173, respectively (Fig. 1). The data strongly suggest that insulin mediates via bumetanide-sensitive cell swelling (35), swelling-dependent integrin and subsequent c-Src kinase activation (15) followed by trans-activation of the EGFR by phosphorylation at Tyr845, which is a known target of c-Src (30, 31).
FIGURE 3.
Insulin-induced hepatocyte swelling triggers EGFR and MAP kinase activation. Rat livers were perfused as described under “Experimental Procedures.” When indicated, bumetanide (5 μmol/liter) was added 30 min prior to insulin addition (35 nmol/liter) to the perfusate to inhibit insulin-induced hepatocyte swelling by blocking the Na+/K+/2Cl− co-transporter. Liver samples were taken at the time points indicated, and phosphorylation of EGFR Tyr845, Tyr1015, and Tyr1173 (A) and phosphorylation of the MAP kinases ERK1/2 and p38MAPK (B) was analyzed using phospho-specific antibodies. Total EGFR, ERK1/2, and p38MAPK served as respective loading control. Western blots were analyzed densitometrically. Phosphorylation level at t = 0 min (i.e. immediately before insulin addition) was arbitrarily set to 1. Representative Western blots of three independent perfusion experiments are shown. Bumetanide blunted insulin-induced EGFR Tyr845, Tyr1173, ERK1/2, and p38MAPK phosphorylation, indicating the insulin-induced hepatocyte swelling is a prerequisite for activation of those kinases. Error bars, S.E. of the mean.
Hypoosmotic EGFR Activation in Perfused Rat Liver
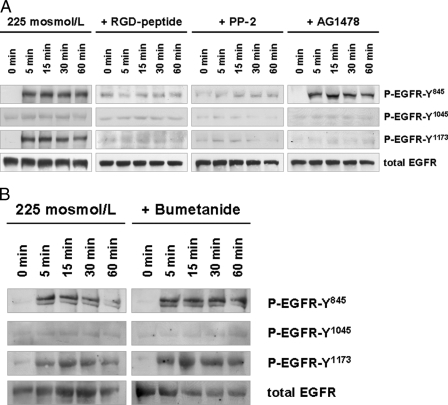
In line with a swelling-induced EGFR activation by insulin, hypoosmotic cell swelling also induced within 5 min an activation of the EGFR in the perfused rat liver (Fig. 4). The EGFR phosphorylation profile was similar to that observed in response to insulin (i.e. phosphorylation at Tyr845 and Tyr1173, but not at Tyr1045; compare Figs. 1 and 4). Hypoosmolarity-induced EGFR phosphorylation was sensitive to inhibition of the integrin system by an RGD peptide and PP-2, whereas AG1478 inhibited only EGFR autophosphorylation at Tyr1173, but not phosphorylation at Tyr845 (Fig. 4). As for control and in contrast to insulin-induced EGFR activation (Fig. 3A), bumetanide did not affect hypoosmotically induced EGFR phosphorylation (Fig. 4B).
FIGURE 4.
Hypoosmolarity-induced EGFR phosphorylation in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), AG1478 (1 μmol/liter) (A) or bumetanide (5 μmol/liter) (B) was added 30 min prior to the institution of hypoosmolarity (225 mosmol/liter). Liver samples were taken at the time points indicated, and phosphorylation of EGFR Tyr845, Tyr1015, and Tyr1173 was analyzed by use of phospho-specific antibodies. Total EGFR served as loading control. Representative Western blots of three independent perfusion experiments are shown. Within 5 min hypoosmolarity induced an RGD peptide- and PP-2-sensitive phosphorylation of EGFR residues Tyr845 and Tyr1173, whereas no phosphorylation at position Tyr1045 became detectable within 60 min (A). AG1478 blunted insulin-induced Tyr1173 phosphorylation, whereas Tyr845 phosphorylation remained unchanged (A), suggestive of an EGFR Tyr845 transphosphorylation leading to EGFR Tyr1173 autophosphorylation as observed upon insulin perfusion (Fig. 1). In contrast to insulin-induced EGFR phosphorylation, bumetanide did not affect hypoosmotic-induced EGFR phosphorylation (B).
