Abstract
Hyaluronidase 2 (Hyal2) is a hyaluronan (HA)-degrading enzyme found intracellularly or/and anchored to the plasma membrane through glycosylphosphatidylinositol (GPI). Normal human bronchial epithelial cells (NHBE) grown at the air-liquid interphase (ALI), treated with PI-specific phospholipase C (PI-PLC), exhibited increased Hyal activity in secretions and decreased protein and activity on the apical membrane, confirming that GPI-anchored Hyal2 is expressed in NHBE cells and it remains active in its soluble form. We have reported that HA degradation was mediated by reactive oxygen species (ROS) in human airways. Here we show that ROS increase Hyal2 expression and activity in NHBE cells and that the p38MAPK signaling pathway is involved in this effect. Hyal2 induction was confirmed by using small interfering RNA (siRNA) expressing lentivirus. These in vitro findings correlated in vivo with smokers, where increased Hyal2 immunoreactivity in the epithelium was associated with augmented levels of HA and the appearance of low molecular mass HA species in bronchial secretions. In summary, this work provides evidence that ROS induce Hyal2, suggesting that Hyal2 is likely responsible for the sustained HA fragmentation in the airway lumen observed in inflammatory conditions associated with oxidative stress.
Keywords: Enzymes, Epithelium, Glycosaminoglycan, Hyaluronate, Lung
Introduction
Hyaluronan (HA)3 is a non-sulfated glycosaminoglycan found in the extracellular matrix, in body fluids, and in secretions of mammals. HA is synthesized by three transmembrane isoenzymes: HAS1, HAS2, and HAS3 at the inner face of the plasma membrane and translocated into the extracellular space (1).
The association with various binding proteins (2, 3) confers HA with a unique plasticity to organize extracellular matrix (ECM) in a tissue specific fashion (for review see Ref. 4), varying from a tightly cross-linked mesh in cartilage to a highly hydrated matrix in dermis and vitreous humor (5).
In addition, HA induces intracellular signaling by binding specific receptors at the cell surface, (6, 7) orchestrating a variety of host responses generally requiring an extensive deposition of a HA in the ECM. This deposition is essential for cumulus oophorus fertilization (8) as well as for proliferation and migration of mesenchymal cells. Airway smooth muscle cells exposed to polyinosinic acid-polycytidylic acid synthesize an abnormal HA matrix with cable-like structures that bind and retains leukocytes (9, 10), suggesting that HA play key roles on host defense against infections as well.
Biological functions of HA are associated with its size (6, 11). For instance, high molecular weight HA (HMWHA) exhibit anti-angiogenic, anti-inflammatory, and immunosuppressive effects while small HA fragments are angiogenic and proinflammatory (12, 13). The contrasting responses elicited by different sizes of HA are exemplified in a recent report in which HMWHA attenuated while low molecular weight HA (LMWHA) increased ozone-induced airway hyperreactivity, in a mouse model of asthma (14).
In human airway epithelium, HA is present at the lumen as a HMW polymer (>1.5 MDa)(15). It is released by submucosal glands and by superficial airway epithelial cells (16, 17) where it serves as an anchor for secreted proteins involved in host defense, preventing their removal by mucociliary clearance (18–20). Tissue kallikrein (TK), a serine protease involved in airway inflammation and mucus hypersecretion (15, 21) is bound to, and inhibited by HMWHA at the airway epithelial surface. Exposure to reactive oxygen species (ROS) depolymerizes HA resulting in the release of active TK (15, 21) and HA fragments that stimulate ciliary beating (22) and induce MUC5B up-regulation (23). Thus, fragmentation of HA at the epithelial surface can profoundly affect cell responses.
HA fragmentation can be achieved by direct action of ROS (24) and/or by the activity of the hyaluronan-degrading enzymes, the hyaluronidases (Hyals) (25). We have recently shown that Hyals 1, 2, and 3 are expressed in airway epithelium as well as in NHBE cells grown at the ALI. We found that Hyal protein expression is increased in the epithelium of asthmatics (26), suggesting that HA fragmentation during inflammatory responses could be due, in addition to ROS (15, 21) to Hyal. In in vitro assays in cell-free conditions, ROS formation is fast and transient (e.g. (27, 28)) and results in a potent and short-lived HA depolymerization. In contrast, when cells are exposed to ROS, HA degradation is sustained for at least 24 h, suggesting that additional mechanisms of degradation are in place. Thus, we hypothesized that our previous observations of HA degradation by ROS was a combination of an immediate direct effect later sustained by Hyal up-regulation.
Hyal2 is a GPI-anchored glycosidase (29, 30) that is also expressed as an intra-lysosomal enzyme (31). Here we report that ROS induce Hyal2 gene and protein expression in NHBE cells grown at the ALI. Using an interfering siRNA-expressing lentivirus system, we confirmed that Hyal2 is a major contributor to apical HA degradation in non-stimulated cells and is responsible for sustained HA depolymerization induced by ROS. We identified p38MAPK as a downstream signaling molecule involved in ROS-induced Hyal2. To explore if these findings were relevant in vivo, as a model of chronic oxidative stress exposure, the expression of Hyal2 was assessed in tracheal tissue sections and secretions obtained from smoker and non-smoker subjects. We found that Hyal2 was highly expressed in the epithelium of smokers, associated with an increased concentration and a decreased molecular mass of soluble HA in bronchial secretions when compared with non-smokers.
In summary, this work provides evidence that Hyal2 is induced by ROS and that Hyal2 is active in its soluble form, suggesting that Hyal2 can potentially determine HA size in airway epithelium from the cell membrane to the periciliary space and mucus layer.
EXPERIMENTAL PROCEDURES
All materials were purchased from Sigma Chemical Co., (St. Louis, MO), unless otherwise specified.
Cell Cultures
Isolation Procedure
Human tracheas and main bronchi, obtained from donor lungs through the University of Miami Life Alliance Organ Recovery Agency with approval from the local Institutional Review Board (IRB) were opened at the membranous portion, and the mucosa was dissected off the cartilage. Mucosal strips were digested with 0.05% protease (type 14) incubated in DMEM (Invitrogen, Carlsbad, CA) and incubated at 4 °C. NHBE cells were released by vigorous shaking and harvested by centrifugation as previously described (15, 32).
