Abstract
Bradyrhizobium japonicum Fur mediates manganese-responsive transcriptional control of the mntH gene independently of iron, but it also has been implicated in iron-dependent regulation of the irr gene. Thus, we sought to address the apparent discrepancy in Fur responsiveness to metals. Irr is a transcriptional regulator found in iron-limited cells. Here, we show that irr gene mRNA was regulated by both iron and manganese, and repression occurred only in the presence of both metals. Under these conditions, Fur occupied the irr promoter in vivo in the parent strain, and irr mRNA expression was derepressed in a fur mutant. Under low iron conditions, the irr promoter was occupied by Irr, but not by Fur, and control by manganese was lost. Fur occupancy of the irr promoter was dependent on manganese, but not iron, in an irr mutant, suggesting that Irr normally interferes with Fur binding. Correspondingly, regulation of irr mRNA was dependent only on manganese in the irr strain. The Irr binding site within the irr promoter partially overlaps the Fur binding site. DNase I footprinting analysis showed that Irr interfered with Fur binding in vitro. In addition, Fur repression of transcription from the irr promoter in vitro was relieved by Irr. We conclude that Fur mediates manganese-dependent repression of irr transcription and that Irr acts as an antirepressor under iron limitation by preventing Fur binding to the promoter.
Keywords: Bacterial Transcription, Chromatin Immunoprecipitation (ChiP), Heme, Iron, Manganese, Bradyrhizobium, Rhizobium
Introduction
Maintenance of metal homeostasis in bacteria involves the activities of transcriptional regulators that directly or indirectly sense the metal to control gene expression. Rhizobia are bacteria that live as free-living organisms or as the endosymbiont of legumes, where they convert atmospheric nitrogen to ammonia within root nodules to fulfill the nutritional nitrogen requirement of the plant host. Recent studies show that iron and manganese homeostasis are regulated very differently in the rhizobia compared with other well studied model systems. Whereas Fur is the major global regulator of iron metabolism in Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis, this function has been replaced largely by Irr or RirA in rhizobia and other α-proteobacteria (1–3). Furthermore, most rhizobia lack the manganese-responsive transcriptional regulator MntR, and some use the Fur homolog instead for manganese metalloregulation.
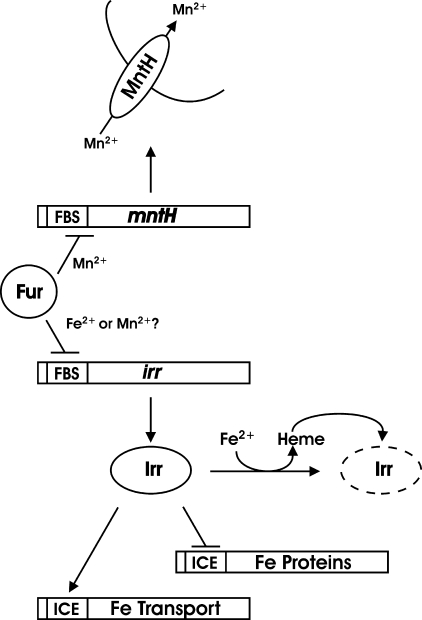
The Irr protein is the primary global regulator of iron homeostasis in Bradyrhizobium japonicum (4) and has been described in other α-proteobacteria as well (5–7). Irr functions under iron limitation and acts as both a positive and negative regulator of gene expression (Fig. 1). Irr recognizes and binds to an iron control element within the promoter of target genes (8). Binding of Irr to the iron control element of a negatively regulated gene is sufficient to repress transcription in vitro (9), but the molecular basis of the positive control is unknown.
FIGURE 1.
Overview of the relationship between Fur, the irr and mntH genes, the proteins they encode, and control by metals and heme. ICE denotes the iron control element recognized by Irr within target genes. FBS denotes the Fur binding site within promoters of target genes. The broken line denotes degraded Irr.
Cellular Irr levels are controlled primarily at the level of protein stability in B. japonicum (10–12). Irr is stable under iron limitation but degrades in response to iron in a heme-dependent manner (11–14). Heme inhibits Irr activity in Rhizobium leguminosarum but does not lead to degradation (6). Manganese contributes to Irr stability in B. japonicum under iron limitation by inhibiting heme binding, thereby raising the threshold heme level necessary to trigger Irr degradation (10). As a result, Irr levels are attenuated under low iron conditions if manganese also is deficient. The post-transcriptional control of Irr results in a general lack of correlation between the Irr protein level and the mRNA level of the gene that encodes it (15–16). Nevertheless, we are interested in the transcriptional control of the irr gene because, as described below, it reveals insights into the function of Irr and of the Fur homolog in this bacterium.
Although Fur is not the primary transcriptional regulator of iron-regulated genes in the rhizobia, homologs are present in most species, and it has been studied in a few of them. In Sinorhizobium meliloti and R. leguminosarum, the Fur homolog is a manganese-responsive regulator and has been renamed Mur (17–21).
