Abstract
Regulated secretion is a fundamental process underlying the function of many cell types. In particular, acrosomal exocytosis in mammalian sperm is essential for egg fertilization. In general, exocytosis is initiated by a cytosolic calcium increase. In this report we show that calcium affects several factors during human sperm acrosomal exocytosis. By using an antibody that specifically recognizes synaptotagmin VI phosphorylated at the polybasic region of the C2B domain, we showed that a calcium-dependent dephosphorylation of this protein occurred at early stages of the acrosomal exocytosis in streptolysin O-permeabilized sperm. We identified the phosphatase as calcineurin and showed that the activity of this enzyme is absolutely required during the early steps of the secretory process. When added to sperm, an inhibitor-insensitive, catalytically active domain of calcineurin was able to rescue the effect of the specific calcineurin inhibitor cyclosporin A. This same domain dephosphorylated recombinant synaptotagmin VI C2B domain, validating this protein as a new substrate for calcineurin. When sperm were treated with catalytically active calcineurin before stimulation, exocytosis was inhibited, an effect that was rescued by the phosphomimetic synaptotagmin VI C2B-T418E,T419E mutant domain. These observations indicate that synaptotagmin must be dephosphorylated at a specific window of time and suggest that phosphorylated synaptotagmin has an active role at early stages of the acrosomal exocytosis.
Keywords: Calcineurin, Calcium, Membrane Fusion, Membrane Trafficking, Protein Phosphatase, Secretion, Spermatozoa
Introduction
Membrane fusion is a common mechanism used for most cells to connect different subcellular compartments; it is also necessary to release the content of secretory granules to the extracellular medium. What makes regulated exocytosis unique is that the membrane fusion process is maintained on hold until the cell is stimulated. The most common secretion trigger is an increase in cytosolic calcium (1). In several secretory cells, calcium causes the fast secretion of a set of granules known as the ready releasable pool (2). In neurons, synaptotagmin is the best characterized calcium sensor protein associated with this fast release (3). Synaptotagmins carry two C2 domains (C2A and C2B) that bind acidic phospholipids in the presence of calcium. However, calcium is required for several other steps of the exocytic process, and several other calcium-binding proteins participating in exocytosis have been identified, including other C2 domain-containing proteins and calmodulin (1).
Not all secreting cells have a ready releasable pool. In many cases, upon stimulation, the secretory granules need to be recruited to the plasma membrane before exocytosis can start (2). To this category belongs acrosomal exocytosis (4). Sperm have a single secretory granule, the acrosome, that must be released at the right time and place to fertilize the egg (5). Several pieces of experimental evidence indicate that the sperm membrane fusion machinery is inactive before the sperm is stimulated: (i) SNAREs are assembled in cis-complexes, resistant to neurotoxins (6); (ii) NSF,5 which is necessary to disassemble these unproductive complexes, is phosphorylated and inactive (7); and (iii) synaptotagmin is phosphorylated (8, 9). At least two synaptotagmin isoforms (VI and VIII) have been implicated in acrosomal exocytosis (9, 10). Several results using an antibody that specifically recognizes a phosphopeptide corresponding to the polybasic region of the C2B domain of synaptotagmin VI indicate that this protein is phosphorylated in resting sperm (9). The polybasic region is highly conserved among synaptotagmins, and it has been implicated in numerous calcium-dependent and -independent interactions with lipids and proteins (11–14). A phosphomimetic mutation in this region greatly reduces the calcium binding properties of the synaptotagmin VI C2B domain and impairs the calcium-dependent binding to liposomes (15). In summary, our results indicate that protein phosphorylation has a central role in keeping acrosomal exocytosis on hold until the sperm contact the egg. Consistent with this idea, inhibition of the Ca2+/calmodulin kinase IIα promotes spontaneous exocytosis in mouse sperm (16).
Calcium is absolutely required for acrosomal exocytosis (4, 17). A first cytosolic calcium increase, coming from the extracellular medium, is necessary to initiate the process, and a second release from intracellular stores, likely the acrosome, is required to complete exocytosis (6, 18). According to our present working model, the second calcium pulse is necessary to activate the interplay between complexin and synaptotagmin that triggers the opening of the fusion pores (15). Interestingly, the first calcium increase can be obviated if Rab3A or cAMP is used to trigger secretion (19, 20). Our aim was to investigate whether calcium has a role during the early events of exocytosis. We found that calcium was necessary for the dephosphorylation of synaptotagmin. The enzyme involved in this process was identified as the calcium-dependent serine/threonine protein phosphatase calcineurin. Synaptotagmin VI was validated as a new substrate for this enzyme. Our results indicate that the activity of calcineurin was an absolute requirement for acrosomal exocytosis.
EXPERIMENTAL PROCEDURES
Reagents
Recombinant streptolysin O was obtained from Dr. S. Bhakdi (University of Mainz, Mainz, Germany). PKCβII, cyclosporin A (CsA), and FK 506 and VIVIT were purchased from Calbiochem (La Jolla, CA). 8-(p-Chlorophenylthio)-2-O-methyladenosine-3,5-cyclic monophosphate (8pCPT) was from Biolog-Life Science Institute (Bremen, Germany). O-Nitrophenyl EGTA-acetoxymethyl ester (NP-EGTA) was from Molecular Probes (Eugene, OR). Prestained molecular mass markers were from Boston BioProducts Inc. (Worcester, MA). A monoclonal anti-calcineurin (α-subunit) antibody produced in mouse (clone CN-A1, ascites fluid) was obtained from Sigma-Aldrich. An antibody that recognizes phosphorylated synaptotagmin VI (antiPStg) was raised against RRLKKKKTTIKKNTL, phosphorylated in the second T, by Genemed Synthesis (San Francisco, CA). Cy3-labeled goat anti-rabbit antibody was from Jackson Immunochemicals (Sero-immuno Diagnostics, Inc., Tucker, GA). Glutathione-Sepharose and nickel-nitrilotriacetic acid-agarose were from GE Healthcare. All other chemicals were purchased from Sigma-Aldrich or Tecnolab (Buenos Aires, Argentina).
Recombinant Proteins
A cDNA encoding human constitutively active calcineurin (CA-CaN; residues 1–347) was synthesized by GenScript Corporation (Piscataway, NJ) and fused to His6 (pQE80L plasmid; Qiagen). Expression in BL21 Escherichia coli cells (Stratagene, La Jolla, CA) was induced overnight at 25 °C with 0.5 mm isopropyl 1-thio-d-galactopyranoside. Constructs encoding αSNAP and NSF in pQE9 were generously provided by Dr. S. Whiteheart (University of Kentucky, Lexington, KY). The light chain of tetanus toxin (pQE3) was generously provided by Dr. T. Binz (Medizinische Hochschule Hannover, Hannover, Germany). These proteins were expressed as described (7). Purification of His6-tagged recombinant proteins was accomplished under native conditions according to QIAexpressionist. A pGEX-2T plasmid encoding human Rab3A was provided by Dr. P. Stahl (Washington University, St. Louis, MO). A plasmid encoding the C2B (residues 361–511) domain of rat synaptotagmin VI fused to GST was kindly provided by Dr. T. Sudhof (University of Texas Southwestern Medical Center, Dallas, TX). The C2B-T418E,T419E (C2BTE) mutant was obtained as described (9). The cDNA-encoding NFAT regulatory domain (residues 4–385) was generously provided by Dr. J. M. Redondo (Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain). Expression in E. coli BL21 was induced overnight at 22 °C with 0.5 mm isopropyl 1-thio-d-galactopyranoside, and recombinant proteins were purified on glutathione-Sepharose following standard procedures. Rab3A was always used prenylated and loaded with GTPγS (6).
