Abstract
p27 is an atypical tumor suppressor that can regulate the activity of cyclin-dependent kinases and G0-to-S phase transitions. More recent studies reveal that p27 may also exhibit its tumor-suppressive function through regulating many other essential cellular events. However, the molecular mechanisms underlying these anticancer effects of p27 are largely unknown. In this study, we found that depletion of p27 expression by either gene knock-out or knockdown approaches resulted in up-regulation of both Hsp27 and Hsp70 expression at mRNA- and promoter-derived transcription as well as protein levels upon arsenite exposure, indicating that p27 provides a negative signal for regulating the expression of Hsp27 and Hsp70. Consistently, arsenite-induced activation of JNK2/c-Jun and HSF-1 pathways was also markedly elevated in p27 knock-out (p27−/−) and knockdown (p27 shRNA) cells. Moreover, interference with the expression or function of JNK2, c-Jun, and HSF-1, but not JNK1, led to dramatic inhibition of arsenite-induced Hsp27 and Hsp70 expression. Collectively, our results demonstrate that p27 suppresses Hsp27 and Hsp70 expression at the transcriptional level specifically through JNK2/c-Jun- and HSF-1-dependent pathways upon arsenite exposure, which provides additional important molecular mechanisms for the tumor-suppressive function of p27.
Keywords: AP-1 Transcription Factor, Cell Cycle, Gene Regulation, Heat Shock Protein, Signal Transduction, Arsenite
Introduction
Heat shock or stress proteins (Hsps),4 also known as molecular chaperones, play essential roles in protein biosynthesis, transport, translocation, and folding and are conserved among different species (1). There are four major families of mammalian Hsps according to their molecular size: Hsp90, Hsp70, Hsp60, and the small Hsps. Hsp90 and Hsp60 are constitutively expressed in mammalian cells, and Hsp27 and Hsp70 are inducible by stress such as heat, oxidative stress, or anticancer drugs (2). Hsp27 and Hsp70 are reported to be highly expressed in malignant cells, causing oncogenesis and chemotherapy resistance (3). Thus, both Hsp27 and Hsp70 attract more attention as pharmacological targets.
p27 (encoded by CDKN1B) is a member of a family of cyclin-dependent kinase inhibitors, which bind to cyclin/cyclin-dependent kinase complexes and cause cell cycle arrest. p27 works as a putative tumor suppressor gene and plays a critical role in the pathogenesis of several human malignant cancers, and its down-regulation has been correlated with poor prognosis of cancer patients (4). However, the molecular mechanisms linking p27 to cancer development remain largely unknown. Thus, elucidation of novel molecular mechanisms involved in its tumor-suppressive function is of high significance.
Arsenite is a well known human carcinogen and is implicated in the development of various cancers, including skin, lung, and urinary bladder (5, 6). Arsenite occurs naturally in the earth's crust and is widely distributed in the environment. Our previous studies have demonstrated that arsenite exposure is able to initiate both cell apoptosis and cell transformation through different signaling pathways, dependent on dosages of arsenite exposure in various cell lines (7–11). In this study, we found, for the first time to our knowledge, that p27 mediated the suppression of Hsp27 and Hsp70 induction by arsenite through inhibiting JNK2/c-Jun- and HSF-1 (heat shock factor 1)-dependent transcriptional activation, which may provide novel molecular mechanisms underlying the tumor-suppressive function of p27 in the arsenite response.
MATERIALS AND METHODS
Cell Culture
Immortalized wild-type p27 (p27+/+) and p27-deficient (p27−/−) mouse embryonic fibroblasts (MEFs) (12), HSF-1+/+ and HSF-1−/− MEFs (13, 14), and their stable transfectants were maintained at 37 °C in a 5% CO2 incubator with DMEM supplemented with 10% FBS (Nova Tech Inc., Grand Island, NE), 2 mm l-glutamine, and 25 μg/ml gentamycin. Mouse epidermal JB6 Cl41 cells and their stable transfectants were cultured in minimum essential medium with 5% FBS. The cultures were dissociated with trypsin and transferred to new 75-cm2 culture flasks (Fisher) twice each week. Sodium arsenite was purchased from Aldrich.
Constructs and Transfection
The shRNA constructs against p27, JNK1, and JNK2 were purchased from Open Biosystems (Thermo Fisher Scientific) and were stably transfected into MEFs or JB6 Cl41 cells. The stable transfectants were established by puromycin selection. The hsp27 promoter-luciferase reporter (−1090 to +15) was kindly provided by Dr. Suzanne A. W. Fuqua (Lester and Sue Smith Breast Center, Baylor College of Medicine, Houston, TX) (15). The hsp70-luciferase reporter (−2589 to +183) was a generous gift from Dr. Alice Liu (Department of Cell Biology and Neuroscience, Division of Life Sciences, Rutgers State University of New Jersey, Piscataway, NJ) (16). A mutant of a putative AP-1-binding site (from +80TGACT+84 to +80GTCTG+84) in human the hsp70 promoter region was generated by gene synthesis and inserted into the pGL3-basic vector following KpnI and BglII digestion and is referred to as hsp70-Luc-AP-1-mutant.
