Abstract
In previous studies, we observed that mice knocked out for the serotonin-2B receptor (5-HT2BR) show defects in bone homeostasis. The present work focuses on the downstream targets relaying the anabolic function of this receptor in osteoblasts. A functional link between the 5-HT2BR and the activity of the tissue-nonspecific alkaline phosphatase (TNAP) is established using the C1 osteoprogenitor cell line. During C1 osteogenic differentiation, both 5-HT2BR and TNAP mRNA translations are delayed with respect to extracellular matrix deposition. Once the receptor is expressed, it constitutively controls TNAP activity at a post-translational level along the overall period of mineral deposition. Indeed, pharmacological inhibition of the 5-HT2BR intrinsic activity or shRNA-mediated 5-HT2BR knockdown prevents TNAP activation, but not its mRNA translation. In contrast, agonist stimulation of the receptor further increases TNAP activity during the initial mineralization phase. Building upon our previous observations that the 5-HT2BR couples with the phospholipase A2 pathway and prostaglandin production at the beginning of mineral deposition, we show that the 5-HT2BR controls leukotriene synthesis via phospholipase A2 at the terminal stages of C1 differentiation. These two 5-HT2BR-dependent eicosanoid productions delineate distinct time windows of TNAP regulation during the osteogenic program. Finally, prostaglandins or leukotrienes are shown to relay the post-translational activation of TNAP via stimulation of the phosphatidylinositol-specific phospholipase C. In agreement with the above findings, primary calvarial osteoblasts from 5-HT2BR-null mice exhibit defects in TNAP activity.
Keywords: Cell/Stem, Eicosanoids, G Proteins/Coupled Receptors (GPCR), Receptors/Neurotransmitters, Signal Transduction, Tissue/Organ Systems/Bone, Tissue-Nonspecific Alkaline Phosphatase, Serotonin
Introduction
Imbalances in bone formation or resorption frequently result in pathological situations, such as osteoporosis, osteopenia, or osteomalacia. Proper skeletal development and bone homeostasis notably necessitate a tight regulation of mineralization. Over the past few years, growing attention has been paid to the involvement of serotonin (5-hydroxytryptamine, 5-HT)2 in bone biology (1). Indeed, beyond its role as a neurotransmitter in brain (2), platelet-stored 5-HT broadly participates to the homeostasis of various tissues. For instance, 5-HT regulates cardiovascular, smooth muscle, and endocrine functions (for review, see Ref. 3).
In the periphery, 5-HT is synthesized exclusively by enterochromaffin cells of the gut and stored by platelets. Thus, because they express the serotonin transporter and diverse serotonergic receptors (4–6), osteoblasts may be directly influenced by circulating 5-HT. In agreement with this idea, inhibition of the serotonin transporter reduces bone formation (7, 8). Moreover, patients receiving selective serotonin reuptake inhibitor antidepressants appear to be at risk for osteoporosis (9). Five types of serotonergic receptors have been depicted in osteoblast primary cultures or osteoblastic cell lines: 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, and 5-HT2B (4, 6, 10, 11). For instance, focusing on the 5-HT1B subtype, Yadav et al. have recently reported that this receptor inhibits osteoblast proliferation and negatively controls bone mass (11). As in the central nervous system (12), the relative affinity of serotonergic receptors for 5-HT and the diversity of their couplings may allow a fine tuned response of osteoblasts depending on the external 5-HT concentration.
In previous works, we identified the 5-HT2B receptor (5-HT2BR) as an important player of bone metabolism. Our first series of observations was gained using the C1 osteoprogenitor cell line (13). These cells are endowed with the capacity to recapitulate osteogenic differentiation within 12–14 days in response to β-glycerophosphate and ascorbate, with a nearly 100% frequency of differentiation (13, 14). C1 osteogenic cells implement a functional 5-HT2BR precisely when extracellular matrix (ECM) mineralization begins (10). No other 5-HT2R subtype is expressed along C1 osteogenic differentiation (10). During the course of the program, the G protein-coupled 5-HT2BR continuously displays constitutive activity toward both the nitric oxide (NO) and the phospholipase A2 (PLA2) pathways. In response to 5-HT, the 5-HT2BR promotes further NO production and PLA2-dependent arachidonic acid (AA) release (10). In Ref. 10, we additionally reported that the 5-HT2BR does not recruit the inositol 1,4,5-trisphosphate pathway, thus excluding a coupling to the Gq/11 family of G proteins, as classically but not systematically described for receptors of the 5-HT2 family (15). The 5-HT2BR contributes to mineralization in C1 cells because incorporation of calcium within the matrix is reduced by 40% upon inhibition of the receptor signaling activity. In addition, 5-HT2BR knock-out mice were shown to display reduced bone density, thus confirming the involvement of the receptor in osteogenesis (6).
In the present study, we establish that the tissue-nonspecific alkaline phosphatase (TNAP), a glycosylphosphatidylinositol-anchored protein involved in ECM mineralization, is a target of the 5-HT2BR signaling pathways in C1 cells. The 5-HT2BR does not interfere with the translation of TNAP mRNA. However, the 5-HT2BR controls the enzymatic activity of TNAP. The regulation of TNAP occurs at a post-translational level, downstream from the PLA2 coupling. During the initial mineralization phase, PLA2-mediated activation of TNAP is relayed by the cyclooxygenase (COX) pathway. Thereafter, the COX pathway is switched off, and the 5-HT2BR/PLA2-dependent control on TNAP activity is exerted by the lipoxygenase (LOX) pathway. Besides, we show that the positive action of the 5-HT2BR on TNAP downstream from eicosanoids requires the activity of the glycosylphosphatidylinositol-solubilizing enzyme phosphatidylinositol-specific phospholipase C (PIPLC). Finally, primary calvarial osteoblasts obtained from 5-HT2BR knock-out mice, which suffer from osteopenia (6), exhibit defects in TNAP activity.
EXPERIMENTAL PROCEDURES
Chemicals
Ritanserin and BW723C86 were synthesized at Hoffmann-La Roche Ltd. All other chemicals were from Sigma. All tissue culture reagents were purchased from Invitrogen.
Cell Culture and Differentiation
The C1 cell line has been described in Ref. 13 and is available upon request, according to the ASBMB editorial policy. C1 cells were first seeded on untreated plastic Petri dishes at 6 × 105 cells/10 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) to form three-dimensional aggregates. After 10 days, C1 aggregates were transferred to DMEM supplemented with 1% FCS depleted for 5-HT (<1 nm) (10). Osteogenic differentiation was induced (day 0) by the addition of 0.25 mm ascorbic acid and 7 mm β-glycerophosphate. Because C1 cells do not synthesize 5-HT (tryptophan hydroxylase activity <0.5 pmol/10 min per mg of protein, 5-HT content <0.2 pmol/mg of protein) (10), the absence of 5-HT is guaranteed, thus ruling out any stimulation of 5-HT2BRs. For thymidine incorporation, see supplemental “Experimental Procedures.”
