Abstract
A new strategy has emerged to improve healing of bone defects using exogenous glycosaminoglycans by increasing the effectiveness of bone-anabolic growth factors. Wnt ligands play an important role in bone formation. However, their functional interactions with heparan sulfate/heparin have only been investigated in non-osseous tissues. Our study now shows that the osteogenic activity of Wnt3a is cooperatively stimulated through physical interactions with exogenous heparin. N-Sulfation and to a lesser extent O-sulfation of heparin contribute to the physical binding and optimal co-stimulation of Wnt3a. Wnt3a-heparin signaling synergistically increases osteoblast differentiation with minimal effects on cell proliferation. Thus, heparin selectively reduces the effective dose of Wnt3a needed to elicit osteogenic, but not mitogenic responses. Mechanistically, Wnt3a-heparin signaling strongly activates the phosphoinositide 3-kinase/Akt pathway and requires the bone-related transcription factor RUNX2 to stimulate alkaline phosphatase activity, which parallels canonical β-catenin signaling. Collectively, our findings establish the osteo-inductive potential of a heparin-mediated Wnt3a-phosphoinositide 3-kinase/Akt-RUNX2 signaling network and suggest that heparan sulfate supplementation may selectively reduce the therapeutic doses of peptide factors required to promote bone formation.
Keywords: Akt PKB, Heparan Sulfate, Heparin, Heparin-binding Protein, Wnt Pathway, Phosphoinositide 3-Kinase, RUNX2, Osteogenesis
Introduction
Improved anabolic strategies are required for clinical therapies that treat skeletal fractures and bone loss. At present, the in vivo efficacy of bone anabolic factors (e.g. BMP2,3 parathyroid hormone, and Wnts) is limited, and new molecular approaches to improve the effectiveness and selectivity of osteo-inductive extracellular ligands are desirable. Results from our group and others suggest that glycosaminoglycans (GAGs) and in particular heparan sulfate (HS) and heparin are potent co-stimulators of osteogenic signaling pathways, including BMP2-Smads and FGF-FGFR-MAPK (1–7). Thus, the opportunity arises to leverage the stimulatory properties of HS-derived compounds as adjuvants for osteo-inductive therapies.
The direct interaction of Wnts with heparin, which has allowed for the affinity purification of Wnt3a (8, 9), strongly suggests that it may also modulate the bone-promoting activity of Wnts. Wnt proteins are a group of cysteine-rich secreted glycolipoproteins of which at least 19 members have been identified in mammals (10). Wnts bind to serpentine receptors of the Frizzled family and their co-receptor, low density lipoprotein receptor-related protein 5/6 (LRP5/6). Several studies have shown that loss-of-function mutations in Wnt signaling components (e.g. LRP5) result in low bone mass and subsequent osteoporosis in both human patients and murine models (11, 12). Conversely, gain-of-function mutations in the LRP5 gene cause increased bone mass (13), whereas loss of the Wnt antagonist sFRP1 (secreted frizzled-related protein 1) increases trabecular bone mineral density and volume (14).
Wnt/Frizzled/LRP5 activation on the plasma membrane initiates a canonical cascade that is characterized by the accumulation and nuclear transportation of β-catenin. Nuclear β-catenin in turn activates the transcription factors lymphoid enhancer binding factor 1 (LEF1)/T cell-specific factor (TCF) (15, 16). The osteogenic ligand Wnt3a induces osteoblast maturation and extracellular matrix mineralization in multipotent progenitor cells via the β-catenin pathway (11, 17). The promoters of ALP (18) and the osteogenic transcription factor RUNX2 each contain LEF1/TCF binding sites (19), indicating that these genes are direct and highly relevant targets of canonical Wnt-β-catenin-LEF1/TCF signaling. Wnt-dependent induction of RUNX2 (19), subsequent binding of RUNX2 to LEF1/TCF proteins (19), and enhanced expression of proteoglycans and HS-modifying enzymes (20, 21) may together provide an effective feed-forward loop that sustains expression of osteogenic biomarkers to stimulate osteogenesis (19).
Cross-talk between Wnts and other ligands, such as the FGFs, BMPs, and insulin-like growth factors (IGF) is important for the progression of osteogenesis. In mesenchymal progenitor cells, the integrity of canonical Wnt (Wnt3a and Wnt1) signaling is required to maintain cellular responsiveness to the osteo-inductive effects of BMP2 (17). Interestingly, FGF1 has been reported to antagonize the Wnt pathway in osteoblasts, which may contribute to the disturbed osteoblast maturation seen in bone pathologies resulting from the aberrant activation of FGF signaling (22, 23). The phosphoinositide 3-kinase (PI3K)/Akt cascade also plays a critical role in osteoblast differentiation and synergizes with Wnt signaling (24, 25). Because Wnts as well as FGFs BMPs, and IGF each interact distinctly with GAGs, it is necessary to establish which signaling pathways respond to exogenous administration of a given ligand (e.g. Wnt) with a given fraction of heparan sulfate.
Heparan sulfates are unbranched polysaccharides consisting of repeating disaccharide units with N-, 2-O-, or 6-O-sulfate residues, the negatively charged groups bound to positively charged proteins. Heparin is a highly sulfated GAG and, thus, avidly binds to a range of growth factors, including Wnts. Heparin-growth factor interactions control the activities of susceptible ligands and in particular their interactions with cognate receptors, so dictating mesenchymal lineage progression and cell behavior. Heparin (and the less sulfated heparan sulfates) facilitates the binding of FGF2 to FGFRs, so augmenting osteoblast proliferation and differentiation (1–5). These GAGs also enhance the activity of BMPs, osteoinductive growth factors that have been clinically approved for spinal fusion, in part by protecting them from enzymatic attack or structural antagonists (6, 7).
Although Wnt signaling and HS/heparin have osteogenic potential and the interaction between Wnts and these sugars is known, no previous studies have provided direct evidence how the Wnt-HS interaction affects bone formation. Moreover, chronic heparin treatment in patients has been linked to osteoporosis, indicating that current models for the postulated biological interactions between Wnt and GAGs during bone formation are fundamentally incomplete. Notably, heparin can be either a positive or a negative regulator of canonical Wnt signaling in non-osseous tissues (26, 27). Our study now clearly resolves the biological function of Wnt-heparin interactions in bone tissue. The data establishes that in addition to canonical β-catenin signaling, Wnt3a and heparin cooperate to invoke a potent osteogenic response that involves the synergistic activation of the non-canonical PI3K/Akt/RUNX2 pathway to promote osteoblastic differentiation. These findings provide an empirical rationale for the development of pre-translational studies that use heparin as an adjuvant therapy for skeletal regeneration.
EXPERIMENTAL PROCEDURES
Chemicals and Inhibitors
LY294002, SU5402, and cycloheximide were obtained from Calbiochem. Protease inhibitor cocktails and other chemicals were purchased from Sigma.
Cell Culture
Murine pre-osteoblast MC3T3-E1 cells and myoblastic C2C12 cells were obtained from ATCC. RUNX2−/− murine calvarial-derived osteoprogenitor cells were established in a previous study (28). MC3T3-E1 and RUNX2−/− cells were maintained in α-minimum essential medium (Invitrogen) supplemented with 10% fetal calf serum (HyClone), antibiotics (Invitrogen), 1 mm sodium pyruvate (Invitrogen), and 2 mm l-glutamine (ATCC). C2C12 cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal calf serum and antibiotics. For assays, sodium pyruvate was removed from the medium for MC3T3-E1 and RUNX2-null cells and the fetal calf serum was reduced to 5% for C2C12 cells. Primary rat calvarial osteoprogenitor cells (rOB) were isolated by collagenase/EDTA digestion as described previously (5). The fraction enriched with osteoprogenitors was maintained in the same medium as that of MC3T3-E1 cells except for additional supplementation with 0.25 mg/ml amphoterin B. In conditions where cells were treated with both Wnt3a (R&D Systems) and heparin (Iduron), these two reagents were incubated in a sterile Eppendorf tube at room temperature for 20 min before application to the cell layer.
