Abstract
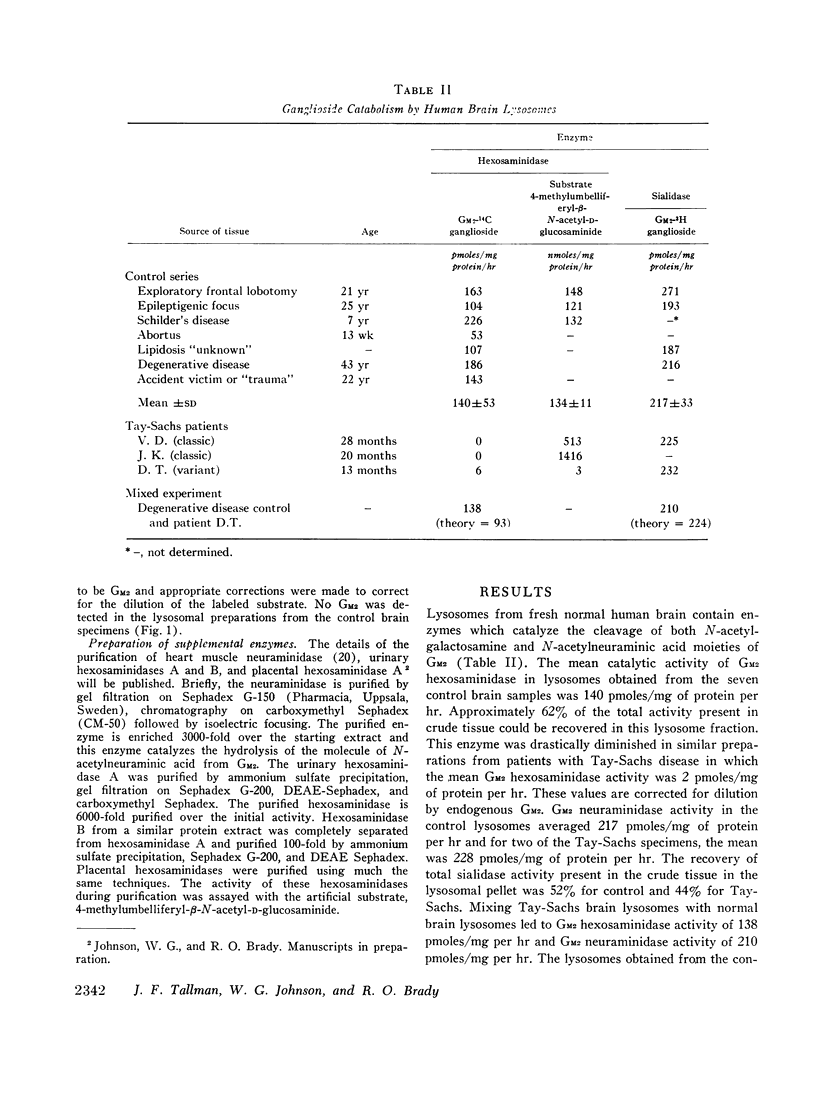
The catabolism of Tay-Sachs ganglioside, N-acetylgalactosaminyl- (N-acetylneuraminosyl) -galactosylglucosylceramide, has been studied in lysosomal preparations from normal human brain and brain obtained at biopsy from Tay-Sachs patients. Utilizing Tay-Sachs ganglioside labeled with 14C in the N-acetylgalactosaminyl portion or 3H in the N-acetylneuraminosyl portion, the catabolism of Tay-Sachs ganglioside may be initiated by either the removal of the molecule of N-acetylgalactosamine or N-acetylneuraminic acid. The activity of the N-acetylgalactosamine-cleaving enzyme (hexosaminidase) is drastically diminished in such preparations from Tay-Sachs brain whereas the activity of the N-acetylneuraminic acid-cleaving enzyme (neuraminidase) is at a normal level. Total hexosaminidase activity as measured with an artificial fluorogenic substrate is increased in tissues obtained from patients with the B variant form of Tay-Sachs disease and it is virtually absent in the O-variant patients. The addition of purified neuraminidase and various purified hexosaminidases exerted only a minimal synergistic effect on the hydrolysis of Tay-Sachs ganglioside in the lysosomal preparations from the control or patient with the O variant of Tay-Sachs disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch G. G. Glycolipid abnormalities in a myoclonic variant of late infantile amaurotic idiocy. J Lipid Res. 1970 May;11(3):241–247. [PubMed] [Google Scholar]
- Brady R. O. The sphingolipidoses. N Engl J Med. 1966 Aug 11;275(6):312–318. doi: 10.1056/NEJM196608112750606. [DOI] [PubMed] [Google Scholar]
- Frohwein Y. Z., Gatt S. Enzymatic hydrolysis of sphingolipids. VI. Hydrolysis of ceramide glycosides by calf brain beta-N-acetylhexosaminidase. Biochemistry. 1967 Sep;6(9):2783–2787. doi: 10.1021/bi00861a019. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M., Svennerholm L. Biosynthesis and biodegradation of rat brain gangliosides studied in vivo. J Neurochem. 1972 Mar;19(3):609–622. doi: 10.1111/j.1471-4159.1972.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Kolodny E. H., Brady R. O., Quirk J. M., Kanfer J. N. Preparation of radioactive Tay-Sachs ganglioside labeled in the sialic acid moiety. J Lipid Res. 1970 Mar;11(2):144–149. [PubMed] [Google Scholar]
- Kolodny E. H., Brady R. O., Volk B. W. Demonstration of an alteration of ganglioside metabolism in Tay-Sachs disease. Biochem Biophys Res Commun. 1969 Oct 22;37(3):526–531. doi: 10.1016/0006-291x(69)90947-4. [DOI] [PubMed] [Google Scholar]
