Abstract
Three groups of male Wistar rats were pair fed NIH-31 diets for 14 days to which were added 30% of calories as corn starch, palm oil, or R-3-hydroxybutyrate-R-1,3-butanediol monoester (3HB-BD ester). On the 14th day, animal brains were removed by freeze-blowing, and brain metabolites measured. Animals fed the ketone ester diet had elevated mean blood ketone bodies of 3.5 mm and lowered plasma glucose, insulin, and leptin. Despite the decreased plasma leptin, feeding the ketone ester diet ad lib decreased voluntary food intake 2-fold for 6 days while brain malonyl-CoA was increased by about 25% in ketone-fed group but not in the palm oil fed group. Unlike the acute effects of ketone body metabolism in the perfused working heart, there was no increased reduction in brain free mitochondrial [NAD+]/[NADH] ratio nor in the free energy of ATP hydrolysis, which was compatible with the observed 1.5-fold increase in brain uncoupling proteins 4 and 5. Feeding ketone ester or palm oil supplemented diets decreased brain l-glutamate by 15–20% and GABA by about 34% supporting the view that fatty acids as well as ketone bodies can be metabolized by the brain.
Keywords: Acetoacetate, Beta-hydroxybutyrate, Brain Metabolism, Ketone Bodies, Ketone Body Metabolism, Malonyl-CoA, Uncoupling Protein
Introduction
The metabolism of ketone bodies in the working perfused heart increased the supply of mitochondrial NADH and the ΔG of ATP hydrolysis (1). Other than the observation that ketone bodies can replace glucose as the major energy substrate in brain (2), little is known about the precise effects of ketone metabolism in brain in vivo. The elevation of ketone bodies (3-hydroxybutyrate and acetoacetate) and free fatty acids through ketogenic diets have been used for almost a century to treat drug refractory epilepsy (3, 4). In addition, it has been suggested that mild ketosis might be an effective treatment for a number of neurodegenerative and other diseases (5–7). We therefore undertook a broad survey of the effects of a ketone ester- and a fat-supplemented diet on several of the pathways of intermediary and energy metabolism in rat brain.
Prevention of postmortal changes, necessary for the accurate determination of in vivo redox and phosphorylation states in brain, require rapid inactivation of tissue, which was accomplished by freeze-blowing (8, 9). In addition to the [lactate]/[pyruvate] ratio, we report differences in the [succinate]/[fumarate] ratio as an additional indicator of hypoxic changes induced by different brain collection methods used in brain metabolism analysis.
Elevation of blood ketone bodies, by either fasting or high fat diets, results in elevation of both blood ketone bodies and free fatty acids. Therefore, prior to this report, it has not been possible to investigate ketone body metabolism in brain independent of the effects induced by elevation of plasma free fatty acids.
More recently, ketogenic diets have been used in the treatment of obesity where it has been shown that high protein, low carbohydrate diets decreased appetite, sensation of hunger, and food intake in hospitalized patients(10). The intraventricular infusion of 3-hydroxybutyrate (11, 12) or intravenous administration of its precursor 1,3-butanediol (13), have previously been shown to decrease food intake in the rat as has intraperitoneal injections of 3-hydroxybutyrate or 1,3-butanediol in the pigmy goat (14). Subcutaneous administration of 3-hydroxybutyrate, but not acetoacetate, has also been shown to decrease food intake in the rat (15). Increased hypothalamic malonyl-CoA associated with administration of the fatty acid synthase inhibitor, C75, decreased food intake (16–19) for 1 day in normal lean mice but for up to 6 days in the obese ob/ob leptin-deficient mouse (20).
Classic ketogenic diets containing minimal amounts of carbohydrate and large amounts of saturated fats are unpalatable, leading to poor patient compliance. More importantly, these diets lead to elevated blood cholesterol (21) and free fatty acids (22, 23), both of which have well documented adverse effects. Therefore, alternative methods of elevating blood ketone body levels were needed. Accordingly, we synthesized a monoester comprised of d-β-hydroxybutyrate and R-1,3-butanediol. R-1,3-butanediol is converted by liver to ketone bodies (24). We report here, for the first time, the use and analysis of a ketone ester-supplemented diet on various pathways of brain intermediary metabolism and contrast these effects with those resulting from a starch or palm oil-supplemented diet.
EXPERIMENTAL PROCEDURES
Animals and Diets
Male Wistar rats weighing 280–310 g (n = 6–12) were obtained from Charles River Laboratories, Wilmington, MA. All experiments were reviewed and approved by the Animal Care and Use Committee of NIAAA, National Institutes of Health. Diets were prepared by grinding NIH-31 fortified rodent diet to powder in a Waring blender and mixing the diet with “sugar free” maltodextrin containing gelatin (Jell-OTM), water, and the various components listed in Table 1. Vitamins and minerals (AIN-93 GMX, Bio-serv) were added according to guidelines set by the American Institute of Nutrition in 1993 (25).
TABLE 1.
Diet composition
The composition of the diet-fed rats during the entire experiment is listed below. The recipe lists components as grams per 100 grams diet.