Immunofluorescence and Confocal Microscopy
Cells grown on chamber slides until reaching 40–50% confluence were treated with 50 ng/ml Wnt3a for 6 h. The cells were then fixed in 4% paraformaldehyde for 20 min, blocked in 2% BBX (0.1% Triton X-100, 0.1% bovine serum albumin, 250 mm NaCl, prepared in phosphate-buffered saline) for 30 min and incubated with a monoclonal antibody against β-catenin (BD Biosciences) followed by another incubation with a fluorescein isothiocyanate-conjugated anti-mouse IgG (Sigma) for 1 h per incubation. Non-specifically bound molecules were removed by rinsing in BBX at each step. Thereafter, 4′,6-diamidino-2-phenylindole nuclear staining (Invitrogen) was performed for 5 min. Labeled cells were visualized using a Zeiss LSM 5 Pascal laser scanning microscope (Carl Zeiss MicroImaging). Images were converted to the tagged information file (TIF) format and prepared with Adobe Photoshop software.
Luciferase Reporter Assay
To determine LEF1/TCF transcription activity, 0.4 μg of the reporter plasmids TOP-FLASH or FOP-FLASH (Upstate Biotechnology) were transfected into 40,000 cells with Lipofectamine 2000 (Invitrogen). 4 ng (1% of TOP-FLASH) of Renilla-LUC reporter vector (Promega) was co-transfected as control for transfection efficiency. Cells were treated 4 h post-transfection as indicated with different ligands for another 24 h before luciferase activity was determined using the Dual-Luciferase assay kit (Promega) according to the manufacturer's instructions. Briefly, cells were lysed in PLB buffer supplied, and the activities of firefly (produced by TOP-FLASH or FOP-FLASH) and Renilla luciferases were measured sequentially in each sample. The TOP-FLASH luciferase activities were calculated as -fold stimulation after normalization to Renilla-LUC activity and FOP-FLASH activity. To determine RUNX2 transcription activity, cells were transfected with 0.4 μg of the reporter plasmid 6xOSE-LUC vector (29) and 4 ng of Renilla-LUC reporter vector for 24 h before the indicated treatments. Luciferase activity was assayed as described above 48 h post-treatment. To assess the transcriptional effects of BMP signaling, cells were transfected with 0.8 μg of Id1 promoter luciferase reporter, which contains a BMP-responsive element (a generous gift from Dr. Bruno Reversade, Institute of Medical Biology, A*STAR, Singapore) and 8 ng of Renilla-LUC vector for 6 h before treatment with Wnt3a and heparin for another 24 h. Luciferase activity was then assayed as described above.
Heparin-Sepharose Bead Precipitation Assay
The interaction between Wnt3a and heparin was detected by the amount of Wnt3a bound to heparin-Sepharose beads. Free heparin or desulfated heparins (Iduron) was used to compete with heparin-Sepharose for Wnt3a binding. Based on information provided by the supplier, the efficiency of desulfation of N-, 2-O-, and 6-O-desulfated heparins is 92, 95, and 90%, respectively. All selectively desulfated heparins retain normal content of other non-targeted sulfate groups, with the exception of 6-O-desulfated heparin, which retains normal content of N- but a 25% loss of 2-O-sulfates. Wnt3a (50 ng) was incubated with 40 μl of 75% heparin-Sepharose CL 6B slurry (Amersham Biosciences) in 100 μl of phosphate-buffered saline in the presence or absence of 50 μg/ml unmodified heparin or selectively desulfated heparins for 20 min at 4 °C. The beads were then washed twice with phosphate-buffered saline, and the bound Wnt3a was eluted by Laemmli buffer (Sigma). Wnt3a was detected using anti-Wnt3a antibody by Western blot analysis.
Heparin-Wnt3a Binding Assay
To investigate Wnt3a-binding properties to heparin, an enzyme-based assay was performed using the specially prepared GAG binding plates (Iduron) as per the manufacturer's instructions. Briefly, the plates were first coated with various amounts of unmodified heparin or selectively desulfated heparins (Iduron) in the standard assay buffer provided. After blocking in 0.2% gelatin, 50 ng/ml Wnt3a was applied to the plate. The heparin-coated wells not subject to Wnt3a served as blank controls. To eliminate the nonspecific binding of Wnt3a to the plates, wells without heparin coating but subject to Wnt3a were also used as blank controls. Wnt3a binding to heparin was then detected by its specific antibody conjugated with biotin (Abcam). Biotin was further bound with ExtrAvidin-AP (alkaline phosphatase) followed by incubation with p-nitrophenyl phosphate, which is the chromogenic substrate of AP and gives a yellow-color product detectable at 405 nm using a Victor3 multilabel plate reader (PerkinElmer Life Sciences). The entire assay was performed at room temperature or 37 °C as instructed and protected from light, with each step followed by extensive washing to remove nonspecific interactions. The experimental readings were adjusted by subtracting background (determined from blank readings) and analyzed.
Western Blot Analysis
Cells were treated as indicated and denatured in Laemmli buffer at 95 °C for 5 min. Protein lysates (20 μl) were separated by SDS-PAGE, and proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk or bovine serum albumin in TBST (10 mm Tris-base, 150 mm NaCl, pH 7.6, 0.1% Tween 20) and then incubated with the corresponding primary antibody and secondary antibody for 1 h at each step. The blot was rinsed with TBST between each incubation to remove any non-specifically bound molecules. Monoclonal antibody against β-catenin, RUNX2, or actin was purchased from BD Biosciences, MBL, or Chemicon International, respectively. Polyclonal antibodies against Wnt3a, p85, phosphorylated p85, Akt, phosphorylated Akt, and phosphorylated glycogen synthase kinase 3β were supplied by Cell Signaling Technology. Anti-mouse or rabbit secondary antibodies conjugated to horseradish peroxidase were from Amersham Biosciences. The horseradish peroxidase-bound protein bands were then visualized using the enhanced chemiluminescence (ECL) kit according to the manufacturer's instructions (Amersham Biosciences). For reblotting, membranes were stripped at 37 °C for 15–30 min in stripping buffer (Pierce). Efficacy of stripping was determined by re-exposure of the membranes to ECL. Clear membranes were reblocked and immuno-labeled as desired. The intensity of protein bands on blots was quantified by densitometric scanning (Epson V500, Epson) and analyzed by Quantity One software (Bio-Rad).
Proliferation Assay
MC3T3-E1 cells were plated in triplicate at 30,000 cells/cm2 in 48-well plates, and the rate of proliferation was determined by monitoring cell number after 3 days using the GUAVA PCA-96 Viacount system (GUAVA Technologies). Briefly, cells were trypsinized at 37 °C for 5 min, trypsin was inactivated, and cells were stained with GUAVA Flex dye (supplied by the manufacturer). The cell suspension was transferred to a 96-well plate with a U-shaped bottom (Nunc) and counted in triplicate using the GUAVA Viacount program.
ALP Activity Assay
Cells seeded in triplicate at 30,000 cells/cm2 were treated as indicated. Cells were then lysed in radioimmunoprecipitation assay buffer (150 mm NaCl, 10 mm Tris, pH 7.4, 2 mm EDTA, 0.5% Nonidet P-40, 0.1% SDS, 1% Triton X-100, protease inhibitor mixture), and the protein concentration was determined using the BCA protein assay kit (Pierce) following the manufacturer's instructions. ALP activity was determined using equal amounts of total protein. Briefly, 20 μg of proteins were incubated with 40 μl of assay buffer including substrate p-nitrophenyl phosphate (Zymed Laboratories Inc.) at 37 °C for 1 h. Calf intestinal alkaline phosphatase (New England Biolabs) was used as positive control. The absorbance at 405 nm was measured using the Victor3 1420 multilabel counter (PerkinElmer Life Sciences).