The Fur protein from B. japonicum originally was identified based on its ability to complement an E. coli fur mutant (22). Although it recognizes a canonical E. coli Fur-binding cis-acting element, it binds a dissimilar sequence in B. japonicum gene promoters (23–24). Currently, irr and mntH are the only genes known to be direct targets of B. japonicum Fur (23–24). Both gene promoters contain a conserved motif of three imperfect direct repeat hexamers necessary for Fur binding and transcriptional repression activity. Moreover, the affinities of Fur for the irr and mntH promoters are very similar to each other. Finally, either Mn2+ or Fe2+ can serve as a cofactor in vitro to confer DNA binding on either promoter (25).3 Despite the similarities between the two promoters, previous reports indicate that irr and mntH are regulated differently (16, 24). The irr gene is modestly regulated by iron at the mRNA level, which is lost in a fur mutant, whereas Fur mediates manganese-dependent control of the mntH gene and is unresponsive to iron. Therefore, the biological activity of Fur is not understood completely in B. japonicum. In this study, we show that Fur mediates manganese-dependent repression of both the irr and mntH genes at the mRNA level. However, Irr binds to its own promoter but not the mntH promoter under iron limitation to relieve Fur-dependent repression. Thus, an explanation for the apparent discrepancy in Fur function is provided, and a novel function for Irr has been identified.
EXPERIMENTAL PROCEDURES
Strains and Media
B. japonicum strains USDA110 and LO are the parent strains used in this study. Strain GEM4 (22) is a mutant derivative of USDA110 in which the fur gene is replaced by an omega-cassette. LODTM5 is a mutant derived from strain LO that contains a transposon Tn5 inserted within the irr gene (15). B. japonicum strains were grown routinely at 29 °C in GSY (glycerol-salts-yeast) medium as described previously (26). For low manganese and iron conditions, modified GSY was used, containing 0.5 g per liter yeast extract instead of 1 g per liter, with no exogenous manganese or iron added. The actual concentrations of manganese and iron in unsupplemented media are 0.2 and 0.3 μm respectively, as determined by atomic absorption using a Perkin-Elmer Life Sciences model 1100B atomic absorption spectrometer. High metal medium was supplemented with either 50 μm MnCl2, 20 μm FeCl3, or both.
Analysis of RNA
Expression levels of selected genes were determined by qPCR4 with an iQTM SYBR Green Supermix (Bio-Rad) using iCycler thermal cycler (Bio-Rad) as described previously (4). RNA was isolated from B. japonicum cells using a hot phenol extraction method as described previously (4). cDNA was synthesized from 5 μg total RNA using an iScriptTM cDNA synthesis kit (Bio-Rad). qPCR reactions were carried out as described previously (24). The data are expressed as the relative starting quantity (SQ) of the respective mRNAs normalized to the housekeeping gene gapA and presented as the average triplicate samples with S.D.
Quantitative in Vivo Cross-linking and Immunoprecipitation
This technique was used to analyze the occupancy of the mntH or irr promoter by Fur or Irr. 200-ml cultures of parent strain USDA110 or fur strain GEM4, and parent strain LO or irr strain LODTM5 were grown under low or high manganese and low or high iron conditions to mid-log phase (A540 0.4–0.6). In vivo cross-linking of DNA to protein and subsequent immunoprecipitation with antibodies specific to Irr or Fur were carried out as described elsewhere (27). Immunoprecipitated DNA (1 μl) was analyzed by qPCR using primers that amplify the promoter regions of interest. The data are expressed as the SQ of immunoprecipitated DNA normalized to the mock pulldown in which primary antibody was omitted from the immunoprecipitation reaction.
DNase I Footprinting Analysis
DNase I footprinting analyses examining the DNA regions protected by increasing concentrations of Fur and Irr in the presence of 100 μm MnCl2 were carried out as described previously (24). Titration experiments were done in the absence of protein or with titrating concentrations of Irr in the presence of 10 nm Fur.
In Vitro Transcription Assay
In vitro transcription of the irr gene from template DNA was performed as described previously (23) in the presence of 100 μm MnCl2, in the presence or absence of 10 nm Fur, and in the presence or absence of 150 nm Irr.