| Component | Starch | Fat | Ketone ester |
|---|---|---|---|
| Diet Recipe (g/100 g diet) | |||
| NIH-31 fortified diet | 25.7 | 25.7 | 25.7 |
| Sugar-free Jell-OTM | 13.4 | 13.4 | 13.4 |
| Water | 43.1 | 52.1 | 46.1 |
| Palm oil | 0 | 7.22 | 0 |
| Corn starch | 16.25 | 0 | 0 |
| Ketone ester | 0 | 0 | 13.3 |
| Salt mix | 1.2 | 1.2 | 1.2 |
| Vitamin mix | 0.3 | 0.3 | 0.3 |
| Total | 100 | 100 | 100 |
| Diet composition (% kcal) | |||
| Carbohydrate | 73.9 | 46.5 | 46.2 |
| Protein | 21.2 | 21.2 | 20.6 |
| Fat | 4.9 | 32.3 | 4.8 |
| Ketone ester | 0 | 0 | 28.8 |
| Energy content, kcal/g | 2.15 | 2.15 | 2.15 |
Feeding Protocol and Sample Collection
One group of animals was meal fed the 3 diets (starch, fat, and ketone ester) ad lib for 3 h and food intake monitored to determine the effects of diets on voluntary food intake. Because the group receiving the ketone ester ate less food in a 3 h ad lib meal, a second group of animals was pair fed for 3 h each morning for 14 days, matching the caloric intake of the starch- and fat-supplemented diets to that of the ketone ester-supplemented diet. Initially, the food intake in the pair fed animals was 10 g per rat per day but increased gradually to 15–20 g per day. In addition, the bodyweight of the animals decreased by about 10% but stabilized in all three diet groups after 4 days. Following the meal on the 14th day, brains were freeze-blown, and blood collected in heparinized syringes. The arterial plasma pH and pCO2 was measured using an i-STAT blood gas analyzer (Abbott Laboratories). The whole blood was centrifuged at 5,000 × g for 10 min at 4 °C. Plasma was collected from supernatant and stored at −80 °C until analysis.
Comparison of Brain Extraction Techniques
Measurements of lactate, pyruvate, succinate, and fumarate from brain samples harvested with the freeze-blowing technique (n = 4) or by rapid removal of the brain followed by immersion in liquid N2 (n = 4) and the calculation of the free cytosolic [NAD+]/[NADH] ratio were performed as previously described (8, 9).
Enzymatic Analysis
Brain samples were prepared by perchloric acid extraction as previously described (8). Enzymatic analyses were performed using methods described by Passonneau and Lowry (26) Glycolytic intermediates, ATP, creatine, phosphocreatine, plasma ketone bodies, and plasma glucose were measured enzymatically (27). All other metabolites were measured enzymatically as described previously (1).
GC-MS Measurements
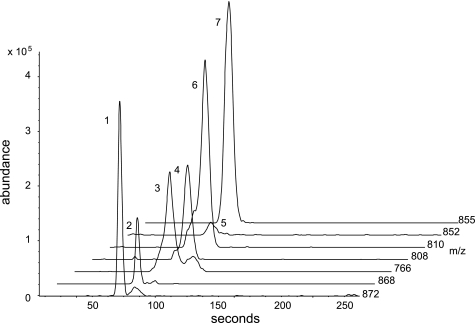
Perchloric acid extracts of frozen brain were used to measure citrate, isocitrate, α-ketoglutarate, succinate, fumarate, malate, glutamate, glutamine, aspartate, N-acetyl aspartate, and γ-amino butyric acid (GABA).2 They were analyzed as the silyl ether derivatives, quantified using 13C-labeled standards for each analyte using gas chromatography-mass spectrometry (GC-MS) as previously described (28). Briefly, the mass spectrometer was operated in the electron impact mode (70 eV) and the quadrupole mass analyzer scanned for ions which corresponded to a loss of 15 mass units (-CH3) from the molecular ion and the base peak of each analyte and its corresponding 13C-labeled internal standard using selected ion monitoring. The ratio of peak area count of the 13C-labeled internal standard to that of the analyte was used to quantify its concentration according to our previous report (28). A sample chromatogram is given in Fig. 1.
FIGURE 1.
CE-MS analysis of rat whole brain extracts for acyl-CoA compounds. Selected ion chromatographic tracings for the molecular anion mass values (M-1−) for: (1) [13C4]succinyl-CoA (m/z: 872), (2) succinyl-CoA (m/z: 868), (3) CoA (m/z:766), (4) acetyl-CoA (m/z:808), (5) malonyl- CoA (m/z: 852), (6) [13C2]acetyl-CoA (m/z: 810) and (7) [13C3]malonyl-CoA (m/z: 855) from rat brain tissues extracted with chloroform and analyzed by capillary electrophoresis mass spectrometry according to previously described methods.
Sample Extraction Procedure and Capillary Electrophoresis Mass Spectral (CE-MS) Determination of Acyl-CoA Compounds and Acetylcholine
Frozen brain tissue samples were extracted using a modified chloroform-methanol extraction procedure and analyzed by CE-MS according to the method of Soga (29) with the addition of 13C-labeled CoA internal standards for quantification as described previously (28).
Plasma Peptide Measurements
Plasma insulin and leptin were measured using a rat plasma EIA kit (from Alpco Diagnostics and Lingo/Millipore, respectively) according to the manufacturer's instructions.
Calculations
The free [Mg2+] (30), phosphorylation potential, free cytosolic [ADP], [AMP], [Pi], [oxaloacetate], the ΔG of ATP hydrolysis, cytosolic and mitochondrial redox states were calculated as previously described (1, 31). The adjustment of the equilibrium constants for tissue pH and free [Mg2+] were done as previously described (32).
Brain Mitochondrial UCP4 and UCP5 Immunoblotting
In each case, 30 μg of total brain protein was loaded onto a 12% polyacrylamide gel, and proteins were separated by running the gel at 100 V before transferring the protein on to a nitrocellulose membrane. Polyclonal rabbit anti-UCP4 and anti-UCP5 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used at concentrations of 1:1000 in 5% milk TBS-Tween. The secondary antibody used in both cases was anti-rabbit IgG peroxidase conjugate polyclonal antibody (Autogen Bioclear, Wiltshire, UK) at a concentration of 1:3500 in 5% milk TBS-Tween. For all blots, the films were scanned and densitometry measurements quantified using Un-Scan-It gel-digitizing software (Silk Scientific, Orem, UT). UCP4 and UCP5 levels were normalized to total protein using Ponceau S stain (Sigma).
Statistical Analysis
The number of samples analyzed for each metabolite, n, varied from 6 to 8. The results are presented as means ± S.E. A non-parametric statistical procedure, Mann-Whitney U test, was used to determine the significance of the difference between means.