Properties of the Pharmacological Drugs Used
Drugs used to target 5-HT receptors include ritanserin and BW723C86. Ritanserin is a nonselective inverse agonist of 5-HT2 receptors (16, 17), which binds 5-HT2A, 5-HT2B, and 5-HT2C receptors. BW723C86 is a selective agonist of 5-HT2B receptors (16, 17). Drugs used to discriminate TNAP from other alkaline phosphatase (ALP) activities include levamisole and vanadate. Levamisole is a noncompetitive inhibitor of TNAP (18). Vanadate is an inhibitor of ALP enzymes with lower affinity toward TNAP than other phosphatases (19). Other drugs used include the nonselective COX-1/COX-2 inhibitor indomethacin (20), the LOX inhibitor MK-886 (21), the NO synthase inhibitor l-N monomethyl arginine (l-NMA), the PIPLC inhibitor U73122 and its neutral congener U73343 (22).
RNA Isolation and Reverse Transcription-PCR Analysis
Total RNA isolation and semiquantitative reverse transcription-PCR were performed as previously described (10). The following specific primers were used: TNAP, forward 5′-GCAGGATTGACCACGGACACTATG-3′ and reverse 5′-TTCTGCTCATGGACGCCGTGAAGC-3′ (432 bp); 5-HT2BR, forward 5′-AGGAATCGAGACTGATGTGAT-3′ and reverse 5′-CTTAGGAAAACTGTGGGCACA-3′ (230 bp); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′ and reverse 5′-CATGTAGGCCATGAGGTCCACCAC-3′ (982 bp), used as internal standard.
5-HT2B Receptor Knockdown
Oligonucleotides coding for short hairpin RNA (shRNA) specifically against 5-HT2BR were cloned into pLenti6 (a lentiviral RNAi expression system; Invitrogen). Lentivirus production and cell infection were done according to the manufacturer's instructions. The following target region of 5-HT2BR was chosen: GCACAACTTCTGAGCACATTT. Stable transduced clones were established by selection with blasticidin (Invitrogen). It was verified in all experiments that a scramble shRNA (sequence GCAACATTCCCATGACTTAGT) yielded values similar to controls (data not shown).
ALP and Eicosanoid Determinations
C1 cells were washed twice with ice-cold PBS and scraped in 10 mm Tris-HCl containing 2 mm MgCl2 and 0.05% Triton X-100, pH 8.2. The cell suspension was treated or not with the indicated drugs before homogenization (MM301 homogenizer; Retsch AG, Haan, Germany) at 4 °C. Aliquots of supernatants were subjected to protein assay with the bicinchoninic acid method (Pierce) and to ALP activity measurement using an Elecsys automat (Roche Diagnostics) with CSPD as substrate. Cell extracts were incubated in the presence of levamisole or vanadate for 10 min prior to the measurement of phosphatase activity. TNAP protein amounts were quantitatively measured using the immunoradiometric assay Tandem-R Ostase (Beckman-Coulter). The accumulations of PGE2 and LTB4 in the C1 cell culture supernatants were determined using high sensitivity PGE2 and LTB4 enzyme immunoassay kits (Cayman Chemicals).
Measurement of PIPLC Activity
PIPLC activity was measured by monitoring the conversion of [3H]phosphoinositides to [3H]glycerophosphoinositol. Briefly, C1 cells were labeled with [3H]phosphatidylinositol 4,5-bisphosphate (5 μm, 200 μCi) for 24 h at 37 °C. After labeling, cells were washed three times in HBSS containing calcium and magnesium and supplemented with 20 mm HEPES and 0.1% BSA (experimental medium). Following exposure to the indicated drugs in experimental medium for 24 h, C1 cells were washed and scraped, and analysis of PIPLC products was carried out as in Ref. 23. Specific PIPLC activity was calculated as the phosphatidylinositol 4,5-bisphosphate-sensitive formation of [3H]glycerophosphoinositol/min per mg of protein.
Calvarial Osteoblast Primary Culture
Primary osteoblasts were enzymatically isolated from calvaria of neonatal (2–3-day old) wild-type (WT) and 5HT2BR−/− mice as described in Ref. 6. Briefly, dissected calvaria were sequentially digested in a PBS collagenase solution containing 0.2% collagenase IV and 0.01% deoxyribonuclease for 70 min at 37 °C. After centrifugation, calvaria cells were collected and expanded for 5 days in α-MEM supplemented with 10% FCS depleted of 5-HT, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were then plated at 105 cells/cm2 in the differentiation medium containing 50 μg/ml ascorbic acid and 10 mm β-glycerophosphate. ALP activity was measured after 7 days of culture.
Data Analysis and Statistics
The nonparametric Kolmogorov-Smirnov test was used for statistical analysis of small groups. All values are given as means ± S.E.
RESULTS
5-HT2BR Intrinsic Signaling Controls the Peak in ALP Activity Associated with Osteogenic Differentiation
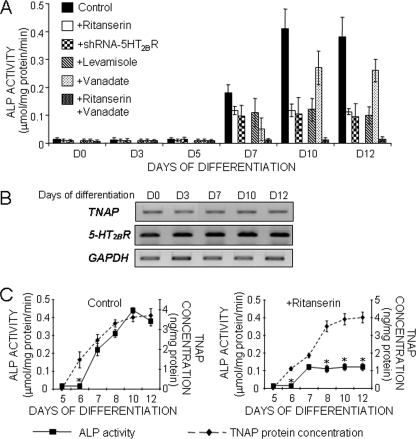
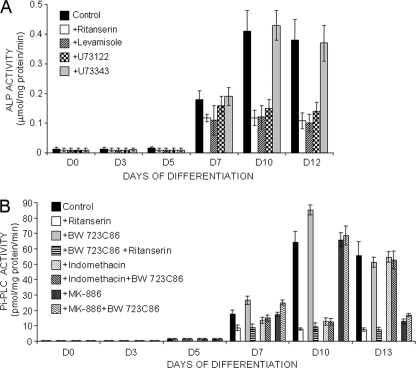
In 5-HT2BR knock-out mice, the age-dependent decrease in bone formation was shown to correlate with low levels of plasma ALP activity (6). Our goal was to delineate the relationship between the 5-HT2BR and ALP further. In a first set of experiments, we exploited the kinetics of C1 osteogenic differentiation to evaluate the dynamics of ALP activity in relation to other steps of the program, and notably the onset of 5-HT2BRs prior to mineral deposition. Total ALP activity was measured in homogenates of C1 nodules during the kinetics of differentiation (Fig. 1A). Before the onset of the receptor, at day 5 of the program, ALP activity was faintly detectable. At day 7, ALP activity became measurable, reaching its maximal value at day 10. Between day 10 and day 12, when C1 cells converted into osteocytes and ceased dividing (supplemental Fig. 1), ALP activity remained stable.
