Abstract
Met, the tyrosine kinase receptor for the hepatocyte growth factor is a prominent regulator of cancer cell invasiveness and has emerged as a promising therapeutic target. Binding of the anti-Met monoclonal antibody DN30 to its epitope induces the proteolytic cleavage of Met, thereby impairing the invasive growth of tumors. The molecular mechanism controlling this therapeutic shedding process has so far been unknown. Here, we report that A Disintegrin And Metalloproteinase (ADAM)-10, but not ADAM-17, is required for DN30-induced Met shedding. Knockdown of ADAM-10 in different tumor cell lines or abrogation of its proteolytic activity by natural or synthetic inhibitors abolished Met down-regulation on the cell surface as well as reduction of Met activation. Moreover, hepatocyte growth factor-induced tumor cell migration and invasion were impaired upon ADAM-10 knockdown. Thus, the therapeutic effect of DN30 involves ADAM-10-dependent Met shedding, linking for the first time a specific metalloprotease to target therapy against a receptor tyrosine kinase.
Keywords: ADAM ADAMTS, Anticancer Drug, Cancer Therapy, Protease, Receptor Tyrosine Kinase, ADAM-10, Antibody, DN30, Met, Proteolytic Cleavage
Introduction
Signaling through the membrane-bound tyrosine kinase receptor Met (1) upon binding of its ligand hepatocyte growth factor (HGF)2 (2) is a crucial pathway regulating the migratory and invasive capacity of cells in physiological and pathological conditions (3). Usually, HGF is produced by cells of mesenchymal origin, whereas Met is mainly expressed by epithelial cells (4–6). This paracrine system is normally tightly regulated (3). However, Met overexpression and increased Met signaling are detected in a variety of cancers (7) and are shown to trigger cancer cell invasion and metastatic progression (8). Consequently, interference with Met overexpression and/or activation has emerged as a promising approach in targeted cancer therapy (9–11).
It has been shown that binding of the monoclonal antibody DN30 to an extracellular epitope of Met reduces Met receptor levels at the cell surface and quenches signal transduction. In vitro, the antibody inhibits anchorage-independent growth as well as HGF-stimulated invasion of cancer cells (10). In vivo, it reduces primary tumor growth and spontaneous metastasis formation in preclinical models (10). The therapeutic effect of DN30 antibody involves shedding (release from the cell surface) of the extracellular domain of Met in proximity of the cell membrane (10). This results in net reduction of functional surface receptors and generation of a soluble extracellular domain acting as an inhibitor (decoy) that binds the HGF ligand. The molecular mechanism responsible for antibody-induced Met shedding is so far unknown.
Membrane-bound metalloproteinases of the ADAM (A Disintegrin And Metalloproteinase) family (12) are major mediators of cell surface protein shedding during tumor progression, thereby interfering with several intracellular signaling pathways (13). We recently suggested ADAM-10 as a protease regulating spontaneous Met shedding because its knockdown leads to increased cell surface levels of Met in vitro (14). ADAM-17 has been proposed as another mediator (15). In the present study, we show by a functional genetic approach that DN30 antibody-induced Met shedding is selectively mediated by ADAM-10. This finding is of interest to understand the molecular mechanism(s) responsible for the therapeutic effect of anti-Met antibodies and will be useful for the further development of DN30 as an anticancer agent.
EXPERIMENTAL PROCEDURES
Generation of Knockdown Cell Lines
Human gastric carcinoma cells (GTL-16) were obtained and cultured as described previously (1). Human non-small cellular lung carcinoma cells (A549) and human ovarian carcinoma cells (SKOV3ip) were cultured according to the provider's instructions (ATCC-LGC Standards, Wesel, Germany). Stable knockdown of ADAM-10 or ADAM-17 in all cell lines was achieved by lentivirus-based RNA interference. Virus particles were produced with ViraPowerTM Lentiviral Expression System (Invitrogen), using plasmids encoding shRNA sequences against human ADAM-10 or ADAM-17, respectively (Sigma-Aldrich). 293T were transfected with LipofectamineTM 2000 according to the manufacturer's protocol (Invitrogen). Target cells were seeded on day 0 (4 × 105 cells/6-well plate) and infected with lentiviral particles on day 1. To prevent interference with adaptation mechanisms, shedding experiments where carried out on day 2 after infection without selection of positive clones (“acute knockdown”).
