Abstract
Abnormalities in very low density lipoprotein (VLDL) assembly and secretion impact intrahepatic lipid homeostasis, plasma lipoprotein profile, and energy metabolism of distal peripheral tissues. We have evaluated the role of the transcriptional coactivator, the peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), in VLDL assembly and secretion. PGC-1α overexpression in HepG2 cells led to diminished rates of triglyceride (TG) synthesis but strongly stimulated VLDL-TG secretion, markedly increasing the efficiency of secretion of newly synthesized TG. PGC-1α overexpression increased the rate of secretion of apoB100 and promoted secretion of larger, less dense VLDL particles. PGC-1α overexpression in intact mouse liver also stimulated rates of VLDL TG secretion and attenuated hepatic TG accumulation resulting from high fat diet feeding. To determine the molecular mechanisms mediating the effect of PGC-1α on VLDL assembly, we evaluated the expression of several candidate mediators known to be involved in VLDL assembly or hepatic lipid homeostasis. Cell death-inducing DFFA-like effector B (CideB) expression was greatly induced by PGC-1α, and siRNA against CideB reversed the effects of PGC-1α on the secretion of TG and VLDL-sized particles by HepG2 cells, indicating that CideB is a critical mediator of stimulatory effects of PGC-1α on VLDL secretion. Collectively, these data suggest that PGC-1α plays an important role in partitioning cytoplasmic TG toward the VLDL secretory compartments and promoting VLDL secretion via transcriptional induction of CideB.
Keywords: Lipid Droplet, Lipid Transport, Lipoprotein Metabolism, Liver Metabolism, Transcription Coactivators, PGC1, VLDL Assembly, apoB Secretion, CideB, Fatty Liver
Introduction
The liver exports triglycerides (TG)2 to extrahepatic tissues through the secretion of very low density lipoproteins (VLDL). Each VLDL particle contains one apolipoprotein B (apoB) molecule, a surface lipid interface composed of phospholipids and free cholesterol, and a hydrophobic core of TG and cholesterol esters (1). Defects in VLDL secretion cause nonalcoholic fatty liver disease in humans (2–4) and mice (5–7). Conversely, overproduction of VLDL is frequently a co-morbidity of obesity, peripheral insulin resistance, and type 2 diabetes mellitus and is a key mechanism leading to the development of dyslipidemia in metabolic syndrome (8–10). The mechanisms governing VLDL assembly and secretion have yet to be fully understood, and new factors playing important roles in these processes continue to emerge.
The biosynthesis of VLDL is generally believed to be a two-step process (11, 12). The initial step involves the translocation of the nascent apoB polypeptide across the endoplasmic reticulum (ER) membranes and the cotranslational lipidation of apoB by the microsomal triglyceride transfer protein (MTP). This first step results in the formation of primordial pre-VLDL particles in the high density lipoprotein (HDL)-density range. The second step involves the bulk addition of triglycerides to the VLDL precursors in the smooth ER (13, 14) and to a lesser extent in the Golgi apparatus (15, 16), through the fusion of pre-VLDL particles with the luminal lipid droplets in these secretory compartments. The formation of mature VLDL particles highly depends on the availability of TG in the secretory compartments, especially the smooth ER (11, 12, 14, 17). Fatty acids used for the biosynthesis of VLDL-TG are mainly derived from TG stored in the cytoplasmic lipid droplets of hepatocytes (18–20). However, increased cytosolic TG accumulation alone does not necessarily result in increased VLDL secretion. For example, whereas liver-specific overexpression of the key triglyceride synthetic enzymes Dgat1 and Dgat2 robustly stimulated hepatic TG synthesis and caused TG accumulation in mouse liver, this was not sufficient to affect rates of hepatic VLDL secretion (21, 22). Similarly, hepatic VLDL secretion was either unchanged (23) or even decreased (24) in leptin-deficient ob/ob mice despite marked hepatic TG accumulation. Identifying factors that control the partitioning of cellular TG between the cytosolic storage and VLDL assembly compartments is critical to our full understanding of regulation of VLDL secretion.
The peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is a transcriptional coactivator that has emerged as a master regulator of hepatic energy metabolism (25, 26). Through its interactions with LXRα, FXRα, PPARα, and HNF4α, PGC-1α plays important roles in modulating cholesterol catabolism, fatty acid oxidation, ketogenesis, and gluconeogenesis in liver (26–32). Hepatic PGC-1α expression is relatively low under most conditions but is highly inducible in response to fasting and in most diabetic rodent models, including streptozotocin-induced insulin deficiency and ob/ob mice (30, 31). Although PGC-1β, the other PGC-1 protein in liver, has been shown to stimulate hepatic VLDL secretion in mice (33–35), the role of PGC-1α in the regulation of VLDL assembly and secretion is less well understood.
We have determined the effects of PGC-1α on TG and VLDL secretion in vitro by using human hepatoma HepG2 cells, primary mouse hepatocytes, and in vivo in intact mice. We demonstrate that PGC-1α overexpression suppresses TG synthesis but stimulates VLDL secretion and corrects the defective VLDL secretion in HepG2 cells. We further demonstrate that the stimulatory effect of PGC-1α on VLDL secretion is mediated, at least in part, by inducing the expression of the cell death-inducing DFFA-like effector B (CideB), a lipid droplet-associated protein recently identified in VLDL maturation in the ER (36). Together, these findings establish an important role for PGC-1α in regulation of VLDL secretion.
EXPERIMENTAL PROCEDURES
Materials
35S-Promix (530 MBq/ml), an l-[35S]Met and l-[35S]Cys metabolic labeling solution, was from Amersham Biosciences. 2-[3H]Glycerol (20 mCi/mmol) was from American Radiolabeled Chemicals, Inc. (St. Louis, MO). Triton WR-1339 (Tyloxapol) and rabbit anti-human albumin were from Sigma. Mouse PGC-1α monoclonal antibody was from Calbiochem. Rabbit anti-human apoB antibodies have been described (37).
Mammalian Cell Culture
HepG2 cells obtained from the American Type Culture Collection were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and 1× antibiotic/antimycotic at 37 °C in 5% CO2. The cells were passaged every 3 to 4 days. Primary cultures of mouse hepatocytes were prepared from C57BL/6 mice and cultured as described previously (5).
