Abstract
Protein kinase Cϵ (PKCϵ), a diacyglycerol- and phorbol ester-responsive serine-threonine kinase, has been implicated in mitogenic and survival control, and it is markedly overexpressed in human tumors, including in prostate cancer. Although prostate cancer cells undergo apoptosis in response to phorbol ester stimulation via PKCδ-mediated release of death factors, the involvement of PKCϵ in this response is not known. PKCϵ depletion by RNAi or expression of a dominant negative kinase-dead PKCϵ mutant potentiated the apoptotic response of PMA and sensitized LNCaP cells to the death receptor ligand TNFα. On the other hand, overexpression of PKCϵ by adenoviral means protected LNCaP cells against apoptotic stimuli. Interestingly, PKCϵ RNAi depletion significantly enhanced the release of TNFα in response to PMA and greatly potentiated JNK activation by this cytokine. Further mechanistic analysis revealed that PMA fails to promote phosphorylation of Bad in Ser112 in PKCϵ-depleted LNCaP cells, whereas PKCϵ overexpression greatly enhanced Bad phosphorylation. This effect was independent of Akt, ERK, or p90Rsk, well established kinases for Ser112 in Bad. Moreover, expression of a S112A-Bad mutant potentiated PMA-induced apoptosis. Finally, we found that upon activation PKCϵ accumulated in mitochondrial fractions in LNCaP cells and that Bad was a substrate of PKCϵ in vitro. Our results established that PKCϵ modulates survival in prostate cancer cells via multiple pathways.
Keywords: Apoptosis, JNK, Protein Kinase C (PKC), Tumor Necrosis Factor (TNF), Tumor Promoter, Bad Phosphorylation, Prostate Cancer Cells
Introduction
Protein kinase C (PKC),3 a family of serine-threonine kinases that comprises the classical (cPKCs α, β, and γ), novel (nPKCs δ, ϵ, η, and θ), and atypical (aPKCs ζ and λ) PKCs, is a key signaling component of growth factor and cytokine pathways. Despite their structural similarities, PKC isozymes have unique modes of regulation as well as differential patterns of cell and tissue expression (1, 2). Only cPKCs and nPKCs are regulated by phorbol esters and diacylglycerol, a lipid second messenger generated upon activation of G protein-coupled receptors and tyrosine kinases (1–3). Phorbol esters are capable of promoting opposite responses in different cell types, such as mitogenesis/survival versus growth arrest/apoptosis. This paradigm of functional diversity is exemplified by the nPKCs: whereas in most cases PKCϵ acts as a mitogenic or antiapoptotic kinase, activation of PKCδ inhibits proliferation or triggers an apoptotic response (4–8). PKCϵ can signal to mitogenesis via Raf/MEK/ERK and cyclin D1 induction (9, 10) or can even transform cells (4, 6, 11). In addition, PKCϵ has been linked to cell survival through the activation of Akt and Bax (12, 13).
Tumor cells display in many cases an altered balance in PKC isozyme expression, potentially reflecting the involvement of PKCs in the etiology and progression of cancer. Most notably, many cancer cells show marked up-regulation of PKCϵ. PKCϵ is elevated in prostate cancer, particularly in high grade tumors, and is implicated in prostate tumor progression and the transition to androgen-independence (14–17). The functional complexity of PKC in prostate cancer cells is nonetheless highlighted by the fact that phorbol esters promote an apoptotic response in androgen-dependent prostate cancer cells (18, 19). Previous work from our laboratory and others established that PKCδ is the major mediator of the death effect of PMA in LNCaP cells (19–21). In this context, the role of PKCϵ remains controversial, as unlike in other cell types, PKCϵ was found to be either dispensable for prostate cancer cell survival or even contribute to the proapoptotic effects of PKC activators (22–24). Given our limited understanding of the mechanistic insights of PKC-driven responses in prostate cancer cells, additional studies would be required to ascertain the specific contribution of PKC isozymes to this paradox.
We have recently demonstrated that PKCδ-mediated prostate cancer cell death involves the activation of an apoptotic autocrine loop through the release of death factors (primarily TNFα), and the subsequent activation of the extrinsic apoptotic cascade and the JNK pathways (20, 25, 26). Whether PKCϵ is implicated or not in the control of this pathway remains to be determined. In the present study we used a series of gain- and loss-of-function approaches to demonstrate that PKCϵ is implicated in survival signaling in prostate cancer cells by modulating the secretion of TNFα and also by acting as an effector of the death factor response. In addition, we identified a major role for PKCϵ in the control of Bad phosphorylation, pointing to multiple mechanisms implicated in the prosurvival response of PKCϵ in prostate cancer cells.
EXPERIMENTAL PROCEDURES
Materials
PMA was purchased from LC Laboratories (Woburn, MA). DAPI (4′,6-diamidino-2-phenylindole) was obtained from Sigma. TNFα was from PeproTech (Rocky Hill, NJ). GF109302X was from BioMol (Plymouth Meeting, PA). Cell culture reagents and media were purchased from ATCC. Bad constructs were kindly provided by Dr. Michael Greenberg (Harvard University). Dominant negative Rsk was a kind gift from Dr. Margaret Chao (Children's Hospital of Philadelphia).
Cell Culture
LNCaP, C4, and DU145 human prostate cancer cells were obtained from ATCC and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C in a humidified 5% CO2 atmosphere.
Western Blotting
Cell harvesting and Western blot analysis were carried out as previously described (20, 26, 27). The following primary antibodies were used at a 1:1000 dilution: anti-PKCϵ (Santa Cruz Biotechnology), phospho-Ser112-Bad, Bad, Bcl-2, phospho-Ser380-p90 Rsk, pan-Rsk, phospho-Thr202/Tyr204-ERK1/2, ERK1/2, phospho-Thr180/Tyr182-p38, p38, phospho-Thr183/Tyr185-JNK, and JNK (Cell Signaling). As loading controls, either β-actin or vinculin levels were determined using specific antibodies (Sigma).