NHBE Cells Grown at the ALI
Human tracheobronchial epithelial cells were counted, and their viability was determined by Trypan Blue exclusion (viability was always > 80%). 1 × 106 freshly prepared cells (referred to as P0) were plated on collagen-coated plastic dishes (100 mm; Corning Costar Corporation, Cambridge, MA), and grown in bronchial epithelial growth medium (BEGM (33)) yielding undifferentiated airway epithelial cells. After reaching confluence, cells were dissociated with trypsin (referred to as P1 (34)), and 5 × 105 cells were plated onto 24-mm transwell-clear culture inserts (Corning), coated with human placental collagen. Cells were fed on the top and bottom with ALI medium composed of 50% DMEM and 50% Lechner and LaVeck (LHC) basal medium supplemented with insulin (5 μg/ml), hydrocortisone (0.072 ng/ml), epidermal growth factor (0.5 ng/ml), triiodothyronine (T3, 6.5 ng/ml), transferrin (10 μg/ml), epinephrine (0.6 μg/ml), phospholethanolamine (0.5 μm), ethanolamine (0.5 μm), bovine pituitary extract (1% v/v), bovine serum albumin (0.5 mg/ml), CaCl2 (0.08 mm), trace elements, penicillin (100 units/ml), streptomycin (100 μg/ml), and retinoic acid (1 μm) (32). Cells were grown in an incubator at 37 °C in ambient air supplemented with 5% CO2. Medium was removed from the apical surface exposing the cells to air as soon as they reached confluence (∼7 days). These conditions were maintained until the cells reached full re-differentiation, as evidenced by ciliary beating and the presence of mucus on the apical surface (∼4 weeks). These fully differentiated cells were used for the experiments described below.
Treatments
To test the effects of ROS, differentiated NHBE cells were exposed apically to 500 μl of PBS (controls) or with 0.6 mm xanthine plus 0.05 units of xanthine oxidase (XXO) in PBS in the presence or the absence of catalase (150 units/ml) at 37 °C for 24 h. After treatments, one set of cells were used for QPCR experiments and other set for estimation of Hyal2 protein levels and activity. Apical washes were collected and cell layers were lysed with 20 mm sodium phosphate, 150 mm NaCl, 5 mm EDTA, 50 mm HEPES, 1% Triton X-100, 50 mm NaF, 1 mm sodium orthovanadate, 5 mm PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin at pH 7.8 (lysis buffer) for 30 min at 4 °C. To remove insoluble material, cell lysates were centrifuged at 12,000 × g for 5 min at 4 °C, and supernatants stored at −20 °C for later analysis. In experiments designed to study p38MAPK signaling, NHBE cells were apically exposed to 500 μl of XXO, XXO plus 10 μm of SKF86002, a p38MAPK inhibitor, and RNA and proteins were isolated after 2, 4, 6, and 24 h after treatments. To determine if p38MAPK activation was sufficient to induce Hyal2 expression, NHBE cultures were treated with 10 μm l-α-lysophosphatydylcholine (LPC) a p38MAPK stimulator. Hyal2 gene and protein expressions were assessed 24 h after treatments. PBS was used for controls in all the experiments described above. In studies aimed at confirming that Hyal2 was attached to the cell membrane by a GPI anchor, NHBE cultures were treated apically with phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus (Invitrogen) in HEPES buffer pH 7.4 for 30 min at 37 °C. Controls were exposed to the same incubation buffer without PI-PLC.
HA-Zymography
Hyal activity was assessed by zymography as described (35). Briefly, samples were electrophoresed in 10% polyacrylamide gels containing 0.17 mg/ml HA from Streptococcus zooepidemicus. Following electrophoresis, gels were washed in 50 mm HEPES buffer pH 7.4 containing 3% Triton X-100 and subsequently incubated overnight in 0.15 m NaCl-0.1 m sodium formate, pH 4.2 at 37 °C. Gels were then stained with Alcian Blue (0.5% in 3% acetic acid), and Hyal activity was visualized as clear bands on the blue background. For the assessment of relative activity, intensities of the bands were recorded using the GelDoc XRS system (Bio-Rad) and analyzed using Quantity One software (Bio-Rad). Results are expressed as % changes in intensity (INT) per surface (mm2).
QPCR
RNA was extracted from NHBE cultures using Trizol reagent (Invitrogen) and reverse transcribed into cDNA using iScriptTM cDNA synthesis kit (Bio-Rad) according to manufacturer's instructions. Quantitative real-time PCR (QPCR) was performed using pre-made TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA) with TaqMan® MGB probes, FAMTM-labeled Hyal1 (ID:Hs00738390_m1), Hyal2 (ID:Hs00186841_m1), and Hyal3 (ID:Hs00185910_m1). The QPCR was performed using TaqMan® Universal PCR Master Mix (Applied Biosystems) following the manufacturer's instructions. Thermal cycling was carried out using ICycler IQ apparatus (Bio-Rad). The comparative CT method (ΔΔCT) was used for relative quantization. All results were normalized using the housekeeping gene GAPDH (Hs99999905_m1).
p38MAPK Phosphorylation
Aliquots of cells lysates containing equal amounts of protein (n = 3 different lung donors) were run on 4–15% Tris-HCl Ready gels (Bio-Rad) and transferred to polyvinylidene fluoride (PVDF, Millipore, Billerica, MA). Visualization of total and phosphorylated p38MAPK was achieved using the PhosphoPlus® p38MAP Kinase (Thr-180/Tyr-182) antibody kit (Cell Signaling Technology, Beverly, MA) according to the manufacturer's instructions). Blots were developed with LumiGlo peroxidase chemiluminescent substrate kit (KPL, Gaithersburg, MD), bands detected using the GelDoc XRS system and quantified using Quantity One software. Assessment of p38MAPK activation was achieved by calculating the ratio between phosphorylated and total p38MAPK.
Hyal2 ELISA
Standards (Hyal2 partial recombinant protein; aa 340–439, Novus Biological, Littleton, CO) and samples (50 μg) where diluted in NaCO2/NaHCO3 buffer (pH 9.6) loaded into a high binding 96-well plate (Corning Costar) and dried overnight at 37 °C. After blocking (2% BSA in TTBS) mouse anti human Hyal2 (20 μg/ml, Novus Biological) diluted in blocking buffer was loaded and incubated for 2 h at room temperature. Plates were washed with TTBS followed by alkaline phosphatase-rabbit anti mouse IgG (KPL). P-nytrophenyl phosphate was used as substrate.
Immunofluorescence
Cell Cultures
After removing culture medium, NHBE cells on filter inserts were fixed in 4% paraformaldehyde in PBS, pH 7.4 for 20 min and permeabilized with 0.05% Triton X-100 in PBS for 20 min at room temperature (RT). After washing with PBS, each filter was blocked with 5% BSA in PBS for 1 h followed by mouse anti human-Hyal2 polyclonal antibody diluted 1/300 in blocking solution. Hyal2 was visualized with Alexa 555-labeled anti-mouse antibody (4 μg/ml, Invitrogen), and nuclei were labeled with 4′6-diamidine-2-phenylindole (DAPI, KPL). Samples were mounted on slides with gel/mount (Biomeda, Foster City, CA), and images were obtained using a Zeiss LSM-510 confocal laser scanning microscope (Zeiss LSM, Thornwood, NY) at the University of Miami Analytical Imaging Core Facility.