In line with previous data (15, 16) and similar to the findings with insulin, hypoosmolarity led to an RGD peptide- and PP-2-sensitive activation of ERKs and p38MAPK (Fig. 5), whereas inhibition of EGFR tyrosine kinase activity by AG1478 only abolished hypoosmotic ERK but not p38MAPK activation (Fig. 5). These findings suggest an involvement of EGFR in hypoosmotic ERK, but not p38MAPK activation.
FIGURE 5.
Hypoosmolarity-induced MAP kinase activation in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), or AG1478 (1 μmol/liter) was added 30 min prior to the institution of hypoosmolarity (225 mosmol/liter). Liver samples were taken at the time points indicated, and phosphorylation of ERK1/2 (A) and p38MAPK (B) was analyzed by use of phospho-specific antibodies. Total ERK1/2 or p38MAPK, respectively, served as loading control. Western blots were then analyzed densitometrically. Phosphorylation level at t = 0 min was arbitrarily set as 1. Representative blots and statistics of three independent perfusion experiments are shown. A, hypoosmolarity induced a significant increase in ERK phosphorylation at t = 5, 15 and 30 min (#, p < 0.05), which was significantly inhibited by RGD peptide, PP-2, and AG1478 (*, p < 0.05). B, hypoosmolarity induced a significant increase in p38MAPK phosphorylation at t = 5 and t = 15 min (#, p < 0.05), which was significantly inhibited by RGD peptide and PP-2 (*, p < 0.05), but not by AG1478 (p > 0.05; n.s. = not significant). Error bars, S.E. of the mean.
Hepatocyte Swelling Triggers EGFR/c-Src Kinase Association
Swelling-induced EGFR trans-activation may require an EGFR/c-Src association because hepatocyte swelling by either insulin or hypoosmolarity induces an integrin- and FAK-mediated activation of c-Src (15, 16), but not of Yes or Fyn (supplemental Fig. 2). EGFR was immunoprecipitated from insulin-treated (Fig. 6, A and C) or hypoosmotically perfused rat liver samples (Fig. 6B). As shown in Fig. 6, both exposure to either insulin (Fig. 6, A and C) or hypoosmolarity (Fig. 6B) induced within 5 min an EGFR/c-Src association. In contrast, no association of the EGFR with either Fyn or Yes, i.e. other members of the Src kinase family, was observed (supplemental Fig. 3). Whereas inhibition of the EGFR tyrosine kinase activity by AG1478 did not affect insulin- (Fig. 6A) or hypoosmolarity-induced EGFR/c-Src association (Fig. 6B), prevention of insulin-induced hepatocyte swelling by bumetanide, inhibition of integrin-dependent cell volume sensing by an RGD peptide, or inhibition of downstream c-Src kinase by PP-2 largely prevented the otherwise observed EGFR/c-Src association (Fig. 6). These data suggest that insulin-induced EGFR trans-activation requires hepatocyte swelling, integrin-mediated c-Src activation, and subsequent EGFR/c-Src association.
FIGURE 6.
Insulin- and hypoosmolarity-induced EGFR/c-Src association in perfused rat liver. Rat livers were perfused as described under “Experimental Procedures.” When indicated, RGD peptide (10 μmol/liter), PP-2 (250 nmol/liter), AG1478 (1 μmol/liter), or bumetanide (5 μmol/liter) was added 30 min prior to the institution of either insulin (35 nmol/liter; A and C) or hypoosmolarity (225 mosmol/liter, B). Liver samples were taken at the time points indicated, and EGFR was immunoprecipitated (IP) as described under “Experimental Procedures.” Samples were then analyzed for EGFR/c-Src association by detection of c-Src. Total EGFR served as a loading control. Within 5 min both insulin (A) and hypoosmolarity (B) induced an EGFR/c-Src association, which lasts for up to 60 min. This EGFR/c-Src association was sensitive to inhibition of the integrin system (RGD peptide) or inhibition of c-Src kinase activity (PP-2), indicating that insulin- (A) or hypoosmolarity-induced (B) and integrin-mediated c-Src-activation (15, 16) is required for EGFR/c-Src association. Bumetanide also inhibited insulin-induced EGFR/c-Src association (C), underlining the importance of insulin-induced hepatocyte swelling for these processes. WB, Western blotting.