Real-time Quantitative PCR Analysis
The mRNA levels of target genes were quantitated using reverse transcription and the TaqMan® real-time PCR method. MC3T3-E1 cells were grown in triplicate and treated with either 50 ng/ml Wnt3a, 50 μg/ml heparin, or their combination for 72 h. Total RNA was isolated using the NucleospinTM RNA extraction kit (Macherey-Nagel) as instructed by the manufacturer. The quality and quantity of total RNA was determined by gel electrophoresis and a GeneQuant spectrophotometer (Amersham Biosciences). Total RNA (1 μg) was reverse-transcribed into cDNA using SuperScript® VILOTM cDNA synthesis kit (Invitrogen). Expression levels of target genes were determined using 20 ng of each cDNA sample assayed in triplicate and amplified with the ABI Prism 7500® sequence detection system (PerkinElmer Life Sciences). The primers and probes for RUNX2, osterix, ALP (liver/bone/kidney isoenzyme), bone sialoprotein, osteocalcin, c-Myc, FGFR1, FGFR2, FGFR3, cyclinB, cyclinD, cyclinE, CDK2, CDK4, p21, p27, p57, and housekeeping gene actin were pre-designed by Applied Biosystems. As a control for the input amount, each cDNA sample was also amplified using the pre-designed primer and probe for actin (Applied Biosystems). Data were analyzed using the Applied Biosystems Sequence Detector software.
Plasmid Construction and Transfection
A human RUNX2 cDNA (30) was subcloned into pcDNA3.1 (−) (Invitrogen), and the integrity of the RUNX2 protein coding sequence was confirmed by DNA sequencing (BSF DNA sequencing facility, A*STAR, Singapore). The pcDNA3-RUNX2 vector (1 μg) was then transfected into RUNX2−/− cells plated at 10,000 cells/cm2 in a 6-well plate using the GeneJammer transfection reagent (Stratagene) as per the manufacturer's instructions. Cells transfected with the empty vector pcDNA3.1 (-) were used as negative control. Cells were re-plated at 24 h post-transfection at a density of 2500 cells/cm2 and then treated for 72 h with 50 ng/ml Wnt3a and/or between 0.5–50 μg/ml heparin as indicated before harvesting.
RNA Interference
MC3T3-E1 cells seeded at 20,000 cells/cm2 in 24-well plates were transfected with 50 pmol of siRNA specific for the target genes or scrambled siRNA as the negative control using Lipofectamine 2000 (Invitrogen). The commercially synthesized siRNAs were purchased from Qiagen (Germany) and used as described in the protocols provided by the manufacturer. For each target gene, two or three siRNAs were tested, and the siRNA with the most potent and specific gene silencing effect was selected for further studies. Cells were treated as indicated 24 h post-transfection with siRNAs.
Statistical Analysis
Each experiment was repeated three times, and data were expressed as the mean ± S.E. Differences among treatments were analyzed by Student's t test or analysis of variance. Significant differences were considered as those with a p value of <0.05.
RESULTS
Wnt3a and Heparin Synergistically Increase ALP Activity
Wnt3a activates osteogenic differentiation in both multipotent mesenchymal cells and pre-committed osteoblasts (17, 31, 32). Stimulation of the canonical Wnt pathway in response to Wnt3a was reflected by a dose-dependent increase in the transcriptional activity of LEF1/TCF that reaches maximal potency at 50 ng/ml (within the dose range tested) as measured by transient transfection assays with the TOP-FLASH reporter in MC3T3-E1 cells (Fig. 1A). Nuclear translocation of β-catenin (labeled with fluorescein isothiocyanate) was evident upon stimulation with 50 ng/ml Wnt3a (for 6 h) as visualized by immunofluorescence and confocal microscopy (Fig. 1B). As expected, β-catenin was detected in the cytoplasm of cells at peripheral sites of cell-cell contact in the absence of Wnt3a, whereas β-catenin was predominantly detected in the nucleus, which was visualized by chromatin staining using 4′,6-diamidino-2-phenylindole in Wnt3a-stimulated cells (Fig. 1B). Exogenous Wnt3a was a potent ligand for induction of ALP activity in mesenchymal progenitor cells (C2C12) but was not effective in stimulating ALP activity in MC3T3-E1 preosteoblasts (Fig. 1C), as had also been observed previously (17). The ineffectiveness of Wnt3a in stimulating ALP in MC3T3-E1 osteoblasts was notable considering that the dose we applied (50 ng/ml) was optimal for the transcriptional activation of β-catenin-LEF1/TCF complexes and nuclear translocation (Fig. 1, A and B). Thus, Wnt3a signaling by itself is ineffective in stimulating ALP activity in MC3T3-E1.
FIGURE 1.
Wnt3a is not sufficient to stimulate ALP activity in MC3T3-E1 cells. A, Wnt3a stimulated LEF1/TCF transcription activity in MC3T3-E1 cells. B, Wnt3a induced nuclear translocation of β-catenin in MC3T3-E1 cells that was assessed by immunofluorescence. Merged images of β-catenin and nuclear 4′,6-diamidino-2-phenylindole (DAPI) staining were shown (green, β-catenin; blue, 4′,6-diamidino-2-phenylindole). Bar, 10 μm. C, Wnt3a stimulated ALP activity in C2C12 but not in MC3T3-E1 cells. Both cells were treated by 50 ng/ml Wnt3a for 72 h before the ALP activity was assayed.
Wnt signaling can be regulated by either HS or heparin (26, 27), GAGs that promote the osteogenic activity of BMP2 and BMP7 and potentiate bone formation (6, 7, 33). Heparin binds to Wnt3a (9) and, thus, may enhance the osteogenic potential of Wnt. Indeed, co-incubation of MC3T3-E1 cells with both exogenous heparin and Wnt3a, which had been preincubated for 20 min to allow complex formation, resulted in a synergistic and dose-dependent elevation of ALP activity (Fig. 2A). Maximal ALP activity was increased by 2–3-fold compared with either Wnt3a or heparin treatment alone. This co-stimulation of ALP activity by Wnt3a and heparin was also observed in C2C12 mesenchymal progenitors and primary calvarial osteoprogenitor (rOB) cells (Fig. 2, B and C).
FIGURE 2.
Wnt3a and heparin synergistically enhanced ALP activity. MC3T3-E1 (A) or C2C12 (B) cells seeded at 30,000 cells/cm2 or rOB cells (C) seeded at 5000 cells/cm2 were treated with either 50 ng/ml Wnt3a, increasing doses of heparin (0.5 to 50 μg/ml) or the combination of 50 ng/ml Wnt3a with indicated doses of heparin. Untreated samples served as the control. ALP activity was determined 72 h after treatment.
As Wnt signaling stimulates osteoblast proliferation, we next examined whether heparin increases the mitogenic effect of Wnt3a while enhancing its osteogenic potential. Wnt3a modestly increased cell number (<1.5-fold) after 3 days of stimulation in cultures of actively growing MC3T3-E1 (supplemental Fig. S1A). However, rather than increasing cell proliferation, heparin reduced Wnt3a-stimulated cell expansion as well as the basal MC3T3-E1 growth rate (supplemental Fig. S1A). Similarly, heparin did not synergize with Wnt3a to increase cyclin/CDK (cyclins A, B, D, and E as well as CDK2 and CDK4) expression or suppress cell cycle inhibitors (p21, p27, p57) (supplemental Fig. S1, B and C). The effect of heparin on selected cell cycle regulators (cyclinA2, B1, p21, or p57) was opposite that observed for Wnt3a, consistent with a moderate antagonism of heparin for Wnt3a-dependent cell growth (supplemental Fig. S1A). In summary, heparin synergizes with Wnt3a to promote its osteogenic activity but not its mitogenic activity.
N-Sulfation of Heparin Is Critical for Its Wnt3a Binding Ability and Biological Activity
The polysaccharide chains of heparin are heavily modified by sulfation on N, 2-O, and 6-O groups (Fig. 3A) (34), which create negative charge patterns that attract particular basic amino acid motifs in heparin-binding proteins (35). To assess the importance of different sulfate groups for the interaction of heparin with Wnt3a, we performed binding assays in which heparins selectively depleted for one type of sulfation were used as binding competitors. Unmodified heparin immobilized to Sepharose beads precipitates Wnt3a upon centrifugation (Fig. 3B). Free unmodified heparin competed with the heparin-Sepharose for Wnt3a binding, thus significantly decreasing the amount of Wnt3a precipitated upon centrifugation (Fig. 3B). Importantly, desulfated heparins were less potent than unmodified heparin in competing for the binding between heparin-Sepharose and Wnt3a (Fig. 3B). N-Sulfate-deficient (Δ-N) heparin does not compete with heparin-Sepharose for Wnt3a binding, indicating that N-sulfation is essential for the Wnt3a-heparin interaction. For comparison, loss of 2-O-sulfate (Δ-2-O) reduced the binding of Wnt3a to heparin-Sepharose, whereas Δ-6-O heparin only partially competed for Wnt3a binding.