Trachea Tissue Sections
Lungs were obtained from non-smoker and smoker organ lung donors. A smoker was defined following two criteria. 1) According to the Centers for Disease Control (CDC): a person who had smoked at least 100 cigarettes or more and was currently a smoker every day or some days (36) and 2) histological criteria: lungs with histological hallmarks of chronic bronchitis (enlargement of tracheobronchial submucosal glands, mucous cells metaplasia, and hyperplasia) (37). None of the tissues had signs of active infection. Smoker lungs were compared with control group of lifelong nonsmokers. Paraffin-embedded sections were hydrated and subjected to heat-induced antigen retrieval with 2 mm EDTA, pH 8.0 for 15 min at 100 °C. Slides were treated with by acetone at −20 °C for 10 min and blocked with an image enhacer, Image-iTTM FX (Invitrogen) following manufacturer's instructions. Immunolocalization was performed as described above in the cell culture section.
Hyal2 Immunoblotting
Cell lysate aliquots containing 25 μg of protein were run on 4–15% Tris-HCl Ready Gels (Bio-Rad) and then transferred to PVDF membranes Visualization of Hyal2 was achieved using the mouse anti-Hyal2 polyclonal antibody (1/500) followed by alkaline phosphatase-conjugated anti-mouse antibodies (1/5,000 (v/v); KPL). Blots were developed with a Lumi-PhosTM WB (Pierce Protein Research Products, Rockford, IL) according to the manufacturer's instructions. A digitized image of each blot was obtained and a relative quantification of proteins was performed as described above.
Hyal2 Knockdown
Because differentiated NHBE cells are refractory to direct transfection with siRNA, we used a third-generation, propagation-deficient HIV-pseudotyped lentivirus expressing Hyal2 targeted shRNA to knock down Hyal2 gene expression. To identify an effective Hyal2 shRNA target sequence, Silencer® Pre-designed shRNA from Ambion (shRNA ID 119198) consisting of siRNAs against 3 different Hyal2 mRNA sequences were purchased and tested in HEK 293T cells. One sequence, CCUAAUGAGGGUUUUGUGA, was found to be highly effective and thus chosen for the construction of lentivirus for transduction and shRNA expression. Oligonucleotides for both strands, including the sense, loop, antisense and terminator, to permit transcription of Hyal2 siRNA to this sequence were synthesized (Sigma-Genosys) and cloned into the SIH-H1-GFP vector using the Clone-it kit (System Biosciences, Mountain View, CA). Same vector without siRNA sequence was used as control (non-insert). Replication-deficient lentivirus were prepared as previously described (38). Briefly, HEK 293T cells were cotransfected with the lentiviral vector and packaging DNAs, pMDLg/pRRE 54 pRSV-Rev and pMDLgVSVG, using CalPhos Mammalian Transfection kit (Clontech, Mountain View, CA) following the manufacturer's instruction. Viruses were collected daily for 3 days beginning 24 h after removing the precipitates and concentrated, if necessary, by polyethylene glycol (38). Virus titers were estimated by measuring p24 by ELISA (Perkin-Elmer, Wellesley, MA). These viruses were used to infect undifferentiated NHBE cells (70% confluent) as described (38). Fully differentiated cells determined by the presence of mucus secretion and beating cilia (21 days on air) were used for the experiments.
Hyal Activity by Agarose Electrophoresis
To confirm that the observed increases in Hyal activity correlated with HA degradation, cultures were assessed as previously described (39) with slight modifications: Samples from Hyal2-siRNA and non-insert controls exposed to ROS (n = 3) were lysed and volumes containing equal amount of proteins incubated with 50 μg of HMW HA from Streptococcus zooepidemicus in 200 μl (final volume) of 1 mm EDTA, 0.2% Triton X-100 sodium formate (pH 4.2) at 37 °C for 24 h. After exposure, HA was electrophoresed in agarose gel as described before (15, 21, 40), mobility changes were assessed using Select-HATM standards (Hyalose, L.L.C., Oklahoma City OK) and visualized using Stains-All®.
Human Tracheal Aspirates (HTA)
Following an IRB-approved protocol, human tracheal aspirates were collected from patients undergoing general anesthesia for elective surgery indicated for non-pulmonary reasons. Respiratory secretions were collected and processed as previously described (16, 41).
HA Content
Aliquots containing equal amounts of proteins were digested with proteinase K (125 μg/ml for 2 h at 60 °C), and centrifuged at 5,000 × g for 5 min. Supernatants were precipitated with ethanol, freeze-dried and prepared for the assessment of HA amounts by fluorophore-assisted carbohydrate electrophoresis (FACE) analysis as previously described (16, 42) or HA size as described below.
HA Average Molecular Size
HA average molecular size was estimated by agarose electrophoresis as described above for Hyal activity (n = 3 for both smokers and non-smokers pooled separately) in agarose with one modification. Before proteinase K digestion, samples were treated with chondroitinase ABC (0.5 IU/ml in 50 mm Tris-buffered pH 7.4, where Hyal activity is negligible (18)) at 37 °C overnight.
Statistical Analysis
Data were expressed as mean ± S.E. Shapiro-Wilk test was used for normality analysis. Differences between multiple groups were tested for significance using a one-way ANOVA followed by Tukey test or Kruskal-Wallis ANOVA on ranks. Significance was accepted at p < 0.05.
RESULTS
ROS Exposure Induces Hyal Activity and Hyal2 mRNA in NHBE Cells
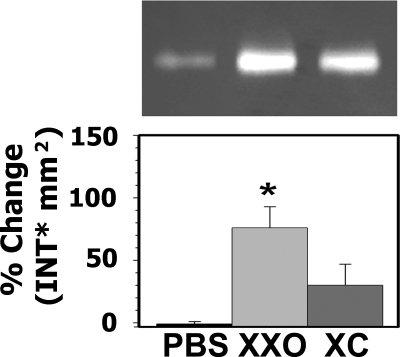
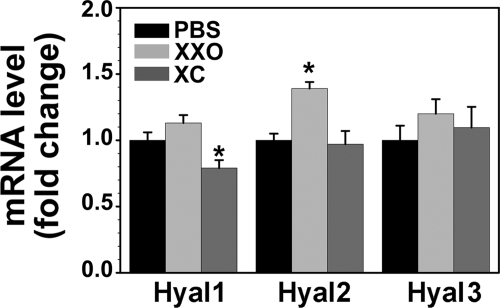
We have previously shown that Hyal1, 2, and 3 are expressed in bronchial epithelium (being Hyal2 the one exhibiting the highest expression), and that Hyal2 was present at the apical membrane of airway epithelium (26). Because oxidative stress induces sustained HA degradation in the airway lumen, we assessed whether a single exposure to ROS resulted in increased Hyal activity and/or expression. ROS exposure significantly increased Hyal activity 80 ± 20% when compared with controls (n = 7, p < 0.05). Addition of catalase partially prevented this effect 20 ± 20% (Fig. 1). To identify the Hyal responsible for the increased activity, mRNA levels of Hyal1, -2, and -3 were assessed by QPCR. As depicted in Fig. 2, no significant increases in Hyal1 or Hyal3 (1.13 ± 0.05; 1.23 ± 0.11-fold, respectively) expression was observed in XXO-treated cells. In contrast, ROS exposure significantly augmented Hyal2 mRNA (1.40 ± 0.05-fold) with respect to PBS control (n = 5; p < 0.05) and catalase blocked this effect (1.10 ± 0.08; p < 0.05). Interestingly, a significant reduction of Hyal1 mRNA expression (0.79 ± 0.06-fold) with respect to PBS controls (p < 0.05) was observed in XXO + catalase (XC)-treated cells. These findings indicate that among the three Hyals expressed in the airways, only Hyal2 expression is up-regulated by ROS.