RESULTS
The irr Gene Is Regulated by Both Iron and Manganese at the mRNA Level
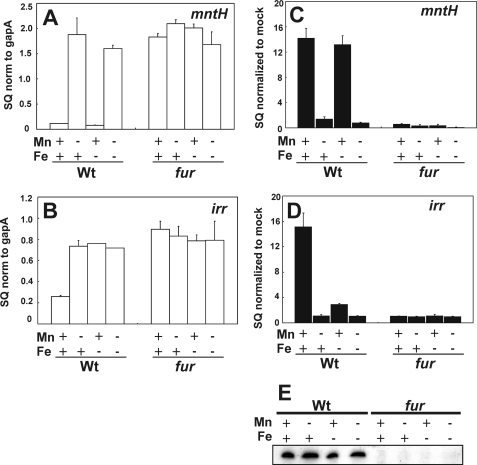
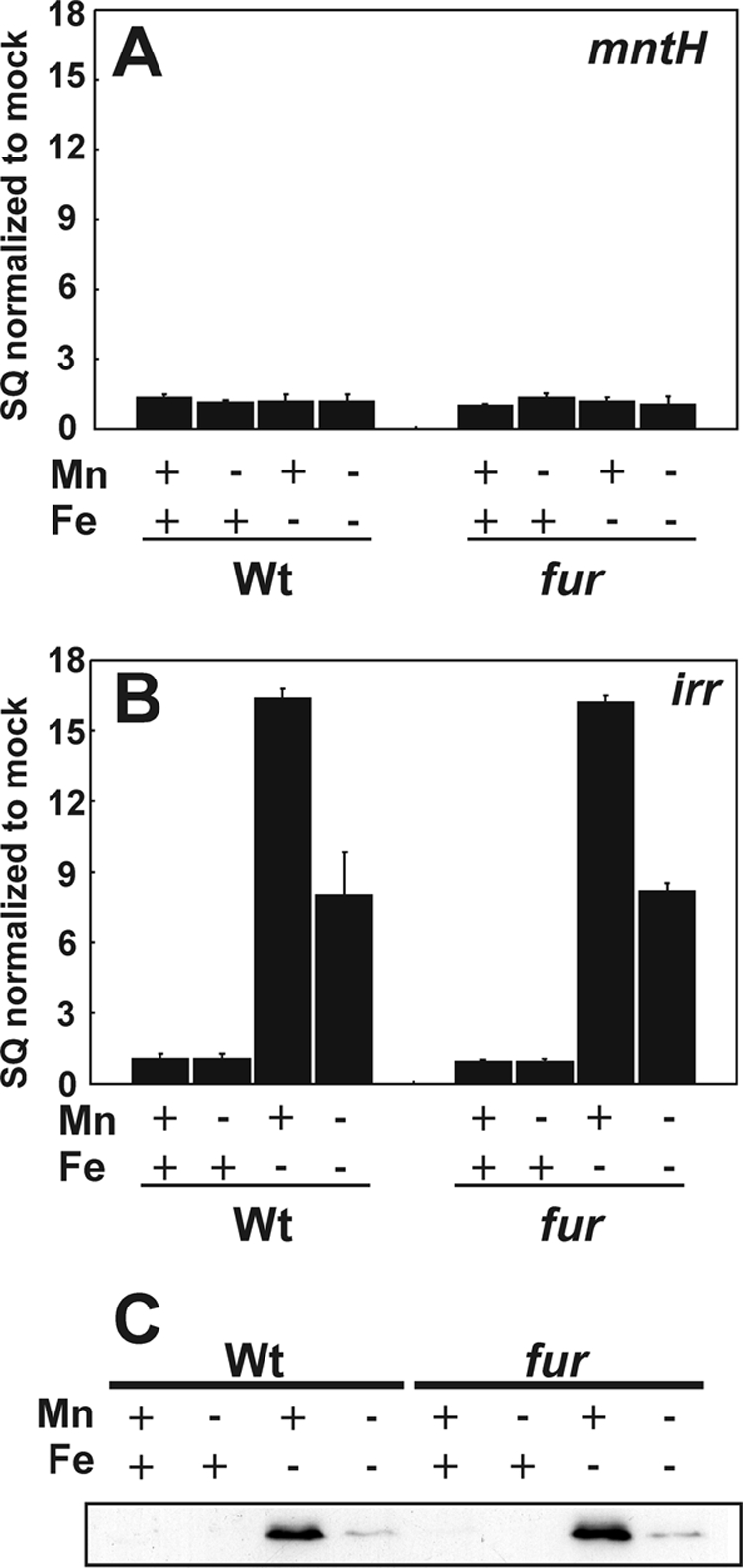
We showed previously that the irr gene is regulated by iron at the RNA level and that this control is lost in a fur mutant (16). More recently, we found that the mntH gene is repressed by Fur in a manganese-responsive manner and is not transcriptionally controlled by iron (24). Furthermore, the promoters of irr and fur contain conserved cis-acting elements that are bound by Fur with similar affinities (23–24). In the previous irr gene analysis, the trace elements routinely added to the growth medium included manganese (16), and therefore, the role of manganese on expression of that gene has not been addressed. Here, we examined irr and mntH mRNA levels by quantitative real-time PCR in cells grown in media containing different combinations of high and low iron and manganese concentrations (Fig. 2, A and B). As shown previously (24), mntH expression was repressed in the presence of manganese independent of the iron status (Fig. 2A). By contrast, irr mRNA expression was repressed by manganese in the presence of iron but remained high under iron limitation regardless of the manganese status (Fig. 1B). Thus, irr mRNA is regulated by both metals, and manganese-dependent repression is lost under low iron conditions.