FIGURE 1.
5-HT2BR intrinsic signaling controls TNAP activity through post-translational events. A, total ALP activity measured during the osteogenic program of control C1 cells, C1 cells treated with ritanserin, an inverse agonist of the 5-HT2BR, or C1 cells transfected with a 5-HT2BR-specific shRNA. TNAP activity was discriminated from other ALP activities by incubating cell extracts with levamisole, a specific inhibitor of TNAP, or vanadate, a potent inhibitor of other phosphatases/ATPases. In ritanserin-treated cell extracts where TNAP activity is abolished, basal ALP activity is quenched by vanadate whatever the stage of differentiation. B, reverse transcription-PCR analysis of mRNA expression for TNAP and 5-HT2BR throughout differentiation. Results were normalized to GAPDH mRNA expression. C, radioimmunoassays analysis of TNAP synthesis during differentiation of C1 cells exposed or not to ritanserin. The amounts of immunoreactive TNAP molecules and ALP activities were measured in the same cell extracts. Chronic exposure to ritanserin does not affect TNAP synthesis but reduces the increase in ALP activity attributed to TNAP. Values are the means ± S.E. (error bars) of four independent experiments.
To assess a functional link between 5-HT2BR and ALP, the receptor constitutive activity was quenched with ritanserin (30 nm) from day 0 (or day 5, not shown) of the osteogenic program. Ritanserin is a nonselective inverse agonist of 5-HT2 receptors, i.e. 5-HT2A, 5-HT2B, and 5-HT2C (16, 17). The absence of 5-HT2A and 5-HT2C receptors along C1 osteogenic differentiation warrants the use of ritanserin to quench the constitutive activity of the 5-HT2BR (10). Although ritanserin does not hinder the conversion of C1 cells into osteocytes, it reduces 45Ca2+ incorporation within the ECM by 40% (10). Upon addition of ritanserin, ALP activity was still turned on at day 7. However, the intensity of phosphatase activity was reduced by 45% and remained constant from day 7 up to day 12 (Fig. 1A). In parallel experiments, we analyzed the impact of shRNA-mediated knockdown of the 5-HT2BR on total ALP activity during the kinetics of C1 differentiation. As shown in Fig. 1A, 5-HT2BR silencing interfered with the raise in ALP activity from day 7 and yielded ALP activity values comparable with those obtained with ritanserin. We therefore conclude that the 5-HT2BR controls a component of ALP activity that normally peaks during matrix mineralization.
To account for this observation, we may assume that at least two distinct ALP enzymatic activities superimpose during differentiation. A first set of phosphatase activity would sustain the basal level of ALP, independently from the onset of 5-HT2BR signaling. A second phosphatase activity would yield the peak of ALP activity that is switched off by ritanserin.
We may also note that the above experiments were performed in the absence of 5-HT (see “Experimental Procedures” and Ref. 10). Thus, the control of ALP activity by the 5-HT2BR between days 7 and 12 has to be ascribed to the intrinsic activity of the receptor.
TNAP Is a Downstream Target of 5-HT2BR Intrinsic Signaling
Although it is not osteoblast-specific, TNAP is considered as a phenotypic marker of osteogenic cells. Its activity is necessary to achieve optimal bone mineralization (24). We therefore evaluated whether the 5-HT2BR-dependent ALP activity identified above could be assigned to TNAP. To this purpose, C1 cell extracts were incubated with levamisole (4.5 mm), an inhibitor of TNAP (18). Under this treatment, ALP activity in samples collected at days 7, 10, and 12 of differentiation remained at the value measured with extracts from ritanserin-treated cells (Fig. 1A). Thus, levamisole-sensitive TNAP activity indeed accounts for the 5-HT2BR-dependent phosphatase activity rising from day 7 onward.
Compared with the other phosphatases expressed in osteogenic cells, TNAP is less sensitive to vanadate addition (19). We thus added this inhibitor to cell homogenates and searched for the threshold concentration able to switch off the basal phosphatase(s) activity that resists levamisole (data not shown). At such a concentration, the peak of ALP activity accompanying differentiation should not be masked. Indeed, we observed that with 0.15 mm vanadate, the peak persisted while being cut down by the basal level of vanadate-sensitive phosphatase activity (Fig. 1A). With ritanserin-treated cell extracts, residual ALP activity was quenched by vanadate throughout differentiation (Fig. 1A). Thus, our experiments identify TNAP as the source of ALP activity under control of the intrinsic coupling of the 5-HT2BR.
5-HT2BR Controls TNAP Activity through Post-translational Mechanisms
The question then arose as to whether, from day 7, TNAP molecules were rendered functional by de novo mRNA transcription or translation or through post-translational maturation. The levels of TNAP transcripts and proteins were followed in C1 cells throughout differentiation (Fig. 1, B and C). TNAP mRNAs were present as soon as day 0, thus indicating that, before their recruitment toward the osteogenic program, C1 mesoblastic cells already transcribe the TNAP gene. The amount of 5-HT2BR and TNAP mRNAs did not vary during the 12 days of differentiation (Fig. 1B). Thus, the 5-HT2BR-mediated TNAP regulation necessarily involves post-transcriptional events.
Anti-TNAP antibodies were then used to follow TNAP synthesis (Fig. 1C). In radioimmunoassays using C1 cell homogenates, the TNAP protein was faintly detectable up to day 5. At day 6, i.e. 1 day before the increase in levamisole-sensitive phosphatase activity, TNAP concentration increased and reached a plateau from day 8 onward (Fig. 1C). Interestingly, TNAP protein expression was not changed upon exposure of C1 cells to ritanserin. This indicates that the 5-HT2BR does not interfere with the translation of TNAP mRNA. The amounts of immunoreactive TNAP molecules and total ALP activity during differentiation are plotted on Fig. 1C. Clearly, de novo TNAP synthesis starts 1 day before ALP activity becomes detectable. Moreover, although it cancels TNAP activity, ritanserin does not change the capacity of C1 cells to synthesize TNAP. Our data thus favor the view that in osteogenic cells, preexisting TNAP molecules are turned on from an inactive to an active state by post-translational controls driven by functional 5-HT2BRs.