Acrosomal Exocytosis in Permeabilized Sperm
Human semen samples were obtained from normal healthy donors. Highly motile sperm were recovered following a swim-up separation in human tubal fluid as formulated by Irvine Scientific (Santa Ana, CA) supplemented with 0.5% BSA for 1 h at 37 °C in an atmosphere of 5% CO2, 95% air. Concentration was adjusted to 5–10 × 106 cells/ml, and incubation proceeded for at least 2 h. Permeabilization was accomplished as described using 1.9 units/ml (6). Sperm were resuspended in ice-cold sucrose buffer (250 mm sucrose, 0.5 mm EGTA, 20 mm Hepes-K, pH 7) containing 2 mm DTT and treated as described in the figure legends. Acrosomal status was evaluated by staining with FITC-coupled Pisum sativum agglutinin (30 min at 20 °C, 50 μg/ml in PBS). At least 200 cells were scored for each condition. Negative (no stimulation) and positive (10 μm free Ca2+) controls were included in all experiments. For each experiment, acrosomal exocytosis index values were calculated by subtracting the number of reacted spermatozoa in the negative control (range, 10–30%) from all values and expressing the resulting values as percentages of the acrosome reaction observed in the positive control (range, 25–40%). The average difference between positive and negative controls was 13% (experiments where the difference was less than 10% were discarded).
SDS-PAGE and Western Blots
The sperm were washed in PBS, and proteins were extracted in ice-cold 20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10% glycerol, 1% Triton X-100, and a protease inhibitor mixture (P2714; Sigma). After sonication for 15 s (three times with 10-s intervals) and incubation for 30 min at 4 °C, the sperm extracts were clarified by centrifugation at 14,000 × g for 20 min and used immediately or stored at −20 °C. The proteins from rat brain and human sperm were separated on 10% Tris-Tricine SDS-PAGE and transferred to 0.22-μm nitrocellulose membranes (Hybond; GE Healthcare). Nonspecific reactivity was blocked by incubation for 2 h at room temperature with skim milk (5% for brain and 0.5% for sperm) dissolved in washing buffer (PBS, 0.1% Tween 20). The blots were incubated with the monoclonal anti-calcineurin antibody (1:5000 in blocking solution) overnight at 4 °C. Horseradish peroxidase-conjugated goat anti-mouse IgG (Kierkegaard & Perry Laboratories Inc., Gaithersburg, MD) was used as secondary antibody (0.25 μg/ml, in washing buffer containing 5% skim milk, 1 h at room temperature). Excess first and second antibodies were removed by washing five times for 7 min in washing buffer. Detection was accomplished with a chemiluminescence system (Western Lightning; PerkinElmer Life Sciences) and subsequent exposure to Pierce CL-XPosure film (Tecnolab) for 1–10 min.
Indirect Immunofluorescence
Nonpermeabilized sperm (7 × 106 cells/ml) treated as described in the figure legends were spotted on poly-l-lysine-covered slides and fixed in 2% paraformaldehyde in PBS for 10 min at 20 °C. After fixation, the sperm were incubated in 50 mm glycine-PBS for 10 min at 20 °C and permeabilized in 0.1% Triton X-100, PBS for 10 min at 20 °C. The samples were then incubated for 1 h at 20 °C in 5% bovine serum, PBS. The cells were labeled with the antiPStg antibody (1 h at 37 °C, 135 nm in 5% bovine serum, PBS) followed by a Cy3-labeled anti-rabbit IgG (1 h at 20 °C, 1:700 in PBS). In some experiments, the antiPStg antibody was preincubated with the phosphorylated peptide (1.35 μm). Finally, the cells were incubated 1 min in cold methanol and stained with FITC-coupled P. sativum agglutinin for 40 min at 20 °C and washed with distilled water for 20 min at 4 °C. Coverslips were mounted in 1% propyl-gallate, 50% glycerol, PBS. The sperm were examined with an Eclipse TE2000 Nikon microscope equipped with a Hamamatsu Orca 100 camera operated by the MetaMorph software package (Universal Imaging Corp.). The presence of immunostaining in the acrosomal region was evaluated in at least 200 cells in five independent experiments.
In Vitro Phosphorylation and Dephosphorylation Assay
For C2B phosphorylation, 20 μm C2B domain was incubated for 40 min at 37 °C in 20 mm Hepes-K, pH 7.4, 2 mm DTT, 100 μm ATP, 5 mm MgCl2, and 100 μm CaCl2 containing 0.6 unit/ml of PKCβII, 140 μm phosphatidylserine, and 325 nm phorbol 12-myristate 13-acetate. The mixture was then filtered through a Sephadex G-25 spin column equilibrated with sucrose buffer to eliminate phorbol 12-myristate 13-acetate and small molecules.
For dephosphorylation assays, 1.8 μm C2B domain bound to glutathione-Sepharose beads was first phosphorylated as described above using 10 μm ATP containing 100 μCi/ml [γ-32P]ATP (PerkinElmer Life Sciences). After washing with 50 mm Tris, pH 7.5, containing 0.1 mg/ml BSA, the beads were incubated for 1 h at 30 °C with 1.8 μm CA-CaN in the same buffer containing 1 mm MnCl2 or 1 mm EGTA. In some experiments (see Fig. 6B), the beads were washed to eliminate unbound proteins. The samples were resolved in 12.5% Tris-Tricine SDS-PAGE; the gels were dried and exposed to Pierce CL-XPosure film (Tecnolab). To test the effect of the antiPStg antibody, 0.5 μm phosphorylated C2B domain bound to beads was incubated with increasing concentrations of antiPStg antibody (0–2.5 μm for 30 min at 37 °C). Finally, the beads were incubated for 1 h at 30 °C with 0.5 μm CA-CaN, and the proteins were resolved as explained above.
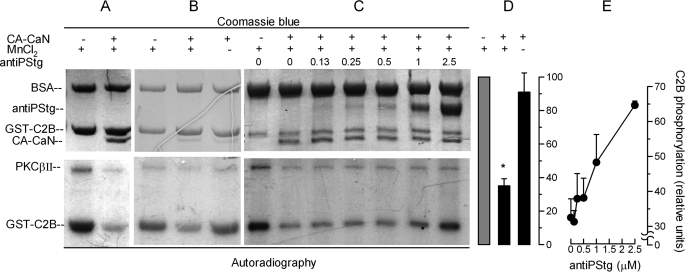
FIGURE 6.
Synaptotagmin is a substrate for calcineurin. A–C, GST-C2B domains immobilized in glutathione-Sepharose beads were incubated for 40 min at 37 °C with PKCβII under activating conditions in the presence of [γ-32P]ATP. The beads were washed and then incubated for 1 h at 30 °C in the presence or absence of the constitutively active catalytic domain of calcineurin (CA-CaN) in a buffer containing 1 mm MnCl2 (MnCl2 +) or 1 mm EGTA (MnCl2 −). In some experiments, increasing concentrations of an antibody that recognizes the phosphorylated polybasic region of the C2B synaptotagmin domain were added (antiPStg, 0–2.5 μm). The samples were then resolved by SDS-PAGE. Total proteins (top panels) are shown by Coomassie Blue stain. Phosphorylated proteins were detected by autoradiography (bottom panels). D, autoradiographies from three experiments as shown in B were quantified and normalized for the protein load. The data represent the means ± S.E. The asterisk indicates a significant difference from incubation without CA-CaN (one-way ANOVA and 99% confidence interval). E, autoradiographies from two experiments as shown in C were quantified. The data represent the means ± range.