FIGURE 2.
Effects of manganese and iron on mntH and irr expression in the wild type and fur mutant, and on Fur occupancy of the mntH and irr promoters in those cells. A and B, steady state mRNA levels of the mntH or irr genes were analyzed by quantitative real-time PCR from cells grown in media supplemented with (+) or without (−) 50 μm MnCl2 and with (+) or without (−) 20 μm FeCl3. The data are expressed as the SQ of the respective mRNAs normalized (norm) to the housekeeping gene gapA and presented as the average triplicate samples plus S.D. C and D, Fur occupancy of the mntH or irr promoter was carried out by cross-linking of cells grown under the iron and manganese conditions described for the qPCR experiments, followed by immunoprecipitation using anti-Fur antibodies or a mock control lacking the primary antibody. Co-immunoprecipitated DNA was quantified utilizing qPCR with primers used to amplify the promoter regions of irr and mntH. The data are expressed as the SQ of immunoprecipitated DNA normalized to the mock pull down. E, Western blot analysis of Fur protein levels in cells of the wild type (Wt) or fur mutant grown under the iron and manganese conditions described above. 50 μg of protein was loaded per lane.
In Vivo Occupancy of the irr Promoter by Fur Is Dependent on the Status of Both Iron and Manganese
Fur regulates mntH and irr (16, 23–24); thus, we examined mRNA levels in cells of a fur mutant strain grown in media with various combinations of high and low iron and manganese concentrations (Fig. 2, A and B). Expression of both genes was derepressed in the fur strain under all conditions tested, as expected for a repressor function for Fur.
In vivo promoter occupancy of the mntH and irr genes by Fur was by assessed by cross-linking/immunoprecipitation analysis (Fig. 2, C and D). Cells were grown to mid-log phase, followed by cross-linking of protein to DNA. DNA that co-precipitated with anti-Fur antibodies in cell extracts was analyzed by qPCR using primers that amplify each promoter region. The total Fur level in cells was constitutive in the wild type under all conditions tested as observed by Western blot analysis (Fig. 2E). The mntH promoter was occupied by Fur only in the presence of manganese regardless of the iron status (Fig. 2C). However, Fur occupancy of the irr promoter was dependent on the status of iron as well as manganese. In that case, high occupancy was observed only under high manganese, high iron conditions (Fig. 2D). Thus, promoter occupancy by Fur corresponds with repression for both genes, but the conditions under which each promoter is occupied differs between mntH and irr. Specifically, Fur fully occupied the mntH promoter, but not the irr promoter, under high manganese, low iron conditions.
Irr Binds the irr Gene Promoter in Vivo and in Vitro
The difference between the expression pattern of irr and mntH gene mRNA was observed under low iron, high manganese conditions. In those cells, mntH was repressed and its promoter occupied by Fur, whereas irr was derepressed and had diminished Fur occupancy of its promoter (Fig. 2). Irr accumulates and functions under low iron conditions, leading us to ask whether Irr may control expression of its own transcript. In addition, bioinformatic analysis predicts an Irr binding site upstream of the irr open reading frame (28).
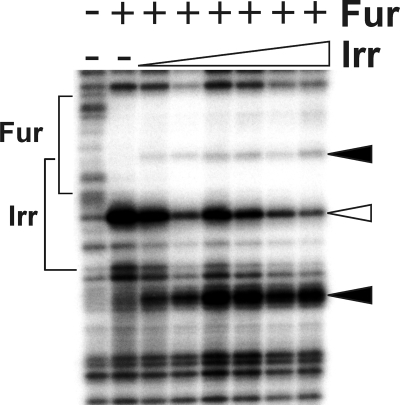
In vivo occupancy of the irr promoter by Irr was examined by cross-linking/immunoprecipitation as described above, except that anti-Irr antibodies were used in the immunoprecipitation step (Fig. 3). Irr occupied the irr promoter in cells grown under iron limitation (Fig. 3), conditions where Irr accumulates and functions. Occupancy was higher in the presence of manganese than in its absence, which agrees with previous observations that Irr levels are attenuated under manganese limitation due to degradation (10) and was confirmed here (Fig. 3C). The occupancy profile in cells grown under the different metal conditions was the same in the wild type and the fur mutant, indicating that Fur does not interfere with Irr binding. In contrast to irr, no Irr occupancy of the mntH promoter was observed compared with the mock control in which antibody was omitted (Fig. 3). These findings indicate that differences in Irr occupancy between the irr and mntH genes may contribute to differences in the regulation of the two genes.
FIGURE 3.
Effect of iron and manganese on Irr occupancy of the mntH and irr promoters in vivo in cells of the wild type and fur mutant strain. A and B, cross-linking and immunoprecipitation of wild type (Wt) or fur mutant cells grown with the various iron and manganese conditions were carried out as described in Fig. 2, except that anti-Irr antibodies were used. The data are expressed as the SQ of immunoprecipitated DNA normalized to the mock pull down. C, Western blot analysis of Irr protein levels in cells of the wild type or fur mutant grown under the iron and manganese conditions described above. 50 μg of protein was loaded per lane.
