Abstract
Cysteine is considered a nonessential amino acid in mammals as it is synthesized from methionine via trans-sulfuration. However, premature infants or patients with hepatic failure may require dietary cysteine due to a lack of cystathionine γ-lyase (CTH), a key trans-sulfuration enzyme. Here, we generated CTH-deficient (Cth−/−) mice as an animal model of cystathioninemia/cystathioninuria. Cth−/− mice developed normally in general but displayed hypercystathioninemia/hyperhomocysteinemia though not hypermethioninemia. When fed a low cyst(e)ine diet, Cth−/− mice showed acute skeletal muscle atrophy (myopathy) accompanied by enhanced gene expression of asparagine synthetase and reduced contents of glutathione in livers and skeletal muscles, and intracellular accumulation of LC3 and p62 in skeletal myofibers; they finally died of severe paralysis of the extremities. Cth−/− hepatocytes required cystine in a culture medium and showed greater sensitivity to oxidative stress. Cth−/− mice exhibited systemic vulnerability to oxidative injury, which became more prominent when they were fed the low cyst(e)ine diet. These results reveal novel roles of trans-sulfuration previously unrecognized in mice lacking another trans-sulfuration enzyme cystathionine β-synthase (Cbs−/−). Because Cbs−/− mice display hyperhomocysteinemia and hypermethioninemia, our results raise questions against the homocysteine-based etiology of CBS deficiency and the current newborn screening for homocysteinemia using Guthrie's method, which detects hypermethioninemia.
Keywords: Antioxidant, Autophagy, Glutathion, Homocysteine, Oxidative Stress, Amino Acid Response, Cystathioninuria, Myopathy, Newborn Screening, Trans-sulfuration
Introduction
Cys is considered a nonessential amino acid because it is provided through diet or the Met cycle/trans-sulfuration in which a sulfur molecule is transferred from Met-derived homocysteine to Ser (1, 2). Two pyridoxal 5′-phosphate-dependent enzymes, cystathionine β-synthase (CBS4; EC 4.2.1.22) and cystathionine γ-lyase (CTH, γ -cystathionase, often abbreviated as CSE; EC 4.4.1.1) play essential roles in trans-sulfuration; the former catalyzes a condensation reaction between homocysteine and Ser to form cystathionine, and the latter catalyzes the hydrolysis of cystathionine to form Cys (2). However, Cys is often considered a semiessential or conditionally essential amino acid because premature infants can require dietary Cys due to a lack of hepatic CTH activity/expression (3–5), and patients suffering from a vitamin B6 deficiency, hepatic failure, or surgical stress may require Cys due to impaired trans-sulfuration (6, 7). Biosynthesized Cys is further metabolized in the liver to yield glutathione and taurine, two major antioxidants (2), and both CBS and CTH are capable of metabolizing cyst(e)ine to produce hydrogen sulfide (H2S), a novel gaseous biological mediator (8, 9), suggesting important physiological roles played by CBS/CTH.
Genetic deletion of CBS is known to cause homocysteinemia (or homocystinuria: MIM 236200), an autosomal recessive inborn error with increased levels of plasma homocysteine and urinary homocystine (10). CBS-deficient patients show a wide variety of clinical symptoms, including atherosclerosis, thrombosis, mental retardation, osteoporosis, ectopia lentis, skeletal abnormality, and hepatic steatosis (10). The mass screening of newborns for homocysteinemia currently is conducted in most advanced countries because adequate therapy right after birth can prevent progression of the disease (11, 12). CBS-deficient (Cbs−/−) mice have been generated as an animal model of homocysteinemia (13) and exhibit some pathophysiological features similar to CBS-deficient patients including endothelial dysfunction, hepatic steatosis, and impaired learning ability (14–16). Cbs−/− mice display profound lethality around the weaning age, severe growth retardation, hepatic dysfunction, and a shortened life, hampering further analysis of the role of CBS in later developmental stages (13, 15), although our trial of genetic background conversion partially rescued Cbs−/− mice from juvenile lethality and ameliorated their growth (16).
Meanwhile, genetic loss of CTH is thought to cause cystathioninemia (or cystathioninuria: MIM 219500) (17), another autosomal recessive inborn error with increased plasma/urinary levels of cystathionine and an estimated incidence of 1 in 73,000–333,000 (10). In contrast to homocysteinemia, cystathioninemia has been considered to be free of any striking clinical/pathological manifestations (10, 18, 19). Here, we generated CTH-deficient (Cth−/−) mice. While the study was underway, Yang et al. (20) reported that their Cth−/− mice appeared normal but displayed age-related hypertension as well as sex-related hyperhomocysteinemia; however, our Cth−/− males and females were both normotensive and hyperhomocysteinemic to similar extents. Our Cth−/− mice also appeared normal but displayed acute lethal myopathy when fed a low-cyst(e)ine diet and greater sensitivity to oxidative injury.