Shedding Experiments
Tumor cells were incubated with or without 80 μg/ml DN30 monoclonal antibody (10) and 1,000 ng/ml recombinant human TIMP-1 (recTIMP-1), 1,000 ng/ml recombinant human TIMP-3 (recTIMP-3) for metalloproteinase broad spectrum inhibition, or 1 to 5 μm ADAM-10-specific inhibitor GI254023X (16). Shedding was stimulated with 100 nm PMA (phorbol-12-myristate-13-acetate) in GTL-16 cells. Supernatants were kept for analysis, and cells were washed twice with ice-cold PBS, incubated with cell lysis buffer (Cell Signaling Technology, Danvers, MA) for 5 min, scratched, transferred to reaction tubes, and sonicated 3 times for 10 s. After centrifugation (14,000 × g, 10 min, 4 °C), supernatants were collected and stored at −80 °C.
Western Blotting
Protein isolation from cells, quantification, electrophoresis, and electroblotting of 60 μg of protein were done as described previously (17). Unspecific binding of antibodies was blocked using 10% (w/v) BSA in TBS. Polyclonal primary antibodies against ADAM-10 (Abcam, Cambridge, MA), ADAM-17 (Cell Signaling Technology), Met (DL-21) (18), and α-tubulin (Calbiochem) as well as horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) were used for detection with Lumi-light (Roche Diagnostics). For densitometric analysis, band intensities of Met were measured using ImageJ software.
Immunoprecipitation
For detection of shedding products, equal amounts of supernatants were incubated for 2 h with Sepharose-protein A beads on a steering wheel. After washing with PBS, Laemmli buffer was added to the beads, samples were boiled for 5 min, and Western blot analysis was performed as described above.
Immunocytochemistry for Phosphorylated Met
GTL-16shNT or GTL-16shADAM-10 cells were seeded on chamber slides and incubated with TIMP-1 and/or DN30 for 5 h. Then, cells were washed with PBS, fixed with ice-cold acetone for 15 min, air-dried for 30 min, rehydrated in TBS, and incubated with an anti-phospho-Met antibody (Assay Designs, Ann Arbor, MI) for 2 h. Detection was carried out with an anti-rabbit Alexa Fluor 488-conjugated antibody (Invitrogen). For densitometric analysis, signal intensities of Alexa Fluor 488 were measured using ImageJ software.
Scatter Assay
A549shNT or A549shADAM-10 cells were seeded at very low density. After colony formation, tumor cells were incubated with 80 μg/ml DN30 for 5 h and then stimulated with 20 ng/ml recombinant human HGF (recHGF). Scattering was microscopically determined.
Invasion Assay
1 × 105 human A549shNT or A549shADAM-10 cells were seeded in the upper chamber of Transwell® of 8-μm pore size (Becton Dickinson), coated with 78 μg/cm2 Matrigel in medium with 1% (w/v) FCS. 80 μg/ml DN30 was added to the upper chamber, and 5 h later 20 ng/ml recHGF was added to the lower chamber. Another 24 h later, Matrigel and cells on the upper side of the membrane were removed using Q-tip, and adherent cells on the lower side of the membrane were stained with DAPI. Invaded cells in 10 fields of view were counted.
Statistical Analysis
Statistical analysis was done using Student's t test when data were normally distributed. Otherwise, the Mann-Whitney U Rank Sum test was used. p < 0.05 was considered significant.
RESULTS
TIMP-1 and TIMP-3 Inhibited DN30-induced Met Shedding
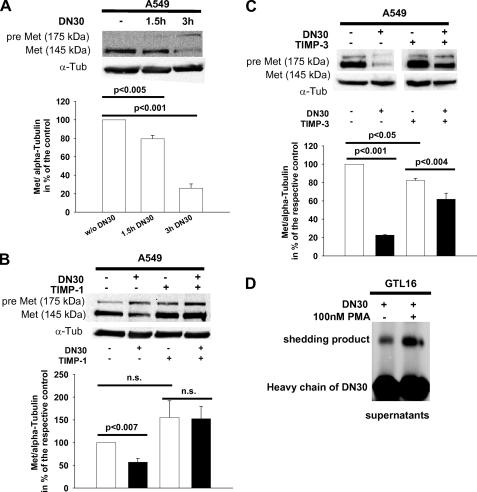
DN30 monoclonal anti-Met antibody induced a time-dependent down-regulation of Met in A549 lung carcinoma cells (Fig. 1A). Because we have previously reported that elevated systemic levels of TIMP-1 increase Met protein levels, possibly by the ability of TIMP-1 to inhibit ADAM-10 (14), we tested whether TIMP-1 interfered with DN30-induced Met shedding. Incubation of A549 cells with DN30 and/or recTIMP-1 revealed down-regulation of mature Met in the presence of DN30 alone (Fig. 1B, first and second lanes), which was prevented upon addition of recTIMP-1 (third and fourth lanes). We also observed a slight increase of mature Met protein levels when recTIMP-1 was added (first lane versus third lane). Because TIMP-3 is known to inhibit ADAM-10 (19, 20), we tested whether TIMP-3 also interfered with DN30-induced Met shedding. Incubation of A549 cells with recTIMP-3 was also effective in inhibiting DN30-induced down-regulation of Met (Fig. 1C). Because PMA can enhance the activity of ADAMs (19, 21), we next tested its effect on DN30-induced Met shedding. To be able to detect sufficient amounts of the shedding product in supernatants, we employed GTL-16 cells, which express large amounts of the receptor (22). We found that DN30-induced shedding was augmented by PMA (Fig. 1C), suggesting the involvement of an ADAM as the responsible protease.