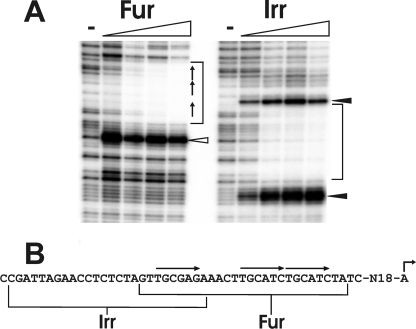
To determine the Irr binding site within the irr promoter, DNaseI footprinting was carried out using purified recombinant Irr and 32P-labeled DNA that corresponds to the irr gene upstream region (Fig. 4). Irr bound to the irr promoter as determined by the protected region in the −64 to −39 region relative to the transcription start site (+1). In addition, two DNase I-hypersensitive sites were observed (filled arrows). Footprinting analysis of the irr gene using purified Fur showed protection in the −47 to −21 region of the promoter and a hypersensitive site (open arrow) (Fig. 4), in good agreement with a previous report (23). Thus, the Irr and Fur binding sites overlap with each other.
FIGURE 4.
DNase I footprinting of the irr promoter with B. japonicum Fur and Irr. A, protection of DNA from DNase I digestion by Fur or Irr was carried out in the presence of MnCl2 and 0, 5, 10, 25, or 50 nm Fur or 0, 25, 150, 250, or 500 nm Irr. DNA was radiolabeled with 32P at the 5′-end of the non-template strand, and thus, the 3′-end is at the top of the gel. Brackets represent the protected regions of Fur and Irr. The open arrowhead indicates a DNase I-hypersensitive site caused by Fur binding. The closed arrowheads represent the DNase I-hypersensitive sites caused by Irr binding. B, the sequence of the irr promoter region protected by Irr or Fur is shown. The bent arrow represents the transcriptional start site of irr. The arrows over the sequence show the three imperfect direct repeat sequences shown previously to be necessary for Fur binding.
Iron-dependent Control of irr mRNA Expression Is Lost in an irr Mutant Strain
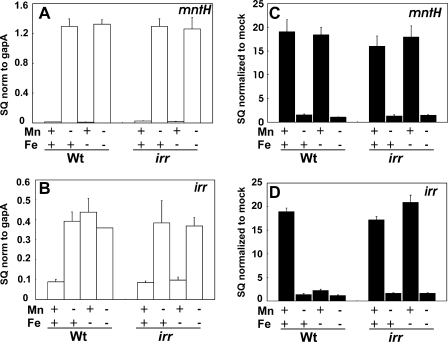
To examine the role of Irr in gene expression, irr and mntH mRNA levels were examined in an irr mutant (Fig. 5, A and B). We have been unable to construct an irr mutant in strain USDA110, but an irr mutant is available derived from strain LO (15). The two parent strains are very similar with regards to global iron-responsive gene expression (4, 29), Irr-responsive gene expression (4, 8), and metal-dependent control of the genes under study herein (see below). The mutant has a transposon inserted within the open reading frame, and there are 113 nucleotides of transcribed DNA between the transcription start site and the transposon insertion. Thus, it was possible to examine the intact irr promoter and transcript synthesized from it in an irr strain. mntH mRNA expression was regulated similarly in the mutant as it was in the parent strain, showing control by manganese, but not iron, in both strains (Fig. 5A). This agrees with the lack of Irr occupancy of the mntH promoter (Fig. 3A) and confirms that mntH is not an Irr-regulated gene. However, iron responsiveness of the irr gene was lost in the irr mutant, but manganese-dependent expression was retained (Fig. 4B). Control of irr in the irr strain was similar to that of mntH in the wild type, and thus differential control of mntH and irr in the parent strain can be attributed to Irr.
FIGURE 5.
Effects of manganese and iron on mntH and irr expression in the wild type and irr mutant and on Fur occupancy of the mntH and irr promoters in those cells. A and B, steady state mRNA levels of the mntH or irr genes were analyzed by quantitative real-time PCR from cells grown with various iron and manganese conditions as described in Fig. 2. The data are expressed as the SQ of the respective mRNAs normalized to the housekeeping gene gapA and presented as the average triplicate samples plus the S.D. C and D, cross-linking and immunoprecipitation of wild type (Wt) or fur mutant were carried out as described in Fig. 2, but anti-Irr antibodies were used for the immunoprecipitation. The data are expressed as the SQ of immunoprecipitated DNA normalized to the mock pull down. norm, normalized.