- Kolodny E. H., Kanfer J., Quirk J. M., Brady R. O. Properties of a particle-bound enzyme from rat intestine that cleaves sialic acid from Tay-Sachs ganglioside. J Biol Chem. 1971 Mar 10;246(5):1426–1431. [PubMed] [Google Scholar]
- Leibovitz Z., Gatt S. Enzymatic hydrolysis of sphingolipids. VII. Hydrolysis of gangliosides by a neuraminidase from calf brain. Biochim Biophys Acta. 1968 Jan 10;152(1):136–143. doi: 10.1016/0005-2760(68)90015-5. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Okada S., Ho M. W., Fillerup D. L., Veath M. L., Adams K. Ganglioside storage diseases. Fed Proc. 1971 May-Jun;30(3):956–969. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
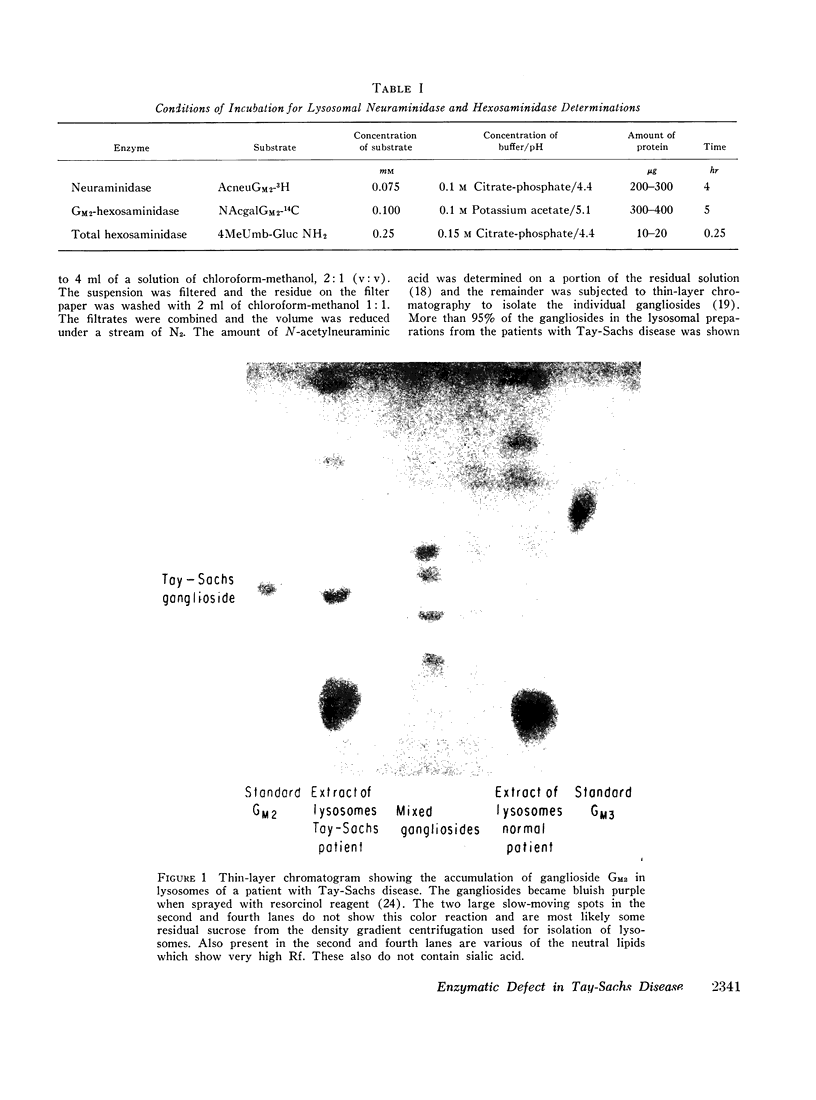
- Penick R. J., Meisler M. H., McCluer R. H. Thin-layer chromatographic studies of human brain gangliosides. Biochim Biophys Acta. 1966 Apr 4;116(2):279–287. doi: 10.1016/0005-2760(66)90010-5. [DOI] [PubMed] [Google Scholar]
- Pilz H., Müller D., Sandhoff K., ter Meulen V. Tay-Sachssche Krankheit mit Hexosaminidase-Defekt. Klinische, morphologische und biochemische Bedunde bei einem Fall mit viszeraler Speicherung von Nierenglobosid. Dtsch Med Wochenschr. 1968 Sep 27;93(39):1833–passim. doi: 10.1055/s-0028-1110836. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. The chemical structure of normal human brain and Tay-Sachs gangliosides. Biochem Biophys Res Commun. 1962 Nov 27;9:436–441. doi: 10.1016/0006-291x(62)90030-x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexozaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Life Sci. 1968 Mar 15;7(6):283–288. doi: 10.1016/0024-3205(68)90024-6. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Sellinger O. Z., Nordrum L. M. A regional study of some osmotic, ionic and age factors affecting the stability of cerebral lysosomes. J Neurochem. 1969 Aug;16(8):1219–1229. doi: 10.1111/j.1471-4159.1969.tb05969.x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Jacob J. C., Suzuki K., Kutty K. M., Suzuki K. Gm2-gangliosidosis with total hexosaminidase deficiency. Neurology. 1971 Apr;21(4):313–328. doi: 10.1212/wnl.21.4.313. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Jr, Brady R. O., Suzuki K. Enzymic activities associated with membranous cytoplasmic bodies and isolated brain lysosomes. J Neurochem. 1971 Sep;18(9):1775–1777. doi: 10.1111/j.1471-4159.1971.tb03754.x. [DOI] [PubMed] [Google Scholar]