FIGURE 1.
A, shedding of pre-Met upon administration of DN30 appeared to be time-dependent. Representative Western blots for pre-Met and Met in A549 cells, incubated with 80 μg/ml DN30 for 1.5 h and 3 h, are shown. α-Tubulin (α-Tub) was used to normalize protein levels. Densitometric analysis (n = 3): without DN30, 100.00%; 1.5 h DN30, 79.4 ± 3.7; 3 h DN30, 25.9 ± 4.7). B, DN30-induced shedding of pre-Met was inhibited by addition of recTIMP-1. Representative Western blots for pre-Met and Met in A549 cells cultured with 1 μg/ml recTIMP-1 for 1 h prior to addition of 80 μg/ml DN30 and further incubation for 5 h with or without 1 μg/ml recTIMP-1 are shown. α-Tubulin was used to normalize protein levels. Densitometric analysis (n = 3): without DN30 without TIMP-1, 100.00%; with DN30 without TIMP-1, 56.8 ± 8.4; without DN30 with TIMP-1, 155.2 ± 36.5; with DN30 with TIMP-1, 152.4 ± 27.5). C, DN30-induced shedding of pre-Met was inhibited by addition of recTIMP-3. Representative Western blots for pre-Met and Met in A549 cells cultured with 1 μg/ml recTIMP-3 for 1 h prior to addition of 80 μg/ml DN30 and further incubation for 5 h with or without 1 μg/ml recTIMP-3 are shown. α-Tubulin was used to normalize protein levels. Densitometric analysis (n = 3): without DN30 without TIMP-3, 100.00%; with DN30 without TIMP-3, 22.5 ± 0.7; without DN30 with TIMP-3, 82.1 ± 2.4; with DN30 with TIMP-3, 61.8 ± 6.6). D, DN30-induced shedding of pre-Met in GTL-16 cells was stimulated by PMA. Cells were incubated with or without 80 μg/ml DN30 and 100 nm PMA for 4 h. Supernatants were analyzed afterward with Western blotting. A representative Western blot of three experiments is shown. n.s., not significant.
ADAM-10 but Not ADAM-17 Was Required for DN30-induced Met Shedding
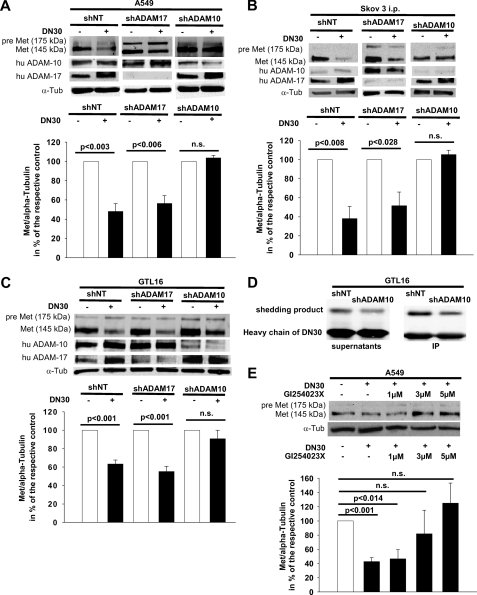
To discriminate the responsible ADAM, we knocked down ADAM-10 or ADAM-17, respectively, in A549, SKOV3ip, and GTL-16 cells. Knockdown of ADAM-10 prevented DN30-induced Met down-regulation in all three cell lines (Fig. 2, A–C, right panels each). In contrast, knockdown of ADAM-17 did not inhibit DN30-induced shedding in any cell line (Fig. 2, A–C, middle versus left panels each). However, in GTL-16 cells, reduction of cell surface Met was not completely abolished by ADAM-10 knockdown (Fig. 2C, middle panel). Next, we analyzed the amount of the Met shedding product in the supernatants of GTL-16 cells. Knockdown of ADAM-10 decreased DN30-induced accumulation of the shedding product (Fig. 2D, left panel). This result was confirmed by immunoprecipitation of the shedding product that was still bound to DN30 (Fig. 2D, right panel). The role of ADAM-10 in DN30-induced Met shedding was confirmed further by co-incubation of A549shNT or A549shADAM-17 cells, respectively, with DN30 and recTIMP-1, which is a strong inhibitor of ADAM-10, but not of ADAM-17 (20, 23). Also, the ADAM-10-specific synthetic inhibitor GI254023X (16) abolished the DN30-induced Met shedding in a dose-dependent manner (Fig. 2E). As secondary effects, we found an up-regulation of ADAM-17 after DN30 administration (Fig. 2, B and C, left panels each) and an increase of ADAM-10 levels upon knockdown of ADAM-17 (Fig. 2, B and C, middle versus left panels each) in SKOV3ip and GTL-16 cells.