Statistical Analysis
The data were evaluated using one-way ANOVA. The Dunnett post hoc test was used for multiple comparisons with a control group. 95 or 99% confidence intervals were used to assess significant differences from a pre-established value (e.g. 100, which corresponds to the positive control).
RESULTS
Role of Calcium during Early Stages of Acrosomal Exocytosis
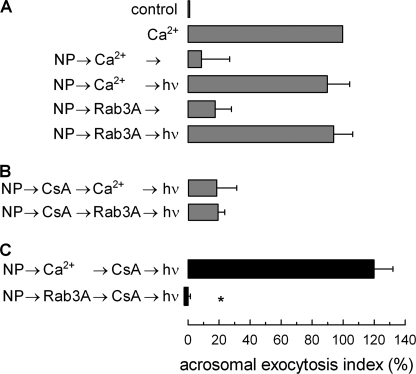
Calcium plays a central role in acrosomal exocytosis. A calcium influx from the extracellular medium is necessary to activate secretion, and a calcium efflux from the acrosome is required for later events, including membrane fusion and the release of the intra-acrosomal content (15). Interestingly, calcium in the extracellular medium is not required when Rab3A (18) or cAMP analogs (20) are used to trigger exocytosis in permeabilized sperm. Similar results are observed when membrane-permeant reagents are used in non-permeabilized cells (21). We wondered whether the early steps accomplished when acrosomal exocytosis was initiated in the presence or absence of calcium were the same. With this aim, several conditions that affect early events were tested in a protocol using the light-sensitive intra-acrosomal calcium chelator NP-EGTA. When permeabilized sperm are incubated with this compound in the dark, the chelator accumulates in the acrosome and depletes intra-acrosomal calcium (18). If exocytosis is then stimulated, the process progresses until the release of calcium from the acrosome is required (6). Secretion is reassumed when the chelator is inactivated by a flash of UV light (Fig. 1A). When exocytosis has progressed to the stage blocked by NP-EGTA, the system becomes resistant to the addition of inhibitors that affect early steps of the process, and secretion is completed in the presence of these inhibitors when NP-EGTA is inactivated (6). We found a striking difference in the presence or absence of calcium when the C2B domain of synaptotagmin VI was tested in a NP-EGTA assay. This recombinant protein inhibited calcium-, Rab3A-, and 8pCPT (an exchange protein directly activated by cAMP (EPAC)-specific cAMP analog)-triggered exocytosis (Fig. 1B). However, when secretion was initiated by calcium with the intra-acrosomal pool depleted by NP-EGTA, the fusion process progressed to a stage where C2B had no effect (Fig. 1C). In contrast, when exocytosis was initiated by Rab3A or by 8pCPT, fusion reached a stage where the C2B domain was still inhibitory (Fig. 1C).
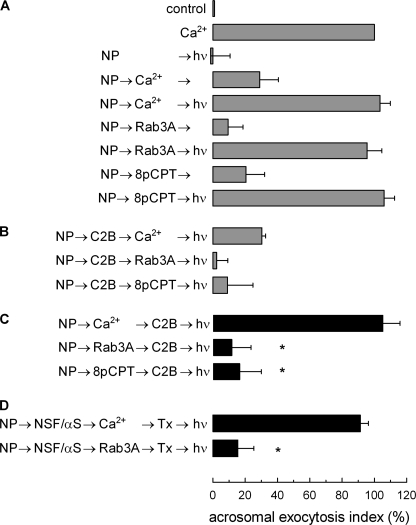
FIGURE 1.
Role of calcium during early stages of acrosomal exocytosis. Permeabilized spermatozoa were loaded with 10 μm NP-EGTA (NP) for 15 min at 37 °C to chelate intra-acrosomal Ca2+. Acrosomal exocytosis (AE) was then initiated by adding 10 μm free Ca2+, 300 nm Rab3A, or 50 μm 8pCPT. After a further 10 min of incubation at 37 °C to allow exocytosis to proceed up to the intra-acrosomal Ca2+-sensitive step, sperm were treated for 10 min at 37 °C with 500 nm synaptotagmin VI C2B domain (C2B). All of these procedures were carried out in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis. Relevant experimental conditions are shown as black bars in C. Control experimental conditions shown in A (gray bars) include background AE in the absence of any stimulation (control); AE stimulated by 10 μm free Ca2+; no effect of the combination of NP and light; inhibitory effect of NP-EGTA in the dark; and the recovery upon illumination when AE was initiated with Ca2+, Rab3A, or 8pCPT. Control conditions in B show the inhibitory effect of the C2B domain when included from the beginning of the experiment. In D, after loading the acrosome with NP-EGTA, the sperm were stimulated in the presence of 310 nm NSF plus 500 nm αSNAP (NSF/αS) with Ca2+ or Rab3A, and then 100 nm tetanus toxin (Tx) was added to cleave SNARE (specifically VAMP) molecules not assembled in SNARE complexes. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis. The sperm were fixed, and AE was measured. The percentage of reacted sperm was normalized as described under “Experimental Procedures.” The data represent the means ± S.E. of at least three independent experiments. The asterisks indicate significant differences from similar conditions stimulated with Ca2+ (p < 0.01, one-way ANOVA and Dunnett test).
The chaperone complex NSF·αSNAP does not disassemble already formed tetanus toxin-resistant trans-SNAREs complexes (6). Moreover, even in the presence of NSF·αSNAP, a toxin-resistant stage was reached when exocytosis was initiated by the addition of calcium (Fig. 1D). In contrast, SNAREs remained toxin-sensitive when exocytosis was triggered by Rab3A. These results indicate that calcium is changing some characteristics of the fusion machinery during the early stages of the exocytic process.
We speculated that calcium during the early steps of exocytosis may be affecting the activity of endogenous synaptotagmin. We have previously observed that synaptotagmin VI is dephosphorylated at an early stage of the acrosome reaction, before the release of calcium from the acrosome (9) and that phosphorylation profoundly affects the biological activity of this protein (15). To assess whether dephosphorylation of synaptotagmin was influenced by the presence of calcium at early stages of the process, the effect of an antibody that recognizes the phosphorylated protein was tested in a protocol similar to the one used in Fig. 1. As reported previously, after a calcium stimulus the antibody was not inhibitory when added at the NP-EGTA-sensitive stage (Fig. 2C). In contrast the antibody was still inhibitory using the same protocol when acrosomal exocytosis was triggered by Rab3A or 8pCPT in the absence of extracellular calcium (Fig. 2C). In control experiments, we corroborated that the binding of the antibody to phosphorylated C2B synaptotagmin VI domain was not influenced by calcium (supplemental Fig. S1). These observations indicate that synaptotagmin is not dephosphorylated during the early stages of acrosomal exocytosis when the process is triggered at resting concentrations of calcium (∼100 nm) (18).
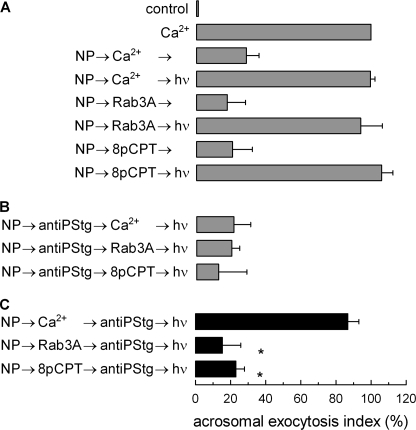
FIGURE 2.