Recombinant Adenovirus Construct and Infection
Recombinant adenoviral (Ad) vectors were constructed using the AdEasy vector system (Quantum Biotechnologies). p27 cDNA was subcloned into the shuttle plasmid pAdTrack, containing a cytomegalovirus-driven GFP marker gene and arms of homology to the left and right ends of the Ad5 genome flanking a plasmid backbone containing the kanamycin resistance gene. Shuttle plasmids were linearized and co-electroporated into the recombinogenic Escherichia coli BJ5183 strain with the 30-kb supercoiled plasmid pAdEasy, containing the Ad genome in an ampicillin-resistant plasmid. Transformants were selected on kanamycin plates, miniprep DNA from resistant colonies was screened by restriction digest, and clones showing the correct restriction pattern were retransformed into E. coli DH10 to prevent recombination. Virus stocks were produced by transfection of recombinant Ad genomes into HEK293 cells. Infected HEK293 cells were lysed by a freeze-thaw protocol. Cell debris was removed by centrifugation, and the supernatants were saved at −80 °C until used. Viral titers were determined using the tissue culture ID50 assay on HEK293 cells. For infection, selective recombinant Ad was added to cells for 24 h, and >80% of the cells showed GFP green fluorescence at 24 h post-infection.
RT-PCR
3, 6, or 12 h post-arsenite exposure, cells were collected, and total RNA was extracted from the cells using TRIzol reagent (Invitrogen). Total cDNAs were synthesized using the ThermoScriptTM RT-PCR system (Invitrogen). The mRNA amount present in the cells was measured by semiquantitative RT-PCR. The primers for mouse hsp70 were 5′-CGA CCT GAA CAA GAG CAT CA-3′ and 5′-ATG ACC TCC TGG CAC TTG TC-3′, and those for mouse hsp27 were 5′-CCT CTT CGA TCA AGC TTT CG-3′ and 5′-CTC AGG GGA TAG GGA AGA GG-3′. The control mouse β-actin mRNA was also detected by RT-PCR using primers 5′-GAC GAT GAT ATT GCC GCA CT-3′ and 5′-GAT ACC ACG CT T GCT CTG AG-3′. The PCR products were separated on 2% agarose gels and stained with ethidium bromide, and the images were scanned under UV light.
Luciferase Reporter Assay
p27+/+ and p27−/− MEFs were transiently transfected with wild-type or mutant hsp27 or hsp70 promoter-luciferase reporter constructs in combination with the pRL-TK vector (Promega) as an internal control. After the cells were subjected to arsenite treatment, the luciferase activities were determined using a luminometer (Wallac 1420 VICTOR2 multilabel counter system) as described previously (17).
Western Blotting
After exposure to arsenite as indicated in the figure legends, the cells were washed twice with ice-cold PBS and collected with cell lysis buffer (10 mm Tris-HCl (pH 7.4), 1% SDS, and 1 mm Na3VO4). The cell extracts were sonicated, denatured by heating at 100 °C for 5 min, and quantified with a DC protein assay kit (Bio-Rad). Equal aliquots of cell extracts were separated on SDS-polyacrylamide gels. The proteins were then transferred to PVDF membranes (Bio-Rad), blocked, and probed with antibody against Hsp27 or HSF-1 (Stressgen, Ann Arbor, MI); Hsp70, non-phosphorylated c-Jun, JNK1/2, p38, phospho-Ser63/Ser73 c-Jun, phospho-Thr183/Tyr185 JNK, phospho-Ser257/Thr261 MKK4, phospho-Ser271/Thr275 MKK7, phospho-Thr308/Ser473 AKT (Cell Signaling Technology, Beverly, MA); JNK1 (BioSource, Invitrogen); p27 (Santa Cruz Biotechnology Inc., Santa Cruz, CA); or β-actin (Sigma). Primary antibody-bound proteins were detected using an alkaline phosphatase-linked secondary antibody and an ECF Western blotting system (Amersham Biosciences).
EMSAs
EMSAs were performed with a LightShift® chemiluminescent EMSA kit (Pierce). Binding reactions were performed for 25 min at room temperature in 100 mm Hepes (pH 7.6), 5 mm EDTA, 50 mm (NH4)2SO4, 5 mm dithiothreitol, 1 μg/ml poly(dI·dC), 20 fmol of biotin 3′-end-labeled double-stranded oligonucleotides, and 5 μg of nuclear extracts. The nucleotide sequences of the double-stranded oligonucleotides were as follows: 5′-AGT ACT GTC TTA GTC AGG ATT T-3′ and 5′-AAA TCC TGA CTA AGA CAG TAC T-3′ for the putative AP-1-binding site in the mouse hsp27 promoter and 5′-GGA CTC TTG ACT CAG AGC ACA-3′ and 5′-TGT GCT CTG AGT CAA GAG TCC-3′ (for the putative AP-1-binding site in the mouse hsp70 promoter). A 30- or 50-fold excess of unlabeled probe was used as a competitor to test the specificity of the signals. The DNA–protein complexes were separated on a native 5% polyacrylamide gel at 100 V and then transferred to a nylon membrane. The positions of the biotin end-labeled oligonucleotides were detected by a chemiluminescent reaction according to the manufacturer's instructions (Pierce).