FIGURE 2.
A–C, ADAM-10, but not ADAM-17, knockdown inhibited DN30-induced shedding of pre-Met. Representative Western blots (of three independent experiments) for pre-Met and Met, ADAM-10, and ADAM-17 in A549 (A), SKOV3ip (B), and GTL-16 (C) cells, cultured 5 h with or without 80 μg/ml DN30 (A–C) are shown. α-Tubulin was used to normalize protein levels. Knockdown cell lines (shADAM-10 or shADAM-17, respectively) and control cell lines (shNT) were generated using shRNAi. Densitometric analysis: A: A549shNT without DN30, 100%; with DN30, 48.0% ± 8.2%; n = 3; shADAM17 without DN30, 100%; with DN30, 56.3% ± 8.1%; n = 3; shADAM10 without DN30, 100%; with DN30, 103.7% ± 2.6%; n = 3. B, SKOV3ipshNT without DN30, 100%; with DN30, 38.1% ± 12.7%; n = 3; shADAM17 without DN30, 100%; with DN30, 51.6% ± 14.3%; n = 3; shADAM10 without DN30, 100%; with DN30, 105.3% ± 4.6%; n = 3. C, GTL16shNT without DN30, 100%; with DN30, 63.4% ± 4.2%; n = 3; shADAM17 without DN30, 100%; with DN30, 55.2% ± 5.6%, n = 3; shADAM10 without DN30, 100%; with DN30, 90.8% ± 9.2%, n = 3. Furthermore, in SKOV3ip (B) and GTL-16 cells (C), elevated levels of ADAM-17 after DN30 administration and increased ADAM-10 protein upon knockdown of ADAM-17 were detected. D, representative Western blot of supernatants and immunoprecipitated protein from supernatants of GTL-16shNT or GTL-16shADAM-10 cells are shown, cultured for 5 h with 80 μg/ml DN30. Knockdown of ADAM-10 reduced Met shedding. E, representative Western blot of A549 cells of three independent experiments cultured with or without 80 μg/ml DN30 and the indicated amounts of GI254023X, showed that ADAM-10-specific inhibition by GI254023X abolished Met shedding. Densitometric analysis: without DN30 without GI254023X, 100%; with DN30 without GI254023X, 42.7% ± 5.6%; with DN30 1 μm GI254023X, 46.6% ± 12.8%; with DN30 3 μm GI254023X, 81.8% ± 33.2%; with DN30 5 μm GI254023X, 125.1% ± 28.2%. n.s., not significant.