RNA Interference (RNAi)
RNAi duplexes were purchased from Dharmacon (Lafayette, CO). Two different ON-TARGET siRNAs for PKCϵ were transfected into LNCaP cells using the Amaxa Nucleofector-II, as described before (27). Transfection of siRNAs into C4 and DU145 cells was achieved with Oligofectamine (Invitrogen), following the manufacturer's protocol (27). The targeting sequences were as follows: PKCϵ RNAi #1, CAGAGGAGAUUAAGACUAU; PKCϵ RNAi #2, GUAAUGAGUCGUCUUUCUA. Control Negative Silencer® siRNA was from Ambion. Experiments were carried out 48 h after transfection.
Apoptosis Assays
The incidence of apoptosis was determined after DAPI staining, as previously described (20, 26, 27).
Infection of LNCaP Cells with PKCϵ Adenovirus
Generation of adenoviruses (AdVs) coding wild-type or dominant negative (L436R) PKCϵ was described elsewhere (28). Two days after plating, LNCaP cells were infected with AdVs at multiplicities of infection (m.o.i.) ranging from 1 to 100 pfu/cell in RPMI 1640 medium supplemented with 2% FBS. After 4 h, viral particles were removed, and cells were incubated for 24 h in RPMI 1640 medium supplemented with 10% FBS.
Preparation of Conditioned Medium and Treatment
Conditioned medium (CM) was prepared as described before (20, 27). Briefly, LNCaP cells growing in 100-mm plates (∼80% confluence) were treated with either vehicle or PMA (100 nm, 1 h). After extensive washing, 7 ml of complete medium was added to the plates. Twenty-four h later, CM was collected and centrifuged. The supernatant was filtered through a 40-μm size pore PVDF filter syringe and added to naïve LNCaP cells growing in 6-well plates.
In Vitro PKCϵ Kinase Assay
Human recombinant Bad (25 ng, Santa Cruz Biotechnology) was incubated with human recombinant PKCϵ (25 ng; Calbiochem) for 15 min at 30 °C in a reaction buffer containing 20 mm HEPES, 10 mm MgCl2, 20 μg of phosphatidylserine vesicles, 100 nm PMA, and 10 μm ATP. Samples were resolved by SDS-PAGE and phospho-Ser112-Bad determined by Western blotting.
Mitochondria Isolation
Mitochondria-enriched fractions were prepared with the Mitochondria Isolation Kit (Pierce) according to the manufacturer's instructions. Vinculin and cytochrome c were used as purity controls for cytosolic and mitochondrial fractions, respectively.
Statistical Analysis
Mean values were compared with an unpaired two-tailed Student's t test using GraphPad Software built-in analysis tools. The confidence interval was set at 95%; p < 0.05 was considered statistically significant.
RESULTS
PKCϵ Depletion or Inhibition Enhances PMA-induced Apoptosis in Prostate Cancer Cells
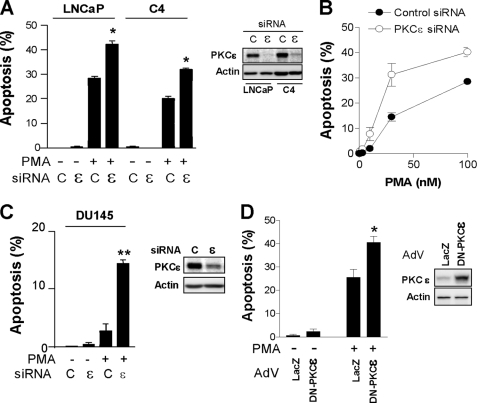
The involvement of PKCϵ in prostate cancer cell apoptosis/survival remains controversial. To begin addressing this issue, we used two approaches. First, different prostate cancer cell lines were transfected with either a PKCϵ or a control siRNA duplex, and the incidence of apoptosis in response to PMA (100 nm, 1 h) was determined. As shown in Fig. 1A, depletion of PKCϵ sensitized LNCaP prostate cancer cells and the C4 LNCaP variant to PMA-induced apoptosis. Similar results were observed with a second PKCϵ RNAi duplex (data not shown). A dose response for the PMA effect in LNCaP cells is shown in Fig. 1B. Notably, DU145 cells, which are resistant to phorbol ester-induced apoptosis, became sensitive to PMA when subjected to PKCϵ RNAi depletion (Fig. 1C). As a second approach, LNCaP cells were infected with an AdV for a dominant negative (L436R, kinase-dead) PKCϵ and subjected to PMA treatment. As shown in Fig. 1D, expression of the dominant negative PKCϵ mutant significantly enhanced the apoptotic response to PMA.
FIGURE 1.
PKCϵ depletion or inhibition sensitizes prostate cancer cells to PMA-induced apoptosis. A, prostate cancer cells transfected with either PKCϵ RNAi duplex #1 (ϵ) or a control RNAi duplex (C), and 48 h later treated with vehicle or PMA (100 nm, 1 h). Apoptosis was determined 24 h later. Inset, PKCϵ levels, as determined by Western blotting. B, concentration-response curve for the apoptotic effect of PMA in LNCaP cells subject to PKCϵ or control RNAi. Inset, PKCϵ levels, as determined by Western blotting. C, LNCaP cells infected with either a control (LacZ) or a DN-PKCϵ AdV (m.o.i. = 10 pfu/cell) and 24 h later treated with PMA (100 nm, 1 h). Apoptosis was determined 24 h later. Inset, PKCϵ levels, as determined by Western blotting. In all cases, data represent the mean ± S.E. (error bars) of three independent experiments. *, p < 0.05; **, p < 0.01 versus control (+PMA).