Adenovirus Constructs
The recombinant adenoviral constructs driving expression of mouse PGC-1α and GFP (Ad-PGC-1α) or GFP alone (Ad-GFP) have been described (38). Adenoviral transductions were carried out by incubating HepG2 cells and hepatocytes with adenoviruses in 5% FBS/DMEM overnight at a multiplicity of infection of 12 and 8, respectively. Experiments were carried out 48–60 h after adenoviral transduction.
Mouse Studies
All animal experiments were approved by the Animal Studies Committee of Washington University School of Medicine. C57BL/6 mice were generated from breeding pairs established at the Washington University mouse facility. Mice used for in vivo studies were 10–12 weeks of age. Male and female mice were used, with no difference noted between the sexes. The mice were maintained in a regular mouse chow diet. A high fat diet (HFD) containing 42% of calories as fat (39, 40) was also used to treat a group of mice. The HFD-treated mice were fed with HFD for 10 days prior to adenoviral infusion as described below and then maintained on this diet throughout the experiment.
To produce hepatic PGC-1α overexpression, mice were intravenously infused with adenoviruses Ad-PGC-1α or Ad-GFP at doses of 2 × 1011 viral particles (∼0.5 × 1010 pfu) for each mouse. Five days after adenoviral transduction, mice were either sacrificed to collect liver tissues and plasma samples for lipid analysis or used for determination of in vivo rates of VLDL-TG secretion by using Triton WR-1339 to inhibit lipoprotein lipolysis, as described previously (5, 23). Plasma TG concentrations were determined at 0, 60, and 120 min after injection of Triton WR-1339, and rates of TG secretion were expressed as milligrams/kg body weight per h.
Metabolic Studies in HepG2 and Mouse Hepatocytes
Rates of fatty acid oxidation by HepG2 cells were measured using [3H]palmitate as described previously (37, 38). To measure rates of synthesis and secretion of TG, HepG2 cells or hepatocytes were incubated with a 2-[3H]glycerol-containing DMEM (5 μCi/ml) for the specified times with or without 0.4 mm oleic acid (OA) complexed to bovine serum albumin (BSA) (6, 37). [3H]TG was isolated from cells and media, and 3H radioactivity was determined as described previously (5). In specified experiments, [14C]oleate (2 μCi/ml) was also used as a labeled substrate to measure TG synthesis and secretion by HepG2 cells. Data for all radiolabeled TG were expressed as dpm/μg of protein/h.
To determine apoB synthesis and secretion, HepG2 cells were pulsed with 35S-Promix for 20 min and chased for the specified times with media containing 100× excess amounts of unlabeled methionine and cysteine as described previously (37, 41) with or without 0.4 mm OA. The 35S-labeled apoB and albumin in cells and media were immunoprecipitated and separated on a gradient SDS gel (3–15%) and quantified as described previously (23, 37, 41).
To determine the density distribution of the secreted apoB-containing lipoproteins, HepG2 cells were continuously labeled with 35S-Promix for 3.5 h in 60-mm dishes in the presence or absence of 0.4 mm OA as described above. The media were then subjected to density gradient ultracentrifugation in a d = 1.006–1.25 g/ml KBr density gradient as described previously (6, 37). Twelve fractions were aspirated and dialyzed; thereafter, they were subjected to immunoprecipitation and 35S-labeled apoBs in each fraction were separated by SDS-PAGE and quantified as described previously (23, 37).
siRNA Oligonucleotides and Transfection
A Silencer siRNA construction kit (Ambion, Austin, TX) was used to synthesize three siRNAs for human apoCIII or CideB and a scrambled, nonspecific sequence siRNA (control). The corresponding targeted nucleotide sequences for human apoCIII are as follows: 5′-AAGGATGCACTGAGCAGCGTG-3′ (hApoCIII-siRNA176); 5′-AAAGACTACTGGAGCACCGTT-3′ (hApoCIII-siRNA257); and 5′-AAGTCCACCTGCCTATCCATC-3′ (hApoCIII-siRNA361). The targeted human CideB nucleotide sequences are as follows: 5′-AACCCCAGTGACTTACTCAGG-3′ (hCideB-siRNA22), 5′-AAAACCCTCGAGACCTCTTTG-3′ (hCideB-siRNA422), and 5′-AAAGTACTCAGGGAGCTCCTT-3′ (hCideB-siRNA517). The siRNAs were transfected onto HepG2 using Lipofectamine 2000 (Invitrogen), and experiments were carried out 48–60 h post-siRNA transfection. Our pilot experiments demonstrated that hApoCIII-siRNA176 and hCideB-siRNA517 produced the highest efficiency of knockdown (data not shown) of apoCIII and CideB, respectively, and therefore, they were chosen for subsequent experiments.
Quantitative Real Time Reverse Transcription (RT)-PCR
Real time RT-PCR was performed using the ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA) and the SYBR Green kit. Arbitrary units of target mRNA were corrected by measuring the levels of 36B4 RNA. The sequence of the oligonucleotides used in quantitative RT-PCR analyses can be found in supplemental Table 1.
Miscellaneous Assays
MTP activity in the adenovirus-infected HepG2 cells was determined using a fluorescent assay kit as described previously (23). Plasma and tissue TG contents were assayed using a commercial enzymatic assay kit (Fisher) as described previously (5, 37). Protein contents of cell or tissue samples were determined using a BCA protein assay kit.
Statistical Analyses
Statistical comparisons were made using analysis of variance or Student's t tests. All data are presented as the means ± S.E., with a statistically significant difference defined as a p value <0.05.
RESULTS
PGC-1α Overexpression Decreases TG Synthesis but Robustly Stimulates TG Secretion in HepG2
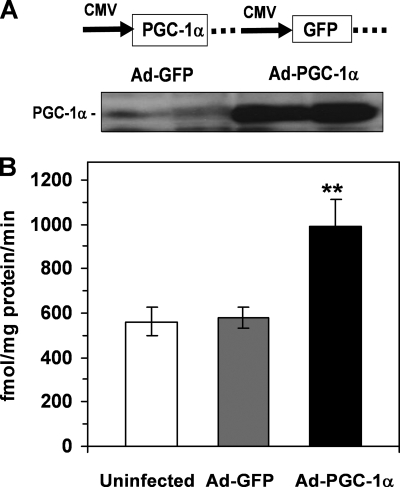
We chose HepG2 cells to evaluate the gain-of-function effect of PGC-1α on VLDL assembly and secretion because previous studies have suggested that this cell line is deficient in PGC-1α activity (42, 43). The effects of a recombinant adenovirus that overexpressed mouse PGC-1α and GFP were compared with an adenovirus expressing GFP alone (Fig. 1A). Western blot analysis showed that infection of HepG2 cells with Ad-PGC-1α led to an ∼10-fold increase in cellular content of PGC-1α (Fig. 1A). As expected, PGC-1α overexpression significantly induced fatty acid oxidation rates (Fig. 1B), demonstrating the functional activity of the overexpressed PGC-1α protein.