DN30-induced ADAM-10-mediated Shedding Reduced Met Activation
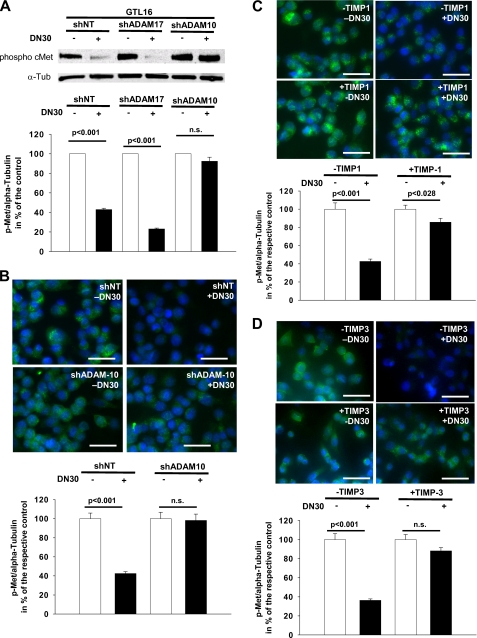
Next, we aimed to elucidate whether ADAM-10 was necessary for the suppression of Met signaling by DN30. We examined Met phosphorylation in GTL-16 cells as Met signaling is constitutively activated in this cell line (22). DN30 treatment of these cells led to a drastic reduction of Met phosphorylation (Fig. 3A, left panel), correlating with the reduced Met protein levels (Fig. 2C, left panel). Knockdown of ADAM-10 prevented the DN30-induced reduction of Met phosphorylation (Fig. 3A, middle panel), whereas knockdown of ADAM-17 did not (Fig. 3A, right panel). This finding was confirmed by immunocytochemical staining for phosphorylated Met in GTL-16 cells as DN30 reduced Met phosphorylation only in the presence of ADAM-10 (Fig. 3B). Moreover, similar results were obtained with the natural ADAM-10 inhibitors TIMP-1 (Fig. 3C) and TIMP-3 (Fig. 3D).
FIGURE 3.
A, representative Western blot for phosphorylated Met in GTL-16 cells, cultured with or without 80 μg/ml DN30 is shown. α-Tubulin was used to normalize protein levels. Knockdown cell lines (shADAM-10 or shADAM-17, respectively) and a control cell line (shNT) were generated using shRNAi. ADAM-10 knockout impaired DN30-induced inhibition of Met phosphorylation, whereas ADAM-17 deficiency had no effect on this inhibition. Densitometric analysis: GTL16shNT without DN30, 100%; with DN30, 42.9% ± 1.3%; n = 3; shADAM17 without DN30, 100%; with DN30, 21.9% ± 0.9%, n = 3; shADAM10 without DN30, 100%; with DN30, 97.1% ± 4.4%; n = 3. B and C, representative images of immunohistochemical staining for phosphorylated Met in GTL-16shNT and GTL-16shADAM-10 cells, respectively, cultured either for 5 h with or without 80 μg/ml DN30 (B) or additionally with or without 1 μg/ml recTIMP-1 (C) are shown. Blue signal, DAPI. Green signal, phosphorylated Met. B, DN30-induced shedding reduced Met phosphorylation only when ADAM-10 was not knocked down. Scale bars, 50 μm. Densitometric analysis: GTL16 shNT without DN30, 100.0% ± 5.8%; with DN30, 42.5% ± 2.0%; n = 15; shADAM10 without DN30, 100.0% ± 6.7%; with DN30, 98.2% ± 6.6%, n = 15. C, incubation with the natural ADAM-10 inhibitor TIMP-1 also impaired DN30-induced reduction of Met phosphorylation. Scale bars, 50 μm. Densitometric analysis: GTL16 without TIMP-1 without DN30, 100.0% ± 6.9%; without TIMP-1 with DN30, 42.6% ± 2.6%; n = 15; with TIMP-1 without DN30, 100.0% ± 4.4%; with TIMP-1 with DN30, 85.6% ± 4.3%, n = 15. Besides, a weak Met phosphorylation upon incubation with recTIMP-1 was detected even after administration of DN30. D, incubation with the natural ADAM-10 and ADAM-17 inhibitor TIMP-3 also impaired DN30-induced reduction of Met phosphorylation. Scale bars, 50 μm. Densitometric analysis: GTL16 without TIMP-3 without DN30, 100.0% ± 6.3%; without TIMP-3 with DN30, 36.3% ± 1.5%; n = 15; with TIMP-3 without DN30, 100.0% ± 5.3%; with TIMP-3 with DN30, 88.2% ± 3.4%; n = 15. n.s., not significant.
ADAM-10 Was Necessary for DN30-induced Reduction of Tumor Cell Migration and Invasion in Vitro
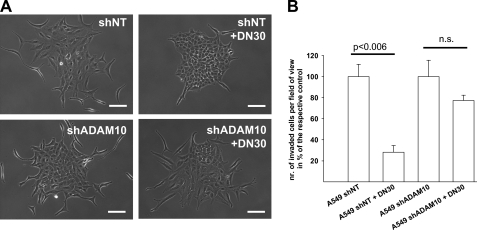
Next, we examined whether ADAM-10 was also necessary for mediating the inhibitory effect of DN30 on HGF-dependent tumor cell migration and invasion. DN30 drastically inhibited HGF-induced scattering of A549 lung carcinoma cells as long as ADAM-10 was present (Fig. 4A). Also, DN30 decreased the HGF-induced cell invasion through a Matrigel matrix (Fig. 4B). In contrast, this inhibitory effect was markedly reduced upon ADAM-10 knockdown (Fig. 4B).