As for control, insulin-induced phosphorylation of IRS-1 was not affected by the RGD peptide, PP-2, AG1478 (supplemental Fig. 4) or bumetanide (35). This is in line with the previous demonstration that RGD peptide and PP-2 were also without effect on Tyr1185 phosphorylation of the β-subunit of the insulin receptor (IR-β) (15) and that hypoosmotic hepatocyte swelling did not induce IR-β or IRS-1 tyrosine phosphorylation (35).
Insulin-induced Proliferation in Primary Rat Hepatocytes
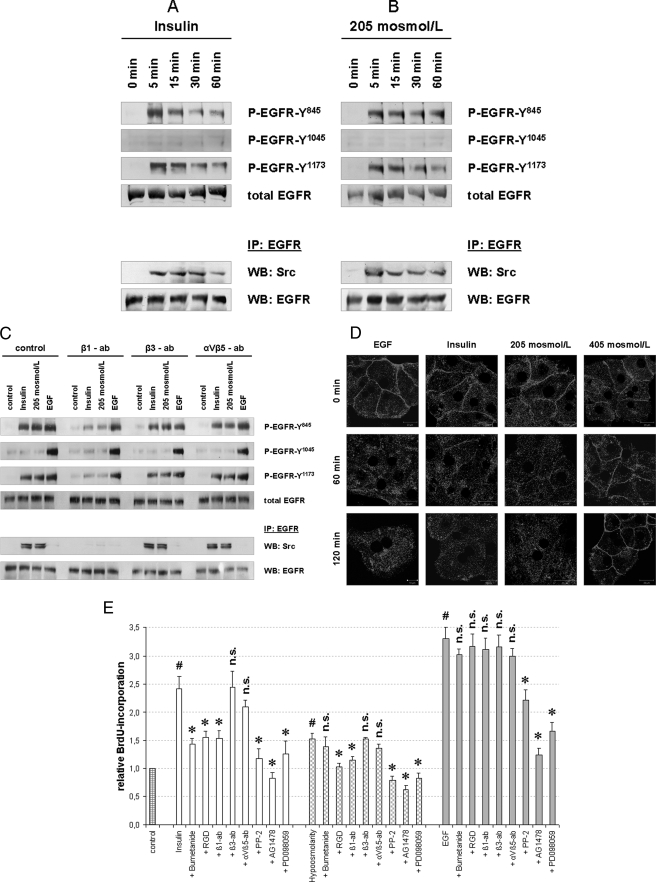
EGFR/c-Src association and EGFR activation in response to insulin (100 nmol/liter) or hypoosmolarity (205 mosmol/liter) were also found in primary rat hepatocytes, which were kept in culture for 48 h (Fig. 7, A and B).
FIGURE 7.
Insulin- and hypoosmolarity-induced EGFR activation and BrdU incorporation in primary rat hepatocytes. Primary rat hepatocytes were isolated as described under “Experimental Procedures” and seeded on collagen-coated wells for 48 h. Insulin- and hypoosmolarity-induced EGFR phosphorylation (A–C) and internalization (D) in primary hepatocytes are shown. Cells were incubated with insulin (100 nmol/liter), hypoosmolarity (205 mosmol/liter), or EGF (50 ng/ml) for the indicated time periods and then analyzed for EGFR phosphorylation at Tyr845, Tyr1015, and Tyr1173 by use of phospho-specific antibodies. Total EGFR served as loading control. In addition, EGFR was immunoprecipitated (IP) from the respective samples as described under “Experimental Procedures” and analyzed for EGFR/c-Src association by detection of c-Src in Western blots (WB). Total EGFR served as loading control. Representative Western blots of three independent experiments are shown. Insulin (A) and hypoosmolarity (B) induced EGFR phosphorylation at Tyr845 and Tyr1173, but not at Tyr1045, and EGFR/c-Src association. C, insulin-, hypoosmolarity- and EGF-induced EGFR phosphorylation and EGFR/c-Src association were tested for integrin blocking antibody sensitivity. Anti-integrin β1 subtype antibody (β1-ab, 1 μg/ml) inhibited insulin-induced (100 nmol/liter, 5 min) and hypoosmolarity-induced (205 mosmol/liter, 5 min) EGFR phosphorylation and EGFR/c-Src association, whereas anti-β3 (β3-ab, 1 μg/ml) and anti-αVβ5 subtype antibodies (αVβ5-ab, 1 μg/ml) were ineffective. As for control, EGF-induced (50 ng/ml, 5 min) EGFR phosphorylation was not affected by the used integrin-blocking antibodies. In another set of experiments, hepatocytes were cultured on glass coverslips and immunostained for EGFR expression as described under “Experimental Procedures.” EGFR localization was visualized by confocal laser scanning microscopy. D, insulin (100 nmol/liter) and hypoosmolarity (205 mosmol/liter) induced an EGFR internalization comparable with that induced by EGF (34, 39), whereas hyperosmolarity (405 mosmol/liter) induced EGFR enrichment at the plasma membrane (39). E, insulin- and hypoosmolarity-induced proliferation of primary hepatocytes is shown. After 48 h of cell culture, culture medium was removed and replaced by culture medium containing BrdU. Then, hepatocytes were stimulated for another 48 h with normoosmotic control medium (305 mosmol/liter), insulin (100 nmol/liter), hypoosmolarity (205 mosmol/liter), or EGF (50 ng/ml) and then analyzed for BrdU uptake. When indicated, bumetanide (10 μmol/liter), RGD peptide (100 μmol/liter), PP-2 (10 μmol/liter), AG1478 (5 μmol/liter), or PD098059 (10 μmol/liter) was instituted 30 min prior to insulin, hypoosmolarity, or EGF incubation. In addition, integrin blocking antibodies, i.e. anti-β1, anti-β3, and anti-αVβ5 antibodies (each 1 μg/ml), respectively, were used to inhibit the respective integrin subtypes. BrdU uptake in hepatocytes kept in normoosmotic control medium was arbitrarily set to 1. Statistical analyses of at least five independent experiments for each condition are shown. No significant inhibition compared with insulin, hypoosmolarity, or EGF incubation is indicated by n.s. (p > 0.05). Insulin, hypoosmolarity, and EGF induced a significant increase in hepatocyte proliferation by means of BrdU uptake (#, p < 0.05). Insulin- and hypoosmotic-induced hepatocyte proliferation was significantly inhibited (*, p < 0.05) by RGD, anti-integrin β1, PP-2, AG1478, and PD098059. Because the insulin-induced proliferation was also bumetanide-sensitive, these data suggest a swelling-dependent, integrin β1-, s-Src-, EGFR- and ERK-mediated hepatocyte proliferation upon either insulin or hypoosmotic stimulation. Error bars, S.E. of the mean.
To determine which RGD peptide-sensitive integrin subtype is responsible for swelling-induced EGFR activation, subtype-specific integrin-blocking antibodies, i.e. anti-integrin β1 (clone 6S6) (22), anti-integrin β3 (clone B3A) (23), and anti-integrin αVβ5 (clone P1F6) (24), were used (each 1 μg/ml). As shown in Fig. 7C, anti-integrin β3 antibodies inhibited insulin- and hypoosmotic-induced EGFR activation, whereas both anti-integrin β3 and anti-integrin αVβ5 antibodies were ineffective. As for control, EGF-induced EGFR activation was not affected by the integrin-blocking antibodies used.
To determine whether the EGFR activated by either insulin (100 nmol/liter) or hypoosmolarity (205 mosmol/liter) is a subject for internalization as has been reported in EGF-treated cells (34, 39), EGFR was stained immunocytochemically in primary rat hepatocytes. As shown in Fig. 7D, both insulin and hypoosmolarity induced EGFR internalization similar to that induced by EGF (10 ng/ml), whereas hyperosmolarity (405 mosmol/liter) induced EGFR enrichment at the plasma membrane as described previously (39).