FIGURE 1.
Adenovirus-mediated overexpression of a mouse PGC-1α cDNA in HepG2 cells. A, increased cellular PGC-1α mass following Ad-PGC-1α infection demonstrated with Western blotting. B, increased palmitic acid oxidation in response to PGC-1α overexpression in HepG2 cells. Rates of palmitic acid oxidation were measured as described under “Experimental Procedures.” Each data point represents mean ± S.E. of five incubations. **, p < 0.01 versus Ad-GFP control.
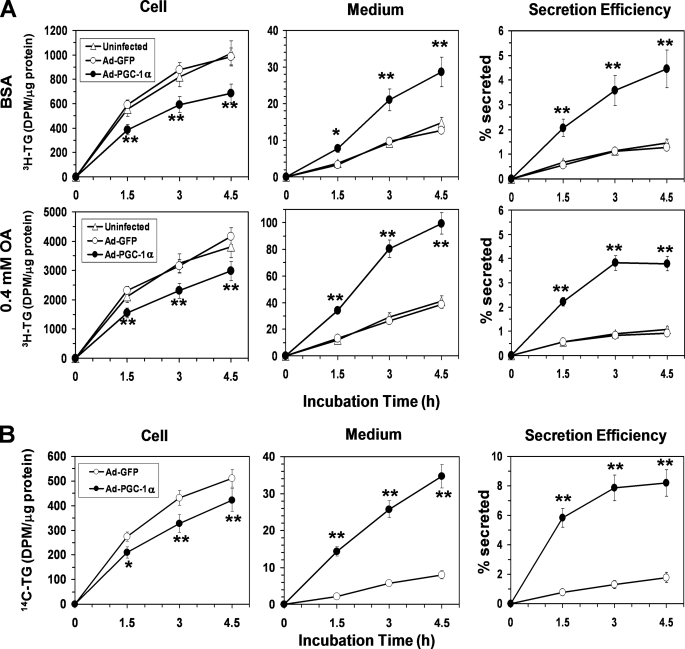
We next examined the effects of PGC-1α overexpression on TG synthesis and secretion using 2-[3H]glycerol as a labeled substrate, in the presence or absence of 0.4 mm exogenous OA. Under both conditions, PGC-1α overexpression caused a significant (25–30%) decrease in [3H]TG synthesis at each time point, as compared with respective Ad-GFP-infected HepG2 controls (Fig. 2A). Cellular TG content was decreased by ∼20% in response to PGC-1α overexpression (data not shown). On the other hand, despite its inhibitory effect on the TG synthesis, PGC-1α increased accumulation of [3H]TG in the medium, with rates of [3H]TG secretion by the PGC-1α overexpressing HepG2 cells increasing ∼3–4-fold over Ad-GFP controls (Fig. 2A). The efficiency of secretion of the [3H]TG (i.e. the percent of total [3H]TG that was secreted into the medium) was increased by ∼4–5-fold in Ad-PGC-1α-infected HepG2 cells, as compared with HepG2 cells infected with Ad-GFP (Fig. 2A). No difference in TG synthesis and secretion was observed between the Ad-GFP-infected and uninfected HepG2 cells (Fig. 2A), indicating TG metabolism was not affected by adenoviral infection.
FIGURE 2.
Overexpression of PGC-1α in HepG2 cells suppresses TG synthesis but strongly stimulates TG secretion. A, changes in [3H]glycerol-labeled TG in the cell and media in response to PGC-1α overexpression under basal (BSA) or OA-supplemented (0.4 mm OA) conditions. B, changes in [14C]oleate-labeled TG in the cell and media in response to PGC-α overexpression in the presence of 0.3 mm OA. A and B, the secretion efficiency of the radiolabeled TG (i.e. the percentage of total labeled TG that was secreted) was also shown. Each data point represents the mean ± S.E. of three independent experiments done in triplicate. *, p < 0.05; **, p < 0.01 versus Ad-GFP controls.
The above data suggest that PGC-1α specifically stimulated the secretion but not the synthesis of [3H]glycerol-labeled TG by HepG2 cells. We further used [14C]OA as a tracer to confirm these observations. In the presence of 0.3 mm OA, PGC-1α overexpression increased secretion of [14C]TG by about ∼4-fold while decreasing TG synthesis by 15–20%, again leading to an ∼5-fold increase in secretion efficiency of [14C]TG (Fig. 2B). Taken together, these results demonstrate that PGC-1α activation in HepG2 cells stimulates TG secretion despite suppressing TG synthesis.
PGC-1α Overexpression Promotes VLDL Production in HepG2 Cells
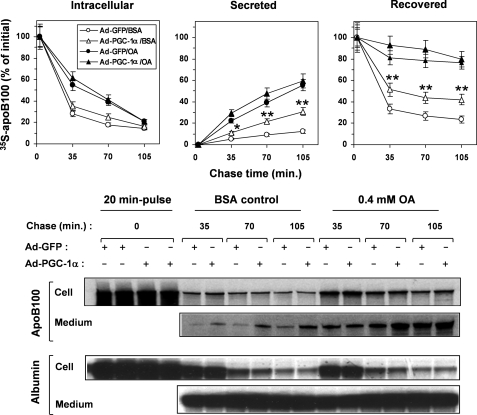
We then sought to determine whether the increased rates of TG secretion by PGC-1α overexpression was achieved by increasing apoB100 secretion, altering the size of the apoB100-containing particles or both mechanisms. We first conducted pulse-chase experiments to determine the effect of PGC-1α on the rates of synthesis, secretion, and presecretory degradation of apoB100 by HepG2 cells. At the end of the initial pulse time point (20 min), similar amounts of intracellular 35S-labeled apoB100 were seen in Ad-PGC-1α- and Ad-GFP-infected HepG2 cells, indicating that PGC-1α did not affect apoB100 synthesis (Fig. 3). After each chase period, when no OA was provided to the cells, most of the 35S-labeled apoB100 was degraded, and little remained inside the cell or was secreted into the medium in the control Ad-GFP-infected HepG2 cells (Fig. 3). PGC-1α overexpression moderately increased the 35S-labeled apoB100 remaining in the cell but induced an ∼2.5-fold increase in the amounts of secreted 35S-labeled apoB100 in the medium compared with the Ad-GFP-infected control (Fig. 3). The recovery of the 35S-labeled apoB100 was increased by 2-fold at the end of each chase period in response to PGC-1α overexpression, indicating an inhibitory effect of PGC-1α on presecretory degradation of the newly synthesized apoB100. When the HepG2 cells were provided with 0.4 mm OA during the chase period, presecretory degradation of apoB100 was greatly reduced, and PGC-1α had little extra effect on apoB100 secretion (Fig. 3). These results also show that under OA-supplemented conditions, the changes in the secretion of apoB100 cannot account for the changes in the TG secretion, indicating that a change in the particle size of the apoB100 lipoproteins might be responsible for the increased TG secretion in response to PGC-1α overexpression.