Irr Interferes with Fur Occupancy of the irr Gene Promoter in Vivo
irr gene mRNA was low in the irr mutant grown in low iron, high manganese media compared with the wild type (Fig. 5B), indicating that Irr is a positive effector of that gene. However, expression levels were high in the irr strain in the absence of manganese. Moreover, the irr promoter contains a binding site for both Irr and Fur but is fully occupied only by Irr under low iron, high manganese conditions, where both regulators are active (Figs. 2 and 3). Collectively, the data suggest that Irr is not an activator of the irr gene but may interfere with the ability of Fur to occupy the irr promoter, thereby preventing repression.
We examined Fur occupancy of the irr and mntH promoters in the irr mutant by cross-linking/immunoprecipitation as described above using anti-Fur antibodies for the immunoprecipitation (Fig. 5, C and D). As expected, occupancy of the mntH promoter in cells grown in various combinations of metal essentially was the same in the wild type and mutant strains (Fig. 5C). By contrast, the irr promoter was fully occupied by Fur in irr mutant cells grown in low iron, high manganese media, whereas occupancy was very low in wild type cells grown under the same conditions (Fig. 5D). These observations correlate well with the aberrantly low expression of irr gene mRNA in the irr mutant (Fig. 5B). We suggest that Irr binding prevents Fur occupancy, thereby derepressing irr transcription.
Irr Inhibits Fur Binding to the irr Gene Promoter in Vitro
The binding sites for Fur and Irr on the irr promoter overlap, and the common region includes the 5′ most direct repeat hexamer of the Fur binding site (Fig. 4). Previous work shows that substitution mutation of that direct repeat DNA is sufficient to abrogate Fur binding to the irr promoter (23). Those observations, along with the in vivo data described above, suggest that Irr binding occludes a portion of the Fur binding site to prevent repression. We examined the effect of Irr on Fur binding to the irr promoter in vitro by DNase footprinting analysis as shown in Fig. 6. Fig. 6 (lanes 1 and 2) shows the unprotected and Fur-protected regions of the DNA, respectively. The Fur-dependent hypersensitive site is indicated with the open triangle. The binding reaction was titrated with increasing amounts of purified Irr, resulting in the diminution of the Fur-hypersensitive site, and the appearance of the two Irr-dependent hypersensitive sites (closed triangles). The data show that Irr binding to the irr promoter inhibits Fur binding and are in good agreement with the in vivo analysis demonstrating that Fur occupancy depends on the status of Irr. Whereas Irr appears to almost completely abrogate Fur binding in vivo (Fig. 5D), the protection was still observed in vitro (Fig. 6) in the presence of Irr. Previous studies suggest that purified recombinant Irr may be less active than that observed in B. japonicum cell extracts (27).
FIGURE 6.
Effect of Irr on Fur binding to the irr gene promoter in vitro. DNase I footprinting analysis was carried out using irr promoter DNA. The binding reactions contained either no protein (lane 1), 10 nm Fur alone (lane 2) or Fur titrated with increasing concentrations of Irr (lanes 3–8). The Irr concentrations used 0, 25, 150, 250, 500, 1000, or 2000 nm. DNA was radiolabeled at the 5′-end of the non-template strand, and thus, the 3′-end is at the top of the gel. The open arrowhead indicates a DNase I-hypersensitive site caused by Fur binding. The closed arrowheads represent the DNase I-hypersensitive sites caused by Irr binding.
Fur-dependent Transcriptional Repression from the irr Promoter Is Relieved by Irr in Vitro
To further address the effects of Fur and Irr on irr gene expression, in vitro transcription analysis from the irr promoter was carried out in vitro using E. coli RNA polymerase (Fig. 7). In the absence of both Fur and Irr, a 157-nucleotide RNA was synthesized from the irr promoter, but transcription was inhibited in the presence of purified Fur. However, in the presence of both Irr and Fur, transcription was restored partially, demonstrating that Irr relieved Fur-mediated repression. Irr alone did not have a substantial effect on transcription. These findings further support the conclusion that Irr is an antirepressor of Fur-mediated repression of irr transcription.
FIGURE 7.
Repression and antirepression of in vitro transcription of irr. In vitro transcription initiated from the irr promoter was carried out using E. coli RNA polymerase in the presence of MnCl2 and the presence (+) or absence (−) of 10 nm Fur or 150 nm Irr. The 157-nucleotide radiolabeled RNA product was visualized on a gel by autoradiography.
DISCUSSION
Previous studies show that B. japonicum Fur is a manganese-responsive transcriptional regulator that controls mntH gene expression (24), but Fur also was implicated in iron-dependent expression of the irr gene (16, 23). This apparent discrepancy was resolved herein by showing that Fur mediates manganese-dependent repression of both mntH and irr, but the irr gene has an additional control mediated by Irr that relieves that repression under iron limitation. Thus, a newly described role for Irr as an antirepressor has been identified. Moreover, we suggest that Fur primarily may be a manganese-responsive regulator in B. japonicum. Finally, the study further implicates the integration of iron and manganese in bacteria.