Agonist-dependent Coupling of 5-HT2BR to TNAP Is Restricted to the Beginning of Matrix Mineralization
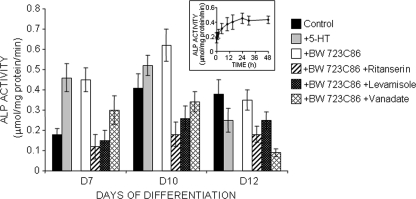
We next evaluated the impact of a 5-HT2BR stimulation on ALP function. Total ALP activity was measured after exposure of C1 cells to BW723C86, a selective agonist of the 5-HT2BR (25). Addition of 1 μm BW723C86 at day 5, i.e. when the receptor is implemented, did not elicit any additional ALP activity. However, upon stimulation at day 6, a 2-fold increase in ALP activity was recorded at day 7 (Fig. 2, inset). Fig. 2 shows the values of ALP activity in homogenates of cells exposed or not to BW723C86 at days 6, 9, or 11. All of the values were measured 1 day after agonist addition. Up to day 9, BW723C86 promoted a significant stimulation of ALP activity. From day 10 onward, ALP values remained at the level measured in the absence of agonist.
FIGURE 2.
TNAP activity is up-regulated by agonist stimulation of 5-HT2BR at the initial period of matrix mineralization. The time-dependent increase in ALP activity following stimulation of 5-HT2BR with BW723C86 in C1 cells at day 6 is shown in the inset. ALP activity was followed during differentiation of C1 cells, 24 h after addition of 1 μm BW723C86. Measurements at days 7 and 10 indicate an up-regulation of TNAP activity in response to 5-HT2BR stimulation. On day 11, the 5-HT2BR loses its ability to regulate TNAP activity (day 12) in an agonist-dependent manner. In parallel experiments, ALP activity was measured in C1 cells exposed to 10 nm 5-HT for 24 h. At days 7 and 10, 5-HT enhances ALP activity similarly to BW723C86, whereas it exerts an opposite effect at day 13. Under combined exposure of C1 cells to BW723C86 and ritanserin (30 nm), all of the effects assignable to the agonist are canceled. Levamisole (4.5 mm), a specific inhibitor of TNAP, or vanadate (0.15 mm), a potent inhibitor of phosphatases/ATPases distinct from TNAP, was added to cell extracts to discriminate TNAP activity from other phosphatases activities. Values are the means ± S.E. (error bars) of four independent experiments.
We further observed that, upon exposure of BW723C86-treated cell extracts to levamisole, ALP activity systematically decreased to values equal to those obtained after inhibition of the 5-HT2BR by ritanserin (Fig. 2). Thus, the agonist-dependent rise in ALP activity may unambiguously be assigned to TNAP.
In a more physiological paradigm, we examined the impact of 5-HT stimulation (10 nm, 24 h) on total ALP activity along the osteogenic differentiation of C1 cells. The value obtained at day 7 was similar to that obtained following the BW723C86 treatment (Fig. 2). At day 10, 5-HT still triggered an increase in ALP activity, although less important than that registered with BW723C86. In contrast, at day 12, 5-HT promoted a reduction of ALP activity to a value similar to that measured in levamisole-treated extracts, which corresponds to non-TNAP phosphatase activity (Fig. 2).
Altogether, the data show that, from day 7 to day 10 of the osteogenic program, agonist- or 5-HT-mediated activation of the 5-HT2BR promotes further recruitment of TNAP. This time window coincides precisely with the very beginning of bone matrix mineralization. When C1 cells convert into osteocyte-like cells (supplemental Fig. 1) (10), i.e. from day 10 onward, the 5-HT2BR loses its ability to stimulate TNAP activity in an agonist-dependent manner. At this stage, the induction of another yet to be identified 5-HT receptor subtype must be postulated to account for the negative action exerted by 5-HT on the 5-HT2BR-mediated control of TNAP activity.
5-HT2BR Turns on TNAP Activity via PLA2
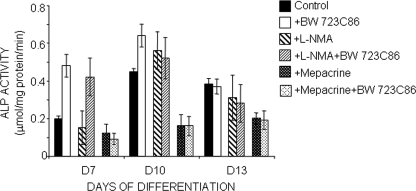
In C1 cells, from day 5 of differentiation onward, the 5-HT2BR is coupled to NO production and PLA2-mediated AA release (10). As shown in Fig. 3, the increase in TNAP enzymatic activity was not affected by l-NMA addition (100 μm) to the culture medium throughout differentiation. Thus, we may exclude an involvement of NO in the 5-HT2BR-mediated TNAP activation. In contrast, addition at day 6 of the PLA2 inhibitor mepacrine (100 nm) abolished the 5-HT2BR-dependent TNAP activation normally observed from day 7 (Fig. 3). Values recorded on days 7, 10, and 13 coincided with those measured after direct inhibition of the 5-HT2BR signaling activity by ritanserin (Fig. 1A). Addition of BW723C86 (1 μm) in combination with mepacrine did not restore any TNAP activity (Fig. 3). We therefore conclude that (i) the autocrine activation of TNAP observed during the overall period of matrix mineralization is elicited by the constitutive PLA2 coupling to the receptor; and (ii) the rise in TNAP activity upon agonist stimulation of the receptor, as observed between day 7 and day 10, is also relayed by the PLA2 pathway.
FIGURE 3.
PLA2/AA signaling pathway mediates activation of TNAP by 5-HT2BR during the overall period of mineral deposition. In C1 osteogenic cells, from day 5 until terminal differentiation, 5-HT2BRs couple to NO synthases and PLA2 in both agonist-dependent and -independent manners. Exposure of C1 cells to l-NMA (100 μm, 24 h), a selective inhibitor of NO synthases, in the presence or absence of 1 μm BW723C86, has no significant impact on the increase in ALP activity accompanying osteogenic differentiation. l-NMA does not affect the basal level of phosphatase/ATPases either. In contrast, exposure of C1 cells to mepacrine (100 nm, 24 h), a specific inhibitor of PLA2, alone or in combination with BW723C86, abrogates the 5-HT2BR-dependent TNAP activation. Values recorded along differentiation coincide with those measured after inactivation of the receptor by ritanserin. Values are the means ± S.E. (error bars) of four independent experiments.
