Abstract
We previously identified Asn331 in transmembrane segment 7 (TM7) as a key residue determining substrate affinity in Hxt2, a moderately high-affinity facilitative glucose transporter of Saccharomyces cerevisiae. To gain further insight into the structural basis of substrate recognition by yeast glucose transporters, we have now studied Hxt7, whose affinity for glucose is the highest among the major hexose transporters. The functional role of Asp340 in Hxt7, the residue corresponding to Asn331 of Hxt2, was examined by replacing it with each of the other 19 amino acids. Such replacement of Asp340 generated transporters with various affinities for glucose, with the affinity of the Cys340 mutant surpassing that of the wild-type Hxt7. To examine the structural role of Asp340 in the substrate translocation pathway, we performed cysteine-scanning mutagenesis of the 21 residues in TM7 of a functional Cys-less Hxt7 mutant in conjunction with exposure to the hydrophilic sulfhydryl reagent p-chloromercuribenzenesulfonate (pCMBS). The transport activity of the D340C mutant of Cys-less Hxt7, in which Asp340 is replaced with Cys, was completely inhibited by pCMBS, indicating that Asp340 is located in a water-accessible position. This D340C mutant showed a sensitivity to pCMBS that was ∼70 times that of the wild-type Hxt7, and it was protected from pCMBS inhibition by the substrates d-glucose and 2-deoxy-d-glucose but not by l-glucose. These results indicate that Asp340 is situated at or close to a substrate recognition site and is a key residue determining high-affinity glucose transport by Hxt7, supporting the notion that yeast glucose transporters share a common mechanism for substrate recognition.
Keywords: Aspartate, Glucose Transport, Membrane Function, Membrane Proteins, Sugar Transport, Yeast, Cysteine-scanning Mutagenesis, Membrane Transport, Substrate Recognition
Introduction
Transport of glucose across the plasma membrane is the first step in glucose utilization by most living cells. The yeast Saccharomyces cerevisiae is able to take up glucose over a wide range of extracellular concentrations with the use of 17 hexose transporters (Hxt1 to Hxt11, Hxt13 to Hxt17, and Gal2) (1, 2) belonging to the major facilitator superfamily (MFS)2 (3). These transporters contain 12 putative transmembrane segments (TMs), intracellular NH2- and COOH termini, and a large intracellular loop between TMs 6 and 7. The members of the MFS include a variety of sugar transporters and transporters of other organic solutes in prokaryotes, archaea, and eukaryotes. Elucidation of the three-dimensional structures of four bacterial MFS transporters, including an oxalate transporter (OxlT) of Oxalobacter formigenes (4) as well as a lactose permease (LacY) (5), glycerol-3-phophate transporter (GlpT) (6), and multidrug transporter (EmrD) (7) of Escherichia coli, has led to the notion that all MFS transporters share a similar topological organization of TMs with a centrally located hydrophilic substrate-translocation pathway.
Among the 17 hexose transporters of S. cerevisiae, the major glucose transporters are Hxt1 to Hxt7 and Gal2, with HXT8 to HXT11 and HXT13 to HXT17 usually being silent and HXT12 being a pseudogene (2, 8–11). Hxt7, Hxt2, and Hxt1 are facilitative glucose transporters with high affinity (Km = 0.67 mm), moderately high affinity (Km = 3.3 mm), and low affinity (Km = 46 mm) for glucose, respectively. The numbers of amino acid residues in each TM and inter-TM loop region of Hxt7 are identical to those in Hxt2 and Hxt1. The three proteins also share ∼65% amino acid sequence identity in these regions, whereas they differ substantially in terms of the size and sequence of their NH2- and COOH-terminal regions. The region responsible for the high-affinity glucose transport of Hxt7 was studied using chimeras constructed with Hxt7 and Hxt1, and it was localized to the latter half of Hxt7 including TM5 to the C-terminal region (12).
We have previously studied which TMs of Hxt2 are important for its moderately high substrate affinity. We adopted a comprehensive chimeric approach (TM shuffling), in which all 12 TMs of Hxt2 were randomly replaced with the corresponding segments of Hxt1, a low-affinity glucose transporter of S. cerevisiae, at the DNA level. We found that TMs 1, 5, 7, and 8 of Hxt2 are necessary for high-affinity glucose transport (13) and that eight amino acid residues in these TMs are important for (five residues) or supportive of (three residues) such activity (14). Among these five important residues, we found that Asn331 in TM7 is a key determinant of glucose affinity by replacing it with each of the other 19 residues, yielding transporters with various affinities (15). In the present study, Asp340 of Hxt7, which corresponds to Asn331 of Hxt2, also was found to be a key residue determining transport affinity. To gain further insight into the structure of TM7 and the common mechanism of substrate recognition by yeast glucose transporters, we performed cysteine-scanning analysis of this TM of Hxt7 in conjunction with exposure to a sulfhydryl-specific chemical reagent. Our results are consistent with the notion that TM7 possesses a hydrophilic outer half and that Asp340, which is located in this region, plays a key role in substrate recognition.