PKCϵ Overexpression Inhibits PMA-induced Apoptosis in LNCaP Cells
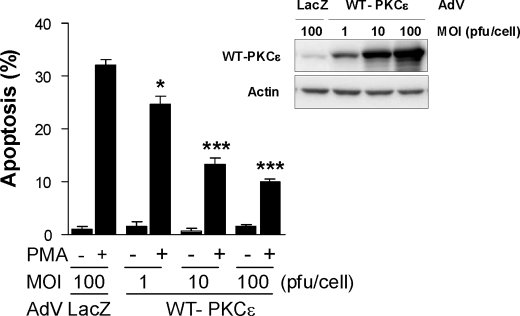
PKCϵ overexpression is a hallmark of prostate cancer, particularly in advanced stages (14, 16). To establish further an antiapoptotic role of PKCϵ in prostate cancer cells, we overexpressed wild-type PKCϵ in LNCaP cells using an adenoviral approach. Overexpression of PKCϵ markedly reduced the apoptotic effect of PMA in LNCaP cells (Fig. 2A). The resistance to apoptosis was proportional to the levels of expression achieved upon increasing m.o.i. (1–100 pfu/cell) of the PKCϵ AdV. On the other hand, a control LacZ AdV (100 pfu/cell) had no effect. Taken together, our results indicate that PKCϵ is a prosurvival kinase in prostate cancer cells.
FIGURE 2.
PKCϵ protects LNCaP prostate cancer cells from PMA-induced apoptosis. LNCaP cells were infected with either a control (LacZ) or a wild-type (WT)-PKCϵ AdV at increasing m.o.i. (1–100 pfu/cell) and 24 h later treated with PMA (100 nm, 1 h). Twenty-four h later, the percentage of apoptotic cells was determined. Lower panels show the levels of PKCϵ by Western blotting. Data represent the mean ± S.E. (error bars) of three independent experiments. *, p < 0.05; ***, p < 0.001 versus LacZ control + PMA.
PKCϵ Modulates PMA-induced Secretion of TNFα from LNCaP Cells
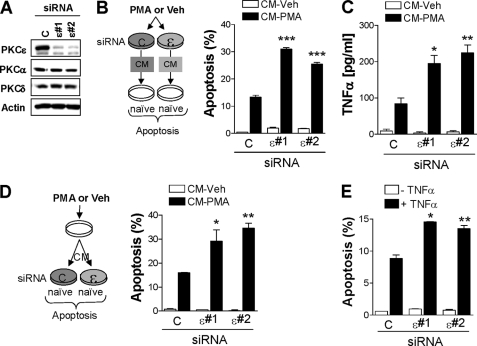
Phorbol esters stimulate the release of autocrine or paracrine factors. For example, CM collected from PKCϵ-overexpressing R6 fibroblasts stimulates DNA synthesis and causes cell transformation (29). Our previous studies identified a proapoptotic autocrine loop triggered by PKCδ activation in prostate cancer cells which is mediated by death receptor ligands, primarily TNFα (20, 30). PKCδ has a dual effect because it promotes the release of death factors and is also required for the death factor response (20). To assess a potential implication of PKCϵ in the autocrine response, we collected CM from LNCaP cells subjected to PKCϵ RNAi depletion or from control cells, either treated with PMA (CM-PMA) or vehicle (CM-Veh). Two different RNAi duplexes that efficiently depleted (>80%) PKCϵ from LNCaP cells were used. Neither of these duplexes altered the expression of other PMA-responsive PKCs present in LNCaP cells, namely PKCα and PKCδ (Fig. 3A). Interestingly, CM-PMA collected from PKCϵ-depleted cells showed a higher apoptotic activity when added to a naïve culture of LNCaP cells (Fig. 3B). As expected, CM-Veh did not induce apoptosis in LNCaP cells.
FIGURE 3.
PKCϵ inhibits TNFα secretion by PMA and modulates TNFα-induced apoptosis. A, LNCaP cells were transfected with either a control or two different PKCϵ RNAi duplexes (#1 and #2). Expression of the phorbol ester-responsive PKC isozymes in LNCaP cells was determined by Western blotting. B, CM was collected 24 h after treatment with either PMA (100 nm, 1 h) or vehicle from LNCaP cells that had been previously subjected to PKCϵ or control RNAi, as described in A. CM was used to assay its apoptogenic activity in naïve LNCaP cells. A schematic representation of the experimental approach is presented. C, TNFα released to CM was determined by ELISA. D, CM prepared from parental LNCaP cells treated with either vehicle or PMA (100 nm, 1 h) was added to LNCaP cells that had been previously subjected to PKCϵ or control RNAi. Apoptosis was determined 24 h later. A schematic representation of the experimental approach is presented. E, LNCaP cells subjected to either PKCϵ or control RNAi were treated with TNFα (10 ng/ml), and the incidence of apoptosis was determined 24 h later. Data are expressed as the mean ± S.E. (error bars) of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control (+CM-PMA or +TNFα).
Because TNFα is the major death factor implicated in PMA-induced apoptosis in LNCaP cells (20, 31), we next determined TNFα levels by ELISA in CM-Veh and CM-PMA from LNCaP cells subject to either PKCϵ RNAi or control RNAi. Notably, although PKCϵ RNAi depletion did not modify basal levels of TNFα in CM-Veh, TNFα was higher in CM-PMA from PKCϵ-depleted cells than in control cells (Fig. 3C), suggesting that activation of PKCϵ inhibits TNFα secretion from LNCaP cells.
We next asked whether PKCϵ modulates the apoptotic response of TNFα in LNCaP cells. To address this issue cells subjected to either PKCϵ RNAi or control RNAi were treated with CM-PMA. Interestingly, we observed that the apoptotic response to CM-PMA doubled when applied to PKCϵ-depleted cells relative to control cells (Fig. 3D). Moreover, PKCϵ-depleted LNCaP cells were more sensitive to TNFα-induced apoptosis than control cells (Fig. 3E). Altogether, these results indicate that PKCϵ not only negatively modulates the secretion of proapoptotic factors from LNCaP cells, but that it is also implicated as an effector of the death factor response.