Calcium affects synaptotagmin phosphorylation during early stages of acrosomal exocytosis. Permeabilized spermatozoa were loaded with 10 μm NP-EGTA (NP) for 15 min at 37 °C to chelate intra-acrosomal Ca2+. AE was then initiated by adding 10 μm free Ca2+, 300 nm of Rab3A, or 50 μm 8pCPT. After a further 10 min of incubation at 37 °C to allow exocytosis to proceed up to the intra-acrosomal Ca2+-sensitive step, the sperm were treated for 10 min at 37 °C with 70 nm anti-phosphosynaptotagmin antibody (antiPStg). All of these procedures were carried out in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis. The relevant experimental conditions are shown as black bars in C. Control experimental conditions shown in A (gray bars) include background AE in the absence of any stimulation (control); AE stimulated by 10 μm free Ca2+; inhibitory effect of NP-EGTA in the dark; and the recovery upon illumination when AE was initiated with Ca2+, Rab3A, or 8pCPT. Control conditions in B show the inhibitory effect of the antiPStg when included from the beginning of the experiment. The sperm were fixed, and AE was measured. The percentage of reacted sperm was normalized as described under “Experimental Procedures.” The data represent the means ± S.E. of at least three independent experiments. The asterisks indicate significant differences from similar conditions stimulated with Ca2+ (p < 0.01, one-way ANOVA and Dunnett test).
Calcineurin Is Present in Human Sperm, and It Is Required at an Early Stage for Acrosomal Exocytosis
Synaptotagmin VI is phosphorylated in one or two threonine residues present in the polybasic region (9). The results in Fig. 2 indicate that a phosphatase activated by calcium dephosphorylates synaptotagmin VI. Calcineurin is a serine/threonine calcium/calmodulin-dependent protein phosphatase with essential roles in cell physiology (22). Calcineurin has been implicated in other exocytic processes (23, 24), but a target for this protein in secretion has not been identified. It was then interesting to evaluate whether this phosphatase was responsible for the calcium-dependent dephosphorylation of synaptotagmin. We first assessed the presence of this protein in human sperm. As shown in Fig. 3A, a single band of the expected molecular mass was detected in sperm. Then we assessed whether the activity of this protein was necessary for acrosomal exocytosis. Secretion was blocked by CsA and FK 506, two specific inhibitors that, in association with their respective immunophilins (cyclophilin and FKBP-12) inactivate calcineurin. These inhibitors do not affect other serine/threonine protein phosphatases such as protein phosphatase 1, 2A, and 2C (25). In addition, an anti-calcineurin antibody that recognizes the α-subunit blocked acrosomal exocytosis (Fig. 3B). In contrast, VIVIT (26), a peptide that specifically inhibits the interaction of calcineurin with NFAT (one of the most prominent targets for this enzyme), even at high concentrations had no effect, indicating that the enzyme is acting on a different protein. Our VIVIT preparation efficiently inhibited the calcineurin/NFAT interaction as assessed by Rodríguez et al. (27) (supplemental Fig. S2). These results are consistent with a role for calcineurin in acrosomal exocytosis independent of NFAT.
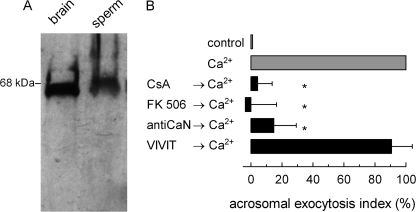
FIGURE 3.
Calcineurin participates in acrosomal exocytosis. A, a post nuclear extract from rat brain (1 μg of proteins, brain) or human sperm (106 cells, sperm) were resolved in 12.5% gels, transferred to nitrocellulose membranes, and probed with an anti-calcineurin antibody. The molecular mass standard is indicated on the left. B, permeabilized spermatozoa were treated at 37 °C for 10 min with 2 μm CsA, 1 μm FK 506, 67 nm anti-calcineurin antibody (antiCaN), or 100 μm VIVIT for 10 min at 37 °C. AE was then initiated by adding 10 μm free Ca2+, and the incubation continued for an additional 15 min (black bars). Controls include (gray bars) background AE in the absence of any stimulation (control) and AE stimulated by 10 μm free Ca2+. The sperm were fixed, and AE was measured. The percentage of reacted sperm was normalized as described under “Experimental Procedures.” The data represent the means ± S.E. of at least three independent experiments. The asterisks indicate significant differences from 100 (one-way ANOVA and 95% confidence interval for each condition).
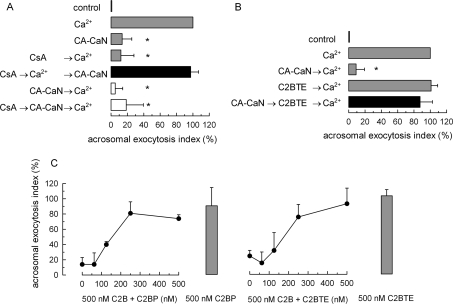
If calcineurin is required for dephosphorylating synaptotagmin in the presence of calcium, its activity should be dispensable at a stage where synaptotagmin is already dephosphorylated. To test this hypothesis, the effect of CsA was assessed in the NP-EGTA protocol. As shown in Fig. 4C, CsA did not affect exocytosis at the stage where the anti-phosphosynaptotagmin had no effect (Fig. 2C). Another corollary of the hypothesis is that calcineurin should be still necessary if sperm are activated without adding calcium; consistent with this prediction, CsA was inhibitory in the same protocol when exocytosis was initiated by the addition of Rab3A (Fig. 4C). In conclusion, our results indicate that calcium activates the calcineurin-dependent dephosphorylation of synaptotagmin VI during the early stages of exocytosis.
FIGURE 4.
Calcium activates a calcineurin-dependent process during the early stages of exocytosis. Permeabilized spermatozoa were loaded with 10 μm NP-EGTA (NP) for 15 min at 37 °C to chelate intra-acrosomal Ca2+. AE was then initiated by adding 10 μm free Ca2+ or 300 nm of Rab3A. After a further 10 min of incubation at 37 °C to allow exocytosis to proceed up to the intra-acrosomal Ca2+-sensitive step, the sperm were treated for 10 min at 37 °C with 2 μm CsA. All of these procedures were carried out in the dark. UV flash photolysis of the chelator was induced at the end of the incubation period (hν), and the samples were incubated for 5 min to promote exocytosis. Relevant experimental conditions are shown as black bars in C. Control experimental conditions shown in A (gray bars) include background AE in the absence of any stimulation (control); AE stimulated by 10 μm free Ca2+; inhibitory effect of NP-EGTA in the dark; and the recovery upon illumination when AE was initiated with Ca2+ or Rab3A. Control conditions in B show the inhibitory effect of the CsA when added before stimulation. The sperm were fixed, and AE was measured. The percentage of reacted sperm was normalized as described under “Experimental Procedures.” The data represent the means ± S.E. of at least three independent experiments. The asterisk indicates significant differences from a similar condition stimulated with Ca2+ (p < 0.01 one-way ANOVA and Dunnett test).