FIGURE 3.
Sulfation on heparin chain is important for its binding affinity and biological activity. A, shown is the structure of disaccharide units of heparin. B, Wnt3a was incubated with heparin-Sepharose in the presence or absence of heparin or desulfated heparin, and the Wnt3a bound to the Sepharose beads was detected by Western blot analysis. The intensity of Wnt3a was quantified by densitometry, and the bar graph plotted was shown above the blot. C, increasing doses of unmodified heparin or desulfated heparin (0.05–150 μg/ml) were immobilized on heparin/GAG binding plates before 50 ng/ml Wnt3a was applied. The amount of Wnt3a bound by these heparins was determined by enzyme-linked immunosorbent assay as described under “Experimental Procedures.” D, MC3T3-E1 cells were stimulated with 50 ng/ml Wnt3a alone or Wnt3a pre-incubated with 50 μg/ml heparin or desulfated heparins for 72 h followed by the determination of ALP activity.
The relative affinity of these sulfate groups to Wnt3a was further determined using a plate with a modified surface that absorbs heparin/GAGs without altering the ligand binding property of these sugars. Unmodified heparin was able to bind 50 ng/ml Wnt3a (A405 ≈ 1.1) (Fig. 3C). In contrast, the maximal binding capacity of either Δ-2-O or Δ-6-O heparin for Wnt3a was only about 30 ng/ml (A405 ≈ 0.6) (Fig. 3C). Therefore, the overall binding ability of heparin is compromised by loss of O-sulfation. More importantly, Δ-N heparin failed to bind Wnt3a even at very high concentrations (150 μg/ml). The absorbance remained around 0.1 regardless of the amount of heparin, indicating background nonspecific binding and the absence of appreciable affinity of Δ-N heparin for Wnt3a. Hence, N-sulfation is crucial for the heparin-Wnt3a interaction. Moreover, our results show that 0.05 μg/ml unmodified heparin suffices for achieving half-maximal binding of Wnt3a, but 10–100-fold higher amounts are required with desulfated heparins (respectively, 0.5 μg/ml Δ-2-O or 1.5–5 μg/ml Δ-6-O heparin; A405 ≈ 0.5 for unmodified heparin; A405 ≈ 0.3 for desulfated heparin). Therefore, the relative affinity of heparin for Wnt3a is decreased by an order of magnitude upon 2-O-sulfate deletion and by up to 2 orders of magnitude upon 6-O-sulfate deletion. Consequently, the relative binding strength of the sulfate groups in heparin for Wnt3a is N- > 6-O- > 2-O-sulfation. However, 6-O-desulfated heparin also bears a ∼25% loss of 2-O-sulfates, which might contribute to its apparent lower affinity to Wnt3a than 2-O-desulfated heparin. A more definitive interpretation of the roles of 6-O versus 2-O sulfation in heparin must await the availability of purer desulfated heparins.
We further examined whether the sulfate groups were also important for the biological activity of heparin. Depletion of any of the three sulfate motifs in heparin resulted in a large decrease of Wnt3a-heparin-induced ALP activity, albeit the loss of N-sulfation had a more pronounced effect than that of 2-O- or 6-O-sulfation (Fig. 3D). These data indicate that N-sulfation, and to a lesser extent O-sulfation, is required for the osteogenic activity of Wnt3a-heparin complexes, consistent with the relative affinities of desulfated heparins as determined by the binding assays.
β-Catenin Contributes to the Synergistic Activation of ALP by Wnt3a and Heparin
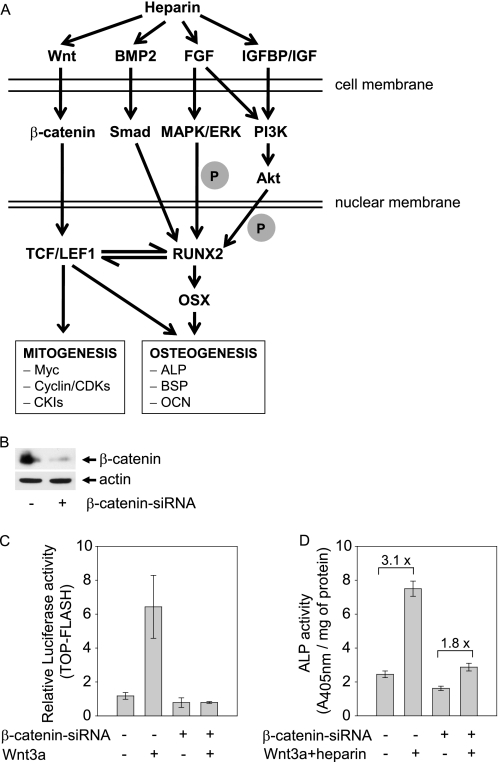
As we have shown that heparin also interacts with Wnt3a to activate ALP, it is necessary to further address whether β-catenin is a key mechanistic component of this pathway (Fig. 4A). Canonical Wnt signals are transduced through β-catenin that contributes to basal ALP activity in MC3T3-E1 cells (17). The interaction of heparin with Wnt3a may enhance the activation of β-catenin and, thus, generate more potent osteogenic signals. We next employed RNA interference of β-catenin to inhibit its expression (Fig. 4B). Wnt3a stimulation of the LEF1/TCF-dependent reporter gene was diminished by preincubation with β-catenin siRNA, establishing the successful suppression of canonical Wnt signaling (Fig. 4C). Basal ALP activity was also decreased in β-catenin-depleted cells (Fig. 4D), consistent with previous findings (17). When β-catenin signaling was reduced, co-stimulation of ALP activity by Wnt3a and heparin was also greatly diminished (Fig. 4D). The Wnt3a-heparin combination remained stimulatory for ALP activity, albeit less potent in the β-catenin-depleted cells (3.1-fold in control cells versus 1.8-fold in siRNA treated cells). We note that Wnt3a and heparin together modestly stimulated ALP activity (1.8-fold) (Fig. 4D) despite the suppression of β-catenin-dependent LEF1/TCF activity (Fig. 4C). However the residual presence of β-catenin in siRNA-treated cells (Fig. 4B) could also account for the 1.8-fold increase in ALP activity (Fig. 4D), thus precluding a definitive interpretation. More importantly, the results of Fig. 4D indicate that the β-catenin pathway is required for co-stimulation by Wnt3a and heparin, although low levels of β-catenin may not suffice for enhancement of ALP activity, and a LEF1/TCF-independent pathway may contribute to the synergistic activity of Wnt3a and heparin. It remains to be determined whether a β-catenin-independent pathway contributes to the synergistic activity of Wnt3a and heparin.
FIGURE 4.
β-Catenin mediated Wnt3a-heparin-stimulated ALP activity. A, shown is an illustration of the interaction of heparin with Wnt and other growth factor signaling pathways that may regulate Wnt dependent osteoblast proliferation and differentiation. IGFBP, IGF-binding protein; BSP, bone sialoprotein; OCN, osteocalcin; OSX, osterix; CKI, cyclin-dependent kinase inhibitor. B, MC3T3-E1 cells were transfected with scramble siRNA or β-catenin siRNA for 24 h. The protein level of β-catenin was assessed by Western blotting. C, cells transfected with β-catenin siRNA or scramble siRNA were further transfected with TOP-FLASH or FOP-FLASH together with Renilla-LUC DNA. 6 h later the cells were exposed to 50 ng/ml Wnt3a for 24 h, and the luciferase activity was measured. D, parallel cultures of β-catenin siRNA-transfected MC3T3-E1 cells were treated with a combination of Wnt3a (50 ng/ml) and heparin (50 μg/ml) for 72 h, and ALP activity assessed.