FIGURE 3.
Pulse-chase analysis of apoB100 in Ad-GFP- or Ad-PGC-1α-infected HepG2 cells. The Ad-GFP- or Ad-PGC-1α-infected HepG2 cells were pulsed with a 35S-Promix for 20 min and chased for the specified times in the presence (OA) or absence (BSA) of 0.4 mm OA. Immunoprecipitation of apoB100 and albumin was carried out, and 35S radioactivity in apoB100 and albumin was quantified as described under “Experimental Procedures.” Each data point represents the mean ±S.E. of three experiments. *, p < 0.05; **, p < 0.001 versus Ad-GFP-infected HepG2/BSA. Representative gel images of immunoprecipitated apoB100 and albumin are shown (lower panels).
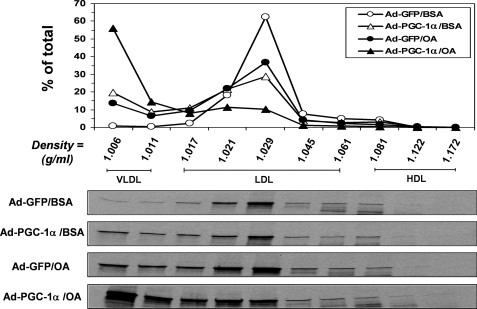
Density gradient ultracentrifugation analyses were carried out to determine the density distributions of apoB100 lipoproteins secreted by the Ad-GFP- or Ad-PGC-1α-infected HepG2 cells under basal and OA-supplemented conditions. Consistent with the well documented defect of VLDL assembly in HepG2 cells (44, 45), the Ad-GFP-infected HepG2 cells secreted very few VLDL-sized particles (Fig. 4), even with OA supplementation (Fig. 4). Importantly, overexpression of PGC-1α rendered HepG2 cells capable of secreting the majority of apoB lipoproteins with a VLDL density range (Fig. 4). Even under basal conditions, nearly 30% of the apoB100 lipoproteins secreted by the Ad-PGC-1α-infected HepG2 cells floated at the VLDL densities (Fig. 4). Thus, the defective VLDL section by HepG2 cells was effectively corrected with PGC-1α overexpression.
FIGURE 4.
Effects of PGC-1α overexpression on density distribution of the apoB100-containing lipoproteins secreted by HepG2 cells. The Ad-GFP- or Ad-PGC-1α-infected HepG2 cells were continuously labeled with a 35S-Promix for 3.5 h under basal (BSA) or OA-supplemented (OA) conditions, and the 35S-labeled apoB100-containing lipoproteins in the media were fractionated and quantified by density gradient ultracentrifugation analysis and immunoprecipitated as described under “Experimental Procedures.” Each data point represents the means of two independent experiments. Representative gel images are shown (lower panels).
PGC-1α Overexpression Also Stimulates VLDL-TG Secretion in Mouse and Attenuates Development of High Fat-induced Hepatic Steatosis
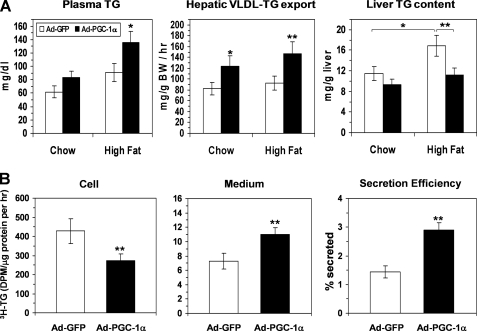
To determine the potential stimulatory effect of PGC-1α on hepatic VLDL secretion in vivo, we intravenously infused Ad-PGC-1α or Ad-GFP into chow-fed mice and short term (10 days) HFD-fed mice. Adenovirus administration via this route leads to PGC-1α overexpression in a liver-restricted manner. Five days after adenoviral infusion, PGC-1α overexpression caused a significant increase in plasma TG in the HFD mouse group but not in the chow-fed group (Fig. 5A). Rates of hepatic VLDL-TG secretion were significantly increased in both Ad-PGC-1α groups as compared with the respective Ad-GFP-infused mice (Fig. 5A). A brief period of high fat diet feeding significantly increased hepatic TG content as compared with chow-fed mice (Fig. 5A), and this increase was reversed in PGC-1α-overexpressing mice (Fig. 5A, right panel).
FIGURE 5.
Effects of PGC-1α overexpression on hepatic TG metabolism in mouse livers. A, changes in plasma TG, hepatic TG secretion, and liver TG content in chow-fed and HDF-treated mice in response to PGC-1α overexpression in the liver. All measurements were carried out on day 5 after adenoviral infusion, and the mice in the HFD-treated group were fed a high fat diet for 10 days prior to adenoviral infection and maintained on this diet throughout the experiment. Each data point represents the mean ± S.E. of five mice. *, p < 0.05; **, p < 0.01 versus the Ad-GFP-infected mice. B, synthesis and secretion of [3H]glycerol-labeled TG by the Ad-GFP or Ad-PGC-1α-infected mouse hepatocytes. The hepatocytes were continuously labeled with [3H]glycerol in the presence of 0.4 mm OA for 3 h, and the [3H]TG in cells and media was determined as described under “Experimental Procedures.” **, p < 0.01 (n = 3).