To analyze insulin- and hypoosmolarity-induced hepatocyte proliferation, BrdU uptake measurements were performed. As shown in Fig. 7E, hypoosmolarity (205 mosmol/liter) as well as insulin (100 nmol/liter) increased BrdU uptake significantly within 48 h, suggestive of a stimulation of hepatocyte proliferation. This swelling-induced hepatocyte proliferation by either insulin (100 nmol/liter) or hypoosmolarity (205 mosmol/liter) was sensitive to inhibition of integrin β1 subtype (RGD peptide, 100 μmol/liter; anti-integrin β1 antibody, 1 μg/ml), c-Src (PP-2, 10 μmol/liter), EGFR tyrosine kinase activity (AG1478, 5 μmol/liter) and ERK1/2 (PD098059, 10 μmol/liter), indicating that swelling-induced EGFR activation mediates hepatocyte proliferation and involves integrin β1, c-Src, EGFR, and ERK. In addition, insulin-induced hepatocyte proliferation was shown to be inhibited by bumetanide (10 μmol/liter), indicating that insulin-induced hepatocyte swelling is necessary for insulin-induced hepatocyte proliferation.
DISCUSSION
Proliferative effects of insulin in liver and other organs were repeatedly described in the past (1–5, 17, 18), but it remained unclear whether EGFR-mediated signaling is involved in these processes. One single study on 3-h cultured primary rat hepatocytes revealed no EGFR activation within 30 s of insulin stimulation (100 nmol/liter) (19), whereas EGF was shown to induce phosphorylation of IRS-1 but not of the IR-β-subunit (19).
As shown in the present study, within 5 min insulin induces an activating EGFR tyrosine phosphorylation at position Tyr845 and an AG1478-sensitive EGFR Tyr1173 autophosphorylation. Several lines of evidence suggest that insulin-induced EGFR activation is mediated by insulin-induced hepatocyte swelling, which involves activation of the loop diuretic-sensitive Na+/K+/2Cl− co-transporter and was characterized in detail previously (13, 15, 35). (i) Bumetanide, which inhibits insulin-induced cell swelling, also inhibits insulin-induced EGFR activation. (ii) Hypoosmotic hepatocyte swelling also activates EGFR and roughly mimics the insulin effects on EGFR. (iii) Inhibition of the volume-sensing integrin β1-subunit (15) by either RGD peptide or blocking anti-integrin β1 antibody prevents insulin- and hypoosmolarity-induced EGFR activation.
Also, insulin effects on hepatic proteolysis are largely mediated by insulin-induced hepatocyte swelling (15, 35), which is sensed by the integrin system (15, 16) and leads to an RGD peptide-sensitive activation of c-Src and downstream ERKs and p38MAPK (14, 15). Accordingly, inhibition of Na+/K+/2Cl− co-transport by loop diuretics such as bumetanide or furosemide largely abolished insulin-induced activation of β1-integrin, c-Src, p38MAPK activation, and inhibition of autophagic proteolysis (15, 35; for review, see 12).
Insulin-induced c-Src activation most likely mediates EGFR activation, as suggested by (i) the PP-2 sensitivity of the process, (ii) the rapid association of c-Src with the EGFR in response to insulin, and (iii) the finding that inhibition of integrin signaling by an RGD peptide abolishes insulin-induced activation of both, c-Src and the EGFR. Furthermore, the EGFR is a known substrate for c-Src tyrosine kinase activity resulting in EGFR trans-activation (30, 31). The insulin- and hypoosmolarity-induced EGFR/c-Src association lasted for up to 60 min. This contrasts the more transient EGFR/Yes association which occurs in response to hepatocyte shrinkage due to hyperosmotic exposure or hydrophobic bile acids and which also results in EGFR phosphorylation at tyrosine residues Tyr845 and Tyr1173 (28, 29). However, the outcome of hyperosmotic Yes-mediated and hypoosmotic c-Src-induced EGFR activation results in proapoptotic and proliferative signaling, respectively. It is likely that these opposing outcomes of shrinkage and swelling-induced EGFR activation are due to different co-signals such as oxidative stress and JNK activation, which occur in response to hepatocyte shrinkage but not hepatocyte swelling (27, 28; for review, see 12, 37). However, the possibility is not excluded that the cell volume-dependent, differential engagement of c-Src and Yes in EGFR activation will contribute.