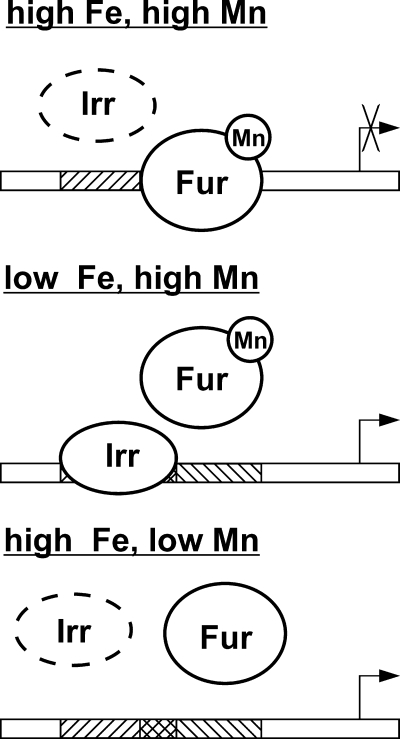
In the presence of iron, Irr is absent, and irr mRNA is manganese-responsive due to Fur binding its promoter (Fig. 8). Under this condition, manganese control of irr and mntH are similar because Fur complexed with Mn2+ recognizes conserved cis-acting elements in both promoters and binds them. Under low iron conditions, Irr is present and bound to the irr promoter. Irr occupancy occludes part of the Fur binding site, thereby preventing Fur occupancy. Therefore, irr mRNA is derepressed under iron limitation independent of the manganese status.
FIGURE 8.
Model for control of irr gene transcription by iron and manganese via Irr and Fur, respectively. The binding of Irr and Fur on their respective binding sites are shown as a function of the iron and manganese conditions. Fur binds DNA to repress transcription only when Fur is complexed with Mn2+. However, Mn2+-complexed Fur cannot bind the irr promoter when Irr is bound, which occurs under low iron conditions, resulting in derepression of the irr gene. The broken line denotes degraded Irr.
Our findings show that Irr acts as an antirepressor of the irr gene rather than an activator. Firstly, the irr mRNA level is high in wild type cells grown in low manganese media regardless of the iron status or Irr occupancy of the promoter (Fig. 2). This is because Fur is inactive under manganese limitation and therefore the status of iron or Irr does not matter. Similarly, Irr is not required for high transcript levels in a fur mutant. Secondly, irr transcript levels remain high in the absence of Irr if the promoter is unoccupied by Fur (Fig. 5), showing that Irr is not an activator but rather an antirepressor. Thirdly, in vitro transcription from the irr promoter proceeds in the absence of Irr, and it becomes necessary only in the presence of Fur (Fig. 7). Collectively, the evidence shows that Irr is necessary for high irr mRNA expression only under high manganese conditions, where Fur is active as a repressor. We do not attempt to extrapolate these findings to other genes positively controlled by Irr, as most of them do not appear to be regulated by Fur (4, 29). Nevertheless, Irr may be an antirepressor of other negative regulators that are yet to be elucidated.
Fur was initially characterized as an iron-responsive regulator in B. japonicum based on its ability to complement an E. coli Fur mutant and aberrant control of numerous iron-dependent genes in a fur strain (16, 29). B. japonicum Fur is both Mn2+- and Fe2+-responsive in vitro with regards to DNA binding and transcriptional repression activities (23, 25). However, irr and mntH are the only genes known to be direct targets of Fur in B. japonicum, and it is now clear that it mediates responsiveness to manganese, not iron, in both genes. The Fur homologs in Sinorhizobium meliloti and R. leguminosarum (named Mur in those organisms) have been described only as Mn2+-responsive regulators (17, 19–20). It is plausible that B. japonicum Fur is solely a Mn2+-responsive transcriptional regulator and that aberrant control of iron-regulated genes is indirect. Recent work showing integration of iron and manganese metabolism lends credence to this idea (10). Because B. japonicum fur can complement an E. coli fur mutant, the differences in metal responsiveness between Fur from E. coli and B. japonicum may be based on different environments of the two cell types. The nickel-responsive transcriptional regulator NmtR from Mycobacterium tuberculosis loses nickel sensitivity but is cobalt responsive in the heterologous host Synechococcus PCC7942 (30).
Although the main purpose of the current study was to explain the apparent discrepancy of Fur responsiveness in regulating two different genes, and the role of Irr in that control, an additional question remains unresolved concerning the rationale for controlling the irr gene in the manner described herein. Irr protein levels are controlled by iron and manganese primarily at the level of protein stability (10–11); thus, the need for transcriptional control remains unclear. One possibility is that a change in irr mRNA under low iron conditions increases the rate of response but does not appreciably affect the steady state level. Alternatively, the transcriptional control may contain an evolutionary vestige. An ancestral form of the fur gene may have been autoregulated in a negative manner, as has been shown in E. coli (31), and irr arose from gene duplication of fur. As Irr changed function, control by Fur was maintained but an additional antirepressor function evolved to maintain basal mRNA level, which is necessary for post-transcriptional control.