To further characterize the effects of PGC-1α overexpression on hepatic TG metabolism, rates of TG synthesis and secretion were determined in Ad-PGC-1α- or Ad-GFP-infected primary mouse hepatocytes. Similar to the findings from HepG2 above, PGC-1α overexpression suppressed TG synthesis, but significantly stimulated TG secretion (Fig. 5B). There was no change in the synthesis and secretion of apoB (data not shown), indicating the increased TG secretion was mainly achieved by increasing the particle size of the apoB lipoproteins secreted by the mouse hepatocytes. Together with findings from the in vivo experiments above, these results strongly suggest a potential role of PGC-1α as a modulator of hepatic VLDL assembly and secretion in vivo.
Effects of PGC-1a on Expression of Selected Genes Involved in Lipogenesis and VLDL Assembly
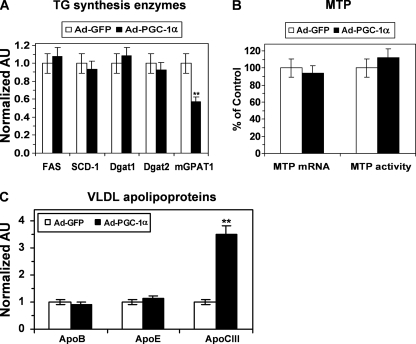
To understand the mechanism responsible for altered TG synthesis and secretion in response to PGC-1α overexpression, we examined the effect of PGC-1α on mRNA levels of the key lipogenic enzyme genes in HepG2 cells. PGC-1α did not alter the mRNA levels for FAS, SCD1, DGAT1, and DGAT2 but significantly reduced GPAM mRNA expression (Fig. 6A). The decrease in mGPAM1 expression is consistent with the reduced TG synthesis rate in PGC-1α-overexpressing cells.
FIGURE 6.
Effects of PGC-1α overexpression on mRNA levels of the key TG lipogenic enzymes, MTP, and VLDL apolipoproteins. A, changes in key lipogenic genes in response to PGC-1α overexpression. B, unaltered MTP mRNA levels and MTP activity in the PGC-1α overexpressed HepG2 cells. C, PGC-1α overexpression did not affect apoB and apoE mRNA levels but induced apoCIII expression. Total RNAs were isolated from HepG2 50–60 h after adenoviral transduction, and the steady-state mRNA level of specified genes was determined by real time PCR as described under “Experimental Procedures.” Each data point represents the mean ± S.E. of three experiments. **, p < 0.001 versus Ad-GFP-infected HepG2. AU, arbitrary units.
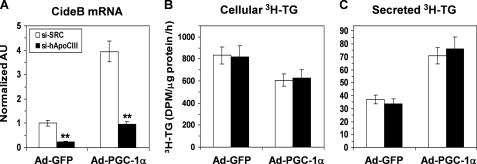
We next examined the effect of PGC-1α on the expression of various genes involved in VLDL particle formation and secretion. Neither the expression nor the activity of MTP was affected by PGC-1α (Fig. 6B). Similarly, PGC-1α did not affect expression of the key VLDL apoproteins apoB and apoE in HepG2 cells (Fig. 6C). In agreement with the findings reported in previous studies (42, 46), PGC-1α induced expression of apoC-III (Fig. 6C). To determine whether this increase in apoCIII expression has a role in mediating the effects of PGC-1α on VLDL secretion, we used siRNA to knock down apoCIII in HepG2 cells. Although our apoC-III siRNA reduced apoCIII mRNA by ∼80% (Fig. 7A), it exerted no effect on TG synthesis and secretion by the Ad-GFP- or Ad-PGC-1α-infected HepG2 cells, suggesting that activation of the apoC-III gene is not required for the induction of VLDL secretion by PGC-1α.
FIGURE 7.
Neutralization of PGC-1α-induced apoCIII expression with an apoCIII siRNA does not affect the stimulatory effect of PGC-1α on TG secretion by HepG2 cells. A, apoCIII mRNA levels in Ad-GFP- or Ad-PGC-1α-infected HepG2 cells transfected with an apoCIII siRNA (si-hApoC3) or a nonspecific scrambled siRNA control (si-SCR). **, p < 0.001 versus si-SCR. AU, arbitrary units. B and C depict the rates of TG synthesis (B) and secretion (C) in the Ad-GFP or Ad-PGC-1a-infected HepG2 cells transfected with si-hApoC3 or si-SCR. The cells were continuously labeled with [3H]glycerol for 3 h in the presence of 0.4 mm OA, and the [3H]TG was isolated and quantified. Each data point represents the mean ± S.E. of three independent experiments done in triplicate.
Identification of CideB as a Potential PGC-1α Target Gene That Mediates the Effects of PGC-1α on VLDL Secretion
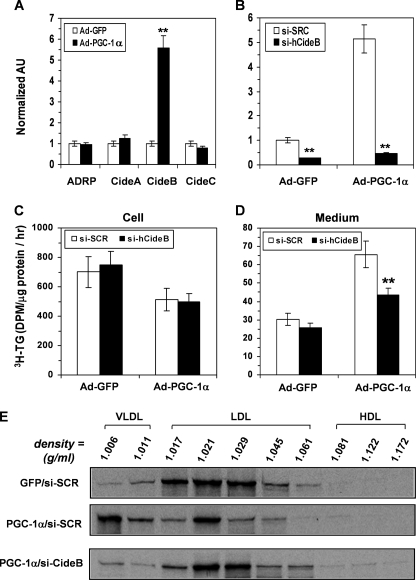
Several lipid droplet-associated proteins have recently been shown to modulate hepatic TG storage or VLDL secretion, including adipose differentiation-related protein (47), CideA (48), CideB (36, 49), and CideC (50). We found that CideB expression was induced by more than 5-fold in response to PGC-1α overexpression (Fig. 8A), with no changes in other lipid-droplet protein mRNAs.
FIGURE 8.