EXPERIMENTAL PROCEDURES
Animals
The Cth genomic DNA was isolated from a mouse 129/SvJ genomic library (21). A 1.5-kb genomic fragment upstream of exon 1, a 4.5-kb lacZ gene (excised from the pβgal-Basic vector; BD Biosciences), a 1.7-kb PGKneo gene (excised from the pFlox vector (22)), and a 7.3-kb genomic fragment downstream of exon 6, were successively subcloned in the pMC1DT-3 vector (23), generating the Cth gene-targeting construct. The vector was linearized with NotI and transfected into 129/Sv embryonic stem cells (Dainippon Sumitomo Pharma, Osaka, Japan) by electroporation. The targeting was completed by homologous recombination under a 200 μg/ml G418-positive selection and a melanocortin-1 promoter-driven diphtheria toxin A subunit-catalyzed negative selection, which replaced the 14.2-kb genome containing Cth exons 1–6 encoding the first 213 amino acids in a 398-amino acid CTH open reading frame with a lacZ–neo cassette. Recombinant Cth+/− embryonic stem cell clones were injected into C57BL/6J (C57BL/6JJcl; CLEA Japan, Tokyo, Japan) blastocysts to produce chimeric male mice, which were then crossed with C57BL/6J females to obtain agouti Cth+/− pups. They were backcrossed for an additional 6–9 generations to achieve >99.2% genetic homogeneity on C57BL/6J background. The Cth+/− males and females produced were bred to obtain wild-type (WT, Cth+/+), Cth+/−, and Cth−/− littermates. Cbs+/− mice (B6.129P2-Cbstm1Unc/J) (13) were obtained from The Jackson Laboratory (Bar Harbor, ME) and backcrossed for 7–12 generations to C57BL/6J. Cbs−/− mice were obtained by Cbs+/− intercrosses on a background close to C57BL/6J (16) for a better comparison between Cbs and Cth mutants.
Diets, Care, and Genotyping of Mice
Mice were housed in an air-conditioned room kept on a 12-h dark/light cycle and allowed free access to the standard dry rodent diet CE-2 (CLEA Japan) and water. In some experiments, a KR (Kojin Rayon®; Tokyo, Japan), KR+Cys, or protein-free amino acid diet (16) was given. The KR diet, which mainly consists of denucleated torula yeast (Oriental Yeast, Tokyo, Japan) and sucrose, was originally formulated as a selenium-deficient diet; but, in this study, it was used as a low cyst(e)ine (selenium-sufficient) diet that contains 0.16% cystine (versus 0.37% in CE-2 or KR+Cys) and 0.164 ppm Na2SeO4 (as a component of the AIN-93G mineral mixture) (16). The protein-free amino acid diet is formulated to contain analytical grade amino acids that quantitatively match the protein-constituent amino acids in CE-2 (e.g. 0.44% Met; 0.37% cystine) (16). Daily food intake was measured using the Metabolic Cage (Asahi Techno Glass, Tokyo, Japan). Cth genotyping was done by PCR using tail genomic DNA and the following three primers: primer 1, 5′-TGCCGACCAATAAGCAGGGC-3′; primer 2, 5′-CCGAGGACTGGCCCGGGAAGT-3′; and primer 3, 5′-CCAGACCGGCAACGAAAATCA-3′. All procedures involving animals were approved by the Animal Care Committee of Keio or Gunma University. When mice became unable to move, drink, or eat, they were euthanized with ether.
Northern Blot Analysis
Mouse tissues were removed quickly and homogenized in TRIzol (Invitrogen). Total RNA (20 μg) was analyzed as described previously (21, 24). Specific probes used were the full-length mouse Cth cDNA (21) and the partial mouse cDNA of the housekeeping GAPDH (24). 18 S rRNA was stained with ethidium bromide as a loading control.
Western Blot Analysis
5 μg of liver and kidney homogenates were analyzed by Western blotting as described previously (21, 25). Mouse CTH was detected using both anti-CTH amino terminus and carboxyl terminus rabbit polyclonal antibodies that recognize amino acids 1–193 and 194–398 of a rat 398-amino acid CTH protein, respectively (21). Mouse CBS was detected with anti-rat CBS rabbit polyclonal antibody (15).