In line with the literature, both insulin and hypoosmolarity induce the activation of ERKs and p38MAPK, which is sensitive to inhibition of the integrin system and c-Src (Figs. 2 and 5) (15, 16). Interestingly, inhibition of EGFR tyrosine kinase activity by AG1478 largely abolished the insulin- or hypoosmolarity-induced ERK activation but had no effect on p38MAPK activation. These findings suggest an involvement of the EGFR tyrosine kinase activity in swelling-induced ERK but not p38MAPK activation. ERKs are known downstream targets of the EGFR and mediators of proliferative signaling (for review, see36, 37). In line with this, insulin and hypoosmolarity induced an AG1478- and PD098059-sensitive hepatocyte proliferation, suggestive of an involvement of the EGFR and ERKs. Swelling-mediated effects of insulin on liver function require an intact cytoskeleton (20) and the physiological interaction between the integrin system and extracellular matrix (ECM) proteins (11, 12). These prerequisites and the fact that insulin-induced net K+ uptake takes several minutes (for review, see 12) may explain why an insulin-induced activation of the EGFR was not found in 3-h cultured hepatocytes within 30 s of insulin exposure (19), whereas the present study gives unequivocal evidence of an insulin-induced and swelling-dependent EGFR activation. Hypoosmotic EGFR activation was also suggested to occur in serum-starved Swiss 3T3 fibroblasts (21). As shown in the present study, swelling-induced EGFR activation requires integrin- and c-Src signaling and can increase via ERKs hepatocyte proliferation. It is, therefore, an interesting speculation whether an altered ECM composition, as it is found in the fibrotic or cirrhotic liver, may confer resistance against insulin-induced and EGFR-mediated proliferation thereby affecting liver regeneration. The present data underline the importance of hepatocyte swelling as a mediator of insulin actions. This may explain why hyperosmotic conditions, cell dehydration, loop diuretics, and possibly altered ECM composition can trigger insulin resistance.
Supplementary Material
Acknowledgments
We thank Elisabeth Winands, Lisa Knopp, and Nicole Eichhorst for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 575 “Experimentelle Hepatologie” (Düsseldorf).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- IRS-1
- insulin receptor substrate-1
- ECM
- extracellular matrix
- EGFR
- EGF receptor
- IR
- insulin receptor
- IR-β
- insulin receptor β-subunit
- RGD
- integrin antagonistic GRGDSP hexapeptide.
REFERENCES
- 1.Diehl A. M., Rai R. M. (1996) FASEB J. 10, 215–227 [DOI] [PubMed] [Google Scholar]
- 2.Hunter T. (1997) Cell 88, 333–346 [DOI] [PubMed] [Google Scholar]
- 3.Avruch J. (1998) Mol. Cell. Biochem. 182, 31–48 [PubMed] [Google Scholar]
- 4.Nystrom F. H., Quon M. J. (1999) Cell. Signal. 11, 563–574 [DOI] [PubMed] [Google Scholar]
- 5.Taha C., Klip A. (1999) J. Membr. Biol. 169, 1–12 [DOI] [PubMed] [Google Scholar]
- 6.White M. F. (1997) Diabetologia 40, 2–17 [Google Scholar]
- 7.White M. F. (1998) Mol. Cell. Biochem. 182, 3–11 [PubMed] [Google Scholar]
- 8.Virkamäki A., Ueki K., Kahn C. R. (1999) J. Clin. Invest. 103, 931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A., Dubé N., Gu F., Tremblay M. L. (2002) Eur. J. Biochem. 269, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
- 11.Cohen P. (2006) Nat. Rev. Mol. Cell Biol. 7, 867–873 [DOI] [PubMed] [Google Scholar]
- 12.Häussinger D., Kubitz R., Reinehr R., Bode J. G., Schliess F. (2004) Mol. Aspects Med. 25, 221–360 [DOI] [PubMed] [Google Scholar]