EXPERIMENTAL PROCEDURES
Vector Construction
We constructed the plasmid Hxt7mnx-pVT to confer expression of HXT7 under the control of the ADH1 promoter in the multicopy plasmid pVT102-U (YEp URA3 bla), following a procedure similar to that used to generate Hxt2mnx-pVT (13). Five restriction enzyme sites were created. The sequence of HXT7 was thus modified by: (i) changing the nucleotides at the start of the open reading frame from ATGTCACAAGAC to ATGTCAGAATTC, thereby creating an EcoRI site and resulting in a change in the encoded amino acids from Met-Ser-Gln-Asp to Met-Ser-Glu-Phe; (ii) creating a ClaI site immediately downstream of the termination codon (TAATTTGC to TAATCGAT); (iii) creating an MroI site in the nucleotide sequence for TM4 (ATTATTTCCGGT to ATTATTTCCGGA); (iv) creating an NheI site in the nucleotide sequence for the loop between TM6 and TM7 (GCATCC to GCTAGC); and (v) creating an XhoI site in the nucleotide sequence for the loop between TM9 and TM10 (CCATCTTCC to CCCTCGAGC). With the exception of the EcoRI site, the creation of the new restriction sites did not affect the encoded amino acids. The EcoRI-ClaI fragment of the modified HXT7 sequence was then ligated into the multicloning site of PVT102-U to yield Hxt7mnx-pVT. Hxt7mnx-pVT was introduced into S. cerevisiae strain KY73 (MATα hxt1Δ::HIS3::Δhxt4 hxt5::LEU2 hxt2Δ::HIS3 hxt3Δ::LEU2::Δhxt6 hxt7Δ::HIS3 gal2Δ::DR ura3-52 MAL2 SUC2 MEL) (16).
Mutagenesis
Replacement of Asp340 in Hxt7 with each of the other 19 amino acids was performed with the use of a PCR-based approach in which the target codon (GAU) was replaced with a specific codon for each of the other 19 residues, as described previously (17). The amplified products were digested with NheI and XhoI and substituted for the corresponding region of HXT7 in Hxt7mnx-pVT. The resulting plasmids were introduced into S. cerevisiae KY73 to yield a series of D340X mutants. The DNA sequence for each of the mutated transporters was confirmed with the use of a DNA sequencer (model 310, Applied Biosystems).
Cysteine-scanning Analysis
Replacement of all 11 cysteine residues of Hxt7 (residues 69, 126, 207, 221, 242, 389, 400, 428, 434, 439, and 501) (supplemental Fig. S1) with alanine resulted in the generation of a mutant with no transport activity. Replacement of Cys389 with Thr and the other 10 cysteines with alanines yielded a functional Hxt7 mutant, designated Cys-less Hxt7. With the use of site-directed mutagenesis, each of the 21 residues in TM7 of Cys-less Hxt7 was individually changed to cysteine, yielding 21 single-Cys mutants. Each of these mutants was designated by the mutated site containing Cys; D340C is the mutant made by replacing Asp340 of Cys-less Hxt7 with Cys. Thus, there are two D340C mutants designated as D340C of Hxt7 (D340X series) and D340C of Cys-less Hxt7.
Transport Assay
Cells harboring plasmids were grown to log phase (optical density at 650 nm, 0.3 to 0.6) at 30 °C in a synthetic liquid medium containing 2% maltose and supplemented with adenine and amino acids but not with uracil (SMal(ura)) (18). Glucose transport by the cells was measured at 30 °C for 5 s in a transport assay medium containing 50 mm MES and 2 mm MgSO4 (pH 6.0), as described previously (19, 20). Transport activities measured at a d-[14C]glucose concentration of 0.1 or 20 mm were expressed as pmol of glucose per 1 × 107 cells per 5 s and were corrected for the background activity determined either in the presence of 0.5 mm HgCl2 or with 0.1 or 20 mm l-[14C]glucose as substrate. Kinetic parameters were measured under the zero-trans entry condition and were determined by nonlinear regression analysis. For examination of the effects of a hydrophilic sulfhydryl reagent, cells were exposed to p-chloromercuri-benzenesulfonate (pCMBS) for at least 5 min at 30 °C before measurement of transport activity. The transport activity of each single Cys mutant in the presence of 0.5 mm pCMBS was expressed as a percentage of the activity obtained in its absence.
Other Assays
A crude membrane fraction was prepared from cells as described (21) and was subjected to immunoblot analysis with rabbit polyclonal antibodies generated in response to a peptide comprising the 13 COOH-terminal residues of Hxt7 coupled to keyhole limpet hemocyanin. Immune complexes were detected with 125I-labeled protein A (IM144, GE Healthcare) and were quantitated with imaging plates (BAS 1800II, Fujifilm) (21) within the intensity range proportional to the amount of protein. Cell number was determined with a particle counter (Z2, Beckman Coulter) after sonication (Branson Sonifier 450 equipped with a cup horn) of cultures for 15 s to disperse aggregated cells. Protein concentration was measured with bicinchoninic acid (Pierce).