5-HT2BR/PLA2 Coupling Contributes to Osteogenic Differentiation of C1 Cells by Controlling the COX and LOX Pathways in a Stepwise Manner
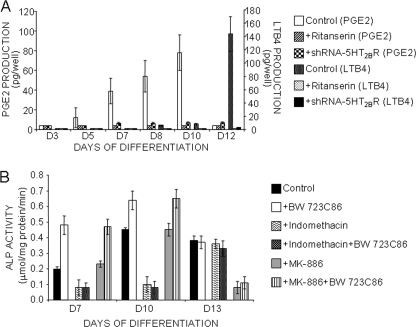
In C1 osteoblasts, the 5-HT2BR is coupled to AA release from day 5 until terminal differentiation. From day 5 to day 10, AA release is followed by a COX-dependent PGE2 synthesis (Fig. 4A) (10). PGE2 production depends on the 5-HT2BR because it is canceled by ritanserin (Fig. 4A) (10) or upon shRNA-mediated down-regulation of the receptor (Fig. 4A). From day 10, we observed a shunt from the COX to the LOX pathway, downstream from the 5-HT2BR/PLA2/AA coupling. Indeed, at this stage, PGE2 production was quenched to the benefit of LTB4 synthesis (Fig. 4A). Again, ritanserin- or 5-HT2BR-specific shRNA canceled LTB4 synthesis, thus demonstrating that the 5-HT2BR intrinsic activity controls the basal production of eicosanoids.
FIGURE 4.
5-HT2BR intrinsic coupling to PLA2 governs TNAP activity by controlling COX and LOX pathways in a stepwise manner. A, PGE2 and LTB4 production in the supernatants of C1 cells was followed along osteogenic differentiation. The capacity of C1 cells to synthesize PGE2 increases from day 5 to day 10. LTB4 production becomes slightly detectable from day 8 to day 10. From day 10 onward, a large increase in LTB4 synthesis coincides with the quenching of COX-dependent PGE2 production. Either PGE2 or LTB4 production is abrogated in the presence of ritanserin or upon shRNA-mediated 5-HT2BR silencing. B, inhibition of COX activity with indomethacin, in combination or not with BW723C86, switches off the 5-HT2BR-dependent TNAP activity at day 7 and day 10. From day 10 onward, AA no longer recruits COX, and as expected, indomethacin does not affect TNAP activity. Addition of MK-886, a LOX inhibitor, switches off the 5-HT2BR-dependent TNAP activity at day 12, but not at earlier stages. Values are the means ± S.E. (error bars) of four independent experiments.
We next evaluated the contribution of COX/LOX signaling on 5-HT2BR-dependent TNAP regulation. First, C1 cells were exposed for 24 h to 5 μm indomethacin, a COX inhibitor, in combination or not with 1 μm BW723C86. As shown in Fig. 4B, blockade of the COX pathway switched off the 5-HT2BR-related TNAP activation observed between day 7 and day 10 of differentiation. As anticipated, ALP activity was insensitive to indomethacin at later stages (Fig. 4B). ALP activity was further measured in C1 cells exposed for 24 h to the LOX inhibitor MK-886 (10 μm). Again, as expected, ALP activity was insensitive to MK-886 up to day 10 (Fig. 4B). Added at day 12, MK-886 abolished the 5-HT2BR-dependent TNAP activity (Fig. 4B).
Altogether, these data highlight that the switch from PGE2 production to leukotrienes synthesis delineates specific stages in the regulation of TNAP activity. The shunt from COX-derived prostaglandins synthesis to LOX-derived leukotrienes production coincides with the conversion of C1 osteoblasts into osteocytes.
Control Exerted by 5-HT2BR on TNAP Is Relayed by PIPLC Activity
The above findings show that the 5-HT2BR turns on TNAP activity by post-translational mechanisms. The report by Ciancaglini et al. that the activity of PIPLC-solubilized TNAP is largely increased (>50-fold) compared with the membrane-bound enzyme (26) prompted us to assess whether PIPLC may contribute to TNAP activation downstream from the 5-HT2BR. First, total ALP activity was measured along differentiation after exposure of C1 cells to 50 nm U73122 (24 h), a selective inhibitor of PIPLC (22), or its neutral congener U73343 (22). With U73122, but not U73343, total ALP activity was reduced to values similar to those measured in cell extracts treated with the TNAP inhibitor levamisole (Fig. 5A). Hence, blockade of PIPLC activity switches off the peak in ALP activity that corresponds to TNAP. We further quantified PIPLC activity along C1 osteogenic differentiation. As shown in Fig. 5B, PIPLC activity was turned on from day 7 and increased further at day 10. Similarly to TNAP activity, PIPLC activity remained stable between days 10 and 13. Of note, whatever the differentiation stage, inhibition of the 5-HT2BR intrinsic activity with ritanserin drastically reduced PIPLC activity to a basal level corresponding to <13% of the maximal value, observed at day 10 (Fig. 5B). On the contrary, agonist stimulation of the 5-HT2BR by addition of BW723C86 up-regulated PIPLC activity between days 7 and 10. If added at day 12, BW723C86 failed to induce any PIPLC activity. Finally, PIPLC activity remained at its basal value, as measured with ritanserin, when C1 cells were exposed to the COX inhibitor indomethacin alone or in combination with BW723C86 up to day 10 (Fig. 5B). As anticipated, indomethacin was without effect when added at day 12. At this stage, PIPLC activity became primarily controlled by the LOX pathway, as inferred by the inhibitory effect of MK-886 (Fig. 5B). As a whole, these results identify PIPLC as a target of the 5-HT2BR downstream from the COX/LOX pathway that mediates the post-translational activation of TNAP.
FIGURE 5.
Control exerted by 5-HT2BR on TNAP activity is relayed by PIPLC. A, addition of the PIPLC inhibitor U73122 (50 nm, 24 h) cancels the peak of ALP activity that accompanies C1 osteogenic differentiation from day 7 and corresponds to TNAP. The structurally related inert analog U73343 behaves neutrally. Values with ritanserin or levamisole are also shown. B, C1 cells implement a PIPLC activity during osteogenic differentiation. The ritanserin-mediated reduction in phospholipase activity indicates a major control of the 5-HT2BR intrinsic activity on PIPLC. Addition of either indomethacin (5 μm, 24 h) or MK-886 (10 μm, 24 h) also reduces PIPLC activity, thus indicating that the control exerted by the 5-HT2BR intrinsic activity on PIPLC is relayed by the downstream eicosanoid effectors. With the agonist BW723C86 (1 μm, 24 h), further PIPLC activity is measured up to day 10, which is sensitive to ritanserin or indomethacin. Values are the means ± S.E. (error bars) of four independent experiments.