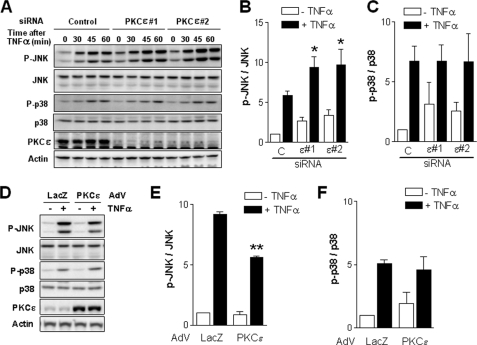
PKCϵ Inhibits JNK Activation by TNFα in LNCaP Cells
We have previously established essential roles for MAPK cascades as modulators of apoptosis downstream of PKCδ (20, 21). Inhibition of JNK blocks phorbol ester-induced apoptosis as well as cell death induced by secreted TNFα in response to PKCδ activation (20, 21). In addition, JNK and p38 are well established effectors of TNFα in LNCaP cells (20, 26, 32). We reasoned that PKCϵ may exert its prosurvival effects in LNCaP cells through the modulation of JNK and/or p38 activation. Remarkably, activation of JNK by TNFα was higher in PKCϵ-depleted cells than in control LNCaP cells, as determined by Western blotting using a phospho-JNK antibody. On the other hand, p38 activation in response to TNFα was similar in PKCϵ-depleted and control LNCaP cells. A representative experiment using the two different siRNAs for PKCϵ (#1 and #2) is shown in Fig. 4A. A densitometric analysis of phospho-JNK and phospho-p38 levels normalized to their corresponding total levels from multiple experiments (for 30 min of TNFα) is presented in Fig. 4, B and C, respectively.
FIGURE 4.
PKCϵ negatively regulates JNK activation by TNFα in LNCaP cells. A, LNCaP cells were transfected with either a control or two different PKCϵ RNAi duplexes (#1 and #2), and 48 h later the activation of JNK and p38 MAPKs in response to TNFα (10 ng/ml, 0–60 min) was analyzed by Western blotting using phospho-Thr183/Tyr185-JNK and phospho-Thr180/Tyr182-p38 antibodies, respectively. A representative experiment is shown. B, densitometric analysis of JNK activation by TNFα (10 ng/ml, 30 min) in control or PKCϵ-depleted cells. C, densitometric analysis of p38 activation by TNFα (30 min) in control or PKCϵ-depleted cells. D, LNCaP cells were infected with either a control (LacZ) or PKCϵ AdV at m.o.i. = 10 pfu/cell, and 24 h later treated with TNFα (10 ng/ml, 30 min). Activation of JNK and p38 was determined by Western blotting using phospho-specific antibodies. A representative experiment is shown. E, densitometric analysis of JNK activation in response to TNFα (30 min) in control or PKCϵ-overexpressing cells. Data are expressed as the mean ± S.E. (error bars) of three independent experiments. *, p < 0.05; **, p < 0.01 versus control (+TNFα). F, densitometric analysis of p38 activation in response to TNFα (30 min) in control or PKCϵ-overexpressing cells.
To corroborate the involvement of JNK in the PKCϵ response, we adenovirally overexpressed wild-type PKCϵ in LNCaP cells and assessed its effect on TNFα-induced activation of JNK. We found that activation of JNK by TNFα was significantly reduced in PKCϵ-overexpressing cells. On the other hand, p38 activation was not affected by PKCϵ overexpression (Fig. 4D; densitometric analysis in Fig. 4, E and F). These results suggest that PKCϵ negatively regulates the activation of JNK in response to TNFα in LNCaP cells.
PKCϵ Induces Phosphorylation of the Proapoptotic Protein Bad at Ser112
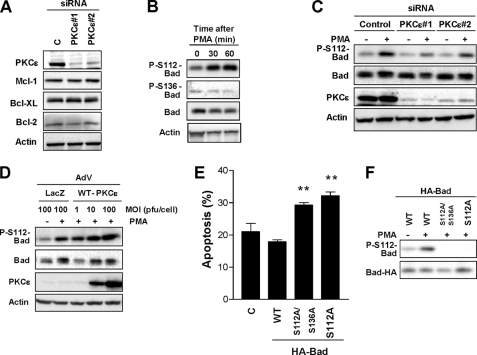
Recent studies found that PKCϵ modulates the levels and/or phosphorylation of Bcl-2 family members. In some cell lines, PKCϵ contributes to Bim down-regulation and Bcl-2 up-regulation, therefore promoting cell survival (33, 34). However, we found that knocking down PKCϵ from LNCaP cells did not appreciably affect the expression of the prosurvival Bcl-2 members Mcl-1, Bcl-XL, or Bcl-2 (Fig. 5A). Studies by Kulik and co-workers have established that phosphorylation of Bad downstream of EGF receptors protects LNCaP cells from apoptosis (35, 36). The proapoptotic activity of Bad can be negatively regulated by phosphorylation at Ser112 and Ser136, which prevents Bad/Bcl-2 or Bad/Bcl-XL interactions (37). We speculated that survival by PKCϵ could be mediated by inactivation of Bad. To this end, we determined phosphorylation of Bad in Ser112 and Ser136 by Western blotting using phospho-specific antibodies. PMA stimulated the phosphorylation of Bad in Ser112 without affecting the phosphorylation status of Bad in Ser136 (Fig. 5B). Interestingly, Bad phosphorylation in Ser112 was impaired upon PKCϵ depletion (Fig. 5C). On the other hand, adenoviral overexpression of PKCϵ significantly potentiated PMA-induced Ser112-Bad phosphorylation (Fig. 5D).
FIGURE 5.
PKCϵ inhibits Bad proapoptotic activity by phosphorylation at Ser112. A, expression of prosurvival Bcl-2 family members was determined in control and PKCϵ-depleted LNCaP cells. B, LNCaP cells were treated with PMA (100 nm, 0–60 min), and the levels of phospho-Ser112-Bad, phospho-Ser136-Bad, and total Bad were determined by Western blotting. C, LNCaP cells transfected with either PKCϵ or control RNAi duplexes were treated with PMA (100 nm, 1 h), and the levels of total and phospho-Ser112-Bad were determined by Western blotting. Three additional independent experiments gave similar results. D, LNCaP cells infected with either control (LacZ) or wild-type (WT)-PKCϵ AdV (m.o.i. = 1–10 pfu/cell) were treated with PMA (100 nm, 1 h), and phospho-Ser112-Bad levels were determined by Western blotting. Two additional independent experiments gave similar results. D, LNCaP cells were transfected with WT-, S112A-, or S112A/S136A-Bad mutants followed by PMA (100 nm, 1 h). The percentage of apoptotic cells was determined 24 h later. Data are expressed as the mean ± S.E. (error bars) of three independent experiments. F, expression and phosphorylation status of wild-type and mutant Bad proteins from experiment in E are shown. **, p < 0.01 versus wild-type Bad.