RESULTS AND DISCUSSION
Expression of D340X Mutants of Hxt7
We generated a yeast multicopy expression plasmid containing HXT7 (Hxt7mnx-pVT) to study the role of Asp340 in the putative TM7 of Hxt7, which corresponds to Asn331 of Hxt2 studied previously (15). We replaced Asp340 with each of the other 19 amino acids to generate the D340X series of mutants and then expressed each modified Hxt7 in S. cerevisiae KY73, in which the genes for the major hexose transporters have been deleted (Δhxt1–7 Δgal2). Expression of each mutant protein was confirmed by immunoblot analysis of a crude membrane fraction with antibodies to Hxt7 (supplemental Fig. S2A). All of the mutant cells yielded a predominant immunoreactive band of ∼49 kDa, corresponding to the position of wild-type Hxt7. The extent of expression of the mutant proteins did not differ markedly from that of wild-type Hxt7, ranging from 66 to 99% of the latter (supplemental Fig. S2B).
Characterization of D340X Mutants of Hxt7
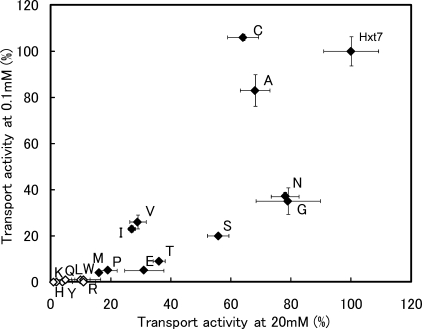
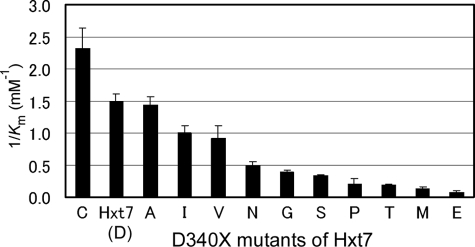
To evaluate differences in the kinetics of glucose transport mediated by the D340X series of mutants, we first measured transport activities with d-glucose at concentrations of 0.1 or 20 mm (Fig. 1). None of the mutants showed a complete loss of transport activity, as revealed by measurement of activity at 20 mm d-glucose. No substantial difference in the pattern of transport activities was apparent between activities normalized by cell number and those normalized by the expression level of each mutant protein as determined by immunoblot analysis (supplemental Table S1). The Km, Vmax, and transport efficiency (Vmax/Km) values for the D340X mutants of Hxt7 were determined under the zero-trans entry condition with 0.1 to 80 mm d-glucose as substrate (supplemental Table S2). The Km, Vmax, and transport efficiency values for d-glucose transport mediated by wild-type Hxt7 expressed in KY73 cells were 0.67 mm, 610 pmol/107 cells per 5 s, and 900 (pmol/107 cells per 5 s)/mm, respectively. The D340X mutants exhibited a wide range of Km values, from 0.43 to 13 mm (Fig. 2), whereas Vmax values normalized by expression level varied from 0.3 to 1.4 (supplemental Table S2). Mutants whose activities were too low for assessment of kinetic parameters included D340L, D340F, D340W, D340Y, D340Q, D340H, D340K, and D340R, with these transporters being expected to show Km values higher than those determined for the other mutants. Replacement of Asp340 with Cys, Ala, Ile, or Val yielded high-affinity glucose transporters. Similar effects of residue replacement at this position in Hxt2 were observed with Val, Ile, and Cys (15), suggesting the existence of a common structure around this site in yeast hexose transporters. These residues all contain medium-sized side chains. Furthermore, the residue of the major human glucose transporter GLUT1 that corresponds to Asp340 of Hxt7 is Ile287, and replacement of this residue with Val or Cys yielded transporters with similar high affinities (22). Among the D340X mutants, D340C showed a higher affinity (Km = 0.43 mm) and higher transport efficiency (Vmax/Km = 1000 (pmol/107 cells per 5 s)/mm) than did wild-type Hxt7.
FIGURE 1.
Glucose transport activities of the D340X series of mutants of Hxt7. The D340X series was created by replacing Asp340 of Hxt7 with each of the other 19 amino acids. KY73 cells expressing each of the D340X mutant transporters were grown to log phase at 30 °C in SMal(ura) medium, after which glucose transport activity was measured for 5 s at 30 °C with d-glucose at 0.1 or 20 mm as substrate. Transport activities were normalized by cell number and expressed as a percentage of the value for wild-type Hxt7. Individual diamonds represent mutant or wild-type Hxt7. Closed diamonds indicate mutants for which kinetic analysis was performed, whereas open diamonds denote those for which transport activity was too low for assessment of kinetic parameters. Data are means ± S.E. (n ≥ 3).