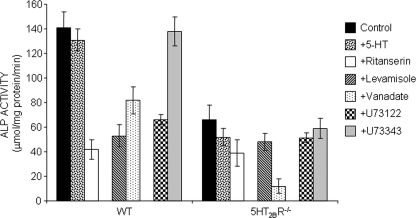
5-HT2BR Controls TNAP Activity in Primary Calvarial Cells
We next took advantage of 5-HT2BR knock-out mice to extend our results to ex vivo cultures. Primary osteoblasts were isolated from calvaria of neonatal WT and 5-HT2BR knock-out mice. As shown in Fig. 6, in a 5-HT2BR-null context, total ALP activity as measured at day 7 of the culture was 50% of that in WT cells. No change in ALP activity was observed upon exposure of WT or 5-HT2BR−/− calvarial cells to 5-HT (10 nm, 24 h) (Fig. 6). This likely relates to the presence of 5-HT in the cultures, despite the use of 5-HT-depleted serum (6). The presence of 5-HT in the medium may thus impair the detection of biochemical effects in response to an addition of extra serotonin. Treatment of WT osteoblasts with the inverse agonist ritanserin (30 nm) from day 0 yielded a 70% reduction in total ALP activity at day 7. This stronger effect upon pharmacological inhibition compared with genetic inactivation of the 5-HT2BR may be accounted for by the expression of 5-HT2AR in calvarial cultures (6). These receptors are absent in C1 osteogenic cells (10). These 5-HT2ARs are also targets of ritanserin (16, 17), and their expression is up-regulated in a 5-HT2BR-null context (6). In agreement with the above idea, we observed that addition of ritanserin to 5-HT2BR−/− mice-derived osteoblasts promoted a 40% reduction in the level of total ALP activity (Fig. 6). This resulting ALP activity compared with that measured in WT calvaria cultures exposed to ritanserin.
FIGURE 6.
TNAP activity is controlled by 5-HT2BR in primary calvarial cells. Total ALP activity, measured in primary WT and 5HT2BR−/− osteoblast cultures on days 7, is decreased by 50% in the absence of 5-HT2BR. The values remain unchanged in cultures treated with 10 nm 5-HT for 24 h. The inverse agonist ritanserin, targeting the 5-HT2BR but also the 5-HT2AR, promotes a stronger reduction in total ALP activity than genetic ablation of the 5-HT2BR. Cell extracts were incubated with levamisole, a specific inhibitor of TNAP, or vanadate, a potent inhibitor of other phosphatases/ATPases, to distinguish TNAP activity from other phosphatases/ATPases. The PIPLC inhibitor U73122, but not its neutral congener U73343, reduces total ALP activities to values close to those attributed to phosphatases distinct from TNAP (levamisole-resistant), in both WT and 5HT2BR−/− osteoblasts. Each value is the mean ± S.E. (error bars) of five independent experiments.
Levamisole (4.5 mm) and vanadate (0.15 mm) were again used to discriminate between TNAP and other ALPs (Fig. 6). Levamisole had no significant impact on the residual ALP activity detected in calvarial cultures from 5-HT2BR−/− mice. This residual activity compares with the levamisole-resistant component of total ALP in WT calvarial cells. It thus corresponds to phosphatases other than TNAP. On the contrary, vanadate-resistant ALP activity, i.e. TNAP, was barely detectable in primary osteoblasts genetically depleted in 5-HT2BR. Finally, exposure of either WT or 5-HT2BR−/− calvarial cells to the PIPLC inhibitor U73122 (50 nm, 24 h) yielded total ALP activity values comparable with the levamisole-resistant components of total ALP in these cells. As anticipated, ALP activity remained insensitive to the neutral analog U73343 (Fig. 6). We may thus conclude that, as in the C1 osteogenic cell line, TNAP activity is under the control of the 5-HT2BR and PIPLC in primary osteoblasts.
DISCUSSION
Despite considerable effort to dissect the molecular pathways sustaining osteogenesis, the mechanisms allowing proper mineralization and bone formation are not fully understood. Critical players of bone biology include the tissue-nonspecific ALP, whose expression with type 1 collagen is currently assumed to be necessary and sufficient to account for the spatial restriction of mineralization to bone and teeth (27). In osteoblasts, TNAP is involved in the control of the Pi/PPi ions ratio. It thereby governs hydroxyapatite crystals formation within the type 1 collagen bone ECM (28–30). Such a role is exemplified by the severe decreases in ECM mineralization observed in TNAP-deficient mice or in human patients with hereditary hypophosphatasia (24, 31, 32). In addition, ectopic TNAP expression in collagen-expressing cells induces pathological mineralization (27).
To gain insight into the mechanisms involved in the implementation and regulation of TNAP activity during mineralization, we took advantage of the C1 mesoblastic cell line, which recapitulates osteogenic differentiation in vitro in a homogeneous and synchronous manner. The data show that the 5-HT2BR signaling plays a role in the control of TNAP activity.
At day 0, condensing C1 mesoblastic cells instruct permissive signals that turn on the production of mRNAs related to osteogenic differentiation, in agreement with in vivo observations (33). TNAP and 5-HT2BR mRNAs are among such transcripts. Their levels remain roughly constant from the stem cell state up to terminal differentiation. However, these transcripts are dormant during the initial stages of differentiation, thus pointing to yet-to-be elucidated signals repressing their maturation and/or translation.
Between day 2 and day 4, C1 cells build up a profuse type 1 collagen matrix (14). The translation of both TNAP and 5-HT2BR mRNAs begins at day 5. Possibly, common signal(s) turn on the translation of the two transcripts. At this stage, C1 cells have elaborated a widespread type 1 collagen matrix. As suggested by the recent work of Hennessy et al. (34), we may propose that activation of collagen-selective integrins recruits downstream signaling cascades that switch on the translation of TNAP and 5-HT2BR mRNAs.
At day 6, 5-HT2BRs functionally couple with NO and PLA2/AA. At variance with the most classic schemes of 5-HT2 receptor signaling, the 5-HT2BR does not function through the Gq/11 family of G proteins in C1 cells (10). Instead, we may consider an involvement of Gα13 (35) or Gαz (36) in the recruitment of the NO and PLA2 pathways, respectively, as observed in neuronal cells. Another possibility would be G protein-independent signaling (35). At this stage, the TNAP protein becomes overtly expressed but still lacks catalytic activity. A post-translational induction of TNAP activity thus appears to be one limiting step during the program. It may account for the delay between ECM formation and mineral deposition, despite the presence of Pi (β-glycerophosphate) in the culture medium from the earliest stages of the program.