BMP or FGF/FGFR Signaling Is Dispensable for Wnt3a and Heparin-stimulated ALP Activity
Heparin may promote osteoblast maturation by enhancing BMP-BMPR-Smads signaling or FGF-FGFR-MAPK signaling that may increase ALP activity and osteoblast differentiation (Fig. 4A). Therefore, we investigated if these signaling pathways were potentiated by exogenous heparin and account for the synergism with Wnt3a that resulted in osteoblast differentiation. We first assessed if co-treatment of Wnt3a and heparin activated BMP signaling as reflected by Smad-dependent activation of the promoter of the Id1 gene, a direct target of BMP signaling (36). We observed that Id1 transcription was dramatically induced by 100 ng/ml BMP2, but Wnt3a and heparin alone or together clearly did not stimulate Id1 promoter activity (supplemental Fig. S2A). Furthermore, ALP activation by Wnt3a and heparin was not diminished by Smad4 siRNA (supplemental Fig. S2, B and C), thus excluding BMP signaling as a pathway that contributes to the co-stimulation of ALP activity by Wnt3a and heparin. In examining FGF signaling, Wnt3a and heparin together were able to increase the expression of FGFR2 and FGFR3, but not FGFR1, at the mRNA or protein levels (supplemental Fig. S2, D and E). However, suppression of the kinase activity of FGFRs using the selective chemical inhibitor SU5402 failed to decrease the ALP activity induced by Wnt3a and heparin (supplemental Fig. S2F). Moreover, the ERK pathway that mediates osteoblast differentiation in response to FGF/FGFR (37, 38) was not activated by Wnt3a and heparin (supplemental Fig. S2G). Collectively, these data indicate that neither BMPs nor FGFs account for osteogenic stimulation by the combination of Wnt3a and heparin.
Wnt3a and Heparin Cooperate to Induce Sustained Activation of the PI3K/Akt Pathway to Stimulate ALP Activity
The PI3K/Akt pathway plays an important role in osteoblast differentiation and synergizes with Wnt signaling to determine osteoblastic cell fate (25, 39). The regulatory subunit of PI3K, p85, is up-regulated within 24 h after a pulse of exogenous Wnt3a and heparin (Fig. 5A). Consistently, the amount of phosphorylated p85 and phosphorylated Akt were both increased, whereas total Akt protein levels remained unchanged (Fig. 5A). Increased phosphorylation of PI3K or Akt upon Wnt3a and heparin administration was not apparent until after 6 h but occurred simultaneously with elevation of p85 protein levels (data not shown), suggesting involvement of an intermediary factor(s) (see “Discussion”). Notably, Wnt3a alone increases the protein level of p85 and subsequent activation of Akt, but the combination of Wn3a and heparin further enhanced the activity of the PI3K/Akt pathway (Fig. 5B). To determine whether the PI3K/Akt pathway mediates the osteogenic activity of Wnt3a and heparin, we disrupted PI3K kinase activity using the chemical inhibitor LY294002. LY294002 completely abolished the phosphorylation of Akt (Fig. 5C) when applied to MC3T3-E1 cells 1 h before co-treatment with Wnt3a and heparin. Notably, inhibition of PI3K activity eliminated Wnt3a-heparin-induced ALP activity (Fig. 5D). Furthermore, silencing of p85 and Akt by their specific siRNAs also disabled Wnt3a-heparin-stimulated ALP activity (Fig. 5, E and F). We conclude that Wnt3a and heparin stimulate the PI3K/Akt pathway via up-regulation of p85-PI3K to enhance ALP activity.
FIGURE 5.
PI3K/Akt pathway is required for Wnt3a-heparin stimulated ALP activity. A and B, p85-PI3K and Akt protein levels as well as their phosphorylation status were measured in MC3T3-E1 cells treated with either 50 ng/ml Wnt3a, 50 μg/ml heparin, or their combination. The relative amount of protein was evaluated by densitometry analysis of the blot in B. C, MC3T3-E1 cells were treated with different concentration of LY294002 for 1 h before the level of phosphorylated Akt (the direct target of activated PI3K) was assessed by Western blotting. D, cells preincubated 1 h with 10 μm LY294002 were stimulated with Wnt3a and heparin for 72 h and then assayed for ALP activity. E and F, MC3T3-E1 cells were transfected with scramble siRNA or siRNA specific for p85 or Akt for 24 h before stimulation with Wnt3a and heparin. The siRNA knock-down efficiency was evaluated by Western blot analysis. ALP activity was measured 72 h post-stimulation.
Wnt3a and Heparin Synergistically Increase the Expression and Activity of RUNX2
We next examined if the combination of Wnt3a and heparin affected other osteogenic biomarkers. RUNX2 and osterix are transcription factors crucial for osteoblast differentiation. ALP, bone sialoprotein, and osteocalcin are osteoblast phenotypic markers up-regulated in a defined temporal sequence. MC3T3-E1 cells treated for 3 days with Wnt3a and heparin expressed higher mRNA levels of bone-related biomarkers compared with either Wnt3a or heparin alone (Fig. 6A), indicating significant stimulation of osteogenic differentiation. Increased ALP mRNA levels corroborated the elevations of ALP activity that we observed throughout our studies (see Fig. 2A). For comparison, in contrast to the overall synergistic up-regulation of osteogenic genes, the mRNA level of c-Myc, a transcriptional regulator controlling cell proliferation, was not affected by Wnt3a and heparin (Fig. 6A). In addition, a panel of genes involved in cell cycle progression exhibited different parallel or opposite patterns of expression compared with mRNA levels for osteogenic biomarkers (see supplemental Fig. S1).
FIGURE 6.
Wnt3a and heparin synergistically increased the expression and activity of osteogenic biomarkers. A, the mRNA levels of several osteogenic transcription factors and osteoblast phenotypic markers were assessed by TaqMan real-time PCR. The samples were prepared in triplicate, and the data represent the mean ± S.E. of three independent experiments. OCN, osteocalcin; OSX, osterix; REU, relative expression unit. B, Western blot analysis of the protein level of RUNX2 after MC3T3-E1 cells were treated with 50 ng/ml Wnt3a, 50 μg/ml heparin, or their combination for 72 h. C, 6xOSE-LUC reporter assay was performed to examine RUNX2 transcription activity as described under “Experimental Procedures.”
RUNX2 plays a critical role in osteoblast differentiation and induces the expression of other genes that regulate or characterize osteoblast phenotypes, including osterix, ALP, bone sialoprotein, and osteocalcin (40, 41). Consistent with its increased mRNA levels, RUNX2 protein and its transcriptional activity on a RUNX2-responsive promoter were also enhanced upon co-stimulation with Wnt3a and heparin (Fig. 6, B and C). Although either Wnt3a or heparin is capable of increasing the expression and activity of RUNX2 to a limited extent, these synergizing agents are a particularly potent stimulus for RUNX2 when administered in combination.
RUNX2 Activity Is Required for Wnt3a and Heparin-stimulated ALP Activity
The central role of RUNX2 in osteoblast differentiation as well as its functional links with Wnt signaling (19) and proteoglycan gene expression (20, 21) suggest that this transcription factor may be an important mediator of the osteogenic activity of Wnt3a and heparin. Upon RUNX2 silencing by specific siRNA in MC3T3-E1 cells, Wnt3a (50 ng/ml) and heparin (50 μg/ml) were unable to stimulate ALP activity (Fig. 7A). A similar loss of their control over ALP was observed in RUNX2−/− osteoprogenitor cells (Fig. 7B), which were derived by immortalization of calvarial osteoblasts from RUNX2 knock-out mice (28, 42). However, forced expression of wild type RUNX2 protein in RUNX2−/− cells recovered the responsiveness of ALP activity to co-stimulation by Wnt3a (50 ng/ml) and heparin (50 μg/ml) (Fig. 7B). Because the responsiveness to different heparin concentrations varied among the cell types, we further conducted a systematic measurement of ALP activity in RUNX2−/− cells or RUNX2−/− cells complemented with wild type RUNX2 after treatment with Wnt3a and/or various doses of heparin (Fig. 7, C and D). In RUNX2−/− cells, either Wnt3a or heparin at different dosage or their combination was unable to stimulate ALP (Fig. 7C). In contrast, upon re-introduction of RUNX2 into this cell line, a dose-dependent increase of ALP activity was observed upon co-treatment with Wnt3a and heparin, and maximal ALP stimulation (≈1.7-fold) was achieved by the combination of 5 μg/ml heparin and 50 ng/ml Wnt3a (Fig. 7D). This robust but quantitatively modest co-stimulatory effect may be less pronounced than in other cell types, because not all cells are expected to express exogenous RUNX2 upon transfection. Wild type primary osteoprogenitor cells derived from rat calvaria (rOB) were also included in this study for comparison with RUNX2−/− cells. Similar to other osteoblastic cells, rOB exhibited enhanced ALP stimulation by Wnt3a and heparin (Fig. 2C). The ability to selectively eliminate and restore osteogenic signaling by Wnt3a and heparin depending on the absence or presence of RUNX2 clearly demonstrates that RUNX2 is a critical and integral part of the Wnt3a-heparin signaling network.