FIGURE 2.
Affinities of the D340X series of Htx7 mutants for d-glucose. KY73 cells expressing the indicated D340X mutants were cultured to log phase at 30 °C in SMal(ura) medium, after which the kinetic parameters of d-glucose transport were measured for 5 s at 30 °C with d-glucose at concentrations of 0.1 to 80 mm as described (20). Affinity is expressed as 1/Km, and data are means ± S.E. (n ≥ 3).
Cysteine-scanning Analysis of TM7
We next generated a functional Cys-less Hxt7 mutant with Km and Vmax values of 1.0 ± 0.0 mm and 450 ± 10 pmol/107 cells per 5 s (means ± S.E., n = 3), respectively. The transport efficiency of the mutant was 430 ± 30 (pmol/107 cells per 5 s)/mm, which is ∼50% of that of wild-type Hxt7. From this Cys-less Hxt7, we then generated a series of 21 mutants of TM7 (L325C to Y345C) by successively changing each residue to cysteine.
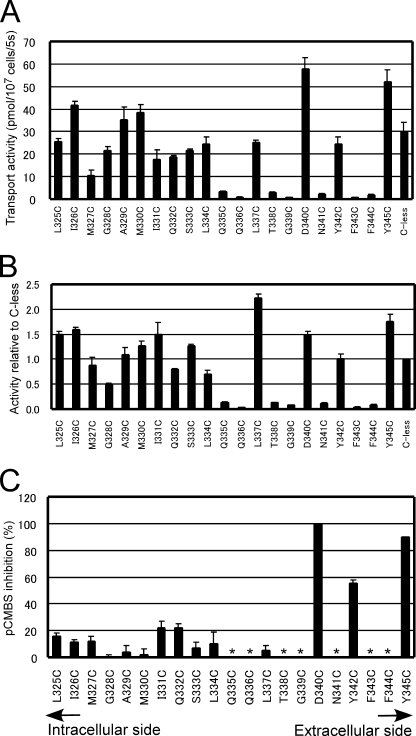
Immunoblot analysis revealed that all the single-Cys mutants were expressed, although the expression levels relative to that of Cys-less Hxt7 varied from 32 to 147% (supplemental Fig. S3). The transport activity of the 21 single-Cys mutants was measured for 5 s at 30 °C with 0.1 mm d-glucose as substrate. Seven single-Cys mutants (Q335C, Q336C, T338C, G339C, N341C, F343C, and F344C) manifested glucose transport activities of <15% of that of Cys-less Hxt7 (Fig. 3A). Given the wide range of expression levels for the mutant proteins, we also normalized transport activity by expression level (Fig. 3B). No substantial differences between the pattern of activities normalized by cell number and that of activities normalized by expression level were apparent for these seven low activity mutants, however. The activities of these mutants were too low for further study of pCMBS sensitivity.
FIGURE 3.
Glucose transport activities of single-Cys mutants and effects of pCMBS. A and B, each residue in TM7 of Cys-less Hxt7 (C-less) was individually replaced with cysteine to yield 21 mutants (L325C to Y345C). KY73 cells harboring plasmids encoding each mutant were grown to log phase at 30 °C in SMal(ura) medium, after which glucose transport activity was measured for 5 s at 30 °C with 0.1 mm d-glucose as substrate. Transport activity was normalized by cell number (A) or was normalized by the expression level of each mutant as determined by quantitative immunoblot analysis and then expressed relative to the value for Cys-less Hxt7 (B). Data are means ± S.E. (n = 4–6). C, KY73 cells expressing individual single-Cys mutants were incubated in the absence or presence of 0.5 mm pCMBS for 5 min at 30 °C before measurement of transport activity with 0.1 mm d-glucose as substrate. Data are expressed as percentage inhibition of transport activity by pCMBS and are means ± S.E. (n = 4–6). Asterisks indicate mutants for which pCMBS inhibition was not determined due to low glucose transport activities.