Day 7 marks the beginning of mineralization. It coincides with the onset of TNAP activity from preexisting TNAP molecules and that of other yet-unidentified ALP activities. The latter phosphatase(s) sustain(s) an unvariant basal level of activity from day 7 up to the end stage of differentiation. Although these phosphatases are not controlled by the 5-HT2BR, the receptor switches on TNAP activity by post-translational mechanisms. TNAP activation involves PIPLC, whose activity is stimulated from day 7 and up to day 10 (i.e. during the initial period of mineralization) by the PLA2/AA/COX/PGE2 pathway. The regulation of TNAP activity is under the control of the receptor intrinsic activity, as shown by either pharmacological or shRNA knock-down approaches. However, additional up-regulation of TNAP activity can be obtained in response to agonist stimulation.
From day 10 onward, the 5-HT2BR continues to control TNAP activity in a cell-autonomous manner. However, it loses its ability to elicit further TNAP activation upon agonist exposure. This time window coincides with a switch in signaling intermediates downstream from the 5-HT2BR-PLA2 coupling, with AA being now metabolized into LTB4 through LOX instead of prostaglandins. Leukotrienes continue to mediate the intrinsic action of the 5-HT2BR on TNAP activity. They also act upstream from PIPLC in the pathway.
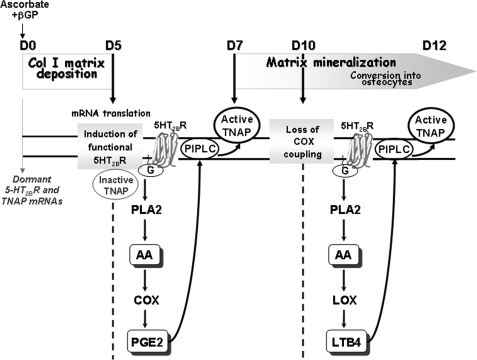
The functional link between the 5-HT2BR and TNAP activity in C1 cells is summarized in Fig. 7. The control exerted by the 5-HT2BR on TNAP activity is imparted by eicosanoids, which in turn activate PIPLC. Eicosanoids are known to be local regulators of bone metabolism (37). Some pathways sustaining the anabolic action of prostaglandin EP2 and EP4 receptors have been recently uncovered (38). Our unpublished results indicate that EP4 receptors are induced along the osteogenic differentiation of C1 cells. The contribution of EP4 receptors to the 5-HT2BR-dependent control on TNAP activity will deserve additional investigation. Further work will also be necessary to solve the mechanisms through which eicosanoids control PIPLC activity.
FIGURE 7.
Dynamic regulation of TNAP activity by 5-HT2BR signaling to PLA2 and PIPLC during osteogenic differentiation. Dormant mRNAs encoding 5-HT2BR and TNAP are present from the mesoblastic stage. Translation occurs once C1 cells are embedded in a type 1 collagen matrix (day 5). The 5-HT2BR is rendered functional on day 5, prior to mineral deposition. At this stage, TNAP is present but in an inactive form. The 5-HT2BR turns on TNAP to an active state through PLA2 signaling from day 7 until terminal differentiation. The osteogenic program is associated with a switch in signaling downstream from the 5-HT2BR-related PLA2 coupling. The shunt from COX-dependent PGE2 to LOX-dependent LTB4 production at day 10 of the osteogenic program delineates two distinct stages of TNAP regulation. Both eicosanoids recruit PIPLC to relay the 5-HT2BR-mediated activation of TNAP.
Our study also shows defects in TNAP activity in primary calvarial osteoblasts derived from 5-HT2BR knock-out mice. Moreover, as observed in C1 cells, the activity of TNAP in WT calvaria is controlled by PIPLC.
In conclusion, our data establish a positive involvement of the 5-HT2BR in mineralization and allow us to relate the osteopenic phenotype of 5-HT2BR knock-out mice (6) to a deficit in TNAP activity. The functional impact of 5-HT signaling in the skeleton is still very much debated because both enhancing and inhibitory effects on bone formation have been depicted in response to serotonin (1). Such diverse effects may be explained by the concurrent expression of several 5-HT receptor subtypes (4, 6, 10, 11) and downstream couplings, which may vary according to the differentiation state of bone-forming cells. For instance, the negative regulation exerted by 5-HT on TNAP activity when C1 cells have reached the osteocyte stage, at variance with the positive effect mediated by 5-HT2BRs at earlier stages, suggests that 5-HT receptors other than the 5-HT2BR counteract the 5-HT2BR-mediated TNAP activation at terminal differentiation. One candidate to be considered to explain the negative regulation exerted by 5-HT on 5-HT2BR-mediated TNAP activation is the 5-HT1B receptor, whose inhibitory action on bone formation has been reported by Yadav et al. (11). Notwithstanding, 5-HT1B receptors have not been detected in calvarial cells by binding experiments (6), in agreement with others (4). In C1 cells, our signal transduction assays allowed us to exclude the presence of functional 5-HT1B receptors up to day 7 of differentiation (10). Whether these receptors are expressed at later stages will deserve further investigation.
Supplementary Material
Acknowledgments
We are grateful to Martine Bühler for skillful technical assistance and to Dr. M. T. Corvol, Dr. F. Rannou, and Prof. S. Blanquet for helpful discussion and critical reading of the manuscript.
This work was supported by grants from Centre National de la Recherche Scientifique and the Institut National de la Santé et de la Recherche Médicale.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” an additional reference, and Fig. 1.
- 5-HT
- serotonin
- 5-HT2AR
- serotonin-2A receptor
- 5-HT2BR
- serotonin-2B receptor
- AA
- arachidonic acid
- ALP
- alkaline phosphatase
- COX
- cyclooxygenase
- ECM
- extracellular matrix
- LOX
- lipoxygenase
- l-NMA
- l-N monomethyl arginine
- LTB4
- leukotriene B4
- PGE2
- prostaglandin E2
- PIPLC
- phosphatidylinositol-specific phospholipase C
- PLA2
- phospholipase A2
- TNAP
- tissue-nonspecific alkaline phosphatase.