ChIP Assay
The ChIP assay was performed with an EZ ChIP kit (Upstate) according to the manufacturer's protocol (17). Briefly, p27+/+ and p27−/− cells were left untreated or treated with arsenite (20 μm) for 9 h, and then genomic DNA and the proteins were cross-linked with 1% formaldehyde for 10 min at room temperature. The cross-linked cells were pelleted, resuspended in lysis buffer, and sonicated to generate 200–500-bp chromatin DNA fragments using a Sonic Dismembrator 550 (Thermo Fisher Scientific) for 2 min at output level 3. After centrifugation, the supernatants were diluted 10-fold and precleared by protein G-agarose. 1% of the supernatant was taken as the input at this step. The rest of the lysis buffer was incubated with anti-c-Jun antibody (Santa Cruz Biotechnology Inc.) at 4 °C overnight. The immune complex was captured with protein G-agarose saturated with salmon sperm DNA and then eluted with elution buffer. DNA–protein cross-linking was reversed by heating at 65 °C for 4 h. DNA was purified and subjected to PCR analysis. To specifically amplify the region containing the putative AP-1-binding sites on the mouse hsp27 promoter, PCR was performed with the following pair of primers: 5′-TTC CAG GTT CGA TGT CTC CT-3′ (forward) and 5′-GTG GAA AGT GTC CGC TGA AT-3′ (reverse). The primers that targeted the non-related region ∼1 kb downstream of the AP-1-binding sites on the hsp27 promoter were also used in the PCR analysis to support the specificity of the ChIP assay: 5′-TCC AGC TAC CGG TAT TAC GC-3′ (forward) and 5′-TAA TGG CAA TGA CCG TCT CA-3′ (reverse). The PCR conditions were as follows: 94 °C for 3 min and 32 cycles at 94 °C for 20 s, 62 °C for 30 s, and 72 °C for 30 s. The primers used to detect the putative AP-1-binding sites on the mouse hsp70 promoter region were 5′-CAT GGT TGT GCT TTC ACT GG-3′ (forward) and 5′-GGC TGT CCT GGA ACT CAC TC-3′ (reverse). The PCR conditions were as follows: 94 °C for 3 min and 32 cycles at 94 °C for 20 s, 61 °C for 30 s, and 72 °C for 8 s. The PCR products were separated on 2% agarose gels with ethidium bromide staining, and the image was scanned under UV light.
Statistical Analysis
The significance of the difference between the treated and untreated groups was determined with the Wilcoxon rank-sum test (one tail). The results are expressed as means ± S.D.
RESULTS
p27 Suppresses Arsenite-induced Hsp27 and Hsp70 Expression
Hsp27 and Hsp70 are inducible chaperones and play essential roles in protein biosynthesis, transport, translocation, and folding (3). To exploit the role of p27 in Hsp27 and Hsp70 induction in the cellular response to arsenite exposure, genetically disrupted cells from p27−/− mouse embryos were employed. As shown in Fig. 1A, in contrast to the marginal induction of Hsp27 and Hsp70 proteins by arsenite in p27+/+ MEFs, much higher induction of both proteins was observed in p27−/− MEFs when the cells were exposed to arsenite under the same experimental conditions, indicating that the presence of p27 expression in p27+/+ MEFs suppresses Hsp27 and Hsp70 induction upon arsenite exposure. To further confirm that the increased Hsp27 and Hsp70 induction in p27−/− cells was due directly to p27 gene deficiency rather than to changes in other genes during establishment of the cells, the full-length p27 gene was transiently expressed in p27−/− cells by an Ad delivery system. As expected, ectopic expression of p27 in p27−/− cells decreased Hsp27 and Hsp70 induction upon arsenite exposure (Fig. 1B). We then verified our findings in p27 knockdown MEFs using p27 shRNA. As shown in Fig. 1C, decreasing p27 expression by shRNA also elevated Hsp27 and Hsp70 induction upon arsenite exposure in comparison with that in cells expressing non-silencing RNAs under the same experimental conditions. However, the increment in Hsp27 and Hsp70 induction observed in knockdown cells was not as dramatic as that in knock-out cells even though the knockdown efficiency was >85% (Fig. 1, A and C). These results suggested that the remaining <15% of the p27 protein in shRNA transfectants still had a strong inhibitory effect on Hsp27 and Hsp70 expression. Of note, the -fold induction of Hsp27 and Hsp70 proteins in p27+/+ MEFs and non-silencing MEFs was different regarding the cellular response to arsenite exposure (Fig. 1, A and C). This could be due to different embryonic development stages when the two MEFs were generated or different animal strain backgrounds (2). Taken together, our results provide direct and clear evidence demonstrating that p27 initiates a strong inhibitory effect on Hsp27 and Hsp70 induction upon arsenite exposure. In contrast to the increase in Hsp27 and Hsp70 induction in p27−/− MEFs, the cell death induced by arsenite was markedly decreased in p27−/− MEFs in comparison with wild-type cells (Fig. 1D). This result was also reproducible in p27 knockdown cells. As shown in Fig. 1E, caspase-3 activation and poly(ADP-ribose) polymerase cleavage, the hallmarks for cell apoptosis, were both attenuated in p27 knockdown cells. Taken together, our results suggest that p27 facilitates arsenite-associated cell death but suppresses Hsp27 and Hsp70 induction.