FIGURE 7.
RUNX2 was required for Wnt3a-heparin stimulated ALP activity. A, MC3T3-E1 cells deficient in RUNX2 by RUNX2 siRNA transient transfection were challenged by the combination of Wnt3a and heparin. Then the efficiency of RUNX2 silencing, and the effect on ALP activity was evaluated after 72 h. B, the responsiveness of ALP activity to Wnt3a and heparin was assessed in RUNX2−/− cells. Wild type RUNX2 vector was then re-introduced into RUNX2−/− cells. The efficacy of forced-expression of RUNX2 was evaluated, and the ALP activity was again examined in RUNX2−/− cells treated with Wnt3a and heparin after recovery of RUNX2 expression. C and D, RUNX2−/− cells or RUNX2−/− cells transfected with wild type RUNX2 were seeded at 2500 cells/cm2 and then treated with either 50 ng/ml Wnt3a, increasing doses of heparin (0.5 to 50 μg/ml), or the combination of 50 ng/ml Wnt3a with the indicated doses of heparin. Untreated samples served as controls. ALP activity was determined 72 h after treatment.
Stimulation of RUNX2 Activity by Wnt3a and Heparin Is Mediated via the PI3K/Akt Pathway
Recent studies have indicated that PI3K/Akt regulates RUNX2 (39, 43), and our study shows that PI3K/Akt is a prominent pathway contributing to the Wnt3a and heparin stimulation of RUNX2. To test the hypothesis that PI3K/Akt is a component of the same signaling axis and rate-limiting for RUNX2 activity, we disrupted PI3K kinase activity using LY294002 at a dose (10 μm) that was sufficient to suppress PI3K signaling (see Fig. 5C). Notably, inactivation of PI3K/Akt eliminated both basal and Wnt3a-heparin induced RUNX2 protein expression (Fig. 8A). This finding was further consolidated using siRNAs to reduce Akt expression. RNA interference for Akt reduced Wnt3a-heparin-dependent induction of RUNX2 (Fig. 8B). Moreover, LY294002 dramatically blocked the RUNX2 transcriptional activity that was normally enhanced by the combination of Wnt3a and heparin (Fig. 8C). For comparison, LY294002 only minimally affects β-catenin-dependent LEF1/TCF activity (Fig. 8D), suggesting that the previously identified Akt/glycogen synthase kinase 3β/β-catenin/LEF axis (25) is not the primary pathway that transduces Wnt3a-heparin signaling in MC3T3-E1 cells. We conclude that Wnt3a and heparin cooperatively promote osteoblast maturation in part by inducing PI3K/Akt signaling and enhancing RUNX2 function.
FIGURE 8.
PI3K/Akt pathway mediated the effect of Wnt3a and heparin on RUNX2 activity in MC3T3-E1 cells. A, 10 μm LY294002 was applied to the cells 1 h before co-treatment with Wnt3a and heparin. The level of RUNX2 protein was determined 72 h later. B, 24 h after cells were transfected with the specific siRNA against Akt or scramble siRNA, they were stimulated with Wnt3a and heparin for 72 h. The reduced level of Akt and RUNX2 was examined by Western blot analysis. C, cells were transfected with 6xOSE-LUC and Renilla-LUC vectors. After 24 h, cells were challenged with 10 μm LY294002 for 1 h preceding the stimulation with Wnt3a and heparin. The luciferase activity assay was then carried out 48 h later. D, TOP-FLASH or FOP-FLASH together with Renilla-LUC vector was forcibly expressed in cells. 6 h later cells were treated with 10 μm LY294002 for 1 h before the co-treatment of Wnt3a and heparin for another 24 h. The luciferase activity was then determined. The doses of Wnt3a and heparin used above were 50 ng/ml and 50 μg/ml, respectively.
DISCUSSION
The control of osteoprogenitor cell growth and differentiation, which is essential for bone formation, is exercised in large part by bone-related growth factors that interact with their cognate plasma membrane receptors. Heparan sulfate proteoglycans, which are abundant on the cell surface and in the extracellular matrix, have emerged as major co-regulators controlling signaling pathways in osteogenic cells (44). Heparan sulfates increase the expression of bone-related biomarkers and maintain BMP signaling, and studies have suggested that this family of sugar-based compounds orchestrates the transition from proliferation to differentiation (3, 33, 44). Supporting this view, our study demonstrates that heparin strongly synergizes with Wnt3a and selectively stimulates the osteogenic but not mitogenic potential of Wnt3a. As natural polysaccharides, heparan sulfates and heparin are chemically stable and well tolerated clinically. Such advantages make their exogenous application an attractive approach to augment the efficacy of osteogenic growth factors during bone tissue wound healing. Increased understanding of their mechanism of action to co-stimulate downstream signaling pathways, which respond to bone related growth factors, is a prerequisite to their exploitation in skeletal regenerative medicine.
Accumulating evidence has demonstrated that different sulfate groups within HS/heparin contribute unequally to their interactions with target proteins, so providing a molecular basis for selective recognition of distinct ligands (33, 45–47). Heparin forms a ternary complex with FGF2 through N- and 2-O-sulfate groups and with FGFRs via 6-O-sulfate groups (1, 48–51). Such interactions enhance the recognition of the cognate FGFRs and subsequent activation of downstream signaling pathways. Interestingly, N-sulfation is also essential for HS binding to both BMP7 and platelet-derived growth factor-BB, whereas O-sulfation (2-O) is less important (33, 45). A key finding of our study is that N-sulfation is more crucial than O-sulfation for heparin-Wnt3a interactions, albeit both are important for maximal binding affinity and biological activity. Although there are no identified heparin-binding domains in the Wnt receptors Frizzled or LRPs, heparin binds to sFRP1 (secreted Frizzled-related proteins 1), which interacts directly with Wnts and Frizzled (52). Thus, it has been proposed that heparin bridges Wnt and Frizzled by forming a ternary complex similar to the FGF-HS-FGFR model (26). Notably, heparin needs O-sulfation but not N-sulfation to regulate sFRP1 (53, 54). Thus, N-sulfate groups might be a universal moiety for heparin-ligand binding, whereas O-sulfate groups (2-O, 6-O, or both) may be more crucial for heparin-receptor binding (Fig. 9B).
FIGURE 9.
A, shown is a diagram of the signaling mechanism depicting the synergistic activation of ALP by Wnt3a and heparin. B, shown is an illustration of the different role of O-sulfate and N-sulfate groups of heparin in binding to Wnts compared with FGF2. The thickness of the line between the sulfate groups of heparin and the ligand indicates the strength of binding affinity: thinner line, lower affinity; thicker line, higher affinity.