Endogenous Synaptotagmin Is Dephosphorylated after Calcium Stimulation
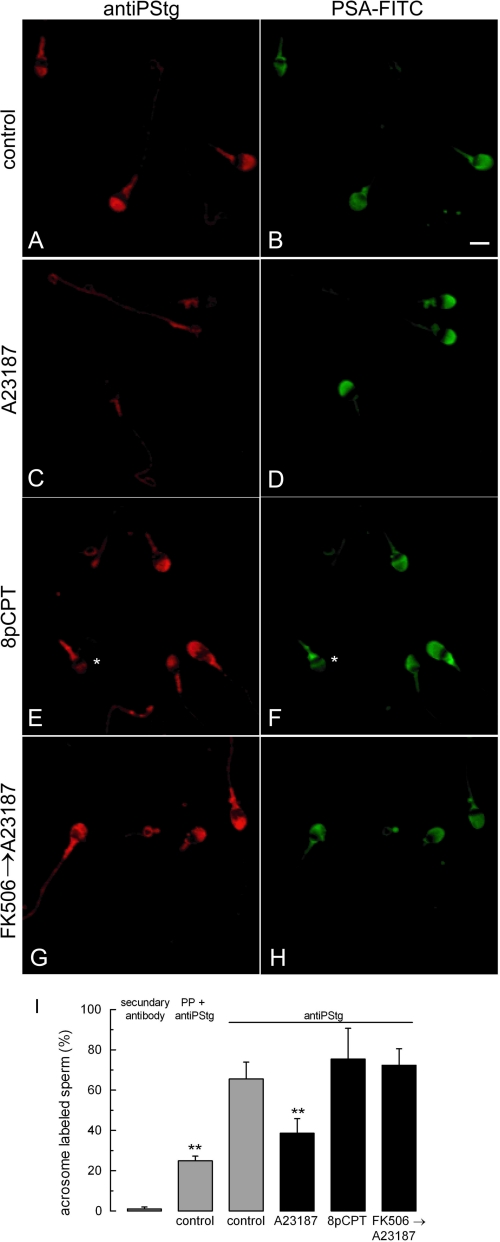
To directly assess whether a rise in cytosolic calcium promotes synaptotagmin dephosphorylation, nonpermeabilized sperm were incubated with the calcium ionophore A23187. To inhibit acrosomal exocytosis, the release of intra-acrosomal calcium was blocked using 2APB, a membrane-permeable IP3-dependent calcium channel inhibitor. The acrosomal content was stained with FITC-coupled P. sativum agglutinin. The degree of synaptotagmin phosphorylation was assessed by immunofluorescence in each cell. Controls for the assay are shown in supplemental Fig. S3. Most cells in resting conditions show a distinct fluorescent mark in the acrosomal region (Fig. 5, A and I). The percentage of cells with acrosomal labeling significantly decreased upon A23187 addition (Fig. 5, C and I). Notice that the label diminution was not due to acrosomal exocytosis, indicating that 2APB efficiently inhibited secretion (Fig. 5D). In agreement with the results in Fig. 2C, sperm stimulation in the absence of calcium (addition of 8pCPT) did not cause synaptotagmin dephosphorylation (Fig. 5, E and I). Finally, the effect of A23187 was blocked by the calcineurin inhibitor FK 508 (Fig. 5, G and I). In conclusion, the immunofluorescence results corroborate that a cytosolic calcium increase promotes the calcineurin-mediated dephosphorylation of synaptotagmin.
FIGURE 5.
Synaptotagmin dephosphorylation depends on calcium and calcineurin. Nonpermeabilized sperm were incubated for 15 min at 37 °C with 100 μm 2-APB and, when indicated, 1 μm FK 506. The cells were then further incubated for 30 min at 37 °C with no additions (control, A and B) or stimulated with 10 μm A23187 (C, D, G, and H) or 50 μm 8pCPT (E and F). The cells were then fixed and double-stained with the antiPStg antibody followed by an anti-rabbit Cy3 (A, C, E, and G) and FITC-coupled P. sativum agglutinin to differentiate between reacted and intact sperm (B, D, F, and H). The asterisk in F shows a reacted sperm with equatorial lectin staining and very faint antiPStg labeling. Bar, 5 μm. In I, at least 200 cells treated as described for A–H were classified as having or lacking distinct acrosomal phosphosynaptotagmin staining. The percentage of immunolabeled sperm in five independent experiments was recorded. As controls, the percentages of labeled cells when the antiPStg antibody was excluded or when it was preblocked with excess phosphorylated peptide are shown (n = 2, supplemental Fig. S3). The data represent the means ± S.E. or the means ± range. The asterisks indicate significant differences from control sperm incubated with unblocked antiPStg antibody (p < 0.01, one-way ANOVA and Dunnett test).
Synaptotagmin Is a New Substrate for Calcineurin
A few specific substrates have been described for calcineurin (22). However, this is the first report suggesting that synaptotagmin can be dephosphorylated by calcineurin. Still, the effect may be indirect, mediated by a different phosphatase activated by calcineurin. Hence, we decided to evaluate whether synaptotagmin is a substrate for calcineurin using purified proteins. With this aim, the catalytic domain of human calcineurin was produced in bacteria. This domain lacks the regulatory region; hence, it does not require calcium/calmodulin for activation and cannot be inhibited by CsA (28). The activity of the constitutively active recombinant protein (CA-CaN) was characterized using p-nitrophenylphosphate as substrate. The protein showed a phosphatase activity similar to that previously reported for a rat calcineurin construct (29). As expected, the activity did not require calcium/calmodulin and was activated by Mn2+ (supplemental Fig. S4). To test whether this phosphatase was capable of dephosphorylating synaptotamin VI directly, the C2B domain was phosphorylated in vitro with PKCβ II in the presence of [γ-32P]ATP as previously described (9). When the phosphorylated C2B domain was incubated in the presence of recombinant calcineurin, a significant dephosphorylation was observed (Fig. 6A). As expected, the effect was Mn2+-dependent (Fig. 6, B and D). The antibody that recognizes the phosphorylated polybasic region of the C2B synaptotagmin domain efficiently inhibited calcineurin-dependent dephosphorylation (Fig. 6, C and E). This observation suggests that phosphorylated threonines in this region are targets for calcineurin and justify the inhibitory effect of the antibody under conditions that should activate the phosphatase (e.g. Fig. 2B, NP → antiPStg → Ca2+ → hν). These results indicate that the phosphorylated polybasic region of the C2B domain of synaptotagmin is a substrate for calcineurin.
Phosphorylated Synaptotagmin Has an Active Role at Early Stages of the Secretory Process
CA-CaN lacks the regulatory domain that is necessary for CsA inhibition. Hence, it can be used to relieve the inhibitory effect of this compound in acrosomal exocytosis to confirm that it affects exocytosis by blocking calcineurin activity. When exocytosis was initiated in the presence of CsA, it presumably progressed to a stage where synaptotagmin dephosphorylation was necessary. Ca-CaN promoted exocytosis when added at this stage (Fig. 7A). Unexpectedly, when CA-CaN was added before stimulation, it inhibited exocytosis, indicating that a premature dephosphorylation of some substrates perturbs exocytosis. This effect was also observed in the presence of CsA because the calcineurin domain used is insensitive to this inhibitor (Fig. 7A).
FIGURE 7.