FIGURE 1.
p27 suppresses Hsp27 and Hsp70 induction upon arsenite exposure. A, immortalized p27+/+ and p27−/− MEFs were exposed to arsenite at different doses and time periods as indicated. Hsp27 and Hsp70 protein expression was determined by Western blot assay. β-Actin was used as a loading control. B, 24 h after p27−/− cells were infected with Ad carrying full-length GFP-tagged p27 cDNA or a GFP vector control, the cells were treated with arsenite for 12 h. The protein expression levels of Hsp27, Hsp70, and p27 were determined by Western blotting. C, mouse p27 shRNA or non-silencing control shRNA was stably transfected into WT MEFs, and the knockdown efficiency was confirmed by Western blot assay (left panel). The same shRNA transfectants were exposed to arsenite for 9 h. The induction of Hsp27 and Hsp70 was evaluated by Western blotting (right panel). D, p27+/+ and p27−/− MEFs were treated with 20 μm arsenite for 12 h, and images were taken under an inverted microscope to record cell death. E, p27 knockdown cells and control cells were exposed to arsenite, and caspase-3 activation and poly(ADP-ribose) polymerase (PARP) cleavage were determined by Western blot assay.
p27 Inhibits hsp27 and hsp70 Gene Transcription in Response to Arsenite
RT-PCR was conducted to determine whether the inhibitory effect of p27 on Hsp27 and Hsp70 induction upon arsenite exposure occurs at the mRNA level. As shown in Fig. 2A, consistent with protein expression, the induction of hsp27 and hsp70 mRNAs was barely observed in p27+/+ cells upon arsenite treatment for the different time points tested, whereas in p27−/− cells, hsp27 and hsp70 mRNAs were markedly induced by arsenite in a time-dependent manner. The basal mRNA level of the hsp70 gene was also higher in p27−/− cells. Next, we used luciferase reporters containing hsp27 and hsp70 promoters to detect the transcriptional activation of these two genes. As shown in Fig. 2B, the transcription of hsp27 and hsp70 was significantly elevated upon arsenite exposure in p27−/− cells, whereas the transcription of hsp27 and hsp70 in p27+/+ cells was relatively low. These results indicate that p27 expression provides a strong suppressing signaling for hsp27 and hsp70 expression at the transcriptional level in the cellular response to arsenite exposure.
FIGURE 2.
p27 down-regulates hsp27 and hsp70 transcriptional induction in response to arsenite. A, after p27+/+ and p27−/− MEFs were treated with 20 μm arsenite for 3, 6, and 12 h, RT-PCR was conducted to determine the mRNA expression levels of hsp27 and hsp70. B, hsp27 and hsp70 promoter-driven luciferase (Luc) reporters were transiently transfected into p27+/+ and p27−/− cells in combination with the pRL-TK vector as an internal control. 48 h post-transfection, the cells were treated with 20 μm arsenite for 12 h. The luciferase activities were then evaluated as described under “Materials and Methods.” The results are presented as luciferase activity relative to the medium control. Each bar indicates the mean ± S.D. of triplicate assay wells. *, significant difference in -fold induction between p27−/− and p27+/+ cells (p < 0.05).
AP-1 and HSF-1 Are Both Involved in Hsp27 and Hsp70 Induction by Arsenite
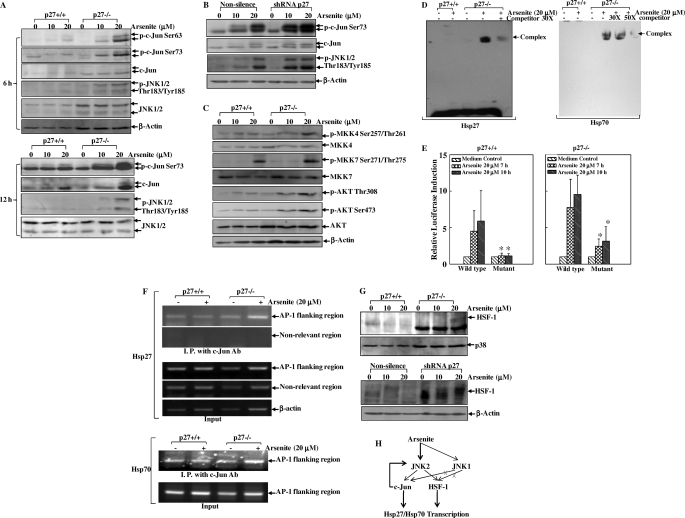
Hsps are subject to transcriptional regulation by transcription factors such as HSF-1 and AP-1. Bioinformatics analysis using TFSEARCH software (Version 1.3) revealed that the promoter region of the mouse hsp27 gene contains the putative DNA-binding site of AP-1 (−1776TTAGTCA−1770, relative to the transcription initial site). There is also a putative AP-1-binding site (−2987TGACTCA−2981) in the mouse hsp70 promoter region. However, to the best of our knowledge, there are few reports regarding the regulatory role of AP-1 in hsp27 and hsp70 transcriptional control. Therefore, we tested whether c-Jun/AP-1 is involved in Hsp27 and Hsp70 induction in response to arsenite by applying TAM67, a dominant-negative mutant form of c-Jun with a deletion of the transactivation domain (18). The validation of TAM67 upon c-Jun activation is confirmed in Fig. 3A, which shows that overexpression