RESULTS
Effect of Ketone Ester on Plasma Measurements of Ketones
Rats fed a starch- or fat-supplemented diet (Table 1) had mean total blood ketone bodies of 0.05–0.08 mm. Rats fed a ketone ester supplemented diet had a mean total ketone body level of 3.5 mm (n = 6) and R-1,3-butanediol concentration of 0.02 mm 3 h after the beginning of the meal.
Effect of Ketone Ester on Arterial Blood pH
The pH of the arterial blood plasma was measured and remained unchanged at 7.35 in all groups with CO2 lowered from 47 mmHg in the starch and fat fed groups to 35 mmHg in the ketone ester fed group.
Effect of Ketone Ester on Plasma Glucose, Insulin, and Leptin
As compared with the fat supplemented diet group, the ketone ester fed group decreased blood glucose from 4.8 mm to 2.8 mm or about 44% even though carbohydrate content of the diets were matched (Table 1). Plasma insulin was also decreased by a factor of 2 (Table 2). The anorexigenic adipose tissue peptide leptin was decreased from 3.1 to 1.8 ng/ml plasma.
TABLE 2.
Blood plasma substrate and leptin levels in rats fed diets containing 30% calories added as starch, fat, or ketone ester
Values are means in mm ± S.E. unless otherwise indicated where n = 6. For leptin measurements, n = 13 for starch, n = 5 for fat, and n = 12 for ketone ester. For insulin measurements, n = 16 for starch, n = 8 for fat, and n = 15 for ketone ester.
| Substrate | Starch | Fat | Ketone ester |
|---|---|---|---|
| Glucose | 4.83 ± 0.15 | 5.24 ± 0.25 | 2.81 ± 0.27a |
| d-β-hydroxybutyrate | 0.018 ± 0.004 | 0.031 ± 0.009 | 2.80 ± 0.19a |
| Acetoacetate | 0.037 ± 0.002 | 0.045 ± 0.005 | 0.71 ± 0.09a |
| Total ketones | 0.055 | 0.076 | 3.51 |
| Stearic acid (18:0) | 0.327 ± 0.042 | 0.396a ± 0.023 | 0.315 ± 0.013 |
| Total free fatty acids | 0.579 ± 0.45 | 0.693a± 0.039 | 0.571 ± 0.025 |
| Plasma leptin ng/ml | 3.12 ± 0.46 | 2.65 ± 0.87 | 1.83 ± 0.19a |
| Plasma insulin ng/ml | 0.544 ± 0.069 | 0.135 ± 0.038a | 0.263 ± 0.055a |
a p < 0.05 by Mann-Whitney U test comparing starch vs. fat or ketone ester diet.
Effect of Diets on Daily Food Intake
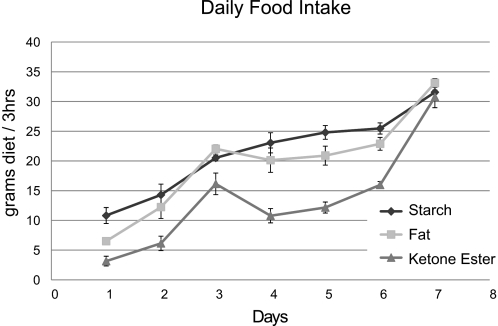
Rats fed the ketone ester supplemented diet ad lib consumed significantly less food in a 3 h meal compared with rats fed either starch- or fat-supplemented diets on days 2–6 as judged by Mann Whitney U test (p > 0.04) n = 6. Furthermore, on day 1, both ketone ester- and fat-supplemented diet groups consumed significantly less than the starch supplemented diet groups (Fig. 2).
FIGURE 2.
Food intake of 3 diets in ad lib fed rats. Food intake of starch-, fat-, and ketone-supplemented diet animal groups. All animals were fed ad lib for 3 h every day, and their food intake was measured for 6 days. Data are presented as means ± S.E. (n = 6).
Comparison of Metabolite Levels in Freeze Blown and Rapidly Frozen Brains
When comparing the reduced substrates from different brain harvesting procedures (freeze-blown versus quick-frozen), there was a 1.5-fold increase in lactate and a 4-fold increase in succinate in brains that were dropped into liquid N2 compared with freeze-blown brains (Table 3). There were no differences in the levels of the oxidized substrates, pyruvate and fumarate.
TABLE 3.
Comparison of metabolite levels in freeze-blown and rapidly frozen brains
Metabolite concentrations of brain extract from quick-frozen (n = 4) samples and brain-blowing (n = 4) samples. Values given in μmol/g wet weight ± S.E.
| Substrate | Freeze-blown brain | Quick-frozen brain |
|---|---|---|
| Lactate | 1.41 ± 0.17a | 2.12 ± 0.10 |
| Pyruvate | 0.064 ± 0.002 | 0.065 ± 0.005 |
| Lactate/Pyruvate | 22.4 ± 3.0a | 33.0 ± 2.7 |
| Free cytosolic [NAD+]/[NADH] | 271 ± 43.6 | 176 ± 14.4 |
| Succinate | 0.119 ± 0.002a | 0.415 ± 0.011 |
| Fumarate | 0.121 ± 0.005 | 0.142 ± 0.012 |
| Succinate/Fumarate | 0.99 ± 0.05a | 2.99 ± 0.32 |
a p < 0.05 between freeze-blown and quick-frozen brain.
Effect of Ketone Ester Diet on Brain Glycolytic Intermediates
Brain glycolytic intermediates were measured in the freeze-blown brains. The concentrations of brain glucose and the glycolytic intermediates 3-phosphoglycerate and l-lactate were significantly decreased in rats fed the ketone ester diet (Table 4). The [lactate]/[pyruvate] ratio was significantly decreased in the ketone ester fed group as compared with starch group. Feeding a diet supplemented with 30% of calories as fat significantly increased brain [lactate] concentration (Table 4).
TABLE 4.