Phosphorylated synaptotagmin has an active role at early stages of the secretory process. A, permeabilized spermatozoa were treated with 2 μm CsA and then stimulated with 10 μm free Ca2+ for 15 min at 37 °C to allow exocytosis to proceed to the calcineurin-dependent step, and finally the inhibition of CsA was relieved by adding 50 nm CA-CaN (black bar). Control experimental conditions (gray bars) include background AE in the absence of any stimulation (control), AE stimulated by 10 μm free Ca2+, and the inhibitory effect of CsA. Two conditions, initially thought as controls, gave unexpected results. When sperm were treated with CA-CaN before activating with calcium (irrespective of the presence of CsA), exocytosis was inhibited (white bars). B, permeabilized spermatozoa were treated with 50 nm CA-CaN for 15 min at 37 °C, then 500 nm C2BTE were added, and finally AE was stimulated with 10 μm free Ca2+ for 15 min at 37 °C (black bar). Control experimental conditions (gray bars) include background AE in the absence of any stimulation (control), AE stimulated by 10 μm free Ca2+, the inhibitory effect of CA-CaN when added before stimulation, and the lack of effect of C2BTE in calcium-triggered exocytosis. C, permeabilized spermatozoa were treated with 500 nm wild type C2B domain in the presence of increasing concentrations of phosphorylated C2B domain (C2BP, left panel) or the phosphomimetic C2B domain (C2BTE, right panel) and stimulated with 10 μm free Ca2+ for 15 min at 37 °C. AE in the presence of 500 nm C2BP or C2BTE is shown as gray bars. The sperm were fixed, and AE was measured. The percentage of reacted sperm was normalized as described under “Experimental Procedures.” The data represent the means ± S.E. of at least three independent experiments. The asterisks indicate significant differences from 100 (one-way ANOVA and 95% confidence interval for each condition).
The observation that an early dephosphorylation (i.e. a preincubation with CA-CaN) inhibits acrosomal exocytosis can be due to different reasons: (i) CA-CaN may dephosphorylate and inactivate some unknown factor necessary for acrosomal exocytosis; (ii) early synaptotagmin dephosphorylation may promote the interaction of the protein with the wrong targets (for example, it may bind lipids or SNAREs in cis and become inactive for fusion-competent interactions in trans); and (iii) phosphorylated synaptotagmin may not only be protected from interacting with wrong targets but may also have a positive role in exocytosis. If the third hypothesis is correct, the presence of phosphorylated synaptotagmin during the early stage of exocytosis may be necessary. We decided to test whether the addition of the phosphomimetic C2B mutant could overcome the inhibitory effect of CA-CaN. As shown in Fig. 7B, C2BTE did not affect calcium-triggered exocytosis but efficiently reactivated the process after an early addition of CA-CaN. This domain did not inhibit the phosphatase activity of CA-CaN when tested in a dephosphorylation assay using purified proteins (supplemental Fig. S5), ruling out the possibility that C2BTE rescued exocytosis by inactivating CA-CaN. These observations are consistent with the idea that the principal target for CA-CaN is synaptotagmin and suggest an active role for the phosphorylated state of this protein in exocytosis.
Previous results were consistent with the idea that phosphorylated synaptotagmin is inactive. The mutant is unable to inhibit acrosomal exocytosis and cannot relieve the block imposed by an excess of complexin (9, 15). Moreover, the phosphomimetic mutant has a strongly reduced calcium-dependent association to liposomes and calcium binding properties (15). C2BTE overcoming the inhibition by CA-CaN is the first active role reported for the phosphomimetic mutant. If C2BTE is not inactive and can promote fusion in the presence of CA-CaN that presumably inhibits secretion by prematurely dephosphorylating synaptotagmin, it may also compete with the inhibitory effect of the early addition of dephosphorylated C2B domain. As shown in Fig. 7C, C2BTE rescued the inhibitory effect of unphosphorylated wild type C2B domain. Moreover, the addition of phosphorylated wild type C2B was also able to overcome the effect of the unphosphorylated protein (Fig. 7C). In conclusion, the experiments with constitutively active calcineurin support the idea that the dephosphorylation of synaptotagmin is a necessary event for acrosomal exocytosis. In addition, they suggest that this event must occur in a specific window of time and that phosphorylated synaptotagmin VI has an active role in the early stages of the secretory process.
DISCUSSION
Calcium plays several roles in the cellular physiology; in particular it is the more common stimulus for regulated exocytosis. Calcium is necessary not only for triggering membrane fusion during secretion but also for several events upstream from this process (1). A complex pattern of cytosolic calcium increase has been described during the acrosome reaction in mammals (17). We have shown that calcium coming from the extracellular medium can trigger acrosomal exocytosis in permeabilized human sperm but is not sufficient to complete secretion. Calcium from some internal pool, likely the acrosome, is necessary to activate the synaptotagmin/complexin interplay that triggers the opening of fusion pores (15). In fact, exocytosis can be initiated in intact and permeabilized sperm in the presence of resting concentrations of calcium (∼100 nm) in the cytosol by using membrane-permeant cAMP analogs or activated Rab3A (20, 21). However, in this report we show that when exocytosis is initiated at low concentrations of calcium, the early steps of the process are not the same as those accomplished in the presence of stimulatory concentrations of the ion (in the micromolar range). In particular, by using NP-EGTA, a reversible inhibitor that blocks exocytosis at the stage where calcium must be released from the acrosome, we observed that synaptotagmin fails to be dephosphorylated. This result was corroborated by the observation that the percentage of cells showing acrosomal phosphosynaptotagmin labeling significantly decreased upon stimulation with calcium but remained unchanged when a cAMP analog (8pCPT) was used. A significant percentage of cells maintained a distinct labeling even upon calcium stimulation. They may represent sperm that are unable to exocytose upon stimulation. Synaptotagmins are a family of calcium-binding proteins with a central role in membrane fusion (30). These proteins interact with several factors that are important for exocytosis such as acidic phospholipids, RIM, calcium channels, and calmodulin (2, 31, 32). Synaptotagmin I binds individual SNAREs (33) or SNAREs assembled in binary (34–36) or ternary complexes (37, 38). We have previously shown that synaptotagmin VI can be phosphorylated in the polybasic region of the C2B domain by protein kinase C (9) and that this modification changes the functional and biochemical properties of this protein (15). We have also shown that synaptotagmin is phosphorylated in resting sperm and that it is dephosphorylated upon calcium-dependent exocytosis stimulation (9). These observations prompted us to test whether calcineurin, a calcium-dependent phosphatase, was responsible for the dephosphorylation of synaptotagmin at early stages of exocytosis.
Calcineurin is a calcium-dependent serine/threonine phosphatase that is found throughout the phylogenic tree and is present in most mammalian tissues, including testis (39) and sperm (40). Calcineurin is mostly known for being the main target of the immunosuppressive agents CsA and FK 506, which interfere with T-cell signaling by preventing activation of the transcription factor NFAT. However, this phosphatase has several other important functions (22). In intracellular transport, calcineurin, in association with dynamin, has a central role in calcium-stimulated endocytosis in synaptosomes (41). Stimulatory (24, 42, 43) and inhibitory (44, 45) roles for calcineurin in regulated exocytosis have also been reported. A role in acrosomal exocytosis in fowl sperm has been suggested by using deltamethrin and fenvalerate, two calcineurin inhibitors (46). However, the target for the phosphatase activity of calcineurin in exocytosis has not been identified (47). The exocytosis of lytic granules by cytotoxic T lymphocytes (24) and natural killer cells (23) is inhibited by CsA and FK 506, but not by VIVIT, a peptide that specifically inhibits the interaction of calcineurin with NFAT, suggesting that the target for the phosphatase activity is a protein different from this transcription factor. Consistent with a role for calcineurin in human acrosomal exocytosis independent of NFAT, the secretion was sensitive to CsA, FK 506, and an anti-calcineurin antibody but resistant to VIVIT. Moreover, the block imposed by the immunosuppressive agents was rescued by the addition of an inhibitor-insensitive, constitutively active calcineurin domain. Finally, the calcium-dependent decrease in acrosomal phosphosynaptotagmin labeling was also sensitive to FK 506.