of TAM67 blocked c-Jun phosphorylation at Ser73, the requisite for its activation. More importantly, overexpression of TAM67 impaired Hsp27 and Hsp70 induction by arsenite (Fig. 3B), indicating that c-Jun/AP-1 is required for Hsp27 and Hsp70 induction in response to arsenite exposure. Because JNKs are the major kinases responsible for the phosphorylation and activation of c-Jun in the cellular response to stress conditions, we further used shRNAs targeting JNK1 and JNK2 to knock down endogenous JNK1 and JNK2 expression, respectively, to investigate their roles in Hsp27 and Hsp70 induction upon arsenite exposure. The efficiencies of shRNAs are shown in Fig. 3C. Introduction of shRNA against JNK2 inhibited c-Jun phosphorylation at Ser73 (Fig. 3C) and consequently attenuated Hsp27 and Hsp70 induction upon arsenite exposure (Fig. 3D), whereas knockdown of JNK1 by shRNA did not show obvious effects on either c-Jun phosphorylation (Fig. 3C) or Hsp27/Hsp70 induction (Fig. 3D). Therefore, our results suggest that JNK2, rather than JNK1, is required for Hsp27 and Hsp70 induction in the cellular response to arsenite exposure. HSF-1 is reported to participate in Hsp induction under many conditions (19). In this study, we also used HSF-1-deficient cells (HSF-1−/− MEFs) (13, 14) and found out that HSF-1 was also essential for both Hsp27 and Hsp70 induction in the cellular response to arsenite exposure (Fig. 3E).
FIGURE 3.
Induction of Hsp27 and Hsp70 by arsenite requires the JNK2/c-Jun pathway and HSF-1 activation. A and B, the TAM67 construct or the empty vector was transfected into mouse epidermal JB6 Cl41 cells, and the stable transfectants were established by G418 selection. The phosphorylation of c-Jun at Ser73 at 16 h after arsenite exposure (A) and the expression levels of Hsp27 and Hsp70 at 24 h after arsenite exposure (B) were detected by Western blotting. C and D, mouse JNK1 or JNK2 shRNA or non-silencing control shRNA was transfected into JB6 Cl41 cells, and the stable transfectants were established by puromycin selection. The phosphorylation of c-Jun at Ser73 (C) and the induction of Hsp27 and Hsp70 (D) in those stable transfectants upon arsenite exposure were determined. E, HSF-1+/+ and HSF-1−/− MEFs were exposed to arsenite for 12 h, and Hsp27 and Hsp70 induction was detected by Western blotting.
Cross-talk between the JNK2/c-Jun Pathway and HSF-1 Activation in the Arsenite Response
Under normal physiological conditions, HSF-1 localizes in the cytoplasm in the form of a non-DNA-binding inactive monomer. Upon stress, HSF-1 undergoes trimerization, hyperphosphorylation, translocation to the nucleus, and binding to heat shock elements and thereby activates heat shock gene transcription (20). Thus, HSF-1 activation could be indicated by the retarded migration bands in the Western blot assay due to its hyperphosphorylation. As shown in Fig. 4A, in JNK2 shRNA cells, the hyperphosphorylation of HSF-1 was attenuated compared with that in non-silencing cells or JNK1 shRNA cells upon arsenite exposure, indicating that JNK2 might be specifically responsible for regulation of HSF-1 activation through facilitating its phosphorylation. Overexpression of TAM67 also led to the inhibition of HSF-1 hyperphosphorylation (Fig. 4B). Because TAM67 attenuated JNK activation at the same time (Fig. 4C), it was anticipated that c-Jun might not affect HSF-1 activation directly, instead forming a feedback loop on JNKs, in turn regulating HSF-1 phosphorylation. This was also supported by published findings that c-Jun up-regulates the expression of p75-Ras-GRF1, a guanine nucleotide exchange factor that results in an increase in the activity of GTP-Ras and PI3K (21), which are the well documented upstream activators of the JNK pathway (22). Collectively, our results suggest that HSF-1 activation is regulated by JNK2, not JNK1, in the cellular response to arsenite exposure (Fig. 5H). On the other hand, knock-out of HSF-1 did not show any obvious inhibition of JNK/c-Jun activation (Fig. 4D), suggesting that HSF-1 has no obvious effect on activation of the JNK/c-Jun pathway upon arsenite exposure.
FIGURE 4.