To establish the functional significance of this phosphorylation event, we next investigated the effect of expressing a nonphosphorylatable Bad mutant (Ser to Ala in position 112) on PMA-induced cell death. We also expressed a double mutant (S112A/S136A-Bad), wild-type Bad, or empty vector. Expression of these mutants in LNCaP cells has an inhibitory effect on growth factor-induced survival (36). Although the three Bad proteins were readily detected by Western blotting, a phospho-Ser112-Bad antibody only detected wild-type Bad, as expected (Fig. 5E). Notably, both S112A-Bad and S112A/S136A-Bad enhanced the apoptotic response to the phorbol ester (Fig. 5F). Essentially identical results were observed by expressing GST-Bad mutant constructs (data not shown). These results indicate that PKCϵ is implicated in Ser112-Bad phosphorylation in response to PMA.
PKCϵ Depletion Does Not Affect the Regulation of Akt, ERK, and Rsk by PMA
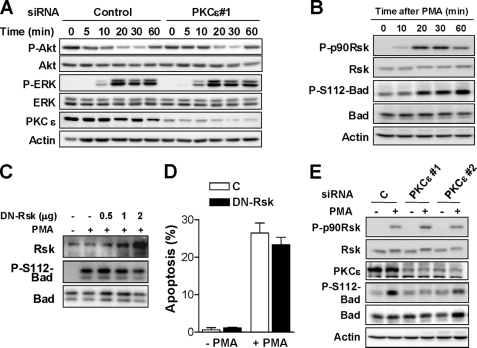
In the next series of experiments, we investigated the potential involvement of PKCϵ in the control of prosurvival pathways known to converge on Bad. We have previously established that activation of the ERK pathway counterbalances the apoptotic effect of PMA in LNCaP cells, as determined by the ability of a MEK inhibitor to enhance the death response of the phorbol ester (21). PMA treatment also causes Akt dephosphorylation in LNCaP cells via activation of a protein phosphatase 2A (PP2A), and PMA-induced apoptosis is rescued by expression of constitutively active (Myr) Akt1 (21). Because both Akt and ERK have been implicated in Bad phosphorylation (35, 38), one possibility is that the prosurvival effect of PKCϵ relates to a differential activation of these pathways. However, we found that PKCϵ-depleted and control LNCaP cells had essentially identical patterns of activation of ERK and inactivation of Akt in response to PMA (Fig. 6A), suggesting that the survival effect of PKCϵ is independent of either pathway.
FIGURE 6.
PKCϵ depletion does not affect the regulation of Akt, ERK, and Rsk by PMA in LNCaP cells. A, LNCaP cells transfected with either PKCϵ #1 or control RNAi duplexes were treated with PMA (100 nm) for various times (0–60 min). Activation of Akt and ERK was determined by Western blotting using phospho-specific antibodies. B, LNCaP cells were treated with PMA (100 nm) for various times (0–60 min). Phospho-Ser380-p90Rsk and total Rsk levels were determined by Western blotting. C, LNCaP cells co-expressing HA-Bad and either a control empty vector (-Rsk) or increasing amounts of a dominant-negative Rsk (DN-Rsk) were treated with PMA (100 nm, 1 h). Phospho-Ser112-Bad levels were determined by Western blotting D, percentage of apoptotic cells in response to PMA was determined in LNCaP cells transfected with either a plasmid encoding DN-Rsk or empty vector. Data are expressed as mean ± S.E. (error bars). E, LNCaP cells transfected with either PKCϵ (#1 and #2) or control RNAi duplexes were treated with PMA (100 nm, 30 min), and phospho-Ser380-p90Rsk levels were determined by Western blotting. In all cases, a representative experiment is shown. Similar results were observed in at least three independent experiments.
The MAPK-activated p90 ribosomal S6 kinase (p90Rsk) can also phosphorylate Bad at Ser112 in response to phorbol esters in certain cell types (39). Although we found that Rsk becomes phosphorylated in LNCaP cells in response to PMA (Fig. 6B), expression of increasing amounts of a dominant negative (DN)-Rsk mutant did not affect PMA-induced phosphorylation of Ser112-Bad (Fig. 6C). Moreover, expression of DN-Rsk (4 times higher than endogenous Rsk) did not alter apoptosis induced by PMA (Fig. 6D). Consistent with this result, we observed that PKCϵ depletion did not affect the phosphorylation of p90Rsk in response to PMA (Fig. 6E). The conclusions of these experiments are two: first, Rsk activation in response to PMA is independent of PKCϵ; and second, Rsk is not involved in the apoptotic effect of PMA in LNCaP cells.
PKCϵ Phosphorylates Bad in Ser112 in Vitro
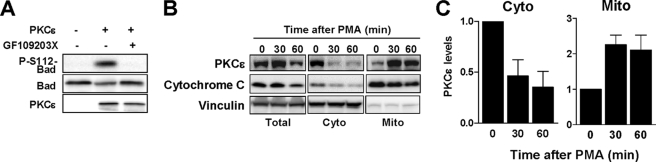
We hypothesized that Bad could be a PKCϵ substrate in LNCaP cells. Using the NetPhosK web server we were able to identify a PKC consensus phosphorylation site at Ser112 of Bad. The score of 0.63 for PKCϵ was in a similar range as for other well established kinases for that site (Akt, 0.79; PKA, 0.62; Rsk, 0.58). To test whether Bad is a direct PKCϵ substrate, an in vitro phosphorylation assay using recombinant proteins was carried out. As shown in Fig. 7A, PKCϵ strongly phosphorylated Bad in Ser112. This effect was abolished by the pan-PKC inhibitor GF109203X. Therefore, Bad is a PKCϵ substrate.
FIGURE 7.