Effects of pCMBS on Single-Cys Mutants of TM7
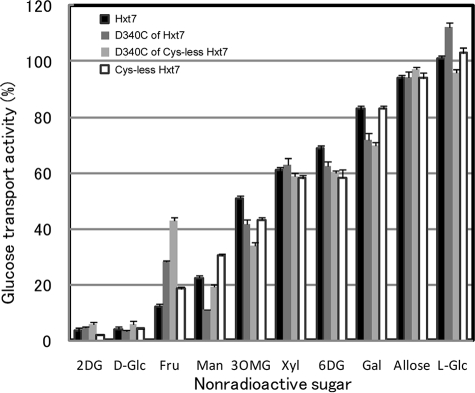
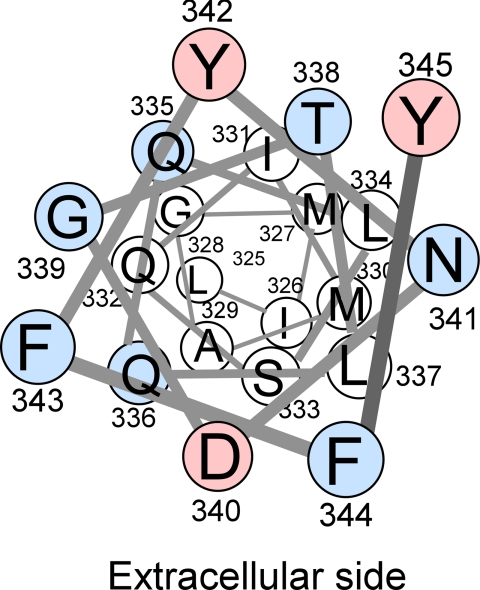
We examined the effects of 0.5 mm pCMBS on the glucose transport activity of the remaining 14 single-Cys mutants with 0.1 mm d-glucose as substrate (Fig. 3C). Three mutants of Cys-less Hxt7, D340C, Y342C, and Y345C, were sensitive to pCMBS. The transport activities of D340C and Y345C were inhibited almost completely (≥90%) by pCMBS, whereas that of Y342C was reduced by about half. Given that Tyr345 is located at the extracellular edge of TM7, the water accessibility of this residue may not indicate that it faces the central translocation pathway. We measured the median inhibitory concentration (IC50) of pCMBS for D340C of Cys-less Hxt7 and wild-type Hxt7 and found that the sensitivity to pCMBS was markedly increased for D340C; with an IC50 of 3.8 ± 0.3 μm (mean ± S.E., n = 3), its sensitivity to pCMBS was ∼70-fold that of wild-type Hxt7 (Fig. 4). The glucose transport activity of Cys-less Hxt7 was inhibited by <10% in the presence of 0.5 mm pCMBS. Protection from pCMBS inhibition by substrate was observed with the D340C mutant. The addition of 20 mm d-glucose or 2-deoxy-d-glucose, but not that of l-glucose (a nontransportable sugar), thus protected D340C from pCMBS inhibition (Table 1). This protection was not observed with the other two pCMBS-sensitive mutants of Cys-less Hxt7, Y342C and Y345C. Finally, we examined the substrate specificity of three mutants (D340C of Hxt7, D340C of Cys-less Hxt7, and Cys-less Hxt7) together with wild-type Hxt7. No marked differences in substrate specificity were observed with the exception of activities measured with d-fructose (Fig. 5).
FIGURE 4.
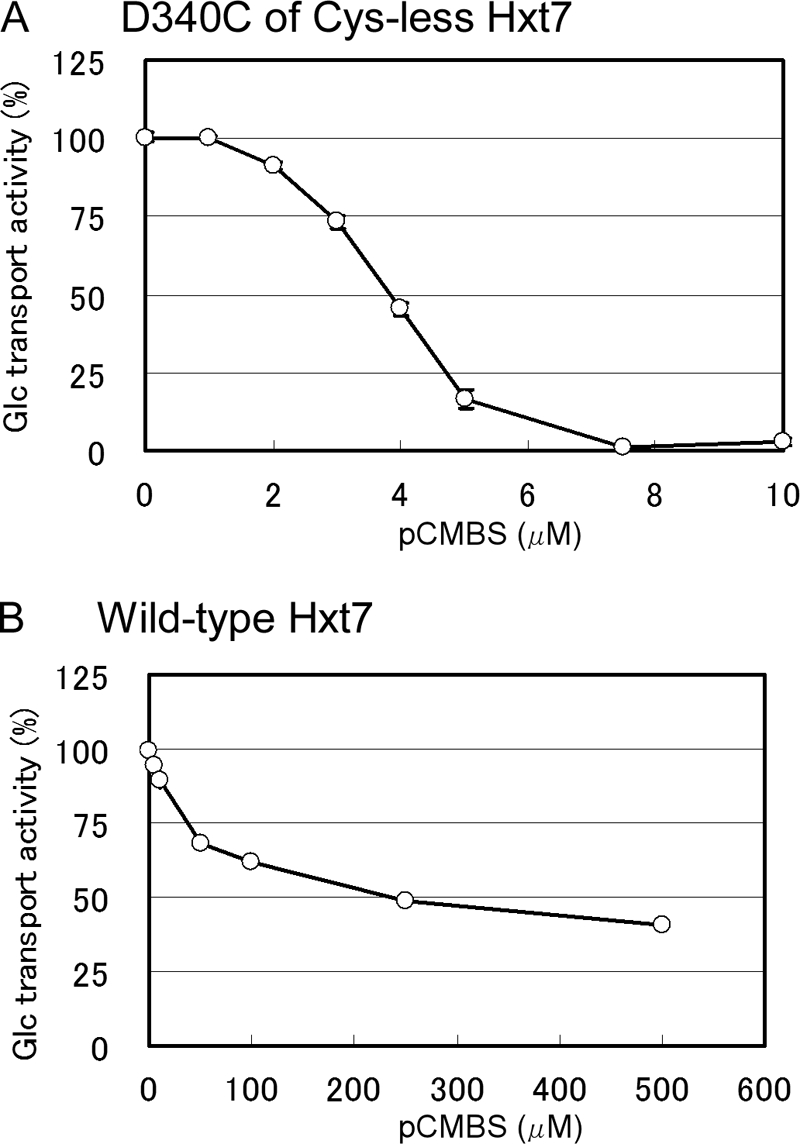
Inhibition of the glucose transport activities of the D340C mutant of Cys-less Hxt7 and of wild-type Hxt7 by pCMBS. Cells expressing either D340C (A), a mutant of Cys-less Hxt7 in which Asp340 is replaced with Cys, or wild-type Hxt7 (B) were incubated for 5 min at 30 °C with the indicated concentrations of pCMBS before measurement of transport activity for 5 s at 30 °C with 0.1 mm d-glucose as substrate. Glucose transport activities are expressed as a percentage of that determined without pCMBS and are means ± S.E. (n ≥ 3).