FIGURE 1.
ROS exposure results in increased Hyal-like activity in NHBE cells. Primary cultures of NHBE cells (n = 7) were exposed to PBS (control) or xanthine plus XXO with or without of catalase (XC) for 24 h. Upper panel, a representative HA zymogram from apical secretions. Volumes were normalized to cell lysates protein (50 μg of proteins). Bottom panel, band intensities were analyzed using Quantity One software and relative Hyal activity was expressed as % change in band intensity (INT) mm2 compared with PBS; mean ± S.E. from seven different lung donors. * indicates p < 0.05 compared with PBS controls.
FIGURE 2.
ROS induce Hyal2 gene expression. NHBE cells (n = 5) were exposed to PBS, XXO, or XC and Hyals mRNA levels were assessed by QPCR 24 h after treatments. Results were normalized to GAPDH and expressed as fold changes with respect to PBS. Bars represent mean ± S.E. from five different lung donors. * indicates p < 0.05 compared with PBS controls.
ROS-induced Hyal Activity Is Mediated by p38MAPK Signaling
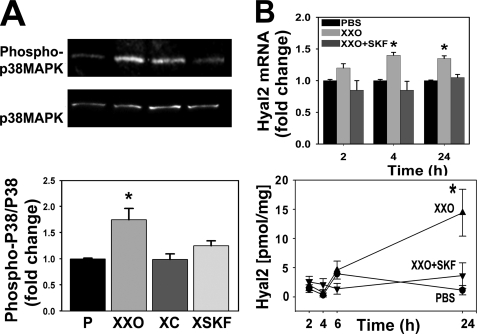
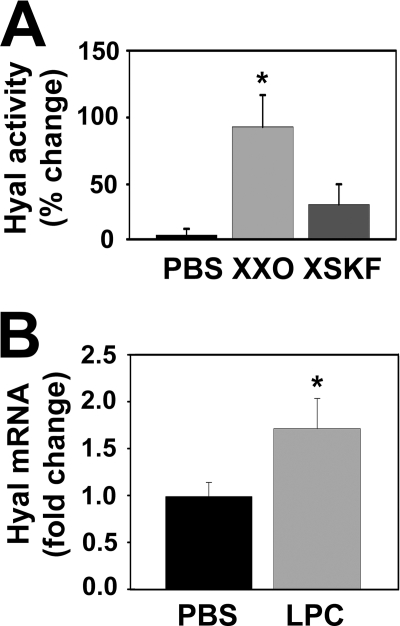
The p38MAPK signaling cascade is involved in cellular mechanisms of airway inflammation induced by environmental stimuli such as tobacco smoke, and by endogenous signals such as cytokines, growth factors, and inflammation-derived oxidants (43). To assess whether ROS-mediated Hyal2 induction was mediated by p38MAPK activation in NHBE cells, p38MAPK phosphorylation was assessed in cells exposed to PBS or XXO in the presence or absence of catalase or the p38MAPK inhibitor, SKF86002 (44). As shown in Fig. 3A, XXO significantly induced p38MAPK phosphorylation (1.75 ± 0.22-fold) as compared with PBS while both catalase (p < 0.05, n = 3) and SKF86002 prevented this effect (0.99 ± 0.11 and 1.25 ± 0.09-fold). Hyal2 mRNA and protein expression were evaluated at 2, 4, 6, and 24 h after treatments. Fig. 3B shows that Hyal2 mRNA levels were significantly increased 4 and 24 h after ROS exposure 1.4 ± 0.05 and 1.3 ± 0.1 (n = 6, p < 0.05). Changes in mRNA were followed with subsequent increases in protein expression at 6 and 24 h (1.0 ± 0.5 to 4.5 ± 1.0 and 14.0 ± 4.0 pg/mg protein (6 and 24 h, respectively p < 0.05). These increases were prevented by SKF86002, (1.0 ± 0.5 and 3.5 ± 2.0 pg/mg 6 and 24 h, respectively). To assess if increased mRNA and protein expression resulted in changes in enzymatic activity, samples (n = 3, from 24 h treatments) were analyzed by HA zymography. A representative zymogram and densitometry analysis (obtained from three separate experiments from different lung donors) depicted in Fig. 4A, evidenced that SKF86002 partially prevented ROS-induced increases in Hyal activity (91 ± 24 versus 35 ± 15%). To further examine if p38MAPK activation was sufficient to induce Hyal2 mRNA expression, we treated NHBE cells with the p38MAPK activator LPC (45). Fig. 4B shows that Hyal2 mRNA levels were significantly increased in LPC-treated cells with respect to controls (1.71 ± 0.32-fold, n = 5, p < 0.05), confirming that p38MAPK signaling is involved in ROS-induced Hyal2.
FIGURE 3.
p38MAPK signaling pathway participates in ROS-induced Hyal2 up-regulation. NHBE cells were treated with P, XXO, XC, or XXO plus p38MAPK inhibitor SKF86002 (XSKF) for 30 min. A, upper panel, WB of phosphorylated and total p38MAPK (n = 3). Lower panel, quantification of the p38/phospho-p38 ratios. B, upper panel, time course of Hyal2 mRNA levels assessed by QPCR (n = 5) at 2, 4, and 24 h after treatments. Lower panel, time course graph shows Hyal2 protein concentration in cell extracts determined by ELISA using partial recombinant Hyal2 for calibration and commercial mouse anti Hyal2 polyclonal antibody for detection (mean ± S.E. from three and five different lung donors, respectively; * indicates p < 0.01 compared with PBS).
FIGURE 4.
p38MAPK activation induces Hyal2 up-regulation. A, NHBE cells (n = 3) were exposed to PBS, XXO, or XSKF and Hyal-like activity was determined in apical washes 24 h after treatments. B, NHBE cells (n = 5) were treated with a p38MAPK activator LPC for 24 h. Hyal2 mRNA levels are expressed as fold change compared with PBS (mean ± S.E. from five different lung donors; * indicates p < 0.05).