Brain glycolytic intermediates
Values are means in μmol/g wet weight ± S.E. where n = 12.
| Substrate | Starch | Fat | Ketone ester |
|---|---|---|---|
| Glucose | 1.03 ± 0.051 | 0.945 ± 0.028 | 0.452 ± 0.116a,b |
| Glucose 6-phosphate | 0.072 ± 0.003 | 0.071 ± 0.003 | 0.066 ± 0.004 |
| DHAP | 0.017 ± 0.001 | 0.019 ± 0.002 | 0.016 ± 0.001 |
| 3-P glycerate | 0.034 ± 0.004 | 0.029 ± 0.002 | 0.025 ± 0.001a |
| Pyruvate | 0.086 ± 0.004 | 0.073 ± 0.005 | 0.085 ± 0.006 |
| l-Lactate | 1.69 ± 0.08 | 1.75 ± 0.08b | 1.43 ± 0.06a |
| Lactate/Pyruvate | 17.7 ± 1.0 | 21.0 ± 1.6 | 15.3 ± 0.8b |
a p < 0.05 between ketone ester and starch.
b p < 0.05 between ketone ester and fat, all as judged by Mann-Whitney U test.
Brain Krebs Cycle and CoA Intermediates
The concentration of Krebs cycle intermediates in brain was unchanged by any of the three diets tested (Table 5). Malonyl-CoA concentration alone was increased by feeding a ketone ester-supplemented diet, but not by a fat-supplemented diet.
TABLE 5.
Brain Krebs cycle and CoA intermediates
Values are means in μmol/g wet weight ± S.E. with n = 12 given in parentheses. CoA is given in nmol/g wet weight.
| Substrate | Starch | Fat | Ketone ester |
|---|---|---|---|
| Citrate | 0.205 ± 0.006 | 0.213 ± 0.005 | 0.229 ± 0.010 |
| Isocitrate | 0.0109 ± 0.0011 | 0.0100 ± 0.0006 | 0.0121 ± 0.0011 |
| α-Ketoglutarate | 0.138 ± 0.005 | 0.152 ± 0.006 | 0.142 ± 0.007 |
| Succinyl-CoA nmol/g | 0.836 ± 0.050 | 0.805 ± 0.120 | 0.930 ± 0.144 |
| Succinate | 0.077 ± 0.002 | 0.079 ± 0.004 | 0.078 ± 0.004 |
| Fumarate | 0.074 ± 0.003 | 0.078 ± 0.004 | 0.074 ± 0.004 |
| l-Malate | 0.195 ± 0.009 | 0.205 ± 0.011 | 0.180 ± 0.011 |
| Calc Oxaloacetate | 0.003 ± 0.0003 | 0.002 ± 0.0001 | 0.003 ± 0.0004 |
| Acetyl-CoA nmol/g | 6.86 ± 0.93 | 7.52 ± 0.75 | 6.23 ± 0.36 |
| Malonyl-CoA nmol/g | 0.970 ± 0.037 | 1.02 ± 0.10 | 1.20 ± 0.08a |
a p < 0.05 between ketone ester and starch as judged by Mann-Whitney U test.
Brain Amino Acids and Neurotransmitter Content
Feeding a ketone ester diet and a fat-supplemented diet both resulted in a decrease of about 40% in brain l-glutamate and GABA when compared with a starch-supplemented diet. There were no significant differences in brain aspartate or acetylcholine between the three diets.
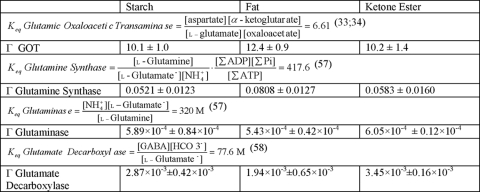
The Equilibrium Constants, Keq and Measured Brain Tissue Metabolite Ratios, Γ, for Enzyme Reactions with Glutamate or Glutamine as Substrates
From the values in Tables 5 and 6, an in vivo ratio of metabolites (Γ) was calculated for glutamic oxaloacetic transaminase, glutamine synthase, glutaminase, and glutamate decarboxylate and compared with the Keq measured in vitro for each reaction. The metabolite ratio measured in tissue, Γ, for the reactants of the glutamic oxaloacetic transaminase reaction (EC 2.6.1.1) was 10–12 and agreed well with the Keq of this reaction, 6.61 (33, 34). In contrast, the other reactions, in which l-glutamine or GABA are substrates, had tissue ratios that were different from Keq by two or more orders of magnitude.
TABLE 6.
Brain amino acids and neurotransmitters
Values are means in μmol/g wet weight ± S.E. with n = 12. Acetylcholine is in nmol/g wet weight.
| Substrate | Starch | Fat | Ketone ester |
|---|---|---|---|
| l-Glutamate | 13.4 ± 0.62 | 11.3 ± 0.62a | 10.5 ± 0.61b |
| l-Glutamine | 6.45 ± 0.48 | 6.12 ± 0.33 | 5.65 ± 0.44 |
| GABA | 1.86 ± 0.13 | 1.22 ± 0.08a | 1.20 ± 0.10b |
| NH4+ | 0.371 ± 0.027 | 0.312 ± 0.020 | 0.319 ± 0.063 |
| l-Aspartate | 1.81 ± 0.03 | 1.73 ± 0.12 | 1.52 ± 0.14 |
| Acetylcholine nmol/g | 27.4 ± 1.5 | 25.4 ± 1.1 | 22.9 ± 1.8 |
a p < 0.05 between fat and starch as judged by Mann-Whitney U test.
b p < 0.05 between ketone ester and starch.
Brain High Energy Intermediates
There were small, but non-significant decreases in total brain ATP and P-creatine in rats fed the fat-supplemented diet (Tables 7 and 8) in comparison to the group fed the starch-supplemented diet. The ketone ester fed group showed a significant decrease in both P Creatine and N-acetyl-aspartate content when compared with the starch-supplemented diet group (Table 8).
TABLE 7.
Keq and tissue metabolite ratios, Γ
Keq and Γ for GOT, glutamine synthase, glutaminase, and glutamate decarboxylate reactions in brain.
TABLE 8.