TABLE 1.
Protection of the single-Cys mutants of Cys-less Hxt7 from pCMBS inhibition by substrate
KY73 cells expressing single-Cys mutants, D340C, Y342C, and Y345C of Cys-less Hxt7 were grown to log phase at 30 °C in SMal(ura) medium and then washed three times with transport assay medium consisting of 50 mm MES and 2 mm MgSO4 (pH 6.0). Portions of the cell suspension were then incubated with or without the indicated sugars (20 mm) for 5 min at room temperature, after which pCMBS was added to indicated concentration (pCMBS(+)) or not added (pCMBS(−)), and the cells were incubated for an additional 10 min at room temperature. The cells were then washed twice with 100 volumes of transport assay medium before measurement of glucose transport activity (3 × 107 to 5 × 107 cells) at 30 °C for 5 s with 0.1 mm d-[14C]glucose as substrate. Data are means ± S.E. (n = 3–5). d-Glc, d-glucose; 2DG, 2 deoxy-d-glucose; l-Glc, l-glucose.
| Single-Cys mutant | IC50 | Addition |
d-Glucose transport |
||||
|---|---|---|---|---|---|---|---|
| pCMBS (−) |
pCMBS (+) |
||||||
| μm | pmol/107 cells/5 s | % | pmol/107 cells/5 s | % | μm | ||
| D340C | 3.8 ± 0.3 | ||||||
| None | 25.6 ± 0.4 | 100 | 3.1 ± 0.1 | 12 | 5 | ||
| d-Glc | 24.9 ± 0.2 | 97 | 16.3 ± 0.4 | 64 | 5 | ||
| 2DG | 20.4 ± 0.3 | 80 | 16.8 ± 0.3 | 66 | 5 | ||
| l-Glc | 23.4 ± 0.3 | 91 | 3.3 ± 0.1 | 13 | 5 | ||
| Y342C | 7.1 ± 0.5 | ||||||
| None | 24.2 ± 0.7 | 100 | 6.4 ± 0 | 27 | 25 | ||
| d-Glc | 23.5 ± 0.7 | 97 | 3.7 ± 0.2 | 15 | 25 | ||
| 2DG | 15.3 ± 0.1 | 63 | 2.5 ± 0.1 | 10 | 25 | ||
| l-Glc | 20.8 ± 0.6 | 86 | 5.9 ± 0.1 | 24 | 25 | ||
| Y345C | 1.8 ± 0 | ||||||
| None | 36.6 ± 0.6 | 100 | 3.8 ± 0.5 | 10 | 5 | ||
| d-Glc | 37.5 ± 0.3 | 103 | 4.3 ± 0.5 | 12 | 5 | ||
| 2DG | 27.9 ± 1.2 | 76 | 6.3 ± 0.9 | 17 | 5 | ||
| l-Glc | 33.7 ± 1.2 | 92 | 8.1 ± 2.8 | 22 | 5 | ||
FIGURE 5.
Substrate specificity of wild-type Hxt7 and D340C of Hxt7, D340C of Cys-less Hxt7, and Cys-less Hxt7. Transport activities were measured at 30 °C for 5 s with 0.1 mm d-[14C]glucose as substrate in the presence of a 200-fold excess of the indicated nonradioactive sugars. Glucose transport activity for each transporter was expressed as a percentage of that determined in the presence of 20 mm sorbitol. Data are means ± S.E. (n ≥ 4). 2DG, 2-deoxy-d-glucose; d-Glc, d-glucose; Fru, d-fructose; Man, d-mannose; 3OMG, 3-O-methyl-d-glucose; Xyl, d-xylose; 6DG, 6-deoxy-d-glucose; Gal, d-galactose; Allose, d-allose; l-Glc, l-glucose.