GPI-anchored and Soluble Hyal2 Forms Are Present Apically in NHBE Cells
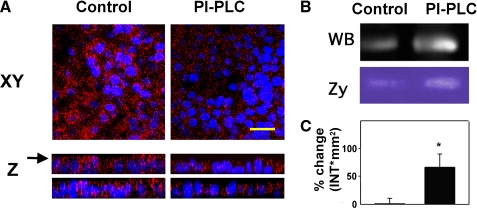
We and others (26, 46) have previously reported that Hyal2 is localized at the apical membrane of the tracheal epithelium. Here we confirmed that Hyal2 is associated with the cell membrane of NHBE cells through a GPI anchor, and limited to the luminal surface (Fig. 5). Cell cultures were treated with PI-specific phospholipase C (PI-PLC), and Hyal2 was assessed by immunofluorescence (Fig. 5A), Western blot (Fig. 5B), and zymography (Fig. 5D). As observed in Fig. 5A, Hyal2 immunoreactivity is markedly decreased after PI-PLC treatment in the apical compartment. This decrease in membrane-bound Hyal2 correlated with increases in protein (Fig. 5C) and activity (Fig. 5D) in apical washes. These data confirm that the Hyal2 observed in surface epithelium is anchored to the cell membrane trough GPI and provides evidence that when released, retains its enzymatic activity.
FIGURE 5.
Hyal2 is GPI-anchored to the apical pole of NHBE cells. NHBE cultures were treated with PI-PLC or buffer control. A, immunofluorescence, Hyal2 was visualized with mouse anti-Hyal2 followed by Alexa 555-labeled anti mouse IgG (red) and nuclei with DAPI, (blue). Upper panels, X-Y planes of NHBE cells after treatments, yellow bar = 35 μm; lower panels, Z-Y and Z-X z-stack reconstructions of XY planes (using a 63X/1.4 NA lens). Arrow points the level of XY. B, released Hyal2 in apical washes, evaluated by WB and HA zymography (Zy). C, relative Hyal activity (mean ± S.E. from four different lung donors; *, p < 0.05).
Hyal2 Knockdown Results in Decreased Enzymatic Activity and a Lack of Response to ROS
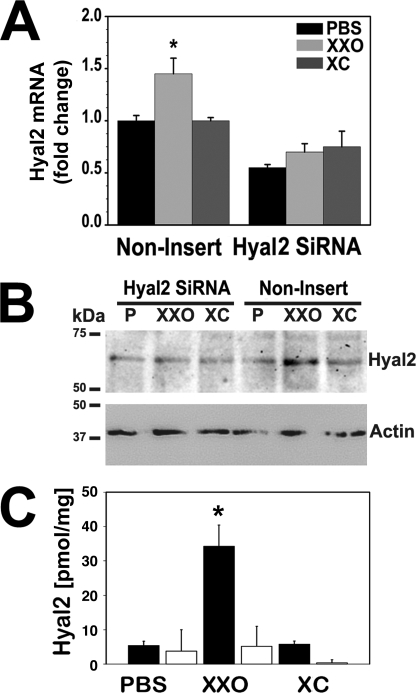
To confirm the role of Hyal2 and to determine its relative contribution to HA degradation induced by ROS, NHBE cultures were infected with lentivirus-expressing Hyal2-siRNA and eGFP as described under “Experimental Procedures.” After full differentiation, QPCR experiments showed that only Hyal2 mRNA expression was decreased (∼50%) while neither Hyal1 nor Hyal3 mRNA levels were affected by Hyal2 shRNA (see supplemental data S1).
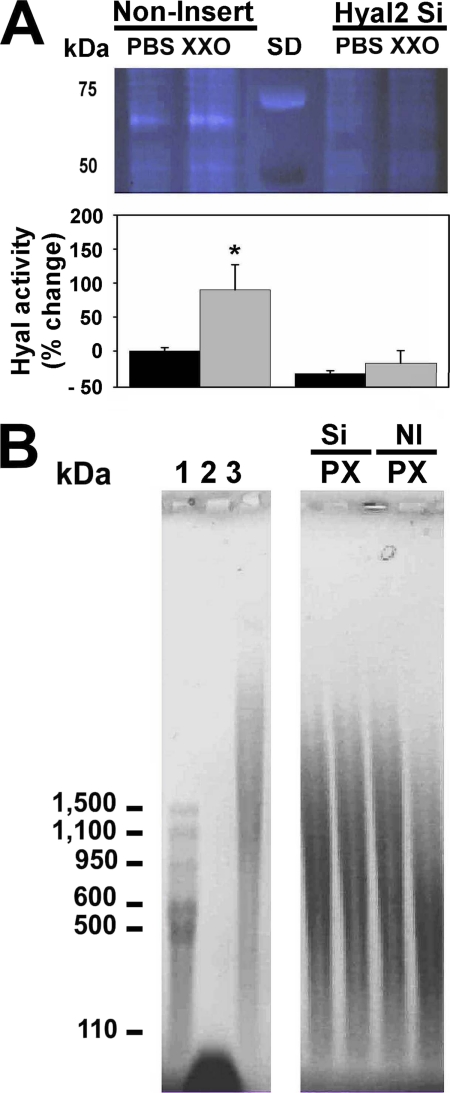
Hyal2-siRNA and control lentivirus-infected cells were treated with PBS or XXO as described under “Experimental Procedures.” Non-insert (NI) lentivirus infection did not affect ROS-induced Hyal2 up-regulation response while Hyal2 mRNA (Fig. 6A) and protein expression (WB, Fig. 6B, and ELISA, Fig. 6C) were abrogated in cells with Hyal2 siRNA. Reduction in gene and protein expression resulted in loss of glycosidase activity (Fig. 7). Hyal2-siRNA samples were analyzed by HA zymography (Fig. 7A), and the ability of these samples to degrade HMWHA (Fig. 7B) in a cell-free system as described under “Experimental Procedures.” Fig. 7A also shows that Hyal2-siRNA infected cells are refractory to induction of enzyme activity by ROS. NI control cells showed an increase in activity while siRNA-infected cells have lower baseline and do not respond to ROS treatment. In accordance to this, we observed a decrease in HA average molecular mass when HMWHA was incubated in vitro with extracts from NI cells exposed to ROS (from ∼825 to ∼180 kDa), whereas lysates obtained from Hyal2-siRNA-infected cells did not affect HA molecular distribution (Fig. 7B).
FIGURE 6.
Hyal2 knockdown prevents induction by ROS. NHBE cells (n = 4) with Hyal2-siRNA or non-insert lentivirus controls exposed to PBS, XXO, or XC for 24 h. A, Hyal2 mRNA expression were assessed by QPCR and expressed as fold changes with respect to PBS. B, representative immunoblot of Hyal2. C, Hyal2 protein concentration measured by ELISA in Hyal2-siRNA (light bars) and in non-insert (dark bars) cells. Bars represent mean ± S.E. obtained from three different lung donors. * indicates p < 0.05 compared with corresponding control.