IGFs are powerful ligands that activate the PI3K/Akt pathway to promote osteoblast differentiation. However, heparin does not enhance IGF-1-stimulated ALP activity in MC3T3-E1 cells (supplemental Fig. S3A); it appears that heparin potentiates neither IGF-1 signaling at the cell surface nor IGF-1-related intracellular cross-talk with Wnt/β-catenin. Rather, heparin synergizes with Wnt3a in a co-stimulation of MC3T3-E1 differentiation by up-regulating PI3K protein, and both compounds additively induce Akt phosphorylation after an initial delay (i.e. 6–24 h post-stimulation). Akt activation was diminished by cycloheximide administration (supplemental Fig. S3B), suggesting that new protein synthesis (i.e. generation of a proxy factor) is involved. Indeed, Wnt3a but not heparin acts to increase p85 protein, although the Wnt3a-dependent increase is enhanced by heparin and results in a prominent elevation of Akt phosphorylation. We propose that heparin magnifies Wnt3a activity by sensitizing p85-PI3K/Akt signaling that together with β-catenin-LEF1/TCF signaling promotes osteoblast maturation.
We have observed that heparin, even on its own, increases the phosphorylation of Akt. There are several possible mechanisms that could account for how heparin/heparan sulfate activates PI3K/Akt signaling. Heparin can bind to integrins in vitro and ex vivo (55). Integrins, as the receptors that mediate cell attachment to the extracellular matrix and in some cases to other cells, associate with focal adhesion kinase to induce several intracellular signaling cascades and so transduce the signals between the cell and its environment. In osteoblasts, the PI3K/Akt pathway functions downstream of the extracellular stimuli that activate integrin/focal adhesion kinase (56). Alternatively, heparin may interact with heparin binding epidermal growth factor to activate the PI3K pathway, as has been found in other cell/tissue types (57, 58). Further investigations are required to distinguish among these possibilities.
Unlike C2C12 myoblastic mesenchymal progenitor cells that are sensitive to the osteogenic activity of Wnt3a, pre-committed MC3T3-E1 osteoblasts exhibit only a minimal increase in ALP activity upon Wnt3a stimulation. Our results show that β-catenin signaling is required for the basal maintenance and co-stimulation of ALP activity by Wnt3a and heparin in MC3T3-E1 cells. Heparin enlarges the osteogenic potential of Wnt3a/β-catenin by synergistically stimulating the PI3K/Akt/RUNX2 axis. Akt may increase β-catenin levels by inhibiting glycogen synthase kinase 3β (25), although we ourselves did not observe this reciprocal feedback regulation. We, therefore, postulate that PI3K/Akt and β-catenin represent two separate mechanisms that are capable of promoting osteoblast differentiation. In support, a recent study has also described the existence of the β-catenin-independent Wnt signaling that activates RUNX2 in osteoblasts (59).
In summary, we have demonstrated that soluble extracellular glycosaminoglycans such as heparin can strongly and selectively potentiate Wnt signaling to promote maturation of cells within the osteoblast lineage. The maximal osteogenic activity of Wnt3a-heparin requires both canonical β-catenin and non-canonical PI3K/Akt/RUNX2 signaling. This novel role of heparin in specifically enhancing Wnt-dependent osteogenic differentiation offers an opportunity to develop new strategies for the healing of bone defects.
Supplementary Material
Acknowledgments
We thank all members of our laboratories and especially Murali Sadasivam and Diah Bramono as well as Jitesh Pratap, Nadiya Teplyuk, Jane Lian, and Janet Stein for stimulating discussions, biological reagents, and technical advice. We thank Suk-Chul Bae for providing the 6xOSE-LUC vector and Bruno Reversade for Id1-LUC vector.
This work was supported by funding from the Singapore Agency for Science Technology and Research (A*STAR), the Biomedical Research Council of Singapore, and the Institute of Medical Biology, Singapore.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- BMP
- bone morphogenetic protein
- GAG
- glycosaminoglycan
- RUNX2
- Runt-related transcription factor
- Wnt
- Wingless and Integration
- FGF
- fibroblast growth factor
- FGFR
- FGF receptor
- CDK
- cyclin-dependent kinase
- ERK
- extracellular signal-regulated kinase
- HS
- heparan sulfate
- LRP
- low density lipoprotein receptor-related protein
- LEF1
- lymphoid enhancer-binding factor 1
- TCF
- T cell-specific factor
- rOB
- rat calvarial osteoprogenitor cells
- ALP
- alkaline phosphatase
- MAPK
- mitogen-activated protein kinase
- PI3K
- phosphoinositide 3-kinase
- siRNA
- small interfering RNA
- IGF
- insulin-like growth factor.
REFERENCES
- 1.Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. (1993) J. Biol. Chem. 268, 23906–23914 [PubMed] [Google Scholar]
- 2.Ishihara M., Shaklee P. N., Yang Z., Liang W., Wei Z., Stack R. J., Holme K. (1994) Glycobiology 4, 451–458 [DOI] [PubMed] [Google Scholar]
- 3.Jackson R. A., Murali S., van Wijnen A. J., Stein G. S., Nurcombe V., Cool S. M. (2007) J. Cell. Physiol. 210, 38–50 [DOI] [PubMed] [Google Scholar]
- 4.Lee J. Y., Choo J. E., Choi Y. S., Lee K. Y., Min D. S., Pi S. H., Seol Y. J., Lee S. J., Jo I. H., Chung C. P., Park Y. J. (2007) J. Biomed Mater. Res. A 83, 970–979 [DOI] [PubMed] [Google Scholar]
- 5.Ling L., Murali S., Dombrowski C., Haupt L. M., Stein G. S., van Wijnen A. J., Nurcombe V., Cool S. M. (2006) J. Cell. Physiol. 209, 811–825 [DOI] [PubMed] [Google Scholar]
- 6.Takada T., Katagiri T., Ifuku M., Morimura N., Kobayashi M., Hasegawa K., Ogamo A., Kamijo R. (2003) J. Biol. Chem. 278, 43229–43235 [DOI] [PubMed] [Google Scholar]
- 7.Zhao B., Katagiri T., Toyoda H., Takada T., Yanai T., Fukuda T., Chung U. I., Koike T., Takaoka K., Kamijo R. (2006) J. Biol. Chem. 281, 23246–23253 [DOI] [PubMed] [Google Scholar]
- 8.Nadanaka S., Ishida M., Ikegami M., Kitagawa H. (2008) J. Biol. Chem. 283, 27333–27343 [DOI] [PubMed] [Google Scholar]
- 9.Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R., 3rd, Nusse R. (2003) Nature 423, 448–452 [DOI] [PubMed] [Google Scholar]
- 10.Wodarz A., Nusse R. (1998) Annu. Rev. Cell Dev. Biol. 14, 59–88 [DOI] [PubMed] [Google Scholar]
- 11.Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Jüppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M. J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L. (2001) Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 12.Kato M., Patel M. S., Levasseur R., Lobov I., Chang B. H., Glass D. A., 2nd, Hartmann C., Li L., Hwang T. H., Brayton C. F., Lang R. A., Karsenty G., Chan L. (2002) J. Cell Biol. 157, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyden L. M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M. A., Wu D., Insogna K., Lifton R. P. (2002) N. Engl. J. Med. 346, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 14.Bodine P. V., Zhao W., Kharode Y. P., Bex F. J., Lambert A. J., Goad M. B., Gaur T., Stein G. S., Lian J. B., Komm B. S. (2004) Mol. Endocrinol. 18, 1222–1237 [DOI] [PubMed] [Google Scholar]
- 15.Daniels D. L., Weis W. I. (2005) Nat. Struct. Mol. Biol. 12, 364–371 [DOI] [PubMed] [Google Scholar]