Measurement of CTH Activity
Measurement of Total Homocysteine and Free Amino Acids
Serum levels of total homocysteine (homocysteine and all its derivatives that give rise to the thiol homocysteine after reductive cleavage of disulfide bonds (26)) were measured using an Azwell autohomocysteine kit (Alfresa Pharma, Osaka, Japan), which is based on a highly sensitive assay applicable to various clinical automatic colorimetric analyzers (27). Free amino acids in serum or urine were derivatized with 4-fluoro-7-nitrobenzofurazan (Dojindo, Kumamoto, Japan) and measured using a HPLC system as described previously (16).
Measurement of Blood Pressure (BP)
Systolic BP (as well as heart rates and diastolic BP) was measured using the BP-98A tail-cuff system (Softron, Tokyo, Japan) without anesthesia.
Measurement of Serum Biochemical Parameters
Levels of albumin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, total bilirubin, blood urea nitrogen, creatine kinase, creatinine (serum and urine), and lactate dehydrogenase were measured using the Dri-Chem 3500 system (FujiFilm, Tokyo, Japan). Serum levels of total and free cholesterol, triglyceride, nonesterified fatty acid, and phospholipid, were measured with enzymatic assay kits from Wako (Osaka, Japan) (15, 16). Serum lecithin-cholesterol acyltransferase activity was measured using the Anasolve lecithin-cholesterol acyltransferase (Sekisui Medical, Tokyo, Japan) (15, 16).
Histochemistry
Anesthetized mice were perfused through the heart with PBS followed by 4% paraformaldehyde in PBS. Tissues (except skeletal muscles) were dissected out, post-fixed overnight in 4% paraformaldehyde in PBS, and then cryoprotected in 30% sucrose in PBS. After sinking, they were embedded in Tissue-Tek optical cutting temperature compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned with a cryostat at 10 μm. Skeletal muscles were dissected out, frozen in isopentane/liquid nitrogen, and sectioned at 10 μm. Sections were stained with Mayer's hematoxylin/eosin Y solutions (as H&E staining) for most tissues and Luxol first blue/cresyl violet solutions (as Kluver-Barrier staining) for spinal sections. Skeletal muscle sections were immunolabeled with goat anti-dystrophin polyclonal (Santa Cruz Biotechnology), rabbit anti-LC3 polyclonal (MBL, Nagoya, Japan) (or sheep anti-LC3 polyclonal (Osenses, Flagstaff Hill, Australia)), and rabbit anti-p62 polyclonal (MBL) antibodies. Alexa Fluor 488 or 568-conjugated antibodies (Molecular Probes) were used as secondary antibodies, and sections were mounted in a ProLong Gold antifade reagent with DAPI (Invitrogen). A TUNEL assay was performed using an in situ apoptosis detection kit (Takara Bio, Tokyo, Japan). Sections were examined with a BZ-9000 fluorescence microscope (Keyence, Osaka, Japan) fitted with a Plan Apo 40× objective (Nikon).
RT-PCR
Total RNA isolated with TRIzol was further purified with a PureLink RNA mini kit (Invitrogen). Two micrograms of the RNA was used to produce the first-strand cDNA with a Superscript VILO cDNA synthesis kit (Invitrogen). A total of 10 ng of cDNA from each sample was amplified via RT-PCR using SYBR Premix Ex TaqII (Takara Bio), primer sets for mouse asparagine synthetase gene (Asns) (5′-CTGTACGGATGAACCATTGC-3′ and 5′-GCCTCCTTGAGTTGCTTCA-3′) and hypoxanthine guanine phosphoribosyl transferase 1 gene (Hprt1) (5′-GACTGATTATGGACAGGACTG-3′ and 5′- GACTGATCATTACAGTAGCTC-3′), and an 7300 real-time PCR system (Applied Biosystems). Asns and Hprt1 mRNA levels were quantified using a comparative CT method with Hprt1 levels for normalization.