FIGURE 7.
Hyal-2 knockdown in NHBE cells results in a decrease of baseline and blocks ROS-induced increases in enzyme activity. NHBE cells with Hyal2-siRNA or non-insert control lentivirus were exposed to PBS or XXO for 24 h. A, upper panel, HA zymography measured in apical secretions and normalized to cell lysate protein (20 μg of protein). SD, MW standards. B, lower panel, quantization of band intensities and relative Hyal activity, expressed as % change compared with non-insert control PBS. HA molecular size distribution after incubation of HMWHA with lysates obtained from cells transfected Hyal2-siRNA and non-insert controls in cell-free conditions. HA molecular weight standards: 1-Select-HATM standards, 2- LMW HA (<100 kDa), and 3-non-digested HMWHA (>2000 kDa).
Hyal2 Expression Is Increased in the Airway Epithelium of Smokers
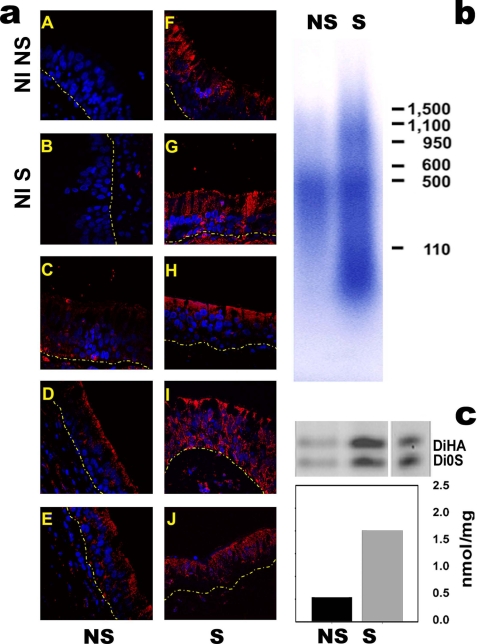
We have previously reported that Hyal mRNA and protein expression were up-regulated in the airways of asthmatics (26). As a model of in vivo oxidative stress, tracheal sections obtained from smokers (n = 8) and non-smokers (n = 6) were used to visualize Hyal2 expression by immunofluorescence. As depicted in Fig. 8a, Hyal2 (red) is increased in smokers when compared with non-smokers at superficial airway epithelium. These results were associated with an increased concentration of soluble HA in HTA samples obtained from smokers (2.0 ± 0.1 versus 0.5 ± 0.05 nmol/mg of proteins) (Fig. 8c) and decreased molecular mass (∼75 kDa, compared with ∼480 kDa in non-smokers, Fig. 8b). These data overall suggest that chronic exposure ROS (cigarette smoke) is associated with increased HA degradation at the airway lumen, and that this effect is likely due to Hyal2 induction by ROS.
FIGURE 8.
Hyal2 is increased in tracheal epithelial cells in smokers. a, depicts four tracheal sections obtained from non-smokers (panels C–E) and four smokers (panels F–J), Hyal2 was detected with mouse anti-human Hyal2 polyclonal antibody and visualized with Alexa 555-conjugated anti-IgG antibody red). Control sections (non-immune, NI) from a non-smoker (NI S) and smoker (NI NS) were incubated with non-immune mouse IgG. Nuclei were visualized with DAPI, and images obtained by confocal microscopy (using a 63X/1.4 NA lens). Pooled secretions obtained from non-smokers and smokers (n = 4) were used to assess HA molecular mass distribution by agarose electrophoresis (b) and HA concentration by FACE (c).
DISCUSSION
In the present work we explored Hyal2 expression and regulation in airway epithelium and its possible role in HA depolymerization in conditions associated with oxidative stress.
We have previously reported that ROS induce HA fragmentation (21, 23), and that Hyal1, 2, and 3 are expressed in the airway epithelium (26). We and others (26, 47) have shown that Hyal2 is selectively increased in inflammatory conditions and that TNF-α and IL-1β induce Hyal2 gene and protein expression in NHBE cells (26). Here we further explored the link between ROS and Hyals.
We found that in NHBE cells, exposure to ROS results in increased Hyal activity and Hyal2 mRNA and protein expression through p38MAPK signaling. Its role was confirmed by pretreating cells with a p38MAPK inhibitor or activator. These findings are not surprising, because p38MAPK is upstream of IL-1β signaling, which we have previously shown to be involved in Hyal2 up-regulation, and it is involved in stress-related responses including oxidative stress, ultraviolet light, and osmotic shock (48, 49).
Hyal2 can be expressed as both, a lysosomal (50) and a membrane GPI-anchored enzyme (30). Here we confirmed that, as previously reported by us and others (26, 30, 46), GPI-anchored and soluble forms of Hyal2 are restricted to the apical compartment of airway epithelium, suggesting a specific luminal function, unrelated to the turnover of HA in the bronchial subepithelial tissue.
GPI-anchored proteins, such as SSp-4 antigen and sialidases in Trypanosoma cruzi, and p76 serine protease in Plasmodium sp (51) remain active after their cleavage by PI-PLC. It has been suggested that this treatment may relax conformational constraints on the active site of the enzyme which exists when it is anchored in the lipid bilayer, thus resulting in increased affinity for the substrate (52). Similar to other GPI-anchored proteins (53), membrane-bound Hyal2 is susceptible to specific GPI-phospholipase D (GPI-PLD) (54), induced in inflammatory and oxidative stress conditions (55, 56); thus it is tempting to speculate that membrane bound and soluble forms may perform different biological functions. For instance, GPI-anchored Hyal2 is likely involved in normal HA turnover, where Hyal2 depolymerize HA into ∼20 kDa (∼50 disaccharides units) fragments, delivering them for further lysosomal degradation by Hyal1 (39, 50). Soluble Hyal2 could be specifically released by GPI-PLD during inflammation, generating excess HA fragments that overwhelm HA catabolic rates, resulting in the triggering of RHAMM- and CD44-mediated intracellular signaling (22, 23). It is reasonable to argue then, that GPI-Hyal2 serves constitutive physiological functions, (39, 57, 58), while the soluble form actively participates in host defense (22, 23).
Additional functions in health and disease have been previously described. Extracellular acidosis (Hyal2 activity is optimal at pH 4.5–6.5 (39, 59)) is commonly observed in the airways in conditions such asthma, COPD (60) and in smokers (61). In agreement with previous report (62), we found that the increases in soluble HA in secretions obtained from smoker was associated with the appearance of small molecular weight species. It has also been shown that, in addition to appropriate pH, Hyal2 became active through its interaction with CD44 (39). However, de la Motte et al. (63) have recently shown that GPI-anchored Hyal2 remains active in human platelets where CD44 could not be detected. In airway epithelial cells, CD44 is localized in the basolateral compartment, and thus, unlikely to participate in Hyal2 activity regulation. Perhaps other proteins or cofactors might also modulate Hyal2 and/or be required for Hyal2 activity (64).