- 16.Li L., Yuan H., Weaver C. D., Mao J., Farr G. H., 3rd, Sussman D. J., Jonkers J., Kimelman D., Wu D. (1999) EMBO J. 18, 4233–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawadi G., Vayssière B., Dunn F., Baron R., Roman-Roman S. (2003) J. Bone Miner Res. 18, 1842–1853 [DOI] [PubMed] [Google Scholar]
- 18.Matsuzaki E., Takahashi-Yanaga F., Miwa Y., Hirata M., Watanabe Y., Sato N., Morimoto S., Hirofuji T., Maeda K., Sasaguri T. (2006) J. Bone Miner. Res. 21, 1307–1316 [DOI] [PubMed] [Google Scholar]
- 19.Gaur T., Lengner C. J., Hovhannisyan H., Bhat R. A., Bodine P. V., Komm B. S., Javed A., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) J. Biol. Chem. 280, 33132–33140 [DOI] [PubMed] [Google Scholar]
- 20.Haupt L. M., Murali S., Mun F. K., Teplyuk N., Mei L. F., Stein G. S., van Wijnen A. J., Nurcombe V., Cool S. M. (2009) J. Cell. Physiol. 220, 780–791 [DOI] [PubMed] [Google Scholar]
- 21.Teplyuk N. M., Haupt L. M., Ling L., Dombrowski C., Mun F. K., Nathan S. S., Lian J. B., Stein J. L., Stein G. S., Cool S. M., van Wijnen A. J. (2009) J. Cell. Biochem. 107, 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambrosetti D., Holmes G., Mansukhani A., Basilico C. (2008) Mol. Cell. Biol. 28, 4759–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansukhani A., Ambrosetti D., Holmes G., Cornivelli L., Basilico C. (2005) J. Cell Biol. 168, 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh-Choudhury N., Abboud S. L., Nishimura R., Celeste A., Mahimainathan L., Choudhury G. G. (2002) J. Biol. Chem. 277, 33361–33368 [DOI] [PubMed] [Google Scholar]
- 25.Raucci A., Bellosta P., Grassi R., Basilico C., Mansukhani A. (2008) J. Cell. Physiol. 215, 442–451 [DOI] [PubMed] [Google Scholar]
- 26.Ai X., Do A. T., Lozynska O., Kusche-Gullberg M., Lindahl U., Emerson C. P., Jr. (2003) J. Cell Biol. 162, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombres M., Henríquez J. P., Reig G. F., Scheu J., Calderón R., Alvarez A., Brandan E., Inestrosa N. C. (2008) J. Cell. Physiol. 216, 805–815 [DOI] [PubMed] [Google Scholar]
- 28.Bae J. S., Gutierrez S., Narla R., Pratap J., Devados R., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B., Javed A. (2007) J. Cell. Biochem. 100, 434–449 [DOI] [PubMed] [Google Scholar]
- 29.Kim H. J., Kim J. H., Bae S. C., Choi J. Y., Kim H. J., Ryoo H. M. (2003) J. Biol. Chem. 278, 319–326 [DOI] [PubMed] [Google Scholar]
- 30.Zaidi S. K., Pande S., Pratap J., Gaur T., Grigoriu S., Ali S. A., Stein J. L., Lian J. B., van Wijnen A. J., Stein G. S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19861–19866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Si W., Kang Q., Luu H. H., Park J. K., Luo Q., Song W. X., Jiang W., Luo X., Li X., Yin H., Montag A. G., Haydon R. C., He T. C. (2006) Mol. Cell. Biol. 26, 2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu X., Joeng K. S., Nakayama K. I., Nakayama K., Rajagopal J., Carroll T. J., McMahon A. P., Long F. (2007) Dev. Cell 12, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irie A., Habuchi H., Kimata K., Sanai Y. (2003) Biochem. Biophys. Res. Commun. 308, 858–865 [DOI] [PubMed] [Google Scholar]
- 34.Esko J. D., Lindahl U. (2001) J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turnbull J., Powell A., Guimond S. (2001) Trends Cell Biol. 11, 75–82 [DOI] [PubMed] [Google Scholar]
- 36.Katagiri T., Imada M., Yanai T., Suda T., Takahashi N., Kamijo R. (2002) Genes Cells 7, 949–960 [DOI] [PubMed] [Google Scholar]
- 37.Kim H. J., Lee M. H., Park H. S., Park M. H., Lee S. W., Kim S. Y., Choi J. Y., Shin H. I., Kim H. J., Ryoo H. M. (2003) Dev. Dyn 227, 335–346 [DOI] [PubMed] [Google Scholar]
- 38.Miraoui H., Oudina K., Petite H., Tanimoto Y., Moriyama K., Marie P. J. (2009) J. Biol. Chem. 284, 4897–4904 [DOI] [PubMed] [Google Scholar]
- 39.Fujita T., Azuma Y., Fukuyama R., Hattori Y., Yoshida C., Koida M., Ogita K., Komori T. (2004) J. Cell Biol. 166, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y. J., Lee M. H., Wozney J. M., Cho J. Y., Ryoo H. M. (2004) J. Biol. Chem. 279, 50773–50780 [DOI] [PubMed] [Google Scholar]
- 41.Matsubara T., Kida K., Yamaguchi A., Hata K., Ichida F., Meguro H., Aburatani H., Nishimura R., Yoneda T. (2008) J. Biol. Chem. 283, 29119–29125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. (1997) Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
- 43.Byon C. H., Javed A., Dai Q., Kappes J. C., Clemens T. L., Darley-Usmar V. M., McDonald J. M., Chen Y. (2008) J. Biol. Chem. 283, 15319–15327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumarasuriyar A., Murali S., Nurcombe V., Cool S. M. (2009) J. Cell. Physiol. 218, 501–511 [DOI] [PubMed] [Google Scholar]
- 45.Abramsson A., Kurup S., Busse M., Yamada S., Lindblom P., Schallmeiner E., Stenzel D., Sauvaget D., Ledin J., Ringvall M., Landegren U., Kjellén L., Bondjers G., Li J. P., Lindahl U., Spillmann D., Betsholtz C., Gerhardt H. (2007) Genes Dev. 21, 316–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faham S., Hileman R. E., Fromm J. R., Linhardt R. J., Rees D. C. (1996) Science 271, 1116–1120 [DOI] [PubMed] [Google Scholar]
- 47.Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. (1992) J. Biol. Chem. 267, 10337–10341 [PubMed] [Google Scholar]
- 48.Jemth P., Kreuger J., Kusche-Gullberg M., Sturiale L., Giménez-Gallego G., Lindahl U. (2002) J. Biol. Chem. 277, 30567–30573 [DOI] [PubMed] [Google Scholar]
- 49.Pye D. A., Vives R. R., Turnbull J. E., Hyde P., Gallagher J. T. (1998) J. Biol. Chem. 273, 22936–22942 [DOI] [PubMed] [Google Scholar]
- 50.Rusnati M., Coltrini D., Caccia P., Dell'Era P., Zoppetti G., Oreste P., Valsasina B., Presta M. (1994) Biochem. Biophys. Res. Commun. 203, 450–458 [DOI] [PubMed] [Google Scholar]
- 51.Schlessinger J., Plotnikov A. N., Ibrahimi O. A., Eliseenkova A. V., Yeh B. K., Yayon A., Linhardt R. J., Mohammadi M. (2000) Mol. Cell 6, 743–750 [DOI] [PubMed] [Google Scholar]
- 52.Chong J. M., Uren A., Rubin J. S., Speicher D. W. (2002) J. Biol. Chem. 277, 5134–5144 [DOI] [PubMed] [Google Scholar]
- 53.Finch P. W., He X., Kelley M. J., Uren A., Schaudies R. P., Popescu N. C., Rudikoff S., Aaronson S. A., Varmus H. E., Rubin J. S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 6770–6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong X., Desilva T., Lin L., Bodine P., Bhat R. A., Presman E., Pocas J., Stahl M., Kriz R. (2007) J. Biol. Chem. 282, 20523–20533 [DOI] [PubMed] [Google Scholar]
- 55.Sobel M., Fish W. R., Toma N., Luo S., Bird K., Mori K., Kusumoto S., Blystone S. D., Suda Y. (2001) J. Vasc. Surg. 33, 587–594 [DOI] [PubMed] [Google Scholar]
- 56.Lee D. Y., Li Y. S., Chang S. F., Zhou J., Ho H. M., Chiu J. J., Chien S. (2010) J. Biol. Chem. 285, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahtouk K., Jourdan M., De Vos J., Hertogh C., Fiol G., Jourdan E., Rossi J. F., Klein B. (2004) Blood 103, 1829–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta V. B., Besner G. E. (2005) J. Immunol. 175, 1911–1918 [DOI] [PubMed] [Google Scholar]
- 59.McCarthy T. L., Centrella M. (2010) Mol. Endocrinol. 24, 587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.