JNK2/c-Jun pathway regulates HSF-1 activation due to arsenite exposure. A and B, mouse JNK1 or JNK2 shRNA or non-silencing control shRNA stable transfectants of JB6 Cl41 cells were exposed to arsenite as indicated. HSF-1 activation was compared in those transfectants by detecting the retarded migration band in Western blotting. C and D, the phosphorylation of JNKs and/or c-Jun induced by arsenite was determined by Western blotting in the indicated cell lines. The loading controls (β-actin) were the same as in Fig. 3 (A and E, respectively).
FIGURE 5.
p27 inhibits activation of the JNK/c-Jun and HSF-1 pathways in the cellular response to arsenite. A and B, the phosphorylation of JNKs and c-Jun induced by arsenite was determined in p27+/+ and p27−/− MEFs (A) or p27 shRNA and non-silencing transfectants (B) as indicated by Western blotting. The loading control (β-actin) for the lower panel in A is the same as in Fig. 1A (12 h). C, The phosphorylation of the AKT/MKK pathway induced by arsenite for 6 h was determined in p27+/+ and p27−/− MEFs as indicated by Western blotting. D, the biotin-labeled probes −1785AGTACTGTCTTAGTCAGGATTT−1764 (for the mouse hsp27 promoter) and −2994GGACTCTTGACTCAGAGCACA−2974 (for the mouse hsp70 promoter) were incubated with nuclear extracts from arsenite-treated or untreated p27+/+ and p27−/− MEFs, and a 30- and/or 50-fold molar excess of the unlabeled probe was used in the competition analysis. E, both hsp70-Luc-WT and hsp70-Luc-AP-1-mutant were transiently transfected into p27+/+ and p27−/− cells. The Hsp70 transcriptional induction was analyzed by luciferase assay upon arsenite treatment. The results are presented as luciferase activity relative to the medium control. Each bar indicates the mean ± S.D. of three independent experiments. *, significant decrease in the luciferase induction in comparison with the wild-type luciferase reporter (p < 0.05). F, soluble chromatin prepared from arsenite-treated and untreated p27+/+ and p27−/− MEFs was subjected to a ChIP assay using anti-c-Jun antibody (Ab). I.P., immunoprecipitation. G, the activation of HSF-1 was compared in the indicated cells in the cellular response to arsenite exposure. H, shown is a proposed model for p27 suppression of Hsp27 and Hsp70 expression upon arsenite exposure.
Activation of JNK/c-Jun and HSF-1 Pathways by Arsenite Is Elevated in p27-deficient MEFs
Activation of the JNK/c-Jun pathway was tested in both p27+/+ and p27−/− cells. As shown in Fig. 5A, the phosphorylation of c-Jun and JNKs was elevated in p27−/− cells compared with p27+/+ cells. These findings were further verified in p27 knockdown cells (Fig. 5B). The investigation of upstream kinase activation indicated that the activation of MKK4, rather than MKK7, was inhibited in p27+/+ cells upon arsenite exposure, whereas a deficiency in p27 expression led to an increase in MKK4 activation upon arsenite exposure (Fig. 5C). Consistently, the phosphorylation of AKT, a well documented upstream kinase for MKK/JNK pathway activation (23), was also negatively regulated by p27 in the arsenite response (Fig. 5C). p27 has been reported to interact with Grb2, by which p27 competes with the Ras guanine nucleotide exchange factor Sos for binding to Grb2 to prevent Ras activation (24, 25). Therefore, inhibiting Ras activation by interacting with Grb2 might be a possible mechanism for p27 suppression of the AKT/MKK/JNK pathway.
Furthermore, the nuclear proteins extracted from the arsenite-treated p27−/− MEFs showed a very high level of binding to the probe containing the putative AP-1-binding sites in the mouse hsp27 promoter region (−1785AGTACTGTCTTAGTCAGGATTT−1764) and in the mouse hsp70 promoter region (−2994GGACTCTTGACTCAGAGCACA−2974) in EMSA in comparison with those from the p27+/+ cells under the same experimental conditions (Fig. 5D). Moreover, a mutant luciferase reporter containing the putative AP-1-binding site in the human hsp70 promoter region (from +80TGACT+84 to +80GTCTG+84) was generated and designated hsp70-Luc-AP-1-mutant. Both hsp70-Luc-WT and hsp70-Luc-AP-1-mutant were transiently transfected into p27+/+ and p27−/− cells. The hsp70 transcriptional induction was analyzed by luciferase assay. As shown in Fig. 5E, mutation of the AP-1-binding site in the hsp70 promoter region reduced its transcriptional induction upon arsenite exposure in both types of cells, indicating that AP-1 plays an important role in hsp70 transcriptional regulation in the arsenite response. To further confirm the results from EMSA experiments, we performed a ChIP assay using the c-Jun-specific antibody. As shown in Fig. 5F, the c-Jun antibody co-immunoprecipitated with the targeted hsp27 promoter region DNA in both p27+/+ and p27−/− cells under untreated conditions, indicating that c-Jun might participate in the constitutive hsp27 transcriptional regulation under normal physiological conditions. Upon arsenite treatment, the binding of c-Jun to the hsp27 promoter was enhanced in p27−/− cells compared with p27+/+ cells, suggesting the inducible recruitment of c-Jun to the endogenous hsp27 promoter in p27−/− MEFs under the simulated conditions. The specificity of this ChIP assay was demonstrated by the inability to detect the occupancy of c-Jun in a non-related region ∼1 kb downstream of the putative AP-1-binding site on the hsp27 promoter. The efficient promoter amplification from the input samples was used as a control. Consistently, the binding of c-Jun to the hsp70 promoter was also elevated in p27−/− cells in response to arsenite treatment compared with that in wild-type cells. Thus, these results demonstrate that c-Jun consistently binds to the promoter regions of hsp27 and hsp70 and regulates their basal level of transcription under normal conditions, whereas p27 negatively modulates c-Jun recruitment to the endogenous hsp27 and hsp70 promoters upon arsenite exposure.