PGC-1α overexpression induces CideB expression, and siRNA-mediated knockdown of CideB blunts the stimulatory effect of PGC-1α on VLDL secretion by HepG2 cells. A, effects of PGC-1α overexpression on adipose differentiation-related protein (ADRP), CideA, CideB, and CideC mRNA levels in HepG2. **, p < 0.001 versus Ad-GFP. AU, arbitrary units. B, CideB mRNA levels in Ad-GFP- or Ad-PGC-1α-infected HepG2 cells transfected with an CideB siRNA (si-hCideB) or si-SCR control. **, p < 0.001 versus si-SCR. C and D, effects of si-CideB on rates of TG synthesis (C) and secretion (D) in the Ad-GFP- or Ad-PGC-1α-infected HepG2 cells. The cells were continuously labeled with [3H]glycerol for 3.5 h in the presence of 0.4 mm OA, and the [3H]TG was isolated and quantified. Each data point represents the mean ± S.E. of three independent experiments done in triplicate. **, p < 0.001 versus si-SCR control. E, density distributions of 35S-labeled apoB100 secreted by the Ad-GFP-infected and si-SCR-transfected HepG2 (GFP/si-SCR) and Ad-PGC-1a-infected HepG2 cells transfected with si-SCR (PGC-1a/si-SCR) or si-hCideB (PGC-1a/si-hCideB). The cells were continuously labeled with 35S-Promix for 3.5 h in the presence of 0.4 mm OA, and 35S-apoB100-containing lipoproteins were fractionated and analyzed as described in legends to Fig. 4. Gel images from a representative experiment are shown in lower panels.
To pursue the role of alterations in CideB expression in mediating the effects of PGC-1α on VLDL assembly and secretion, we created a human CideB-specific siRNA that blocked the induction of CideB mRNA expression in PGC-1α-overexpressing HepG2 cells (Fig. 8B). Knockdown of CideB had no effect on TG synthesis by Ad-GFP- or Ad-PGC-1α-infected HepG2 cells (Fig. 8C) nor did it affect TG secretion by the Ad-GFP HepG2 cells (Fig. 8D). However, CideB knockdown significantly reduced TG secretion by the Ad-PGC-1α-overexpressing HepG2 cells and blunted the effect of PGC-1α on TG secretion (Fig. 8D). Pulse-chase experiments showed that knockdown of CideB did not affect apoB100 secretion (data not shown). However, density distribution analysis of apoB lipoproteins in the medium demonstrated that siRNA against CideB blunted the stimulatory effect of PGC-1α on the secretion of VLDL-sized particles by the Ad-PGC-1α-infected HepG2 cell (Fig. 8E; PGC-1α/si-CideB versus PGC-1α/si-SCR). These data provide evidence suggesting that activation of CideB is an important mechanism for PGC-1α to induce VLDL assembly and lipidation in HepG2 cells.
DISCUSSION
Overproduction of VLDL is a common feature of metabolic derangements in people with obesity, type II diabetes, and metabolic syndrome. A full understanding of the mechanisms regulating hepatic VLDL assembly and secretion has important implications for the treatment of the atherogenic dyslipidemia associated with these diseases. Although an increasing body of evidence suggests that PGC-1α is a critical regulator of fatty acid metabolism in the liver, its role in the regulation of hepatic VLDL assembly and secretion has been less well studied. In this study, we provide several lines of evidence demonstrating an important role for PGC-1α in the control of VLDL production. First, overexpression of PGC-1α stimulates TG secretion and promotes VLDL assembly in HepG2 cells. Second, adenovirus-mediated hepatic overexpression of PGC-1α stimulates VLDL-TG export and attenuates hepatic TG accumulation due to HFD feeding in mice. Finally, our data suggest activation of CideB gene expression as a potential mechanism whereby PGC-1α stimulates VLDL secretion. Together, these findings provide new insights into the biological function of PGC-1α as well as the regulatory mechanism of hepatic VLDL assembly and secretion.
The HepG2 cell is a popular model used for studying lipoprotein secretion. However, it is well recognized that this cell line exhibits very low rates of VLDL assembly (44, 45). Although the underlying molecular defect is not well established, recent data suggest an inherent deficiency in PGC-1α levels and activity in HepG2 cells (42, 43), which in turn leads to impairment in HNF4α function in this cell line (42). Given the important role of HNF4α in regulating expression of genes involved in hepatic VLDL assembly and metabolism (51), we suspected that this impairment in PGC-1α/HNF4α function might be responsible for defective VLDL assembly in HepG2 cells. Indeed, we found that ectopic expression of PGC-1α in HepG2 cells elicited a 3–4-fold increase in TG secretion and produced a shift in the hydrated density distribution of the apoB lipoproteins accumulated in the media from the LDL-HDL ranges toward the VLDL density range. These findings suggest that PGC-1α plays a critical role in regulating VLDL assembly in HepG2 cells and that the inherently low level of PGC-1α represents at least one molecular mechanism underlying the defective VLDL assembly in this cell line. To extend these findings to in vivo settings, we infused Ad-PGC-1α to mice fed a chow diet or a high fat diet. Compared with Ad-GFP-infected mice, overexpression of PGC-1α significantly increased hepatic TG export in mice maintained on either dietary regimen. Hepatic overexpression of PGC-1α also significantly increased plasma TG and reduced hepatic TG levels in high fat diet-treated mice. Together, our findings from HepG2 cells and mice in vivo provide evidence supporting a physiological role for PGC-1α in regulation of VLDL production.
PGC-1β has been shown to regulate VLDL at several levels, including by stimulating lipogenesis (33, 35). In contrast, PGC-1α induced VLDL secretion despite its inhibitory effect on TG synthesis. This finding strongly suggests that the PGC-1α-mediated effects differ from those of PGC-1β at a mechanistic level and that PGC-1α plays a critical role in the control of trafficking and/or recruitment of newly synthesized TG for VLDL assembly. Enhanced recruitment of TG for VLDL assembly could be due to an expanded function of the VLDL assembly machinery. However, we found no effect of PGC-1α on expression of the key proteins in the VLDL assembly pathway, including apoB, apoE, and MTP. On the other hand, PGC-1β activates expression of these proteins, especially MTP (33). Other recent work in HepG2 cells has indicated that apoCIII and apoAIV are up-regulated in HepG2 cells by PGC-1α overexpression (42, 46). However, it has previously been shown that apoAIV overexpression is not sufficient to drive TG secretion in liver cells (52, 53), and our studies excluded the potential involvement of the induced apoCIII expression in mediating the increased VLDL secretion. Taken together, our study suggests that PGC-1α stimulates VLDL secretion by promoting the partitioning of the cytosolic TG toward the secretion-coupled compartments rather than by modulating the VLDL assembly machinery. This conclusion is consistent with findings reported by other investigators that suggest diminished delivery of newly synthesized TG to the VLDL secretory compartments as a key mechanism underlying the defective VLDL assembly in HepG2 cells (54, 55).