The addition of constitutively active calcineurin before stimulation inhibits exocytosis, indicating that synaptotagmin must be dephosphorylated at the right time during the early stages of exocytosis. Several reports using proteoliposomes with well defined composition have shown that synaptotagmin promotes SNARE-dependent membrane fusion (48). However, in this experimental system, the full-length protein in combination with calcium has an inhibitory effect when conditions are set to promote the cis-interaction between its cytoplasmic domain and the membrane where it is anchored through the transmembrane domain (49, 50). Phosphorylation in the polybasic region of synaptotagmin, which affects calcium-dependent binding to membranes (15), may then prevent interactions in cis when the two membranes are still not close enough for trans-interactions. Phosphorylated synaptotagmin may also interact with different effectors to promote the proper assembly of the fusion machinery. Two pieces of evidence suggest that phosphorylated synaptotagmin may have a positive role in exocytosis: (i) the phosphomimetic mutant reverted the inhibitory effect of CA-CaN, and (ii) the phosphorylated C2B domain of synaptotagmin VI or the phosphomimetic mutant competed with the inhibitory effect of the unphosphorylated C2B domain.
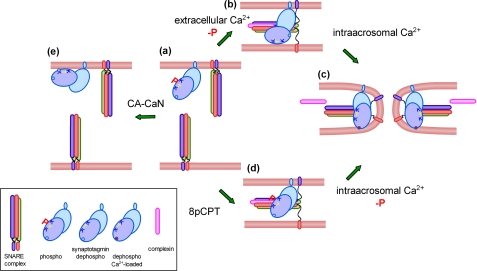
Our observations can be integrated in a model depicted in Fig. 8. In resting sperm, the fusion machinery is inactive, and synaptotagmin is phosphorylated. If synaptotagmin is dephosphorylated at this stage, its premature activation may engage the protein in unproductive interactions (e.g. binding to the membrane in cis). In contrast, when sperm exocytosis is stimulated by a calcium increase in the cytosol, the acrosomal granule swells, favoring the contact of the plasma membrane with the protruding edges of deep invaginations of the acrosomal membrane (51). At some stage calcineurin, in combination with calmodulin and calcium, dephosphorylates synaptotagmin, which can now interact in trans with the opposite membrane (14, 52). According to previous results, at this stage SNAREs are assembled in loose trans-complexes, a process that is facilitated by complexin (15, 51). Calcium coming from the extracellular medium is, however, not enough to complete exocytosis, and a release of calcium from intracellular stores, presumably the acrosome, increases calcium locally, allowing synaptotagmin to liberate the fusion machinery from the complexin clump promoting the full zippering of SNARE complexes (15). When fusion is initiated at low concentrations of calcium in the cytosol, SNAREs are activated, and the process also progresses to the formation of loose trans-complexes (6). However, synaptotagmin remains phosphorylated, and the fusion machinery retains sensitivity to anti-phosphosynaptotagmin antibodies, calcineurin inhibitors, and recombinant C2B domains. However, the release of calcium from the acrosome triggers exocytosis under these conditions, indicating that this calcium may be used to dephosphorylate synaptotagmin at this stage and reassume the membrane fusion process.
FIGURE 8.
Working model for the Ca2+/calcineurin-dependent dephosphorylation of synaptotagmin during acrosomal exocytosis. In resting sperm, SNAREs are assembled in inactive cis-complexes, and synaptotagmin is phosphorylated (a). Upon activation, Ca2+ coming from the extracellular medium triggers SNARE complex disassembly and calcineurin-dependent synaptotagmin dephosphorylation. Acrosomal swelling and deformation of the granule membrane favor the tethering with the plasma membrane. SNAREs can then reassemble in trans in combination with complexin; dephosphorylated synaptotagmin is incorporated into the prefusion complex probably interacting with phospholipids in the opposite membrane through the polybasic region (b). We speculate that the calcium concentration at this stage is not sufficient to insert the aspartic-rich Ca2+-binding loops of synaptotagmin into the membrane and to relieve the complexin clamp. These events must wait for the local influx of calcium coming from the acrosome to promote full SNARE complex assembly and membrane fusion (c). When sperm are activated at low calcium concentrations (e.g. with the cAMP analog 8pCPT), the system progresses to the stage where loose trans-complexes are assembled, but synaptotagmin remains phosphorylated (d). The influx of calcium from the acrosome is sufficient to dephosphorylated synaptotagmin at this stage, and the membrane fusion process is completed (c). Unexpectedly, when constitutively active calcineurin is added to resting sperm at low calcium concentrations, synaptotagmin is dephosphorylated, and exocytosis is inhibited (e). The protein may be engaged in unproductive cis-interactions that prevent exocytosis.
Supplementary Material
Acknowledgments
We thank M. Furlán and A. Medero for excellent technical assistance; Drs. C. Tomes and M. Michaut for critically reading the manuscript; and Drs. T. Binz, T. C. Sudhof, P. D. Stahl, S. W. Whiteheart, and J. M. Redondo for plasmids.
This work was supported by grants from Universidad Nacional de Cuyo, Consejo Nacional de Investigaciones Científicas y Técnicas, and Agencia Nacional de Promoción Científica y Tecnológica (Argentina).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
- NSF
- N-ethylmaleimide-sensitive factor
- 8pCPT
- 8-(p-chlorophenylthio)-2-O-methyladenosine-3,5-cyclic monophosphate
- antiPStg
- anti-phosphosynaptotagmin antibody
- NP-EGTA
- O-nitrophenyl EGTA acetoxymethyl ester
- αSNAP
- soluble NSF attachment protein α
- CsA
- cyclosporin A
- CA-CaN
- constitutively active calcineurin
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- ANOVA
- analysis of variance
- NFAT
- nuclear factor of activated T-cells
- AE
- acrosomal exocytosis.