In addition, our results also showed that HSF-1 hyperphosphorylation was elevated in p27−/− cells and knockdown cells in comparison with p27+/+ cells and parental non-silencing cells (Fig. 5G). Collectively, our results demonstrate that p27 exerts a strong inhibitory effect on activation of the JNK2/c-Jun and JNK2/HSF-1 pathways, which in turn leads to the suppression of hsp27 and hsp70 transcriptional activation in the cellular response to arsenite exposure (Fig. 5H).
DISCUSSION
The p27 protein was first recognized as the inhibitor of cyclin·CDK2 complexes regulating the arrest in the G1 phase of the cell cycle (26). Recent studies showed that p27 might have additional roles such as potential assembly factors, regulators of apoptosis or cell migration, and transcription cofactors (27). In this work, we have demonstrated that p27 had an inhibitory effect on activation of the JNK/c-Jun and HSF-1 pathways, by which p27 exhibited suppression of hsp27 and hsp70 transcriptional expression and in turn resulted in the reduction of Hsp27 and Hsp70 protein expression in the cellular response to arsenite exposure. This might be an additional novel mechanism responsible for the anticancer effect of p27.
Previous studies have shown that Hsp27 and Hsp70 have strong cytoprotective effects and act as molecular chaperones for many other proteins in cells (2). Hsp27 and Hsp70 are subject to transcriptional regulation upon different stresses. HSF-1 is reported to be responsible for Hsp transcriptional regulation in many experimental systems. In our study, we found that HSF-1 was indeed required for Hsp27 and Hsp70 induction based on the observation that the induction of Hsp27 and Hsp70 by arsenite in HSF-1−/− MEFs was attenuated compared with that in HSF-1+/+ MEFs. Bioinformatics analysis indicated that the putative AP-1-binding sites are located in the promoter regions of the mouse hsp27 and hsp70 genes. AP-1 is a master transcription factor and contributes to the regulation of gene expression in the cellular response to many environmental stresses. In this study, we further found that overexpression of TAM67, the dominant-negative form of c-Jun, blocked AP-1 activation and consequently impaired Hsp27 and Hsp70 induction upon arsenite exposure. Furthermore, our results showed that knockdown of JNK2, but not JNK1, had a similar effect. Therefore, our results suggest that the JNK2/c-Jun pathway is required for the transcriptional regulation of Hsp27 and Hsp70 in response to arsenite. More interesting, in p27−/− cells, activation of the JNK2/c-Jun and JNK2/HSF-1 pathways was markedly elevated, indicating that p27 might have an inhibitory effect on these pathways.
Consistent with our findings, there are several reports showing the regulation between p27 and AP-1. Chamovitz and Segal (28) reported that in early G1 phase, p27 binds to Jab1 in the cytoplasm, which prevents the association between Jab1 and AP-1 and leads to inhibition of the activity of AP-1. This might provide one molecular mechanism for the inhibitory effect of p27 on AP-1 activation. Grb2 has been reported to interact with the C-terminal proline-rich domain of p27 (amino acids 90–96), by which p27 competes with the Ras guanine nucleotide exchange factor Sos for binding to Grb2, thereby preventing Ras activation (24, 25). Ras activates multiple signaling cascades and is a potent inducer of cell viability. Therefore, inhibiting Ras activation by interacting with Grb2 might be another possible mechanism for AP-1 pathway repression by p27. In addition, our previous study demonstrated that through regulating GADD45/MKK4/JNK activation, the IκB kinase/NFκB p50 pathway mediates the cellular apoptotic process upon arsenite exposure (10). The IκB kinase/NFκB p50/GADD45/JNK pathway might be another possible linkage for p27 regulation of JNK/c-Jun-dependent Hsp27 and Hsp70 transcription.
In summary, we have demonstrated that p27 exhibits a strong inhibitory signal on the regulation of Hsp27 and Hsp70 expression at the transcriptional level in a JNK2/c-Jun- and JNK2/HSF-1-dependent manner in the arsenite response. Our results provide experimental evidence showing that p27 might exert its tumor-suppressive function by modulating transcription factor activation, leading to alteration of cellular gene expression.
Acknowledgments
We thank Dr. Hector R. Wong for providing HSF-1+/+ and HSF-1−/− cells, Dr. Suzanne A. W. Fuqua for providing the hsp27-luciferase reporter construct, and Dr. Alice Liu for providing the hsp70-luciferase reporter plasmid.
This work was supported in part by National Institutes of Health Grants CA112557 and CA119028-05S110 from NCI and Grants ES012451 and ES010344 from NIEHS.