PKCϵ localizes to mitochondrial fractions upon PMA treatment and phosphorylates Bad in Ser112in vitro. A, PKCϵ kinase reactions were carried out using recombinant proteins as described under “Experimental Procedures.” Phosphorylation of Bad in Ser112 was analyzed by Western blotting. The pan-PKC inhibitor GF109203X was used at 5 μm. B, mitochondria were isolated from PMA-treated cells, and the levels of PKCϵ in cytosolic and mitochondrial fractions were determined by Western blotting. C, densitometric analysis of four independent experiments is expressed as mean ± S.E. (error bars).
PKCϵ Accumulates in Mitochondria upon PMA Stimulation
Because unphosphorylated Bad localizes to the mitochondria (37), we speculated that PKCϵ should relocalize to mitochondria in response to phorbol ester stimulation to phosphorylate Bad. LNCaP cells were treated with PMA for different times, followed by subcellular fractionation to isolate mitochondria and determination of PKCϵ levels by Western blotting. As a purity control for the mitochondrial fraction, we used cytochrome c. Remarkably, PMA treatment led to a significant accumulation of PKCϵ in the mitochondrial fraction with a concomitant reduction of cytosolic PKCϵ (Fig. 7B). A densitometric analysis of PKCϵ levels in mitochondrial and cytosolic fractions from multiple experiments is presented in Fig. 7C. Thus, PKCϵ redistributes to mitochondria upon activation, suggesting its access to mitochondrial Bad.
DISCUSSION
It has been known for years that pharmacological activation of PKC leads to apoptosis of androgen-dependent prostate cancer cells and sensitizes cells to antitumor agents and radiation (23, 40). Studies from several laboratories, including ours, established PKCδ as the key mediator of phorbol ester-induced cell death in LNCaP cells (19–21, 24). Because phorbol esters do not discriminate between cPKCs and nPKCs, other approaches were needed to unmask isozyme-specific effects. The role of PKCϵ was controversial, as this PKC was shown to either inhibit or stimulate apoptosis in prostate cancer cells (17, 22–24). Early studies by Terrian and co-workers demonstrated that overexpression of PKCϵ in LNCaP cells promotes growth and the transition from androgen-dependence to androgen-independence (17). On the other hand, studies by Powell's group showed that overexpression of PKCϵ had no effect on PMA-induced apoptosis of LNCaP cells or could even inhibit apoptosis (23, 24). Moreover, it has been shown that PKCϵ overexpression sensitizes LNCaP cells to the apoptotic effect of the PKC ligand bryostatin 1. The fact that overexpression of PKCϵ has been recently shown to modulate the action of chemotherapeutic agents in prostate cancer cells (41) and the identification of a microRNA that exerts tumor suppressive functions in human prostate through down-regulation of PKCϵ (42) argue for the need of a better understanding of the effects of this nPKC in prostate cancer cells. Emerging evidence suggests that PKCϵ contributes to tumorigenesis in prostate cancer through its stimulatory effects on proliferation, anchorage-independent growth, transition to androgen independence, and invasiveness, as well as via its inhibitory effects on cell death (17, 22, 43). Our studies using RNAi, dominant negative, and wild-type overexpression approaches unambiguously demonstrated that PKCϵ acts as a prosurvival kinase in LNCaP cells. Indeed, PKCϵ depletion by RNAi potentiates PMA-induced apoptosis, arguing that PKCϵ counterbalances the apoptotic function of PKCδ. Moreover, PKCϵ depletion sensitizes LNCaP cells to the death effect of TNFα, the primary effector of PKCδ-induced apoptosis (a model is presented in Fig. 8). It is interesting that PKCϵ depletion from DU145 cells, which do not undergo apoptosis in response to PMA, sensitizes these cells to apoptosis induced by the phorbol ester. PKCϵ RNAi depletion also sensitizes prostate cancer cells to the killing effect of ionizing radiation.4 Thus, together with studies in different cellular models by other groups (43), we conclude that PKCϵ has a broader survival function in response to physiological, pharmacological, and irradiation stimuli. For example, PKCϵ has been shown to protect different cell types from apoptosis induced by ceramide, cisplatin, TNFα, TRAIL, and TPA. Furthermore, the levels of PKCϵ were associated with chemoresistance (43).
FIGURE 8.
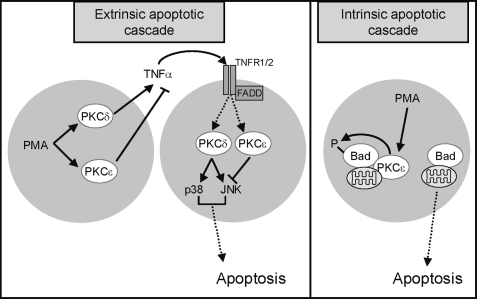
Model for signaling events involved in PKCϵ-mediated survival in LNCaP cells. PKCϵ is implicated both in the modulation of the extrinsic and intrinsic apoptotic cascades in LNCaP prostate cancer cells. PKCδ and PKCϵ have opposite effects on TNFα release and the subsequent activation of TNFα receptor signaling. Additionally, PKCϵ operates downstream of this death receptor by regulating JNK activation. PKCϵ also modulates the apoptotic intrinsic cascade through phosphorylation (and inactivation) of proapoptotic Bad.
Previous studies from our laboratory demonstrated that PKCδ activation in response to PMA promotes the autocrine release of death receptor ligands from prostate cancer cells and the subsequent activation of the extrinsic apoptotic cascade (20). PKCδ mediates PMA-induced release of TNFα, which promotes the activation of death receptors in LNCaP cells. Moreover, activation of p38 and JNK, well established effectors of TNFα, is observed in LNCaP cells in response to either PMA or CM collected from PMA-treated LNCaP cells. We found that PKCϵ is required for the release of TNFα, and consistently, the apoptotic activity of CM-PMA collected from PKCϵ-depleted LNCaP cells was higher than CM-PMA collected from control LNCaP cells. Our studies strongly argue for a dual negative role of PKCϵ in cytokine release and as a mediator of the death effect. Thus, in prostate cancer cells PKCϵ and PKCδ exert opposite effects both in death factor release and as a downstream effector of death factor receptors. Although death receptors do not couple directly to diacylglycerol generation, it has been shown that PKCs can become activated downstream of TNFα in neutrophils, pancreatic acinar cells, and intestinal cells (44–46). Ongoing studies in our laboratory are addressing the mechanistic basis of the PKC regulation downstream of death receptors in prostate cancer cells. Importantly, we found that activation of JNK downstream of TNFα was elevated in PKCϵ-depleted cells, whereas overexpression of PKCϵ diminished the activation of JNK. Consistent with these results, JNK is a major mediator of apoptosis by phorbol esters and other stimuli in prostate cancer cells (26, 47).