- 13.Häussinger D., Lang F. (1992) Trends Pharmacol. Sci. 13, 371–373 [DOI] [PubMed] [Google Scholar]
- 14.vom Dahl S., Schliess F., Reissmann R., Görg B., Weiergräber O., Kocalkova M., Dombrowski F., Häussinger D. (2003) J. Biol. Chem. 278, 27088–27095 [DOI] [PubMed] [Google Scholar]
- 15.Schliess F., Reissmann R., Reinehr R., vom Dahl S., Häussinger D. (2004) J. Biol. Chem. 279, 21294–21301 [DOI] [PubMed] [Google Scholar]
- 16.Häussinger D., Kurz A. K., Wettstein M., Graf D., vom Dahl S., Schliess F. (2003) Gastroenterology 124, 1476–1487 [DOI] [PubMed] [Google Scholar]
- 17.Fausto N., Laird A. D., Webber E. M. (1995) FASEB J. 9, 1527–1536 [DOI] [PubMed] [Google Scholar]
- 18.Michalopoulos G. K., DeFrances M. C. (1997) Science 276, 60–66 [DOI] [PubMed] [Google Scholar]
- 19.Fujioka T., Ui M. (2001) Eur. J. Biochem. 268, 25–34 [DOI] [PubMed] [Google Scholar]
- 20.vom Dahl S., Stoll B., Gerok W., Häussinger D. (1995) Biochem. J. 308, 529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasantes-Morales H., Lezama R. A., Ramos-Mandujano G., Tuz K. L. (2006) Am. J. Med. 119, 4–11 [DOI] [PubMed] [Google Scholar]
- 22.Somanath P. R., Kandel E. S., Hay N., Byzova T. V. (2007) J. Biol. Chem. 282, 22964–22976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crean J. K., Finlay D., Murphy M., Moss C., Godson C., Martin F., Brady H. R. (2002) J. Biol. Chem. 277, 44187–44194 [DOI] [PubMed] [Google Scholar]
- 24.Carlson T. R., Feng Y., Maisonpierre P. C., Mrksich M., Morla A. O. (2001) J. Biol. Chem. 276, 26516–26525 [DOI] [PubMed] [Google Scholar]
- 25.Reinehr R., Graf D., Fischer R., Schliess F., Häussinger D. (2002) Hepatology 36, 602–614 [DOI] [PubMed] [Google Scholar]
- 26.Sies H. (1978) Methods Enzymol. 52, 48–59 [DOI] [PubMed] [Google Scholar]
- 27.Reinehr R., Schliess F., Häussinger D. (2003) FASEB J. 17, 731–733 [DOI] [PubMed] [Google Scholar]
- 28.Reinehr R., Becker S., Höngen A., Häussinger D. (2004) J. Biol. Chem. 279, 23977–23987 [DOI] [PubMed] [Google Scholar]
- 29.Reinehr R., Becker S., Wettstein M., Häussinger D. (2004) Gastroenterology 127, 1540–1557 [DOI] [PubMed] [Google Scholar]
- 30.Boerner J. L., Biscardi J. S., Silva C. M., Parsons S. J. (2005) Mol. Carcinog. 44, 262–273 [DOI] [PubMed] [Google Scholar]
- 31.Biscardi J. S., Maa M. C., Tice D. A., Cox M. E., Leu T. H., Parsons S. J. (1999) J. Biol. Chem. 274, 8335–8343 [DOI] [PubMed] [Google Scholar]
- 32.Poppleton H. M., Wiepz G. J., Bertics P. J., Patel T. B. (1999) Arch. Biochem. Biophys. 363, 227–236 [DOI] [PubMed] [Google Scholar]
- 33.Ravid T., Sweeney C., Gee P., Carraway K. L., 3rd, Goldkorn T. (2002) J. Biol. Chem. 277, 31214–31219 [DOI] [PubMed] [Google Scholar]
- 34.Haj F. G., Verveer P. J., Squire A., Neel B. G., Bastiaens P. I. (2002) Science 295, 1708–1711 [DOI] [PubMed] [Google Scholar]
- 35.Schliess F., vom Dahl S., Häussinger D. (2001) Biol. Chem. 382, 1063–1069 [DOI] [PubMed] [Google Scholar]
- 36.McCubrey J. A., Steelman L. S., Chappell W. H., Abrams S. L., Wong E. W., Chang F., Lehmann B., Terrian D. M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A. M., Franklin R. A. (2007) Biochim. Biophys. Acta 1773, 1263–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinehr R., Häussinger D. (2009) Biol. Chem. 390, 1033–1037 [DOI] [PubMed] [Google Scholar]
- 38.Xu J. C., Lytle C., Zhu T. T., Payne J. A., Benz E., Jr., Forbush B., 3rd (1994) Proc. Natl. Acad. Sci. U.S.A. 15, 2201–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eberle A., Reinehr R., Becker S., Häussinger D. (2005) Hepatology 41, 315–326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.