Knockdown studies using lentivirus-expressing Hyal2-shRNA confirmed that Hyal2 is up-regulated under oxidative stress. These in vitro findings correlated in vivo with observations on tissue sections and secretions obtained from smoker where we found Hyal2 immunoreactivity increased at the airway epithelium and elevated levels of soluble HA in secretions when compared with non-smokers. Although smoking results in hypersecretion, and thus increases in macromolecules including HA is expected, the decreased average molecular size of HA in tracheal aspirates is consistent with the notion that Hyal2 contributes to the increased depolymerization and release of epithelial-bound HA that takes place in the airway lumen of smokers. Although the possibility of additional HA degrading enzymes, such as chondroitinase, cannot be ruled out, it is unlikely they are major contributors, since our knockdown studies demonstrated that Hyal2 is mainly responsible for HA depolymerization in these conditions.
Hyal2 may also perform functions not associated with enzymatic activity. Specifically in sheep airway epithelium, GPI-anchored Hyal2 function as a receptor for oncovirus such as Jaagsiekte retrovirus and enzootic nasal tumor virus (64). Recently, roles in the formation of the glycocalyx and control of CD44-ERM (ezrin-radixin-moesin) interactions have been described (65). Such non-enzymatic functions were not explored in the current work.
In summary, our studies strongly suggest that Hyal2 plays a key role in HA depolymerization at the airway lumen in physiological and pathological conditions, and that ROS and Hyal2 operate in a coordinated fashion to depolymerize HA. The apical localization of Hyal2 and its broad range of influence on the bronchial surface from the cell membrane to the mucus layer, makes Hyal2 an ideal candidate to orchestrate inflammatory responses associated with HA fragmentation, particularly in conditions associated with oxidative stress.
Supplementary Material
Acknowledgments
We thank the University of Miami Imaging Core Facility and Drs. Gregory Conner and Matthias Salathe for continuous support.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-073156. This work was also supported by a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (FAMRI) (to M. E. M.), a Bridge Award from the James and Esther King Foundation (JEK12292), and a Clinical Innovator Award from FAMRI (to R. M. F.); a Scientist Development Grant from AHA and JEK (12324) (to S. M. C.-M.), a Career Development Award from the American Lung Association of Florida, and a Clinical Innovator Award from FAMRI (to N. S. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental data S1.
- HA
- hyaluronan
- XXO
- xanthine oxidase
- ROS
- reactive oxygen species
- GPI
- glycosylphosphatidylinositol
- ALI
- air-liquid interphase
- LPC
- l-α-lysophosphatydylcholine
- IRB
- Institutional Review Board.
REFERENCES
- 1.Weigel P. H., Hascall V. C., Tammi M. (1997) J. Biol. Chem. 272, 13997–14000 [DOI] [PubMed] [Google Scholar]
- 2.Day A. J., Prestwich G. D. (2002) J. Biol. Chem. 277, 4585–4588 [DOI] [PubMed] [Google Scholar]
- 3.Day A. J., Sheehan J. K. (2001) Curr. Opin. Struct. Biol. 11, 617–622 [DOI] [PubMed] [Google Scholar]
- 4.Day A. J. (2001) Glycoforum, http://www.glycoforum.gr.jp/science/hyaluronan/HA16/HA16E.html
- 5.Blundell C. D., Seyfried N. T., Day A. J. (2004) in Chemistry and Biology of Hyaluronan (Garg H. G., Hales C. A. eds) Elsevier Press, Amsterdam [Google Scholar]
- 6.Pure E., Assoian R. K. (2009) Cell Signal 21, 651–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toole B. P. (2004) Nat. Rev. Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 8.Salustri A., Camaioni A., Di Giacomo M., Fulop C., Hascall V. C. (1999) Hum. Reprod. Update 5, 293–301 [DOI] [PubMed] [Google Scholar]
- 9.Lauer M. E., Fulop C., Mukhopadhyay D., Comhair S., Erzurum S. C., Hascall V. C. (2009) J. Biol. Chem. 284, 5313–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauer M. E., Mukhopadhyay D., Fulop C., de la Motte C. A., Majors A. K., Hascall V. C. (2009) J. Biol. Chem. 284, 5299–5312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern R., Asari A. A., Sugahara K. N. (2006) Eur. J. Cell Biol. 85, 699–715 [DOI] [PubMed] [Google Scholar]
- 12.Pardue E. L., Ibrahim S., Ramamurthi A. (2008) Organogenesis 4, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang D., Liang J., Noble P. W. (2007) Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 14.Garantziotis S., Li Z., Potts E. N., Kimata K., Zhuo L., Morgan D. L., Savani R. C., Noble P. W., Foster W. M., Schwartz D. A., Hollingsworth J. W. (2009) J. Biol. Chem. 284, 11309–11317 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Casalino-Matsuda S. M., Monzón M. E., Forteza R. M. (2006) Am. J. Respir. Cell Mol. Biol. 34, 581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monzon M. E., Casalino-Matsuda S. M., Forteza R. M. (2006) Am. J. Respir. Cell Mol. Biol. 34, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basbaum C. B., Finkbeiner W. E. (1988) Horm. Metab Res. 20, 661–667 [DOI] [PubMed] [Google Scholar]
- 18.Forteza R., Lieb T., Aoki T., Savani R. C., Conner G. E., Salathe M. (2001) Faseb J. 15, 2179–2186 [DOI] [PubMed] [Google Scholar]
- 19.Turino G. M., Cantor J. O. (2003) Am J. Respir. Crit Care Med. 167, 1169–1175 [DOI] [PubMed] [Google Scholar]
- 20.Forteza R. M., Conner G. E., Salathe M. (2002) Glycoforum, http://www.glycoforum.gr.jp/science/hyaluronan/HA25/HA25E.html
- 21.Casalino-Matsuda S. M., Monzon M. E., Conner G. E., Salathe M., Forteza R. M. (2004) J. Biol. Chem. 279, 21606–21616 [DOI] [PubMed] [Google Scholar]