FIGURE 4.
A, representative microscopic view of A549shNT or A549shADAM-10 cells in culture. Cells were seeded at low density and incubated with or without 80 μg/ml DN30 for 5 h. After colony formation, cells were stimulated with 20 ng/ml recHGF. ADAM-10 appeared to be essential for DN30-induced inhibition of tumor cell scattering. Scale bars, 50 μm. B, analysis of the invasiveness of A549shNT and A549shADAM-10 cells, incubated with or without 80 μg/ml DN30 for 5 h, was analyzed in a Transwell® invasion assay. ADAM-10 was essential for DN30-induced inhibition of tumor cell invasion. A549shNT, 100.00 ± 20.24; A549shNT + DN30, 28.13 ± 11.13; A549shADAM-10, 100.00 ± 26.96; A549shADAM-10 + DN30, 77.29 ± 7.25. Error bars, S.E., n = 3 each. n.s., not significant.
DISCUSSION
In this study, we show that ADAM-10 is the protease responsible for DN30 antibody-induced Met shedding and the therapeutic effect mediated by down-regulation of Met surface receptors, impaired tyrosine phosphorylation, and the subsequent inhibition of cell migration and invasion. These findings demonstrate for the first time the existence of a functional interplay among an antibody, a receptor tyrosine kinase, and a protease in an envisaged antiinvasive therapy approach.
ADAMs are major mediators of cell surface protein shedding during tumor progression (13). Our present observation that DN30-induced Met shedding was enhanced by PMA, a known activator of ADAMs (19, 21), suggests that this protease family is involved in antibody-induced Met shedding. ADAM-10 and ADAM-17, two members of this family, share common substrates (20, 24, 25), including Met (14, 15). Here, we identified ADAM-10 as the protease responsible for the DN30-induced Met shedding. Abrogation of its expression through shRNA prevented the down-regulation of the receptor in three different human cancer cell lines including gastric carcinoma (GTL-16), non-small cellular lung carcinoma (A549), and ovarian carcinoma (SKOV3ip). The mechanism is rather selective because knockdown of ADAM-17 (another member of the family) was ineffective. The selective involvement of ADAM-10 was confirmed by the use of TIMP-1, a strong inhibitor of ADAM-10 but not of ADAM-17 (19, 20), as well as by treatment with the ADAM-10-specific synthetic inhibitor GI254023X (16). TIMP-3, which is an inhibitor of both ADAM-10 and ADAM-17 (19, 20), did not block Met shedding more efficiently than TIMP-1 underlining the importance of ADAM-10 in this process. In addition, whenever ADAM-17 was up-regulated upon treatment with DN30, Met shedding was not impaired. Although the repertoire of protein substrates for ADAM-10 and ADAM-17 is partially overlapping (13) and the molecular mechanism of substrate recognition is still unknown (26), a certain degree of substrate specificity among the members of the ADAM family exists (13). Binding of an antibody to its epitope can lead to a conformational change of the whole antigenic protein (27): one can hypothesize that the interaction between DN30 and Met unmasks specific determinants for substrate recognition by ADAM-10, but not by ADAM-17 (28). However, the finding that ADAM-10 is crucial for the DN30-induced shedding of the Met receptor does not exclude the involvement of ADAM-17 in Met shedding under normal conditions (15) as ADAM-10 and ADAM-17 are known to share various substrates (20, 24), including the Met receptor (14, 15).
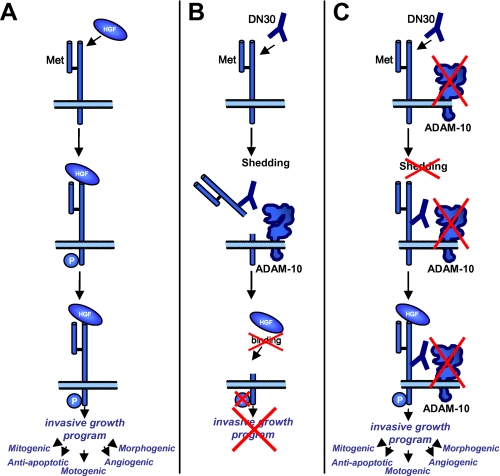
This paper identifies ADAM-10 as a critical mediator of the described DN30 therapeutic effect (10) as ADAM-10 intervenes with the outside-in signaling transduction by prometastatic HGF (Fig. 5). In a variety of human cancers, Met overexpression and increased Met signaling are detected (7). Binding of the ligand HGF to Met was shown to induce receptor dimerization and phosphorylation of Tyr1234 and Tyr1235 (29). This results in activation of the complex invasive growth program (30) (Fig. 5A). Binding of DN30 to Met induces shedding and subsequent decrease of receptor molecules on the cell surface (Fig. 5B), leaving the ligand HGF with fewer interaction partners (Fig. 5B). Consequently Met phosphorylation per cell is abrogated, and the invasive growth program cannot be induced. We here showed that such a therapeutic antiinvasive effect of DN30 was mediated by ADAM-10 (Fig. 5C). In the case of broad spectrum metalloprotease inhibition, including ADAM-10, the antibody is rendered inefficient because cancer cells can still respond to the HGF trigger (Fig. 5C). Taken together, cooperation of DN30 and ADAM-10 is necessary for efficient interference with the first step of Met signaling activation.