REFERENCES
- 1.Barclay J. W., Morgan A., Burgoyne R. D. (2005) Cell Calcium 38, 343–353 [DOI] [PubMed] [Google Scholar]
- 2.Burgoyne R. D., Morgan A. (2003) Physiol. Rev. 83, 581–632 [DOI] [PubMed] [Google Scholar]
- 3.Südhof T. C., Rothman J. E. (2009) Science. 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayorga L. S., Tomes C. N., Belmonte S. A. (2007) IUBMB Life 59, 286–292 [DOI] [PubMed] [Google Scholar]
- 5.Yanagimachi R. (1994) in The Physiology of Reproduction (Knobil E., Neill J. D. eds) pp. 189–317, Raven Press, New York [Google Scholar]
- 6.De Blas G. A., Roggero C. M., Tomes C. N., Mayorga L. S. (2005) PLoS Biol. 3, e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarelli V. E., Ruete M. C., Roggero C. M., Mayorga L. S., Tomes C. N. (2009) J. Biol. Chem. 284, 10491–10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaut M., De Blas G., Tomes C. N., Yunes R., Fukuda M., Mayorga L. S. (2001) Dev. Biol. 235, 521–529 [DOI] [PubMed] [Google Scholar]
- 9.Roggero C. M., Tomes C. N., De Blas G. A., Castillo J., Michaut M. A., Fukuda M., Mayorga L. S. (2005) Dev. Biol. 285, 422–435 [DOI] [PubMed] [Google Scholar]
- 10.Hutt D. M., Baltz J. M., Ngsee J. K. (2005) J. Biol. Chem. 280, 20197–20203 [DOI] [PubMed] [Google Scholar]
- 11.Loewen C. A., Lee S. M., Shin Y. K., Reist N. E. (2006) Mol. Biol. Cell 17, 5211–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borden C. R., Stevens C. F., Sullivan J. M., Zhu Y. (2005) Mol. Cell Neurosci. 29, 462–470 [DOI] [PubMed] [Google Scholar]
- 13.Rickman C., Jiménez J. L., Graham M. E., Archer D. A., Soloviev M., Burgoyne R. D., Davletov B. (2006) Mol. Biol. Cell 17, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo W., Herrick D. Z., Ellena J. F., Cafiso D. S. (2009) J. Mol. Biol. 387, 284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roggero C. M., De Blas G. A., Dai H., Tomes C. N., Rizo J., Mayorga L. S. (2007) J. Biol. Chem. 282, 26335–26343 [DOI] [PubMed] [Google Scholar]
- 16.Ackermann F., Zitranski N., Borth H., Buech T., Gudermann T., Boekhoff I. (2009) J. Cell Sci. 122, 4547–4557 [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Gonzalez C., Michelangeli F., Harper C. V., Barratt C. L., Publicover S. J. (2006) Hum. Reprod. Update 12, 253–267 [DOI] [PubMed] [Google Scholar]
- 18.De Blas G., Michaut M., Treviño C. L., Tomes C. N., Yunes R., Darszon A., Mayorga L. S. (2002) J. Biol. Chem. 277, 49326–49331 [DOI] [PubMed] [Google Scholar]
- 19.Michaut M., Tomes C. N., De Blas G., Yunes R., Mayorga L. S. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9996–10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branham M. T., Mayorga L. S., Tomes C. N. (2006) J. Biol. Chem. 281, 8656–8666 [DOI] [PubMed] [Google Scholar]
- 21.Lopez C. I., Belmonte S. A., De Blas G. A., Mayorga L. S. (2007) FASEB J. 21, 4121–4130 [DOI] [PubMed] [Google Scholar]
- 22.Rusnak F., Mertz P. (2000) Physiol. Rev. 80, 1483–1521 [DOI] [PubMed] [Google Scholar]
- 23.Pores-Fernando A. T., Gaur S., Doyon M. Y., Zweifach A. (2009) Cell. Immunol. 254, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grybko M. J., Bartnik J. P., Wurth G. A., Pores-Fernando A. T., Zweifach A. (2007) J. Biol. Chem. 282, 18009–18017 [DOI] [PubMed] [Google Scholar]
- 25.Sieber M., Baumgrass R. (2009) Cell Commun. Signal 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu H., van Berkel T. J., Biessen E. A. (2007) Cardiovasc. Drug Rev. 25, 175–187 [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez A., Martínez-Martínez S., López-Maderuelo M. D., Ortega-Pérez I., Redondo J. M. (2005) J. Biol. Chem. 280, 9980–9984 [DOI] [PubMed] [Google Scholar]
- 28.Clipstone N. A., Fiorentino D. F., Crabtree G. R. (1994) J. Biol. Chem. 269, 26431–26437 [PubMed] [Google Scholar]
- 29.Liu P., Huang C., Jia Z., Yi F., Yu D. Y., Wei Q. (2005) Biochimie 87, 215–221 [DOI] [PubMed] [Google Scholar]
- 30.Craxton M. (2004) BMC Genomics 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman E. R. (2008) Annu. Rev. Biochem. 77, 615–641 [DOI] [PubMed] [Google Scholar]
- 32.Brachya G., Yanay C., Linial M. (2006) BMC Neurosci. 7, (Suppl. 1) S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kee Y., Scheller R. H. (1996) J. Neurosci. 16, 1975–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickman C., Archer D. A., Meunier F. A., Craxton M., Fukuda M., Burgoyne R. D., Davletov B. (2004) J. Biol. Chem. 279, 12574–12579 [DOI] [PubMed] [Google Scholar]
- 35.Weninger K., Bowen M. E., Choi U. B., Chu S., Brunger A. T. (2008) Structure 16, 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Wit H., Walter A. M., Milosevic I., Gulyás-Kovács A., Riedel D., Sørensen J. B., Verhage M. (2009) Cell 138, 935–946 [DOI] [PubMed] [Google Scholar]
- 37.Bowen M. E., Weninger K., Ernst J., Chu S., Brunger A. T. (2005) Biophys. J. 89, 690–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang J., Maximov A., Shin O. H., Dai H., Rizo J., Südhof T. C. (2006) Cell 126, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 39.Moriya M., Fujinaga K., Yazawa M., Katagiri C. (1995) Cell Tissue Res. 281, 273–281 [DOI] [PubMed] [Google Scholar]
- 40.Tash J. S., Krinks M., Patel J., Means R. L., Klee C. B., Means A. R. (1988) J. Cell Biol. 106, 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai M. M., Hong J. J., Ruggiero A. M., Burnett P. E., Slepnev V. I., De Camilli P., Snyder S. H. (1999) J. Biol. Chem. 274, 25963–25966 [DOI] [PubMed] [Google Scholar]
- 42.Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. (1987) J. Cell Biol. 105, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raufman J. P., Malhotra R., Raffaniello R. D. (1997) Biochim. Biophys. Acta 1357, 73–80 [DOI] [PubMed] [Google Scholar]
- 44.Gromada J., Høy M., Buschard K., Salehi A., Rorsman P. (2001) J. Physiol. 535, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Høy M., Bokvist K., Xiao-Gang W., Hansen J., Juhl K., Berggren P. O., Buschard K., Gromada J. (2001) J. Biol. Chem. 276, 924–930 [DOI] [PubMed] [Google Scholar]
- 46.Ashizawa K., Wishart G. J., Ranasinghe A. R., Katayama S., Tsuzuki Y. (2004) Reproduction 128, 783–787 [DOI] [PubMed] [Google Scholar]
- 47.Pores-Fernando A. T., Zweifach A. (2009) Immunol. Rev. 231, 160–173 [DOI] [PubMed] [Google Scholar]
- 48.Bhalla A., Chicka M. C., Tucker W. C., Chapman E. R. (2006) Nat. Struct. Mol. Biol. 13, 323–330 [DOI] [PubMed] [Google Scholar]
- 49.Stein A., Radhakrishnan A., Riedel D., Fasshauer D., Jahn R. (2007) Nat. Struct. Mol. Biol. 14, 904–911 [DOI] [PubMed] [Google Scholar]
- 50.Lee H. K., Yang Y., Su Z., Hyeon C., Lee T. S., Lee H. W., Kweon D. H., Shin Y. K., Yoon T. Y. (2010) Science 328, 760–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanetti N., Mayorga L. S. (2009) Biol. Reprod. 81, 396–405 [DOI] [PubMed] [Google Scholar]
- 52.Herrick D. Z., Kuo W., Huang H., Schwieters C. D., Ellena J. F., Cafiso D. S. (2009) J. Mol. Biol. 390, 913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.