This work was supported in part by National Institutes of Health Grant R01 GM067966 to (M. R. O'B.).
T. H. Hohle, unpublished observations.
- qPCR
- quantitative PCR
- SQ
- relative starting quantity.
REFERENCES
- 1.Johnston A. W., Todd J. D., Curson A. R., Lei S., Nikolaidou-Katsaridou N., Gelfand M. S., Rodionov D. A. (2007) Biometals 20, 501–511 [DOI] [PubMed] [Google Scholar]
- 2.Rudolph G., Hennecke H., Fischer H. M. (2006) FEMS Microbiol. Rev. 30, 631–648 [DOI] [PubMed] [Google Scholar]
- 3.Small S. K., Puri S., O'Brian M. R. (2009) Biometals 22, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J., Sangwan I., Lindemann A., Hauser F., Hennecke H., Fischer H. M., O'Brian M. R. (2006) Mol. Microbiol. 60, 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez M., Ugalde R. A., Almirón M. (2005) Microbiology 151, 3427–3433 [DOI] [PubMed] [Google Scholar]
- 6.Singleton C., White G. F., Todd J. D., Marritt S. J., Cheesman M. R., Johnston A. W., Le Brun N. E. (2010) J. Biol. Chem. 285, 16023–16031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd J. D., Sawers G., Rodionov D. A., Johnston A. W. (2006) Mol. Genet. Genomics 275, 564–577 [DOI] [PubMed] [Google Scholar]
- 8.Rudolph G., Semini G., Hauser F., Lindemann A., Friberg M., Hennecke H., Fischer H. M. (2006) J. Bacteriol. 188, 733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangwan I., Small S. K., O'Brian M. R. (2008) J. Bacteriol. 190, 5172–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri S., Hohle T. H., O'Brian M. R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 10691–10695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi Z., Hamza I., O'Brian M. R. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Ishimori K., O'Brian M. R. (2005) J. Biol. Chem. 280, 7671–7676 [DOI] [PubMed] [Google Scholar]
- 13.Qi Z., O'Brian M. R. (2002) Mol. Cell 9, 155–162 [DOI] [PubMed] [Google Scholar]
- 14.Yang J., Panek H. R., O'Brian M. R. (2006) Mol. Microbiol. 60, 209–218 [DOI] [PubMed] [Google Scholar]
- 15.Hamza I., Chauhan S., Hassett R., O'Brian M. R. (1998) J. Biol. Chem. 273, 21669–21674 [DOI] [PubMed] [Google Scholar]
- 16.Hamza I., Qi Z., King N. D., O'Brian M. R. (2000) Microbiol. 146, 669–676 [DOI] [PubMed] [Google Scholar]
- 17.Díaz-Mireles E., Wexler M., Sawers G., Bellini D., Todd J. D., Johnston A. W. (2004) Microbiology 150, 1447–1456 [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Mireles E., Wexler M., Todd J. D., Bellini D., Johnston A. W., Sawers R. G. (2005) Microbiology 151, 4071–4078 [DOI] [PubMed] [Google Scholar]
- 19.Chao T. C., Becker A., Buhrmester J., Pühler A., Weidner S. (2004) J. Bacteriol. 186, 3609–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platero R., Peixoto L., O'Brian M. R., Fabiano E. (2004) Appl Environ Microbiol. 70, 4349–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Platero R., de Lorenzo V., Garat B., Fabiano E. (2007) Appl Environ Microbiol. 73, 4832–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamza I., Hassett R., O'Brian M. R. (1999) J. Bacteriol. 181, 5843–5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman Y. E., O'Brian M. R. (2003) J. Biol. Chem. 278, 38395–38401 [DOI] [PubMed] [Google Scholar]
- 24.Hohle T. H., O'Brian M. R. (2009) Mol. Microbiol. 72, 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman Y. E., O'Brian M. R. (2004) J. Biol. Chem. 279, 32100–32105 [DOI] [PubMed] [Google Scholar]
- 26.Frustaci J. M., Sangwan I., O'Brian M. R. (1991) J. Bacteriol. 173, 1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small S. K., Puri S., Sangwan I., O'Brian M. R. (2009) J. Bacteriol. 191, 1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodionov D. A., Gelfand M. S., Todd J. D., Curson A. R., Johnston A. W. (2006) PLoS Comput Biol. 2, e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J., Sangwan I., O'Brian M. R. (2006) Mol. Genet. Genomics 276, 555–564 [DOI] [PubMed] [Google Scholar]
- 30.Cavet J. S., Meng W., Pennella M. A., Appelhoff R. J., Giedroc D. P., Robinson N. J. (2002) J. Biol. Chem. 277, 38441–38448 [DOI] [PubMed] [Google Scholar]
- 31.Escolar L., Pérez-Martín J., de Lorenzo V. (1999) J. Bacteriol. 181, 6223–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]