Comparison with Other Transporters
Our homology model for Hxt2, based on the crystal structure of GlpT, showed that Asn331 in TM7 faces the central pore (15). Our present finding that a Cys mutant of Asp340 of Hxt7, which corresponds to Asn331 of Hxt2, was sensitive to pCMBS provides experimental evidence in support of a similar structure for Hxt7. All the residues in the outer half of TM7 in Hxt7, with the exception of Leu337, were sensitive either to Cys replacement or to pCMBS (Fig. 3C, Fig. 6). In line with our findings, Yan and Maloney (23) observed with cysteine scanning mutagenesis of a E. coli glucose 6-phosphate/phosphate antiporter, UhpT, a close relative of GlpT that seven consecutive single cys mutants in the outer half of TM7 were highly sensitive to pCMBS. In contrast to our observation that only D340C of Cys-less Hxt7 among three pCMBS-sensitive mutants of Hxt7 was protected from pCMBS inhibition by substrates, in the case of UhpT all pCMBS-sensitive single-Cys mutants in the middle part of TM7 were protected by glucose 6-phosphate. In LacY (24), similar ligand protected inhibition by sulfhydryl reagents in single-Cys mutants in the outer half of TM7 was observed. A previous study analyzed all 12 TMs of human GLUT1 by cysteine-scanning mutagenesis in conjunction with pCMBS treatment (25). In most TMs, pCMBS-sensitive residues clustered to make one face of hydrophilic residues; however, in TM7, pCMBS-sensitive residues were located at the extracellular side (26), in good agreement with our present observations with Hxt7. Isoleucine-287 of GLUT1, which corresponds to Asp340 of Hxt7, was sensitive to pCMBS, but, in contrast to our findings, it was not facing the central pore in a structure model (25). In the crystal structures of four bacterial MFS transporters (4–7), this region of TM7 was found to be unusually bent toward the central pore, possibly explaining the unique pCMBS sensitivity of Hxt7. Our model indicates that Tyr342, the Cys mutant of which was moderately sensitive to pCMBS, is located at the opposite side of the central pore from Asp340 in TM7 of Hxt7, suggesting that Tyr342 might interact with a residue in another TM adjacent to TM7. We found previously that Ile287 of GLUT1 is a key residue determining substrate affinity and is located at or near the exofacial binding site, as revealed with the use of the specific inhibitors cytochalasin B and phloretin (22). Our present results indicate that Asp340 is located at or very close to a substrate binding site, but it does not seem to contribute to a strong direct interaction with substrate for several reasons: (i) a common feature of residues conferring high-affinity glucose transport activity at this position is the possession of a medium-sized side chain; (ii) sequence alignment with a wide variety of transporter homologs also shows that residues with a medium-sized side chain are present at this position (supplemental Fig. S4); and (iii) the low-affinity glucose transporter Hxt1 also possesses Asp at the corresponding position, suggesting that Asp340 of Hxt7 is not the sole residue determining substrate affinity. Our results thus support the notion that Asp340 is located in a position that affects the structure of a substrate binding site or that it plays a major role, together with other residues, in the coordinated formation of a binding site. In accordance with the present study, analysis of a plant monosaccharide transporter, Chlorella HUP1, showed that substitution of Asn for Ile303, which corresponds to Asp340 of Hxt7, increased the Km for glucose from 1.5 × 10−5 to 6 × 10−3 m without affecting Vmax (27).
FIGURE 6.
Helical wheel representation of TM7 of Hxt7 shown from the extracellular side. Red circles indicate residues whose single-Cys mutants were inhibited by pCMBS, whereas blue circles represent residues for which Cys substitution resulted in mutants with low activities (<15% of that of Cys-less Hxt7). With the exception of Leu337, all pCMBS-accessible residues and residues sensitive to Cys replacement are located in the outer half of TM7.
The role of TM7 has been studied intensively in members of the mammalian GLUT family of transporters. Studies with GLUT2 and GLUT3 suggested that the QLS motif present in the middle of TM7 in GLUT3 interacts with the C-1 position of d-glucose (28), and Ile314 situated at the outer edge of TM7 of GLUT7 was shown to be required for fructose transport (29). TM7 has also been shown to contribute to the substrate translocation pathway in the crystal structures of the bacterial MFS transporters LacY and GlpT (5, 6). Further efforts to clarify the relation between these previous structural and functional studies and our present study are warranted.
CONCLUSION
Our present data have shown that Asp340 of Hxt7, like Asn331 of Hxt2, is a key residue for determining substrate affinity. By replacing Asp340 with Cys, we generated a transporter with an affinity and transport efficiency higher than those of wild-type Hxt7. Furthermore, by examining the accessibility of substituted cysteine with the hydrophilic sulfhydryl reagent pCMBS, we revealed that the outer half of TM7 of Hxt7 is sensitive either to Cys replacement or to pCMBS and that Asp340 located in this region is readily accessible to water. In addition, protection from pCMBS inhibition by substrate indicated that Asp340 is located at or very close to a substrate recognition site.