Cytosolic TG stores are a major source of TG substrates for the biogenesis of VLDL in liver cells (18, 19). It is believed that TG stored in the cytosol is mobilized and delivered to the secretory compartments (especially the ER) via a process involving a lipolysis/esterification cycle (19). The enzymes and the cofactors governing this process are poorly understood. However, it is now clear that inducing lipolysis or recycling of the cytosolic stores alone is not sufficient to increase VLDL secretion, because ectopic expression of hormone-sensitive lipase or adipose TG lipase in the mouse liver does not stimulate VLDL secretion (56). Similarly, accelerating TG synthesis by overexpression of Dgat1 or Dgat2 does not affect VLDL secretion in vivo (21, 22). Alternatively, several proteins that coat cytosolic lipid droplets are emerging as important regulators of TG availability for VLDL assembly. Adipose differentiation-related protein is a major cytosolic lipid droplet-associated protein in the liver, and it has been shown to exert an inhibitory effect on VLDL assembly (47). Conversely, it has recently been reported that another liver lipid-droplet protein, CideB, which can be associated with both cytosolic lipid droplets and ER membranes, has a critical role in mediating provision of TG for the lipidation and maturation of VLDL particles (36). Although the CideN protein family was originally characterized for effects on apoptotic signaling, more recent data have revealed their important roles in regulating fatty acid metabolism (36, 50, 57). CIDEB is known to be an HNF4α target gene and is expressed most highly in liver and intestine in vivo (49, 58). The current findings suggest that CIDEB is also a PGC-1α target gene. The demonstration that CideB-siRNA blunted the effects of PGC-1α on VLDL secretion and particle size represents direct evidence for CideB as a crucial mediator for the PGC-1α-induced VLDL assembly and secretion.
In summary, this study demonstrates that PGC-1α promotes the secretion of VLDL despite inhibiting TG synthesis. These findings suggest that PGC-1α is partitioning TG toward the secretory pathway in hepatocytes. The identification of CIDEB as a PGC-1α target gene provides a new mechanism whereby PGC-1α can influence hepatic lipid homeostasis.
Supplementary Material
Acknowledgment
We thank Dr. Nick Davidson for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK078187 (to B. N. F.) and R01 HL73939 (to Z. C.). This work was also supported by the core services of the Digestive Diseases Research Core Center Grant P30 DK52574 and the Nutrition Obesity Research Center Grant P30 DK56341 at Washington University School of Medicine.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- TG
- triglyceride
- ER
- endoplasmic reticulum
- MTP
- microsomal triglyceride transfer protein
- h
- human
- OA
- oleic acid.
- HFD
- high fat diet.
REFERENCES
- 1.Hamilton R. L. (1972) Adv. Exp. Med. Biol. 26, 7–24 [DOI] [PubMed] [Google Scholar]
- 2.Schonfeld G. (2003) J. Lipid Res. 44, 878–883 [DOI] [PubMed] [Google Scholar]
- 3.Schonfeld G., Patterson B. W., Yablonskiy D. A., Tanoli T. S., Averna M., Elias N., Yue P., Ackerman J. (2003) J. Lipid Res. 44, 470–478 [DOI] [PubMed] [Google Scholar]
- 4.Cuchel M., Bloedon L. T., Szapary P. O., Kolansky D. M., Wolfe M. L., Sarkis A., Millar J. S., Ikewaki K., Siegelman E. S., Gregg R. E., Rader D. J. (2007) N. Engl. J. Med. 356, 148–156 [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Fitzgerald R. L., Averna M. R., Schonfeld G. (2000) J. Biol. Chem. 275, 32807–32815 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z., Fitzgerald R. L., Schonfeld G. (2002) J. Biol. Chem. 277, 14135–14145 [DOI] [PubMed] [Google Scholar]
- 7.Raabe M., Véniant M. M., Sullivan M. A., Zlot C. H., Björkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. (1999) J. Clin. Invest. 103, 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamba V., Rader D. J. (2007) Gastroenterology 132, 2181–2190 [DOI] [PubMed] [Google Scholar]
- 9.Adiels M., Olofsson S. O., Taskinen M. R., Borén J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1225–1236 [DOI] [PubMed] [Google Scholar]
- 10.Goldberg I. J. (2001) J. Clin. Endocrinol. Metab. 86, 965–971 [DOI] [PubMed] [Google Scholar]
- 11.Blasiole D. A., Davis R. A., Attie A. D. (2007) Mol. Biosyst. 3, 608–619 [DOI] [PubMed] [Google Scholar]
- 12.Chen Z., Davidson N. O. (2006) in Physiology of the Gastrointestinal Tract (Johnson L. R. ed) 4th Ed., pp. 1711–1734, Academic Press, Boston [Google Scholar]
- 13.Yamaguchi J., Gamble M. V., Conlon D., Liang J. S., Ginsberg H. N. (2003) J. Biol. Chem. 278, 42643–42651 [DOI] [PubMed] [Google Scholar]
- 14.Alexander C. A., Hamilton R. L., Havel R. J. (1976) J. Cell Biol. 69, 241–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swift L. L., Valyi-Nagy K., Rowland C., Harris C. (2001) J. Lipid Res. 42, 218–224 [PubMed] [Google Scholar]
- 16.Tran K., Thorne-Tjomsland G., DeLong C. J., Cui Z., Shan J., Burton L., Jamieson J. C., Yao Z. (2002) J. Biol. Chem. 277, 31187–31200 [DOI] [PubMed] [Google Scholar]
- 17.Sniderman A. D., Cianflone K. (1993) Arterioscler. Thromb. 13, 629–636 [DOI] [PubMed] [Google Scholar]
- 18.Yang L. Y., Kuksis A., Myher J. J., Steiner G. (1995) J. Lipid Res. 36, 125–136 [PubMed] [Google Scholar]
- 19.Wiggins D., Gibbons G. F. (1992) Biochem. J. 284, 457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuzil J., Christison J. K., Iheanacho E., Fragonas J. C., Zammit V., Hunt N. H., Stocker R. (1998) J. Lipid Res. 39, 354–368 [PubMed] [Google Scholar]