Our studies also provide evidence for the involvement of the intrinsic apoptotic pathway in the PMA response (see model in Fig. 8). PMA treatment leads to Bad phosphorylation in Ser112 in LNCaP cells. Phosphorylation of Bad, which is known to modulate Bad interaction with Bcl-XL, is lost in PKCϵ-depleted cells. Accordingly, expression of the nonphosphorylatable mutant S112A-Bad potentiates the apoptotic effect of PMA. Expression of this mutant is known to reduce the survival effect of growth factors in LNCaP cells (36). Moreover, the results provided here suggest that in LNCaP cells Bad phosphorylation in response to PMA is independent of well established Bad kinases, including Akt, ERK, and p90Rsk. In Jurkat T cells, overexpression of constitutively active mutants of PKCα, PKCθ, and PKCϵ induces elevated levels of phospho-Ser112-Bad (48), thus preventing Fas-induced apoptosis. It might be interesting to determine whether additional levels of regulation of Bad by PKCϵ contribute to protection from apoptosis.
In summary, our studies provide evidence that PKCϵ is a pro-survival kinase in LNCaP prostate cancer cells via modulation of TNFα secretion and JNK activation, as well as through the regulation of Bad phosphorylation. PKCϵ protects LNCaP cells against several apoptotic stimuli, such as phorbol ester treatment and death receptor activation. As PKCϵ is markedly overexpressed in human prostate tumors, our results suggest a potential role for this PKC in the progression of the disease. Pharmacological targeting of PKCϵ may have therapeutic potential for prostate cancer or other cancers in which this PKC is overexpressed or hyperactive.
Acknowledgments
We thank Dr. Jeff Field (University of Pennsylvania) and Dr. Michael Greenberg (Harvard University) for the Bad-encoding plasmids. M. C. C. thanks Dr. Gonzalez-Guerrico for technical training.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-CA89202 (to M. G. K.).
H. Wang and M. G. Kazanietz, unpublished observations.
- PKC
- protein kinase C
- cPKC
- classical protein kinase C
- nPCK
- novel PKC
- aPKC
- atypical PKC
- AdV
- adenovirus
- DN
- dominant negative
- m.o.i.
- multiplicity of infection
- Veh
- vehicle.
REFERENCES
- 1.Blumberg P. M. (1988) Cancer Res. 48, 1–8 [PubMed] [Google Scholar]
- 2.Griner E. M., Kazanietz M. G. (2007) Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 3.Mellor H., Parker P. J. (1998) Biochem. J. 332, 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borner C., Ueffing M., Jaken S., Parker P. J., Weinstein I. B. (1995) J. Biol. Chem. 270, 78–86 [DOI] [PubMed] [Google Scholar]
- 5.Brodie C., Blumberg P. M. (2003) Apoptosis 8, 19–27 [DOI] [PubMed] [Google Scholar]
- 6.Mischak H., Goodnight J. A., Kolch W., Martiny-Baron G., Schaechtle C., Kazanietz M. G., Blumberg P. M., Pierce J. H., Mushinski J. F. (1993) J. Biol. Chem. 268, 6090–6096 [PubMed] [Google Scholar]
- 7.Fukumoto S., Nishizawa Y., Hosoi M., Koyama H., Yamakawa K., Ohno S., Morii H. (1997) J. Biol. Chem. 272, 13816–13822 [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M., Oliva J. L., Kothapalli D., Fournier A., Assoian R. K., Kazanietz M. G. (2005) J. Biol. Chem. 280, 33926–33934 [DOI] [PubMed] [Google Scholar]
- 9.Kampfer S., Windegger M., Hochholdinger F., Schwaiger W., Pestell R. G., Baier G., Grunicke H. H., Uberall F. (2001) J. Biol. Chem. 276, 42834–42842 [DOI] [PubMed] [Google Scholar]
- 10.Schönwasser D. C., Marais R. M., Marshall C. J., Parker P. J. (1998) Mol. Cell. Biol. 18, 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiroshita N., Musashi M., Sakurada K., Kimura K., Tsuda Y., Ota S., Iwasaki H., Miyazaki T., Kato T., Miyazaki H., Shimosaka A., Asaka M. (2001) J. Pharmacol. Exp. Ther. 297, 868–875 [PubMed] [Google Scholar]
- 12.Lu D., Huang J., Basu A. (2006) J. Biol. Chem. 281, 22799–22807 [DOI] [PubMed] [Google Scholar]
- 13.Okhrimenko H., Lu W., Xiang C., Hamburger N., Kazimirsky G., Brodie C. (2005) Cancer Res. 65, 7301–7309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz M. H., Manoharan H. T., Church D. R., Dreckschmidt N. E., Zhong W., Oberley T. D., Wilding G., Verma A. K. (2007) Cancer Res. 67, 8828–8838 [DOI] [PubMed] [Google Scholar]
- 15.Cornford P., Evans J., Dodson A., Parsons K., Woolfenden A., Neoptolemos J., Foster C. S. (1999) Am. J. Pathol. 154, 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koren R., Ben Meir D., Langzam L., Dekel Y., Konichezky M., Baniel J., Livne P. M., Gal R., Sampson S. R. (2004) Oncol. Rep. 11, 321–326 [PubMed] [Google Scholar]