Supplementary Material
Acknowledgments
We thank A. L. Kruckeberg for yeast strain KY73 and M. Maeda for technical assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from Teikyo University.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1–S4.
- MFS
- major facilitator superfamily
- TM
- transmembrane segment
- pCMBS
- p-chloromercuribenzenesulfonate
- MES
- 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Kruckeberg A. L. (1996) Arch. Microbiol. 166, 283–292 [DOI] [PubMed] [Google Scholar]
- 2.Boles E., Hollenberg C. P. (1997) FEMS Microbiol. Rev. 21, 85–111 [DOI] [PubMed] [Google Scholar]
- 3.Saier M. H., Jr., Tran C. V., Barabote R. D. (2006) Nucleic Acids Res. 34, D181–D186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirai T., Heymann J. A., Maloney P. C., Subramaniam S. (2003) J. Bacteriol. 185, 1712–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abramson J., Smirnova I., Kasho V., Verner G., Kaback H. R., Iwata S. (2003) Science 301, 610–615 [DOI] [PubMed] [Google Scholar]
- 6.Huang Y., Lemieux M. J., Song J., Auer M., Wang D. N. (2003) Science 301, 616–620 [DOI] [PubMed] [Google Scholar]
- 7.Yin Y., He X., Szewczyk P., Nguyen T., Chang G. (2006) Science 312, 741–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reifenberger E., Boles E., Ciriacy M. (1997) Eur. J. Biochem. 245, 324–333 [DOI] [PubMed] [Google Scholar]
- 9.Diderich J. A., Schuurmans J. M., Van Gaalen M. C., Kruckeberg A. L., Van Dam K. (2001) Yeast 18, 1515–1524 [DOI] [PubMed] [Google Scholar]
- 10.Ozcan S., Johnston M. (1999) Microbiol. Mol. Biol. Rev. 63, 554–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L., Berden J. A., van Dam K., Kruckeberg A. L. (2001) Yeast 18, 1257–1267 [DOI] [PubMed] [Google Scholar]
- 12.Elbing K., Larsson C., Bill R. M., Albers E., Snoep J. L., Boles E., Hohmann S., Gustafsson L. (2004) Appl. Environ. Microbiol. 70, 5323–5330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara T., Kasahara M. (2003) Biochem. J. 372, 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasahara T., Ishiguro M., Kasahara M. (2006) J. Biol. Chem. 281, 18532–18538 [DOI] [PubMed] [Google Scholar]
- 15.Kasahara T., Maeda M., Ishiguro M., Kasahara M. (2007) J. Biol. Chem. 282, 13146–13150 [DOI] [PubMed] [Google Scholar]
- 16.Ye L., Kruckeberg A. L., Berden J. A., van Dam K. (1999) J. Bacteriol. 181, 4673–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasahara T., Ishiguro M., Kasahara M. (2004) J. Biol. Chem. 279, 30274–30278 [DOI] [PubMed] [Google Scholar]
- 18.Amberg D. C., Burke D. J., Strathern J. N. (2005) Methods in Yeast Genetics, pp. 200–201, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19.Nishizawa K., Shimoda E., Kasahara M. (1995) J. Biol. Chem. 270, 2423–2426 [DOI] [PubMed] [Google Scholar]
- 20.Kasahara M., Shimoda E., Maeda M. (1997) J. Biol. Chem. 272, 16721–16724 [DOI] [PubMed] [Google Scholar]
- 21.Kasahara T., Kasahara M. (1996) Biochem. J. 315, 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara T., Maeda M., Boles E., Kasahara M. (2009) Biochim. Biophys. Acta 1788, 1051–1055 [DOI] [PubMed] [Google Scholar]
- 23.Yan R. T., Maloney P. C. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5973–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkatesan P., Kwaw I., Hu Y., Kaback H. R. (2000) Biochemistry 39, 10641–10648 [DOI] [PubMed] [Google Scholar]
- 25.Mueckler M., Makepeace C. (2009) Biochemistry 48, 5934–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hruz P. W., Mueckler M. (1999) J. Biol. Chem. 274, 36176–36180 [DOI] [PubMed] [Google Scholar]
- 27.Will A., Grassl R., Erdmenger J., Caspari T., Tanner W. (1998) J. Biol. Chem. 273, 11456–11462 [DOI] [PubMed] [Google Scholar]
- 28.Seatter M. J., De la Rue S. A., Porter L. M., Gould G. W. (1998) Biochemistry 37, 1322–1326 [DOI] [PubMed] [Google Scholar]
- 29.Manolescu A., Salas-Burgos A. M., Fischbarg J., Cheeseman C. I. (2005) J. Biol. Chem. 280, 42978–42983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.