FIGURE 5.
Involvement of ADAM-10 in the DN30-mediated therapeutic effect. A, binding of the ligand HGF to the extracellular portion of Met induces receptor dimerization and phosphorylation of the tyrosine residues 1234 and 1235 thereby leading to the activation of the invasive growth program. B, binding of the monoclonal antibody to the extracellular portion of Met induces ADAM-10-mediated shedding of the receptor thereby reducing the number of available receptors on the cell surface. Activation of the invasive growth program is impaired as HGF can no longer bind to Met. C, under ADAM-10-deficient conditions, DN30 can still bind to Met but cannot induce shedding of the Met receptor. Consequently, receptor levels on the cell surface are not reduced. Met signaling and the invasive growth program can still be activated by HGF.
It is also known that ADAM-10 behaves as a prometastatic protein, and therapeutic strategies based on its inhibition have been envisaged (31, 32). With this knowledge, it is evident that inhibition of ADAM-10 could be detrimental if combined with a DN30-based anti-Met therapy.
This study demonstrates the involvement of a specific endogenous protease in an antibody-induced down-regulation of a receptor tyrosine kinase with oncogenic and prometastatic potential. This newly identified interplay also reveals how inhibitors of metalloproteases for cancer therapy can act as a double-edged sword.
Acknowledgments
We thank Katja Honert, Markus Hardt, Emre Anbarci (all from Technische Universität München, Munich, Germany) and Raffaella Albano and Gigliola Reato (both from Università di Torino, Torino, Italy) for expert technical support.
This work was supported by Framework Programme 7 Project HEALTH-2007-201279, Microenvimet (to C. B., P. M. C., and A. K.) and Deutsche Forschungsgemeinschaft Grant KR2047/1-1 (to A. K.).
- HGF
- hepatocyte growth factor
- ADAM
- A Disintegrin And Metalloproteinase
- rec
- recombinant.
REFERENCES
- 1.Giordano S., Ponzetto C., Di Renzo M. F., Cooper C. S., Comoglio P. M. (1989) Nature 339, 155–156 [DOI] [PubMed] [Google Scholar]
- 2.Naldini L., Weidner K. M., Vigna E., Gaudino G., Bardelli A., Ponzetto C., Narsimhan R. P., Hartmann G., Zarnegar R., Michalopoulos G. K. (1991) EMBO J. 10, 2867–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. (2003) Nat. Rev. Mol. Cell Biol. 4, 915–925 [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg E., Meyer D., Weidner K. M., Birchmeier C. (1993) J. Cell Biol. 123, 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarnegar R., DeFrances M. C. (1993) EXS 65, 181–199 [PubMed] [Google Scholar]
- 6.Di Renzo M. F., Narsimhan R. P., Olivero M., Bretti S., Giordano S., Medico E., Gaglia P., Zara P., Comoglio P. M. (1991) Oncogene 6, 1997–2003 [PubMed] [Google Scholar]
- 7.Danilkovitch-Miagkova A., Zbar B. (2002) J. Clin. Invest. 109, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentile A., Trusolino L., Comoglio P. M. (2008) Cancer Metastasis Rev. 27, 85–94 [DOI] [PubMed] [Google Scholar]
- 9.Comoglio P. M., Giordano S., Trusolino L. (2008) Nat. Rev. Drug Discov. 7, 504–516 [DOI] [PubMed] [Google Scholar]
- 10.Petrelli A., Circosta P., Granziero L., Mazzone M., Pisacane A., Fenoglio S., Comoglio P. M., Giordano S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5090–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigna E., Pacchiana G., Mazzone M., Chiriaco C., Fontani L., Basilico C., Pennacchietti S., Comoglio P. M. (2008) Cancer Res. 68, 9176–9183 [DOI] [PubMed] [Google Scholar]