REFERENCES
- 1.Warden S. J., Robling A. G., Haney E. M., Turner C. H., Bliziotes M. M. (2010) Bone 46, 4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goridis C., Rohrer H. (2002) Nat. Rev. Neurosci. 3, 531–541 [DOI] [PubMed] [Google Scholar]
- 3.Jonnakuty C., Gragnoli C. (2008) J. Cell. Physiol. 217, 301–306 [DOI] [PubMed] [Google Scholar]
- 4.Bliziotes M. M., Eshleman A. J., Zhang X. W., Wiren K. M. (2001) Bone 29, 477–486 [DOI] [PubMed] [Google Scholar]
- 5.Westbroek I., van der Plas A., de Rooij K. E., Klein-Nulend J., Nijweide P. J. (2001) J. Biol. Chem. 276, 28961–28968 [DOI] [PubMed] [Google Scholar]
- 6.Collet C., Schiltz C., Geoffroy V., Maroteaux L., Launay J. M., de Vernejoul M. C. (2008) FASEB J. 22, 418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warden S. J., Robling A. G., Sanders M. S., Bliziotes M. M., Turner C. H. (2005) Endocrinology 146, 685–693 [DOI] [PubMed] [Google Scholar]
- 8.Warden S. J., Nelson I. R., Fuchs R. K., Bliziotes M. M., Turner C. H. (2008) Menopause 15, 1176–1183 [DOI] [PubMed] [Google Scholar]
- 9.Haney E. M., Warden S. J. (2008) J. Musculoskelet. Neuronal Interact. 8, 133–145 [PubMed] [Google Scholar]
- 10.Locker M., Bitard J., Collet C., Poliard A., Mutel V., Launay J. M., Kellermann O. (2006) Cell. Signal. 18, 628–639 [DOI] [PubMed] [Google Scholar]
- 11.Yadav V. K., Ryu J. H., Suda N., Tanaka K. F., Gingrich J. A., Schütz G., Glorieux F. H., Chiang C. Y., Zajac J. D., Insogna K. L., Mann J. J., Hen R., Ducy P., Karsenty G. (2008) Cell 135, 825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millan M. J., Marin P., Bockaert J., la Cour C. M. (2008) Trends Pharmacol. Sci. 29, 454–464 [DOI] [PubMed] [Google Scholar]
- 13.Poliard A., Nifuji A., Lamblin D., Plee E., Forest C., Kellermann O. (1995) J. Cell Biol. 130, 1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poliard A., Ronzière M. C., Freyria A. M., Lamblin D., Herbage D., Kellermann O. (1999) Exp. Cell Res. 253, 385–395 [DOI] [PubMed] [Google Scholar]
- 15.Leysen J. E. (2004) Curr. Drug Targets CNS Neurol. Disord. 3, 11–26 [DOI] [PubMed] [Google Scholar]
- 16.Baxter G., Kennett G., Blaney F., Blackburn T. (1995) Trends Pharmacol. Sci. 16, 105–110 [DOI] [PubMed] [Google Scholar]
- 17.Kleven M. S., Assié M. B., Koek W. (1997) J. Pharmacol. Exp. Ther. 282, 747–759 [PubMed] [Google Scholar]
- 18.Van Belle H. (1976) Clin. Chem. 22, 972–976 [PubMed] [Google Scholar]
- 19.Nakano Y., Beertsen W., van den Bos T., Kawamoto T., Oda K., Takano Y. (2004) Bone 35, 1077–1085 [DOI] [PubMed] [Google Scholar]
- 20.Masferrer J. L., Koki A., Seibert K. (1999) Ann. N.Y. Acad. Sci. 889, 84–86 [DOI] [PubMed] [Google Scholar]
- 21.Kozak W., Fraifeld V. (2004) Front. Biosci. 9, 3339–3355 [DOI] [PubMed] [Google Scholar]
- 22.Bleasdale J. E., Thakur N. R., Gremban R. S., Bundy G. L., Fitzpatrick F. A., Smith R. J., Bunting S. (1990) J. Pharmacol. Exp. Ther. 255, 756–768 [PubMed] [Google Scholar]
- 23.Spyridakis S., Leondaritis G., Nakos G., Lekka M. E., Galanopoulou D. (2010) Am. J. Respir. Cell Mol. Biol. 42, 357–362 [DOI] [PubMed] [Google Scholar]
- 24.Whyte M. P. (1994) Endocr. Rev. 15, 439–461 [DOI] [PubMed] [Google Scholar]
- 25.Baxter G. S. (1996) Behav. Brain Res. 73, 149–152 [DOI] [PubMed] [Google Scholar]
- 26.Ciancaglini P., Simão A. M., Camolezi F. L., Millán J. L., Pizauro J. M. (2006) Braz. J. Med. Biol. Res. 39, 603–610 [DOI] [PubMed] [Google Scholar]
- 27.Murshed M., Harmey D., Millán J. L., McKee M. D., Karsenty G. (2005) Genes Dev. 19, 1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedde K. N., Blair L., Silverstein J., Coburn S. P., Ryan L. M., Weinstein R. S., Waymire K., Narisawa S., Millán J. L., MacGregor G. R., Whyte M. P. (1999) J. Bone Miner. Res. 14, 2015–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hessle L., Johnson K. A., Anderson H. C., Narisawa S., Sali A., Goding J. W., Terkeltaub R., Millan J. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9445–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesch W., Vandenbos T., Roschgr P., Fratzl-Zelman N., Klaushofer K., Beertsen W., Fratzl P. (2003) J. Bone Miner. Res. 18, 117–125 [DOI] [PubMed] [Google Scholar]
- 31.Henthorn P. S., Whyte M. P. (1992) Clin. Chem. 38, 2501–2505 [PubMed] [Google Scholar]
- 32.Narisawa S., Fröhlander N., Millán J. L. (1997) Dev. Dyn. 208, 432–446 [DOI] [PubMed] [Google Scholar]
- 33.MacGregor G. R., Zambrowicz B. P., Soriano P. (1995) Development 121, 1487–1496 [DOI] [PubMed] [Google Scholar]
- 34.Hennessy K. M., Pollot B. E., Clem W. C., Phipps M. C., Sawyer A. A., Culpepper B. K., Bellis S. L. (2009) Biomaterials 30, 1898–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manivet P., Mouillet-Richard S., Callebert J., Nebigil C. G., Maroteaux L., Hosoda S., Kellermann O., Launay J. M. (2000) J. Biol. Chem. 275, 9324–9331 [DOI] [PubMed] [Google Scholar]
- 36.Mouillet-Richard S., Pietri M., Schneider B., Vidal C., Mutel V., Launay J. M., Kellermann O. (2005) J. Biol. Chem. 280, 4592–4601 [DOI] [PubMed] [Google Scholar]
- 37.Hikiji H., Takato T., Shimizu T., Ishii S. (2008) Prog. Lipid Res. 47, 107–126 [DOI] [PubMed] [Google Scholar]
- 38.Minamizaki T., Yoshiko Y., Kozai K., Aubin J. E., Maeda N. (2009) Bone 44, 1177–1185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.