Hepatocyte Culture and Cell Survival Assay
Hepatocytes were prepared from anesthetized 10-week-old male mice. The liver was perfused for 20 min through the portal vein with perfusion buffer A (118 mm NaCl, 25.6 mm NaHCO3, 4.75 mm KCl, 1.19 mm KH2PO4, 1.19 mm MgSO4, 5.55 mm glucose, 4.54 mm pyruvate) saturated with 95% O2, 5% CO2, followed by a 10-min perfusion with the same solution containing 500 μg/ml collagenase and 50 μg/ml trypsin inhibitors, and then by a 10-min perfusion with perfusion buffer B (139 mm NaCl, 3.57 mm NaHCO3, 4.75 mm KCl, 1.19 mm KH2PO4, 1.19 mm MgSO4, 10.1 mm Hepes, 2.5 mm CaCl2). Thereafter, the liver was dispersed in perfusion buffer B, and hepatocytes were filtered through a 70-μm cell strainer (Falcon) and sedimented by low speed centrifugation with 45% Percoll-55% William's E medium (Sigma; containing 5% FBS, 1 nm dexamethasone, 1 nm insulin, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin). The cells were washed three times with William's E medium and dispersed onto collagen I-coated 96-well tissue culture dishes (Corning) at 2 × 104 cells per well. Plates were incubated for cell adhesion for 12 h at 37 °C and in a 5% CO2 incubator. Then, the medium was removed and washed, and the cells were incubated with DMEM (containing 30 mg/liter l-Met and 63 mg/liter l-cystine dihydrochloride) or Met/cystine-free DMEM (Invitrogen), which contained 1 nm dexamethasone, 1 nm insulin, 2 μg/ml aprotinin, 0.1% fatty acid-free BSA, 2 mm glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin with/without Met, cystine, or paraquat. The cells were subjected to the CellTiter-Glo luminescent cell viability assay (Promega) to measure cellular ATP as an indicator of cell survivability.
Measurement of Glutathione and Antioxidant Power
Total glutathione (reduced glutathione (GSH) and oxidized glutathione) levels and GSH/total glutathione molecular ratios were measured using coulometric electrochemical detection as described previously (28). Tissues were homogenized in 5.5% (w/v) metaphosphoric acid using a Micro Smash-100R homogenizing system (Tomy, Tokyo, Japan) and 5-μm diameter Zirconia beads. The homogenates were centrifuged at 16,100 × g for 20 min at 4 °C, and the supernatants were filtrated through an Ultrafree-MC filter (0.45 μm, Durapore PVDF, Millipore). Samples were separated on a reversed-phase C18 column (4.6 mm × 250 mm, LMS, Tokyo, Japan) by acetonitrile gradient elution, and GSH/oxidized glutathione were detected with a CoulArray detector (Model 5600A; ESA, Chelmsford, MA). Total antioxidant (= reduction) power was measured using the total antioxidant power colorimetric microplate assay kit (Oxford Biomedical Research, Oxford, MI); tissue extracts were prepared as described in the manufacturer's instruction manuals.
Statistics
Data are expressed as mean ± S.D. for independent samples. The statistical analysis was performed using Student's t test or one-way ANOVA followed by a Tukey post-test for multiple comparisons using Prism 4 (GraphPad Software). The Kaplan-Meier survival analysis was conducted using Prism 4. A p < 0.05 denotes a statistically significant difference.
RESULTS
No Apparent Abnormality in Cth−/− Mice
The mouse Cth gene consists of 12 exons with 54% of the coding region located in exons 1–6 (21). We deleted this region in embryonic stem cells using homologous recombination (Fig. 1A). CTH protein and its activity were detectable in liver and kidney, but not in brain, heart, lung, thymus, spleen, and skeletal muscle of mice (21). Successful generation of Cth−/− mice from Cth+/− mating was confirmed by Southern blot analysis/PCR of tail genomic DNA (Fig. 1, B and C), Northern blot analysis of liver and kidney RNA (Fig. 1D), as well as Western blot analysis and measurements of CTH activity in liver/kidney extracts (Fig. 1, E and F). CTH expression levels and enzymatic activities in Cth+/− liver/kidney extracts were nearly half of those in corresponding WT extracts (Fig. 1, E and F), whereas the CBS expression in the liver/kidney extracts did not differ among Cth genotypes (Fig. 1E).
FIGURE 1.
Targeted deletion of CTH in mice. A, maps of the WT Cth locus including a total of 12 exons that encode the full ORF, the targeting construct, and the homologously recombined locus. Approximate positions for two Southern probes and three PCR primers are indicated. B, BamH I; pBS, pBluescript; DTA, diphtheria toxin A gene; LacZ, β-galactosidase gene; Neo, neomycin phosphotransferase gene. B, Southern blot analysis of BamH I-digested genomic DNA isolated from WT/recombined embryonic stem cells and tails of Cth mutant mice. Blots were hybridized with either an external or internal probe. C, PCR genotyping using tail DNA and three PCR primers (primers 1–3) detecting the inheritance of WT and recombined Cth alleles (299- and 194-bp, respectively). D, Northern blot analysis of RNA from 8-week-old male livers, kidneys, and brains using the full-length Cth probe. GAPDH blots and ethidium bromide-stained 18 S rRNA are loading controls. E, Western blot analysis of 8-week-old male liver/kidney homogenates using polyclonal antibodies raised against a recombinant CTH amino terminus (N-ter), CTH carboxyl terminus (C-ter), and full-length CBS. F, specific CTH activity in 8-week-old male liver/kidney homogenate. Values are mean ± S.D. (n = 3); *, p < 0.01 and ‡, p < 0.001 in the one-way ANOVA.