- 12.Edwards D. R., Handsley M. M., Pennington C. J. (2008) Mol. Aspects Med. 29, 258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy G. (2008) Nat. Rev. Cancer 8, 929–941 [DOI] [PubMed] [Google Scholar]
- 14.Kopitz C., Gerg M., Bandapalli O. R., Ister D., Pennington C. J., Hauser S., Flechsig C., Krell H. W., Antolovic D., Brew K., Nagase H., Stangl M., von Weyhern C. W., Brücher B. L., Brand K., Coussens L. M., Edwards D. R., Krüger A. (2007) Cancer Res. 67, 8615–8623 [DOI] [PubMed] [Google Scholar]
- 15.Foveau B., Ancot F., Leroy C., Petrelli A., Reiss K., Vingtdeux V., Giordano S., Fafeur V., Tulasne D. (2009) Mol. Biol. Cell 20, 2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hundhausen C., Misztela D., Berkhout T. A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., Kallen K. J., Rose-John S., Ludwig A. (2003) Blood 102, 1186–1195 [DOI] [PubMed] [Google Scholar]
- 17.Arlt M., Kopitz C., Pennington C., Watson K. L., Krell H. W., Bode W., Gansbacher B., Khokha R., Edwards D. R., Krüger A. (2002) Cancer Res. 62, 5543–5550 [PubMed] [Google Scholar]
- 18.Prat M., Crepaldi T., Pennacchietti S., Bussolino F., Comoglio P. M. (1998) J. Cell Sci. 111, 237–247 [DOI] [PubMed] [Google Scholar]
- 19.Amour A., Slocombe P. M., Webster A., Butler M., Knight C. G., Smith B. J., Stephens P. E., Shelley C., Hutton M., Knäuper V., Docherty A. J., Murphy G. (1998) FEBS Lett. 435, 39–44 [DOI] [PubMed] [Google Scholar]
- 20.Amour A., Knight C. G., Webster A., Slocombe P. M., Stephens P. E., Knäuper V., Docherty A. J., Murphy G. (2000) FEBS Lett. 473, 275–279 [DOI] [PubMed] [Google Scholar]
- 21.Kohutek Z. A., diPierro C. G., Redpath G. T., Hussaini I. M. (2009) J. Neurosci. 29, 4605–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponzetto C., Giordano S., Peverali F., Della Valle G., Abate M. L., Vaula G., Comoglio P. M. (1991) Oncogene 6, 553–559 [PubMed] [Google Scholar]
- 23.Amour A., Hutton M., Knäuper V., Slocombe P. M., Webster A., Butler M., Knight C. G., Smith B. J., Docherty A. J., Murphy G. (1999) Ann. N.Y. Acad. Sci. 878, 728–731 [DOI] [PubMed] [Google Scholar]
- 24.Hikita A., Tanaka N., Yamane S., Ikeda Y., Furukawa H., Tohma S., Suzuki R., Tanaka S., Mitomi H., Fukui N. (2009) Biochem. Cell Biol. 87, 581–593 [DOI] [PubMed] [Google Scholar]
- 25.Le Gall S. M., Bobé P., Reiss K., Horiuchi K., Niu X. D., Lundell D., Gibb D. R., Conrad D., Saftig P., Blobel C. P. (2009) Mol. Biol. Cell 20, 1785–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeda S. (2009) Semin. Cell Dev. Biol. 20, 146–152 [DOI] [PubMed] [Google Scholar]
- 27.Benjamin D. C., Williams D. C., Jr., Smith-Gill S. J., Rule G. S. (1992) Biochemistry 31, 9539–9545 [DOI] [PubMed] [Google Scholar]
- 28.Caescu C. I., Jeschke G. R., Turk B. E. (2009) Biochem. J. 424, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naldini L., Vigna E., Ferracini R., Longati P., Gandino L., Prat M., Comoglio P. M. (1991) Mol. Cell. Biol. 11, 1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentile A., Comoglio P. M. (2004) Int. J. Dev. Biol. 48, 451–456 [DOI] [PubMed] [Google Scholar]
- 31.Crawford H. C., Dempsey P. J., Brown G., Adam L., Moss M. L. (2009) Curr. Pharm. Des. 15, 2288–2299 [DOI] [PubMed] [Google Scholar]
- 32.Moss M. L., Stoeck A., Yan W., Dempsey P. J. (2008) Curr. Pharm. Biotechnol. 9, 2–8 [DOI] [PubMed] [Google Scholar]