Brain high energy intermediates
Values are means in μmol/g wet weight ± S.E.
| Substrate | Starch | Fat | Ketone ester |
|---|---|---|---|
| ATP n = 12 | 1.93 ± 0.082 | 1.82 ± 0.08 | 1.75 ± 0.09 |
| P-Creatine n = 12 | 3.41 ± 0.14 | 3.19 ± 0.15 | 2.71 ± 0.21a |
| Creatine n = 12 | 5.12 ± 0.078 | 5.14 ± 0.099 | 5.02 ± 0.197 |
| Pin = 6 | 2.55 ± 0.17 | 2.03 ± 0.28 | 2.10 ± 0.27 |
| N-acetyl-aspartate n = 12 | 5.52 ± 0.12 | 5.32 ± 0.17 | 5.06 ± 0.15a |
a p < 0.05 between ketone ester- and starch-fed animals judged by Mann-Whitney U test.
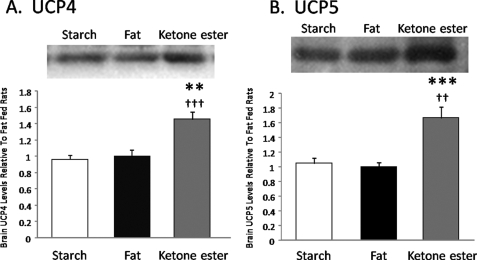
Brain Uncoupling Protein Content
In the brains of ketone diet fed rats, levels of mitochondrial UCP4 (Fig. 3) were 45% greater than in the brains of fat fed rats (p < 0.01) and 50% greater than in the brains of rats fed the starch-supplemented diet (p < 0.001). Similarly, levels of mitochondrial UCP5 (Fig. 3) were elevated in ketone diet fed rat brains by 66% compared with fat-fed rats (p < 0.001) and 59% compared with starch-fed rats (p < 0.01).
FIGURE 3.
Uncoupling proteins 4 and 5 in rat brain fed starch-, palm oil-, or ketone ester-supplemented diets. Levels of mitochondrial UCP4 (panel A) and UCP5 (panel B) in the brains of rats (n = 6 for fat and ketones, n = 7 for starch) fed a diet supplemented with palm oil (fat), starch, or ketone ester. **, p < 0.01; ***, p < 0.001 compared with fat-supplemented diet, ††, p < 0.01; †††, p < 0.001 compared with starch-supplemented diet.
Free Nucleotide Ratios and Concentrations
Feeding ketone ester-supplemented diets led to a significant oxidation in the free cytoplasmic [NAD+]/[NADH] (Table 9) compatible with an increase in UCP 4 and 5 (Fig. 3). There were no changes in either the mitochondrial NAD- or coenzyme Q-couple. The ΔG of ATP hydrolysis was −58.4 to −59.2 in all diet groups estimated from the component of the GAP dehydrogenase-3-phosphoglycerate kinase reactions or the creatine kinase reaction and measured Pi. There was no consistent change in the ΔG of ATP hydrolysis in brain in the three diet groups studied. The free cytosolic [AMP] was not changed in any dietary group (Table 9).
TABLE 9.
Calculated free nucleotide concentrations and ratios
Values are given as means ± S.E. (n = 6 or 12 as indicated). Cytosolic pH was assumed to be 7.2 and equilibrium constants adjusted for free [Mg2+] (32) calculated from the ratio of [citrate]/[ isocitrate] (30). The ΔG of ATP hydrolysis was calculated as previously described (1;31).
| Starch | Fat | Ketone ester | |
|---|---|---|---|
| Free cytosolic [NAD+]/[NADH] from lactate/pyruvate n = 12 | 328 ± 15 | 292 ± 24 | 381 ± 20a |
| Free mitochondrial [NAD+]/[NADH] from αKG×NH4+/Glut n = 6 | 0.68 ± 0.09 | 0.73 ± 0.06 | 0.88 ± 0.19 |
| Free cytosolic [NADP+]/[NADPH] from αKG×CO2/Isocit n = 6 | 0.031 ± 0.002 | 0.030 ± 0.002 | 0.029 ± 0.003 |
| Free [Mg2+] mm from [Cit]/[Isocit] n = 12 | 1.17 ± 0.23 | 1.22 ± 0.11 | 1.06 ± 0.17 |
| Phosphorylation potential m−1 from KGaPDH+3PGKn = 12 | 18,031 ± 1776 | 20,773 ± 1,325 | 24,170 ± 2,653 |
| ΔGATP hydrolysis kJ/mol from KGaPDH+3PGKn = 12 | −58.6 ± 0.4 | −58.4 ± 0.2 | −59.2 ± 0.2a |
| ΔGATP hydrolysis kJ/mol from KCreatKinasen = 6 | −59.2 ± 0.1 | −59.6 ± 0.2 | −59.2 ± 0.1a |
| Free cytosolic ADP mm from KGaPDH+3PGKn = 12 | 0.054 ± 0.001 | 0.046 ± 0.004 | 0.045 ± 0.006 |
| Free cytosolic [AMP] μm from Kmyokinase n = 12 | 2.13 ± 1.00 | 1.18 ± 0.15 | 1.18 ± 0.18 |
a p < 0.05 between ketone ester and fat, as judged by Mann-Whitney U test.
DISCUSSION
Feeding a ketone ester supplemented diet elevated blood ketone bodies to 3.5 mm (Table 2) while decreasing both blood glucose and insulin to about half the value in rats fed a fat- or starch-supplemented diet. This suggests that ketones increase insulin sensitivity as was observed previously in the perfused heart (1). A previous study has demonstrated increased insulin sensitivity in insulin responsive tissues during ketone infusions in man (35).
The ketone ester-supplemented diet group showed a decrease in food intake (Fig. 2) not resulting from an increase in plasma leptin (Table 2), implying a different mechanism for decreased food intake than that produced by increased leptin. Decreased plasma leptin has been observed previously during short term fasting (36), which also is associated with mild ketosis.