- 22.Manzanares D., Monzon M. E., Savani R. C., Salathe M. (2007) Am. J. Respir. Cell Mol. Biol. 37, 160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casalino-Matsuda S. M., Monzon M. E., Day A. J., Forteza R. M. (2009) Am. J. Respir. Cell Mol. Biol. 40, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deguine V., Menasche M., Ferrari P., Fraisse L., Pouliquen Y., Robert L. (1998) Int. J. Biol. Macromol. 22, 17–22 [DOI] [PubMed] [Google Scholar]
- 25.Girish K. S., Kemparaju K. (2007) Life Sci. 80, 1921–1943 [DOI] [PubMed] [Google Scholar]
- 26.Monzón M. E., Manzanares D., Schmid N., Casalino-Matsuda S. M., Forteza R. M. (2008) Am. J. Respir. Cell Mol. Biol. 39, 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Assaf S., Navaratnam S., Parsons B. J., Phillips G. O. (2006) Free Radic Biol. Med. 40, 2018–2027 [DOI] [PubMed] [Google Scholar]
- 28.Frati E., Khatib A. M., Front P., Panasyuk A., Aprile F., Mitrovic D. R. (1997) Free Radic Biol. Med. 22, 1139–1144 [DOI] [PubMed] [Google Scholar]
- 29.Lepperdinger G., Müllegger J., Kreil G. (2001) Matrix Biol. 20, 509–514 [DOI] [PubMed] [Google Scholar]
- 30.Rai S. K., Duh F. M., Vigdorovich V., Danilkovitch-Miagkova A., Lerman M. I., Miller A. D. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4443–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow G., Knudson C. B., Knudson W. (2006) Osteoarthritis Cartilage 14, 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nlend M. C., Bookman R. J., Conner G. E., Salathe M. (2002) Am. J. Respir. Cell Mol. Biol. 27, 436–445 [DOI] [PubMed] [Google Scholar]
- 33.Fulcher M. L., Gabriel S., Burns K. A., Yankaskas J. R., Randell S. H. (2005) Methods Mol. Med. 107, 183–206 [DOI] [PubMed] [Google Scholar]
- 34.Bernacki S. H., Nelson A. L., Abdullah L., Sheehan J. K., Harris A., Davis C. W., Randell S. H. (1999) Am. J. Respir. Cell Mol. Biol. 20, 595–604 [DOI] [PubMed] [Google Scholar]
- 35.Guntenhöner M. W., Pogrel M. A., Stern R. (1992) Matrix 12, 388–396 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (1994) Morb. Mortal Wkly. Rep. 43, 342–346 [Google Scholar]
- 37.Jeffery P. K. (1992) Respiration 59, 13–16 [DOI] [PubMed] [Google Scholar]
- 38.Ransford G. A., Fregien N., Qiu F., Dahl G., Conner G. E., Salathe M. (2009) Am. J. Respir. Cell Mol. Biol. 41, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada H., Takahashi M. (2007) J. Biol. Chem. 282, 5597–5607 [DOI] [PubMed] [Google Scholar]
- 40.Lee H. G., Cowman M. K. (1994) Anal. Biochem. 219, 278–287 [DOI] [PubMed] [Google Scholar]
- 41.Campos M. A., Abreu A. R., Nlend M. C., Cobas M. A., Conner G. E., Whitney P. L. (2004) Am. J. Respir. Cell Mol. Biol. 30, 184–192 [DOI] [PubMed] [Google Scholar]
- 42.Calabro A., Hascall V. C., Midura R. J. (2000) Glycobiology 10, 283–293 [DOI] [PubMed] [Google Scholar]
- 43.Mercer B. A., D'Armiento J. M. (2006) Int. J. Chron. Obstruct. Pulmon. Dis. 1, 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei Z. B., Zhang Z., Jing Q., Qin Y. W., Pei G., Cao B. Z., Li X. Y. (2002) Cardiovasc. Res. 53, 524–532 [DOI] [PubMed] [Google Scholar]
- 45.Bassa B. V., Roh D. D., Vaziri N. D., Kirschenbaum M. A., Kamanna V. S. (1999) Am. J. Physiol. 277, F328–F337 [DOI] [PubMed] [Google Scholar]
- 46.Sinn P. L., Penisten A. K., Burnight E. R., Hickey M. A., Williams G., McCoy D. M., Mallampalli R. K., McCray P. B. (2005) Hum. Gene Ther. 16, 479–488 [DOI] [PubMed] [Google Scholar]
- 47.Dentener M. A., Vernooy J. H., Hendriks S., Wouters E. F. (2005) Thorax 60, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khadaroo R. G., Parodo J., Powers K. A., Papia G., Marshall J. C., Kapus A., Rotstein O. D. (2003) Surgery 134, 242–246 [DOI] [PubMed] [Google Scholar]
- 49.Hsieh C. C., Papaconstantinou J. (2006) Faseb J. 20, 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lepperdinger G., Strobl B., Kreil G. (1998) J. Biol. Chem. 273, 22466–22470 [DOI] [PubMed] [Google Scholar]
- 51.McConville M. J., Ferguson M. A. (1993) Biochem. J. 294, 305–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brewis I. A., Turner A. J., Hooper N. M. (1994) Biochem. J. 303, 633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verghese G. M., Gutknecht M. F., Caughey G. H. (2006) Am. J. Physiol. Cell Physiol. 291, C1258–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Low M. G., Prasad A. R. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 980–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maguire G. A., Gossner A. (1995) Ann. Clin. Biochem. 32, 74–78 [DOI] [PubMed] [Google Scholar]
- 56.O'Brien K. D., Pineda C., Chiu W. S., Bowen R., Deeg M. A. (1999) Circulation 99, 2876–2882 [DOI] [PubMed] [Google Scholar]
- 57.Jadin L., Wu X., Ding H., Frost G. I., Onclinx C., Triggs-Raine B., Flamion B. (2008) Faseb J. 22, 4316–4326 [DOI] [PubMed] [Google Scholar]
- 58.Tammi R., Rilla K., Pienimaki J. P., MacCallum D. K., Hogg M., Luukkonen M., Hascall V. C., Tammi M. (2001) J. Biol. Chem. 276, 35111–35122 [DOI] [PubMed] [Google Scholar]
- 59.Bourguignon L. Y., Singleton P. A., Diedrich F., Stern R., Gilad E. (2004) J. Biol. Chem. 279, 26991–27007 [DOI] [PubMed] [Google Scholar]
- 60.Kostikas K., Papatheodorou G., Ganas K., Psathakis K., Panagou P., Loukides S. (2002) Am J. Respir. Crit. Care Med. 165, 1364–1370 [DOI] [PubMed] [Google Scholar]
- 61.Borrill Z. L., Roy K., Vessey R. S., Woodcock A. A., Singh D. (2008) Int. J. Chron. Obstruct. Pulmon. Dis. 3, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sköld C. M., Blaschke E., Eklund A. (1996) Respir. Med. 90, 523–529 [DOI] [PubMed] [Google Scholar]
- 63.de la Motte C., Nigro J., Vasanji A., Rho H., Kessler S., Bandyopadhyay S., Danese S., Fiocchi C., Stern R. (2009) Am J. Pathol. 174, 2254–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller A. D. (2008) Semin. Cancer Biol. 18, 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duterme C., Mertens-Strijthagen J., Tammi M., Flamion B. (2009) J. Biol. Chem. 284, 33495–33508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.