- 17.Wu D., Foreman T. L., Gregory C. W., McJilton M. A., Wescott G. G., Ford O. H., Alvey R. F., Mohler J. L., Terrian D. M. (2002) Cancer Res. 62, 2423–2429 [PubMed] [Google Scholar]
- 18.Day M. L., Zhao X., Wu S., Swanson P. E., Humphrey P. A. (1994) Cell Growth Differ. 5, 735–741 [PubMed] [Google Scholar]
- 19.Fujii T., García-Bermejo M. L., Bernabó J. L., Caamaño J., Ohba M., Kuroki T., Li L., Yuspa S. H., Kazanietz M. G. (2000) J. Biol. Chem. 275, 7574–7582 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Guerrico A. M., Kazanietz M. G. (2005) J. Biol. Chem. 280, 38982–38991 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka Y., Gavrielides M. V., Mitsuuchi Y., Fujii T., Kazanietz M. G. (2003) J. Biol. Chem. 278, 33753–33762 [DOI] [PubMed] [Google Scholar]
- 22.McJilton M. A., Van Sikes C., Wescott G. G., Wu D., Foreman T. L., Gregory C. W., Weidner D. A., Harris Ford O., Morgan Lasater A., Mohler J. L., Terrian D. M. (2003) Oncogene 22, 7958–7968 [DOI] [PubMed] [Google Scholar]
- 23.Powell C. T., Yin L. (2006) Int. J. Cancer 118, 1572–1576 [DOI] [PubMed] [Google Scholar]
- 24.Yin L., Bennani-Baiti N., Powell C. T. (2005) J. Biol. Chem. 280, 5533–5541 [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Guerrico A. M., Meshki J., Xiao L., Benavides F., Conti C. J., Kazanietz M. G. (2005) J. Biochem. Mol. Biol. 38, 639–645 [DOI] [PubMed] [Google Scholar]
- 26.Xiao L., Eto M., Kazanietz M. G. (2009) J. Biol. Chem. 284, 29365–29375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao L., Caino M. C., von Burstin V. A., Oliva J. L., Kazanietz M. G. (2008) Methods Enzymol. 446, 123–139 [DOI] [PubMed] [Google Scholar]
- 28.Shinohara H., Kayagaki N., Yagita H., Oyaizu N., Ohba M., Kuroki T., Ikawa Y. (2001) Biochem. Biophys. Res. Commun. 284, 1162–1167 [DOI] [PubMed] [Google Scholar]
- 29.Cacace A. M., Ueffing M., Han E. K., Marmè D., Weinstein I. B. (1998) J. Cell Physiol. 175, 314–322 [DOI] [PubMed] [Google Scholar]
- 30.Xiao L., Gonzalez-Guerrico A., Kazanietz M. G. (2009) Mol. Carcinog. 48, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chopra D. P., Menard R. E., Januszewski J., Mattingly R. R. (2004) Cancer Lett. 203, 145–154 [DOI] [PubMed] [Google Scholar]
- 32.Yu R., Mandlekar S., Ruben S., Ni J., Kong A. N. (2000) Cancer Res. 60, 2384–2389 [PubMed] [Google Scholar]
- 33.Sivaprasad U., Shankar E., Basu A. (2007) Cell Death Differ. 14, 851–860 [DOI] [PubMed] [Google Scholar]
- 34.Steinberg R., Harari O. A., Lidington E. A., Boyle J. J., Nohadani M., Samarel A. M., Ohba M., Haskard D. O., Mason J. C. (2007) J. Biol. Chem. 282, 32288–32297 [DOI] [PubMed] [Google Scholar]
- 35.Sastry K. S., Karpova Y., Kulik G. (2006) J. Biol. Chem. 281, 27367–27377 [DOI] [PubMed] [Google Scholar]
- 36.Sastry K. S., Smith A. J., Karpova Y., Datta S. R., Kulik G. (2006) J. Biol. Chem. 281, 20891–20901 [DOI] [PubMed] [Google Scholar]
- 37.Zha J., Harada H., Yang E., Jockel J., Korsmeyer S. J. (1996) Cell 87, 619–628 [DOI] [PubMed] [Google Scholar]
- 38.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. (1997) Cell 91, 231–241 [DOI] [PubMed] [Google Scholar]
- 39.Tan Y., Ruan H., Demeter M. R., Comb M. J. (1999) J. Biol. Chem. 274, 34859–34867 [DOI] [PubMed] [Google Scholar]
- 40.Garzotto M., Haimovitz-Friedman A., Liao W. C., White-Jones M., Huryk R., Heston W. D., Cardon-Cardo C., Kolesnick R., Fuks Z. (1999) Cancer Res. 59, 5194–5201 [PubMed] [Google Scholar]
- 41.Mukherjee S., Bhattacharya R. K., Roy M. (2009) J. Environ. Pathol. Toxicol. Oncol. 28, 269–282 [DOI] [PubMed] [Google Scholar]
- 42.Gandellini P., Folini M., Longoni N., Pennati M., Binda M., Colecchia M., Salvioni R., Supino R., Moretti R., Limonta P., Valdagni R., Daidone M. G., Zaffaroni N. (2009) Cancer Res. 69, 2287–2295 [DOI] [PubMed] [Google Scholar]
- 43.Basu A., Sivaprasad U. (2007) Cell. Signal. 19, 1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang Q., Tepperman B. L. (2003) Br. J. Pharmacol. 140, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilpatrick L. E., Sun S., Korchak H. M. (2004) Am. J. Physiol. Cell Physiol. 287, C633–C642 [DOI] [PubMed] [Google Scholar]
- 46.Satoh A., Gukovskaya A. S., Nieto J. M., Cheng J. H., Gukovsky I., Reeve J. R., Jr., Shimosegawa T., Pandol S. J. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287, G582–G591 [DOI] [PubMed] [Google Scholar]
- 47.Ikezoe T., Yang Y., Taguchi H., Koeffler H. P. (2004) Br. J. Cancer 90, 2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertolotto C., Maulon L., Filippa N., Baier G., Auberger P. (2000) J. Biol. Chem. 275, 37246–37250 [DOI] [PubMed] [Google Scholar]