The ketone ester-supplemented diet led to an increase in brain malonyl-CoA compared with the starch-supplemented group (Table 5). In addition, feeding a palm oil-supplemented diet did not increase malonyl-CoA even though the metabolism of fat should increase the availability of acetyl-CoA in the brain. An increase in brain malonyl-CoA would increase the rate of fatty acid synthesis (37), which could satisfactorily explain why feeding small amounts of β-hydroxybutyrate induced myelination and reversed quadriplegia in multiple acyl-CoA dehydrogenase deficiency patients (38).
Malonyl-CoA is also known to be an anorexigenic metabolite and to be associated with decreased food intake (16, 17). Our data suggest that feeding ketone body esters, which decreased food intake for 6 days (Fig. 2), has a longer lasting effect in normal lean animals than the fatty acid synthase inhibitor C75, which decreased food intake for only 1 day (20).
Because the brain has a high demand for a continuous supply of oxygen to preserve the in vivo energetics, methods of tissue inactivation, extraction, and preservation may alter the cytosolic and mitochondrial redox potentials as well as the phosphorylation potential by altering concentrations of oxygen-sensitive metabolites. Any delay in inactivating brain metabolism leads to an increase in the [lactate]/[pyruvate] and the [succinate]/[fumarate] ratio (Table 3) reflecting a reduction in an NAD and co-enzyme Q linked near equilibrium redox couples. Accurate determination of succinate is of importance since succinate increases hypoxia-inducible factor 1 alpha subunit (HIF1-α) secondary to product inhibition of prolyl hydroxylase (EC 1.14.11.2) (39).
The changes in brain glutamate and GABA after feeding a diet supplemented with palm oil (fat diet, Table 1) are in agreement with earlier reports that palmitate can be taken up and metabolized by rat brain (40). Animals fed a fat-supplemented diet, as ketone-fed animals, had decreased brain GABA, but did not have increased uncoupling protein (Fig. 3) nor increased brain malonyl-CoA (Table 5). This difference is likely due to the duel effects of malonyl-CoA as the immediate precursor of fatty acid synthesis (37) and also the ability of malonyl-CoA to inhibit carnitine palmitoyl-CoA transferase (41) decreasing fatty acid transport into mitochondria to undergo β oxidation. The resultant buildup of fatty acid in the cytoplasm would activate the peroxisome proliferator-activated receptor (PPAR) nuclear transcription factors (42, 43).
Feeding a ketone ester diet compared with starch-fed animals significantly decreased both l-glutamate and GABA in the ketone ester and fat-fed rats but brain l-aspartate was not changed by either diet (Table 6). Our data, therefore, do not support the hypothesis that the antiepileptic action of the ketogenic diet results from increases in the inhibitory brain neurotransmitter, GABA (44–46); rather it suggests that a decrease in the stimulatory metabolite l-glutamate as a potential mechanism for the antiepileptic effects of the ketogenic diet.
Labeling studies suggest that there are two distinct l-glutamate pools in brain: a rapidly turning over l-glutamate pool in glial cell comprising about 10% of brain l-glutamate and a larger neuronal pool comprising about 90% of brain l-glutamate (47, 48). It has also been postulated that the large pool of brain l-glutamate could reflect the mitochondrial redox state of free [NAD+]/[NADH] in brain (49, 50). We show (Tables 6 and 7) that measurements of total brain l-glutamate, when combined with total tissue measurements of the reactants of the aspartate aminotransferase reaction (EC 2.6.1.1), Γ, yielded a value of 10–12, very close to the actual equilibrium constant of the aspartate aminotransferase reaction of 6.6. This suggests that measured brain l-glutamate, l-aspartate, α-ketoglutarate, and oxaloacetate are all localized in a neuronal compartment containing that enzyme.
In contrast, glutamine is synthesized from NH4+ and l-glutamate by glutamine synthase (EC 6.3.1.2), which is exclusively localized in the cytoplasm of astrocytes (51). Our measurements of total tissue contents show that the brain reactions involving l-glutamine, glutaminase (EC 3.5.1.2), and glutamine synthase (EC 6.3.1.2), were all 4–5 orders of magnitude from equilibrium (Table 7). This is compatible with the hypothesis that glutamine synthase reaction is simply out of equilibrium or, more likely, that l-glutamine is largely sequestered in the glial space and absent from neuronal space. The inference that glutamine may be low in neuronal space is compatible with the observation that glutaminase, the enzyme converting glutamine to glutamate and NH4+, is of high activity in neurons (52). This inference is further supported by the observation that the glutaminase reaction appears far from equilibrium in brain tissue. The Γ for the brain glutaminase reaction, despite its high activity, is about 6 orders of magnitude short of near-equilibrium based on whole tissue measurements. This difference between the very low Γ for the glutaminase reaction, compared with the expected Keq, is also compatible with the segregation of glutamine within glia and its exclusion from neurons.
The metabolic effects of the chronic elevation of ketone bodies in brain had effects that varied greatly from the acute effects of the metabolism of ketone bodies observed in perfused working heart. In heart, the metabolism of ketones led to the production of excess reducing equivalents, which in turn caused a decrease in the free [NAD+]/[NADH] ratio in both cytosol and mitochondria, an increase in the metabolites of the first third of the TCA cycle, oxidation of the mitochondrial Q couple, and an increase in the ΔG of ATP hydrolysis (1). None of these effects were observed in brains of rats chronically exposed to ketosis. The striking absence of these effects from ketone body metabolism in brain is most likely due to the increased expression of uncoupling proteins 4 and 5 in brain and the absence of an increase in uncoupling proteins in heart. Uncoupling proteins 4 and 5 have been shown to be expressed primarily in brain (53, 54). Furthermore, uncoupling protein 4 has been shown to reduce mitochondrial membrane potential and act as uncoupling protein 2 (55, 56). Classical administration of high concentrations of the uncoupling agent FCCP result in an increase in free mitochondrial [NAD+]/[NADH] ratio, indicating an increase in the oxidation of the mitochondrial NAD couple, and a decrease in the ΔG of ATP hydrolysis. In this study, where uncoupling was less severe than FCCP-induced uncoupling, the change in free mitochondrial [NAD+]/[NADH] did not reach statistical significance and the ΔG of ATP hydrolysis was not significantly decreased. However, the free cytosolic [NAD+]/[NADH], which is linked to the mitochondrial NAD couple, was significantly oxidized, compatible with an increased utilization of NADH in the respiratory chain.