- 21.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr., Hevener A. L., Farese R. V., Jr. (2007) Cell Metab. 6, 69–78 [DOI] [PubMed] [Google Scholar]
- 22.Millar J. S., Stone S. J., Tietge U. J., Tow B., Billheimer J. T., Wong J. S., Hamilton R. L., Farese R. V., Jr., Rader D. J. (2006) J. Lipid Res. 47, 2297–2305 [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Newberry E. P., Norris J. Y., Xie Y., Luo J., Kennedy S. M., Davidson N. O. (2008) J. Lipid Res. 49, 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X., Grundy S. M., Patel S. B. (1997) J. Lipid Res. 38, 1277–1288 [PubMed] [Google Scholar]
- 25.Lin J., Handschin C., Spiegelman B. M. (2005) Cell Metab. 1, 361–370 [DOI] [PubMed] [Google Scholar]
- 26.Finck B. N., Kelly D. P. (2006) J. Clin. Invest. 116, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberkofler H., Schraml E., Krempler F., Patsch W. (2003) Biochem. J. 371, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Castellani L. W., Sinal C. J., Gonzalez F. J., Edwards P. A. (2004) Genes Dev. 18, 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vega R. B., Huss J. M., Kelly D. P. (2000) Mol. Cell. Biol. 20, 1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herzig S., Long F., Jhala U. S., Hedrick S., Quinn R., Bauer A., Rudolph D., Schutz G., Yoon C., Puigserver P., Spiegelman B., Montminy M. (2001) Nature 413, 179–183 [DOI] [PubMed] [Google Scholar]
- 31.Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., Newgard C. B., Spiegelman B. M. (2001) Nature 413, 131–138 [DOI] [PubMed] [Google Scholar]
- 32.Rhee J., Inoue Y., Yoon J. C., Puigserver P., Fan M., Gonzalez F. J., Spiegelman B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfrum C., Stoffel M. (2006) Cell Metab. 3, 99–110 [DOI] [PubMed] [Google Scholar]
- 34.Lelliott C. J., Ljungberg A., Ahnmark A., William-Olsson L., Ekroos K., Elmgren A., Arnerup G., Shoulders C. C., Oscarsson J., Lindén D. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2707–2713 [DOI] [PubMed] [Google Scholar]
- 35.Lin J., Yang R., Tarr P. T., Wu P. H., Handschin C., Li S., Yang W., Pei L., Uldry M., Tontonoz P., Newgard C. B., Spiegelman B. M. (2005) Cell 120, 261–273 [DOI] [PubMed] [Google Scholar]
- 36.Ye J., Li J. Z., Liu Y., Li X., Yang T., Ma X., Li Q., Yao Z., Li P. (2009) Cell Metab. 9, 177–190 [DOI] [PubMed] [Google Scholar]
- 37.Chen Z., Gropler M. C., Norris J., Lawrence J. C., Jr., Harris T. E., Finck B. N. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S. C., Leone T. C., Wende A. R., Croce M. A., Chen Z., Sherry A. D., Malloy C. R., Finck B. N. (2006) J. Biol. Chem. 281, 19000–19008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finck B. N., Han X., Courtois M., Aimond F., Nerbonne J. M., Kovacs A., Gross R. W., Kelly D. P. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X., Yue P., Xie Y., Davidson N. O., Sakata N., Ostlund R. E., Jr., Chen Z., Schonfeld G. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 289, G146–G152 [DOI] [PubMed] [Google Scholar]
- 41.Chen Z., Fitzgerald R. L., Li G., Davidson N. O., Schonfeld G. (2004) J. Lipid Res. 45, 155–163 [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Jiménez C. P., Gómez-Lechón M. J., Castell J. V., Jover R. (2006) J. Biol. Chem. 281, 29840–29849 [DOI] [PubMed] [Google Scholar]
- 43.Oberkofler H., Schraml E., Krempler F., Patsch W. (2004) Biochem. J. 381, 357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon J. L., Ginsberg H. N. (1993) J. Lipid Res. 34, 167–179 [PubMed] [Google Scholar]
- 45.Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. (1986) J. Lipid Res. 27, 236–250 [PubMed] [Google Scholar]
- 46.Rhee J., Ge H., Yang W., Fan M., Handschin C., Cooper M., Lin J., Li C., Spiegelman B. M. (2006) J. Biol. Chem. 281, 14683–14690 [DOI] [PubMed] [Google Scholar]
- 47.Magnusson B., Asp L., Boström P., Ruiz M., Stillemark-Billton P., Lindén D., Borén J., Olofsson S. O. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1566–1571 [DOI] [PubMed] [Google Scholar]
- 48.Kelder B., Boyce K., Kriete A., Clark R., Berryman D. E., Nagatomi S., List E. O., Braughler M., Kopchick J. J. (2007) Comp. Hepatol. 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J. Z., Ye J., Xue B., Qi J., Zhang J., Zhou Z., Li Q., Wen Z., Li P. (2007) Diabetes 56, 2523–2532 [DOI] [PubMed] [Google Scholar]
- 50.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. (2008) Cell Metab. 7, 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., Gonzalez F. J. (2001) Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao Z., Lauer S. J., Sanan D. A., Fazio S. (1993) Arterioscler. Thromb. Vasc. Biol. 13, 1476–1486 [DOI] [PubMed] [Google Scholar]
- 53.Duverger N., Tremp G., Caillaud J. M., Emmanuel F., Castro G., Fruchart J. C., Steinmetz A., Denèfle P. (1996) Science 273, 966–968 [DOI] [PubMed] [Google Scholar]
- 54.Gibbons G. F., Khurana R., Odwell A., Seelaender M. C. (1994) J. Lipid Res. 35, 1801–1808 [PubMed] [Google Scholar]
- 55.Wu X., Shang A., Jiang H., Ginsberg H. N. (1996) J. Lipid Res. 37, 1198–1206 [PubMed] [Google Scholar]
- 56.Reid B. N., Ables G. P., Otlivanchik O. A., Schoiswohl G., Zechner R., Blaner W. S., Goldberg I. J., Schwabe R. F., Chua S. C., Jr., Huang L. S. (2008) J. Biol. Chem. 283, 13087–13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong J., Sun Z., Li P. (2009) Curr. Opin. Lipidol. 20, 121–126 [DOI] [PubMed] [Google Scholar]
- 58.Da L., Li D., Yokoyama K. K., Li T., Zhao M. (2006) Biochem. J. 393, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.