- Hsp
- heat shock or stress protein
- MEF
- mouse embryonic fibroblast
- Ad
- adenoviral/adenovirus.
REFERENCES
- 1.Kim D., Kim S. H., Li G. C. (1999) Biochem. Biophys. Res. Commun. 254, 264–268 [DOI] [PubMed] [Google Scholar]
- 2.Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. (2001) Biochem. Biophys. Res. Commun. 286, 433–442 [DOI] [PubMed] [Google Scholar]
- 3.Garrido C., Schmitt E., Candé C., Vahsen N., Parcellier A., Kroemer G. (2003) Cell Cycle 2, 579–584 [PubMed] [Google Scholar]
- 4.Sgambato A., Cittadini A., Faraglia B., Weinstein I. B. (2000) J. Cell. Physiol. 183, 18–27 [DOI] [PubMed] [Google Scholar]
- 5.Wang J. P., Qi L., Moore M. R., Ng J. C. (2002) Toxicol. Lett. 133, 17–31 [DOI] [PubMed] [Google Scholar]
- 6.Tseng W. P., Chu H. M., How S. W., Fong J. M., Lin C. S., Yeh S. (1968) J. Natl. Cancer Inst. 40, 453–463 [PubMed] [Google Scholar]
- 7.Zhang D., Song L., Li J., Wu K., Huang C. (2006) J. Biol. Chem. 281, 34113–34123 [DOI] [PubMed] [Google Scholar]
- 8.Zhang D., Li J., Gao J., Huang C. (2009) Toxicol. Appl. Pharmacol. 235, 18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang W., Luo W., Zhang D., Jian J., Ma Q., Li J., Shi X., Chen J., Gao J., Huang C. (2008) Environ. Health Perspect. 116, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L., Li J., Zhang D., Liu Z. G., Ye J., Zhan Q., Shen H. M., Whiteman M., Huang C. (2006) J. Cell Biol. 175, 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J., Li J., Xue C., Wu K., Ouyang W., Zhang D., Yan Y., Huang C. (2006) J. Biol. Chem. 281, 24405–24413 [DOI] [PubMed] [Google Scholar]
- 12.Kiyokawa H., Kineman R. D., Manova-Todorova K. O., Soares V. C., Hoffman E. S., Ono M., Khanam D., Hayday A. C., Frohman L. A., Koff A. (1996) Cell 85, 721–732 [DOI] [PubMed] [Google Scholar]
- 13.McMillan D. R., Xiao X., Shao L., Graves K., Benjamin I. J. (1998) J. Biol. Chem. 273, 7523–7528 [DOI] [PubMed] [Google Scholar]
- 14.Wong H. R., Dunsmore K. E., Page K., Shanley T. P. (2005) Am. J. Physiol. Cell Physiol. 289, C1152–C1158 [DOI] [PubMed] [Google Scholar]
- 15.Oesterreich S., Hickey E., Weber L. A., Fuqua S. A. (1996) Biochem. Biophys. Res. Commun. 222, 155–163 [DOI] [PubMed] [Google Scholar]
- 16.Wu B., Hunt C., Morimoto R. (1985) Mol. Cell. Biol. 5, 330–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L., Li J., Ye J., Yu G., Ding J., Zhang D., Ouyang W., Dong Z., Kim S. O., Huang C. (2007) Mol. Cell. Biol. 27, 2713–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper S. J., MacGowan J., Ranger-Moore J., Young M. R., Colburn N. H., Bowden G. T. (2003) Mol. Cancer Res. 1, 848–854 [PubMed] [Google Scholar]
- 19.Csermely P., Vigh L. (2007) Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks, Springer New York, New York [Google Scholar]
- 20.Prasad K. V., Taiyab A., Jyothi D., Srinivas U. K., Sreedhar A. S. (2007) J. Biosci. 32, 585–593 [DOI] [PubMed] [Google Scholar]
- 21.Leaner V. D., Donninger H., Ellis C. A., Clark G. J., Birrer M. J. (2005) Mol. Cell. Biol. 25, 3324–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J., Ning B., Huang Y., Zhang D., Li J., Chen C. Y., Huang C. (2009) Curr. Cancer Drug Targets 9, 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H. Y., Oh S. H., Suh Y. A., Baek J. H., Papadimitrakopoulou V., Huang S., Hong W. K. (2005) Clin. Cancer Res. 11, 6065–6074 [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama Y., Tomoda K., Tanaka T., Arata Y., Yoneda-Kato N., Kato J. (2001) J. Biol. Chem. 276, 12084–12090 [DOI] [PubMed] [Google Scholar]
- 25.Moeller S. J., Head E. D., Sheaff R. J. (2003) Mol. Cell. Biol. 23, 3735–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kossatz U., Malek N. P. (2007) Cell Res. 17, 832–833 [DOI] [PubMed] [Google Scholar]
- 27.Coqueret O. (2003) Trends Cell Biol. 13, 65–70 [DOI] [PubMed] [Google Scholar]
- 28.Chamovitz D. A., Segal D. (2001) EMBO Rep. 2, 96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]