The effects of ketone metabolism in brain differs from the acute effects of ketone metabolism in heart most significantly by the lack of an increase in mitochondrial reducing power and the absence of a physiologically significant increase in the ΔG of ATP hydrolysis. These differences are compatible with the observed increase in UCP 4 and 5, which most likely results from the ability of malonyl-CoA to both increase fatty acid synthesis and decrease the transport of fatty acids into the mitochondria where they can undergo β oxidation.
This work was supported in part by the Defense Advanced Research Projects Administration, United States Dept. of Defense.
- GABA
- γ-aminobutyric acid
- DHAP
- dihydroxyacetone-phosphate
- UCP
- uncoupling protein
- Pi
- inorganic phosphate
- Cit
- citrate
- Isocit
- isocitrate
- GOT
- glutamic oxaloacetic transaminase
- CE-MS
- capillary electrophoresis mass spectral.
REFERENCES
- 1.Sato K., Kashiwaya Y., Keon C. A., Tsuchiya N., King M. T., Radda G. K., Chance B., Clarke K., Veech R. L. (1995) FASEB J. 9, 651–658 [DOI] [PubMed] [Google Scholar]
- 2.Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr. (1967) J. Clin. Invest. 46, 1589–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conklin H. W. (1922) J. Am. Osteopath. Assoc. 26, 11–14 [Google Scholar]
- 4.Freeman J. M., Kossoff E. H., Hartman A. L. (2007) Pediatrics 119, 535–543 [DOI] [PubMed] [Google Scholar]
- 5.Kashiwaya Y., Takeshima T., Mori N., Nakashima K., Clarke K., Veech R. L. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5440–5444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veech R. L., Chance B., Kashiwaya Y., Lardy H. A., Cahill G. F., Jr. (2001) IUBMB. Life 51, 241–247 [DOI] [PubMed] [Google Scholar]
- 7.Veech R. L. (2004) Prostaglandins Leukot. Essent. Fatty Acids 70, 309–319 [DOI] [PubMed] [Google Scholar]
- 8.Veech R. L., Harris R. L., Veloso D., Veech E. H. (1973) J. Neurochem. 20, 183–188 [DOI] [PubMed] [Google Scholar]
- 9.Lust W. D., Passonneau J. V., Veech R. L. (1973) Science 181, 280–282 [DOI] [PubMed] [Google Scholar]
- 10.Johnstone A. M., Horgan G. W., Murison S. D., Bremner D. M., Lobley G. E. (2008) Am. J Clin. Nutr. 87, 44–55 [DOI] [PubMed] [Google Scholar]
- 11.Davis R. J., Brand M. D., Martin B. R. (1981) Biochem. J. 196, 133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arase K., Fisler J. S., Shargill N. S., York D. A., Bray G. A. (1988) Am. J. Physiol. 255, R974–R981 [DOI] [PubMed] [Google Scholar]
- 13.Carpenter R. G., Grossman S. P. (1983) Physiol Behav. 30, 57–63 [DOI] [PubMed] [Google Scholar]
- 14.Rossi R., Dörig S., Del Prete E., Scharrer E. (2000) J. Vet. Med. A Physiol Pathol. Clin. Med. 47, 9–16 [DOI] [PubMed] [Google Scholar]
- 15.Langhans W., Wiesenreiter F., Scharrer E. (1983) Physiol. Behav. 31, 483–486 [DOI] [PubMed] [Google Scholar]
- 16.Hu Z., Cha S. H., Chohnan S., Lane M. D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12624–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Z., Dai Y., Prentki M., Chohnan S., Lane M. D. (2005) J. Biol. Chem. 280, 39681–39683 [DOI] [PubMed] [Google Scholar]
- 18.Wolfgang M. J., Lane M. D. (2006) J. Biol. Chem. 281, 37265–37269 [DOI] [PubMed] [Google Scholar]
- 19.Wolfgang M. J., Kurama T., Dai Y., Suwa A., Asaumi M., Matsumoto S., Cha S. H., Shimokawa T., Lane M. D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7282–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar M. V., Shimokawa T., Nagy T. R., Lane M. D. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1921–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiterovich P. O., Jr., Vining E. P., Pyzik P., Skolasky R., Jr., Freeman J. M. (2003) JAMA 290, 912–920 [DOI] [PubMed] [Google Scholar]
- 22.Bisschop P. H., de, Metz J., Ackermans M. T., Endert E., Pijl H., Kuipers F., Meijer A. J., Sauerwein H. P., Romijn J. A. (2001) Am. J. Clin. Nutr. 73, 554–559 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki J., Shen W. J., Nelson B. D., Selwood S. P., Murphy G. M., Jr., Kanehara H., Takahashi S., Oida K., Miyamori I., Kraemer F. B. (2002) Am. J. Physiol. Endocrinol. Metab. 283, E94–E102 [DOI] [PubMed] [Google Scholar]
- 24.Veech R. L., Mehlman M. A. (1972) in Energy Metabolism and the Regulation of Metabolic Processes in Mitochondria (Mehlman M. A., Hanson R. W. eds), pp. 171–183, Academic Press, New York [Google Scholar]
- 25.Reeves P. G., Nielsen F. H., Fahey G. C., Jr. (1993) J. Nutr. 123, 1939–1951 [DOI] [PubMed] [Google Scholar]
- 26.Passonneau J. V., Lowry O. H. (1993) Enzymatic Analysis: A Practical Guide, 2nd Ed., Humana Press, Totowa, NJ [Google Scholar]
