Abstract
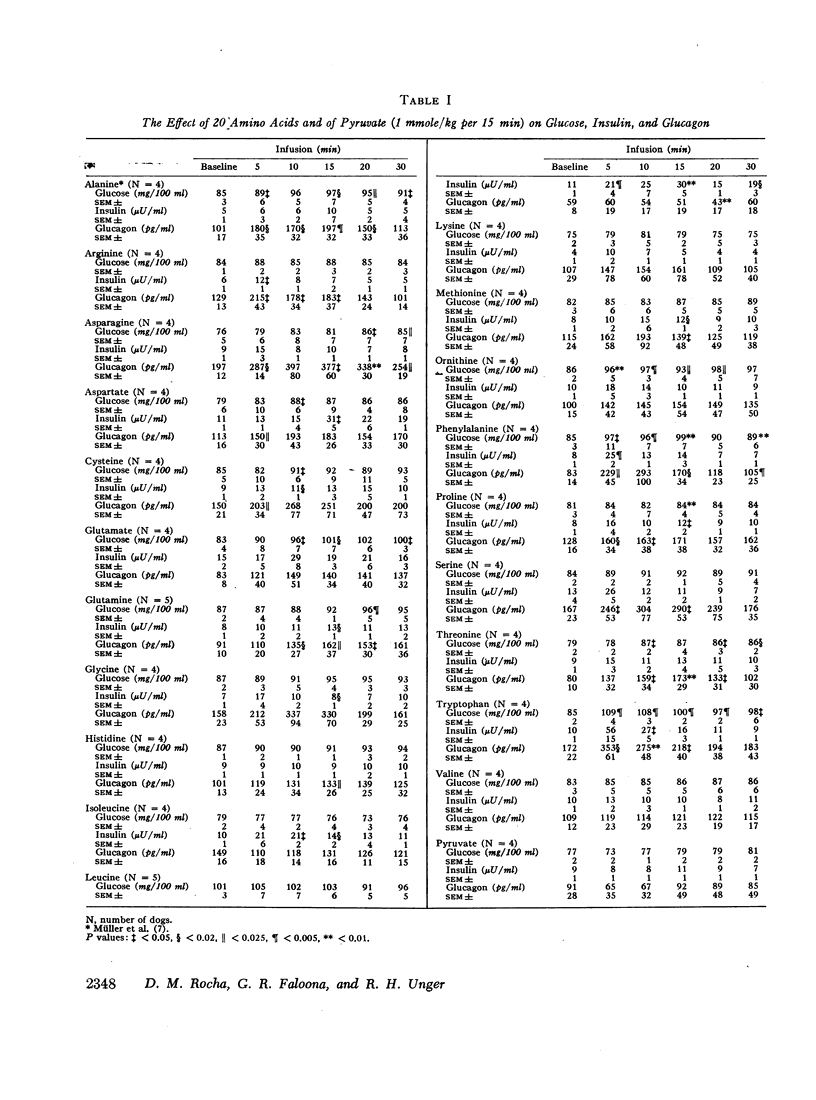
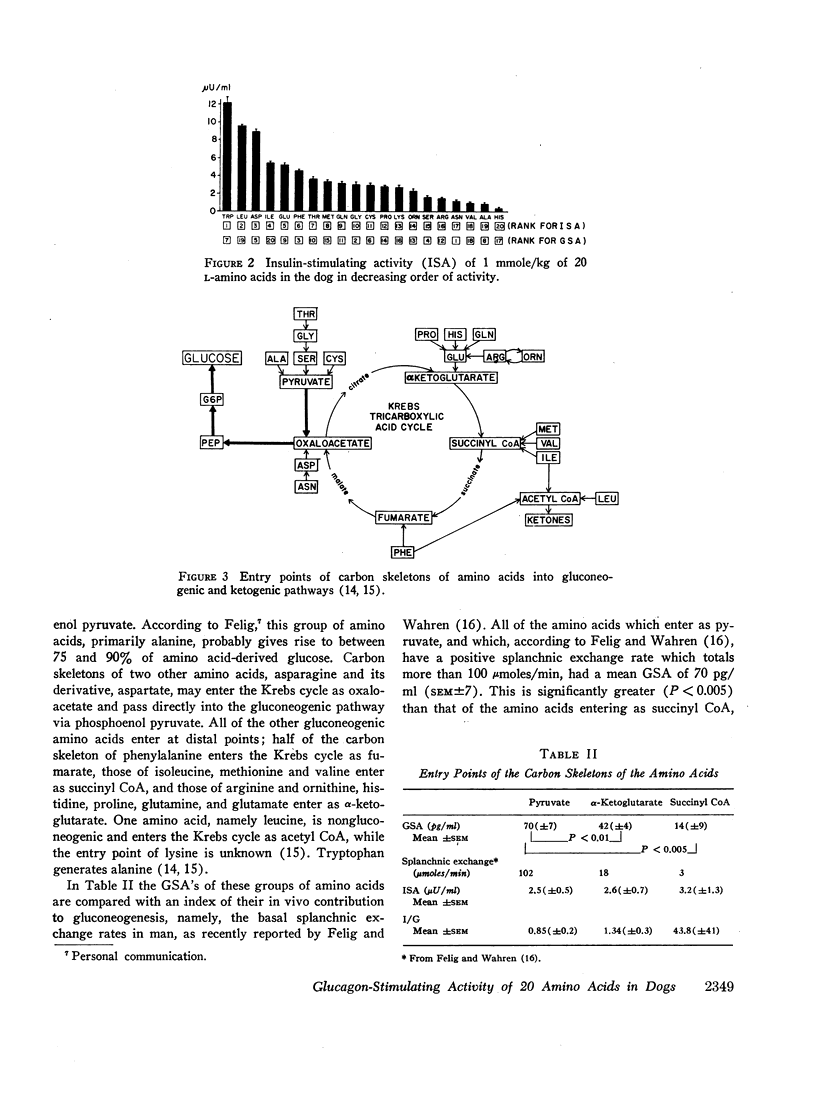
The effect of 20 L-amino acids upon pancreatic glucagon secretion has been studied in conscious dogs. Each amino acid was administered intravenously over a 15 min period in a dose of 1 mmole/kg of body weight to a group of four or five dogs. Pancreatic glucagon and insulin were measured by radioimmunoassay. 17 of the 20 amino acids caused a substantial increase in plasma glucagon. Asparagine had the most glucagon-stimulating activity (GSA), followed by glycine, phenylalanine, serine, aspartate, cysteine, tryptophan, alanine, glutamate, threonine, glutamine, arginine, ornithine, proline, methionine, lysine, and histidine. Only valine, leucine, and isoleucine failed to stimulate glucagon secretion, and isoleucine may have reduced it. No relationship between glucagon-stimulating activity and insulin-stimulating activity was observed. The amino acids which enter the gluconeogenic pathway as pyruvate and, which are believed to provide most of the amino acid-derived glucose, had a significantly greater GSA than the amino acids which enter as succinyl CoA or as α-ketoglutarate. However, pyruvate itself did not stimulate glucagon secretion. The R-chain structure of the amino acid did not appear to be related to its GSA, except that the aliphatic branched chain amino acids, valine, leucine, and isoleucine, were devoid of GSA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Quibrera R., Pek S., Floyd J. C., Jr, Christensen H. N., Conn J. W. Stimulation of insulin release in the dog by a nonmetabollizable amino acid. Comparison with leucine and arginine. J Clin Endocrinol Metab. 1971 Jul;33(1):35–41. doi: 10.1210/jcem-33-1-35. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Conn J. W., Knopf R. F., Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966 Sep;45(9):1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Kaneto A., Kosaka K. Stimulation of glucagon secretion by arginine and histidine infused intrapancreatically. Endocrinology. 1971 May;88(5):1239–1245. doi: 10.1210/endo-88-5-1239. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. A., Faloona G. R., Unger R. H. The influence of the antecedent diet upon glucagon and insulin secretion. N Engl J Med. 1971 Dec 23;285(26):1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]