- 27.Kashiwaya Y., Sato K., Tsuchiya N., Thomas S., Fell D. A., Veech R. L., Passonneau J. V. (1994) J. Biol. Chem. 269, 25502–25514 [PubMed] [Google Scholar]
- 28.Pawlosky R. J., Kashiwaya Y., Srivastava S., King M. T., Crutchfield C., Volkow N., Kunos G., Li T. K., Veech R. L. (2010) Alcohol Clin. Exp. Res. 34, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soga T., Ueno Y., Naraoka H., Ohashi Y., Tomita M., Nishioka T. (2002) Anal. Chem 74, 2233–2239 [DOI] [PubMed] [Google Scholar]
- 30.Veloso D., Guynn R. W., Oskarsson M., Veech R. L. (1973) J. Biol. Chem. 248, 4811–4819 [PubMed] [Google Scholar]
- 31.Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. (1979) J. Biol. Chem. 254, 6538–6547 [PubMed] [Google Scholar]
- 32.Veech R. L., Gates D. N., Crutchfield C. W., Gitomer W. L., Kashiwaya Y., King M. T., Wondergem R. (1994) Alcohol. Clin. Exp. Res. 18, 1040–1056 [DOI] [PubMed] [Google Scholar]
- 33.Krebs H. A. (1953) Biochem. J. 54, 82–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs H. A., Veech R. L. (1969) in The Energy Level and Metabolic Control in Mitochondria (Papa S., Tager J. M., Quagliariello E., Slater E. C. eds), pp. 329–382, Bari, Adriatica Editrice [Google Scholar]
- 35.Amiel S. A., Archibald H. R., Chusney G., Williams A. J., Gale E. A. (1991) Clin. Sci. 81, 189–194 [DOI] [PubMed] [Google Scholar]
- 36.Chan J. L., Heist K., DePaoli A. M., Veldhuis J. D., Mantzoros C. S. (2003) J. Clin. Invest. 111, 1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guynn R. W., Veloso D., Veech R. L. (1972) J. Biol. Chem. 247, 7325–7331 [PubMed] [Google Scholar]
- 38.Van Hove J. L., Grünewald S., Jaeken J., Demaerel P., Declercq P. E., Bourdoux P., Niezen-Koning K., Deanfeld J. E., Leonard J. V. (2003) Lancet 361, 1433–1435 [DOI] [PubMed] [Google Scholar]
- 39.Puchowicz M. A., Zechel J. L., Valerio J., Emancipator D. S., Xu K., Pundik S., LaManna J. C., Lust W. D. (2008) J. Cereb. Blood Flow Metab. 28, 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith Q. R., Nagura H. (2001) J. Mol. Neurosci. 16, 167–172 [DOI] [PubMed] [Google Scholar]
- 41.McGarry J. D., Leatherman G. F., Foster D. W. (1978) J. Biol. Chem. 253, 4128–4136 [PubMed] [Google Scholar]
- 42.Cullingford T. E., Eagles D. A., Sato H. (2002) Epilepsy Res. 49, 99–107 [DOI] [PubMed] [Google Scholar]
- 43.Woods J. W., Tanen M., Figueroa D. J., Biswas C., Zycband E., Moller D. E., Austin C. P., Berger J. P. (2003) Brain Res. 975, 10–21 [DOI] [PubMed] [Google Scholar]
- 44.Erecińska M., Nelson D., Daikhin Y., Yudkoff M. (1996) J. Neurochem. 67, 2325–2334 [DOI] [PubMed] [Google Scholar]
- 45.Yudkoff M., Daikhin Y., Nissim I., Grunstein R. (1997) J. Neurochem. 69, 682–692 [DOI] [PubMed] [Google Scholar]
- 46.Yudkoff M., Daikhin V., Melo T. M., Nissim I., Sonnewald U., Nissim I. (2007) Annu. Rev. Nutr. 27, 413–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berl S., Clarke D. D. (1984) in Glutamine Metabolism in Mammalian Tissues (Haussinger D., Sies H. eds) Springer-Verlag, New York [Google Scholar]
- 48.Cruz F., Cerdán S. (1999) NMR Biomed. 12, 451–462 [DOI] [PubMed] [Google Scholar]
- 49.Hawkins R. A., Miller A. L., Nielsen R. C., Veech R. L. (1973) Biochem. J. 134, 1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller A. L., Hawkins R. A., Veech R. L. (1973) J. Neurochem. 20, 1393–1400 [DOI] [PubMed] [Google Scholar]
- 51.Norenberg M. D., Martinez-Hernandez A. (1979) Brain Res. 161, 303–310 [DOI] [PubMed] [Google Scholar]
- 52.Hogstad S., Svenneby G., Torgner I. A., Kvamme E., Hertz L., Schousboe A. (1988) Neurochem. Res. 13, 383–388 [DOI] [PubMed] [Google Scholar]
- 53.Alán L., Smolková K., Kronusová E., Santorová J., Jezek P. (2009) J. Bioenerg. Biomembr. 41, 71–78 [DOI] [PubMed] [Google Scholar]
- 54.Smorodchenko A., Rupprecht A., Sarilova I., Ninnemann O., Bräuer A. U., Franke K., Schumacher S., Techritz S., Nitsch R., Schuelke M., Pohl E. E. (2009) Biochim. Biophys. Acta 1788, 2309–2319 [DOI] [PubMed] [Google Scholar]
- 55.Ivanova M. V., Hoang T., McSorley F. R., Krnac G., Smith M. D., Jelokhani-Niaraki M. (2010) Biochemistry 49, 512–521 [DOI] [PubMed] [Google Scholar]
- 56.Mao W., Yu X. X., Zhong A., Li W., Brush J., Sherwood S. W., Adams S. H., Pan G. (1999) FEBS Lett. 443, 326–330 [DOI] [PubMed] [Google Scholar]
- 57.Benzinger T., Kitzinger C., Hems R., Burton K. (1959) Biochem. J. 71, 400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koppelman R., Mamdeles S., Hanke M. E. (1958) J. Biol. Chem. 230, 73–80 [PubMed] [Google Scholar]