Cth−/− mice were obtained from Cth+/− intercrosses at the expected frequency (Cth+/+:Cth+/−:Cth−/− = 178:360:181 in 114 litters). Both male and female Cth−/− developed with no apparent abnormalities except for slightly retarded growth in males (not females) (supplemental Fig. S1A); some Cth−/− mice survived >2 years. Systolic (and diastolic; data not shown) BP levels of Cth+/− and Cth−/− mice were not altered from those of WT in either sex (supplemental Fig. S1B). Routine histological examinations of major organs did not reveal any specific abnormality in either Cth+/− or Cth−/− mice (data not shown). Unlike Cbs−/− mice (15, 16), Cth−/− mice were free of hepatic steatosis (data not shown). This observation was supported by serum biochemistry in which normal levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, total and free cholesterol, triglyceride, nonesterified fatty acid, phospholipid, and lecithin-cholesterol acyltransferase activity were obtained; all of the biochemical parameters were dramatically elevated in Cbs−/− mice (supplemental Table S1).
Hypercystathioninemia/Hyperhomocysteinemia but Not Hypermethioninemia in Cth−/− Mice
The vast majority of Cbs−/− mice die before 4 weeks of age (15, 16), and thus, 2-week-old pups derived from heterozygous mating were examined for their amino acid levels in serum (Table 1) or urine. Serum cystathionine levels in Cth−/− were as high as 500 μm, whereas those in others were below detectable levels (Table 1). Cth−/− mice also displayed cystathioninuria (33.1 ± 5.3 mm; n = 7), whereas cystathionine was not detectable (< 5 μm) in either WT or Cbs−/− urine (n = 7 for each). In contrast, homocystine was detected in both Cth−/− and Cbs−/− mice (34.9 and 55.5 μm, respectively) but not in the others (Table 1). Accordingly, total homocysteine levels were remarkably higher in Cth−/− and Cbs−/− mice (145 and 209 μm, respectively) and slightly higher in Cth+/− and Cbs+/− (7.8 and 17.5 μm, respectively) mice than in the respective WT mice (5.9 and 6.1 μm, respectively) (Table 1). Both Cth−/− and Cbs−/− mice displayed homocystinuria (268 ± 125 and 632 ± 283 μm, respectively; n = 7 for each), and homocystine was not detectable (< 5 μm) in WT urine (n = 7). Hypermethioninemia, a characteristic feature of Cbs−/− patients (10, 29), was observed in Cbs−/− (2.64 mm) but not in Cth−/− mice (Table 1). Serum levels of taurine, the most abundant free amino acid in humans that is synthesized from Cys (2, 6), were much lower in both Cth−/− and Cbs−/− mice than in WT (Table 1). Adult Cth−/− males and females also displayed homocysteinemia; total homocysteine levels in 10-week-old males and females were 104 ± 21 (n = 8) and 151 ± 48 μm (n = 9), respectively, whereas those in the respective WT were 3.3 ± 1.0 (n = 8) and 3.9 ± 0.9 μm (n = 11), respectively. The association between a folic acid deficiency and neural tube defects is well known (30), and a molecular basis of folic acid fortification (31) (especially for pregnant women) is a reduced plasma level of homocysteine caused by its remethylation to Met (32). However, no developmental abnormalities (neural tube defects such as spina bifida and anencephaly) were observed in pups born to homocysteinemic Cth−/− dams (supplemental Fig. S2).
TABLE 1.
Hypercystathioninemia, hyperhomocysteinemia, hypotaurinemia, but not hypermethioninemia in Cth−/− mice
Serum samples were collected from 2-week-old WT, Cth, and Cbs mutant mice (both sexes). Concentrations of cystathionine, homocystine, methionine, and taurine were measured by HPLC, and those of total homocysteine were measured by an enzymatic assay. ND, not detectable (<5 μm). Values are mean ± S.D. (n).
|
Cth genotype (in offspring from Cth+/− × Cth+/−) |
Cbs genotype (in offspring from Cbs+/− × Cbs+/−) |
|||||
|---|---|---|---|---|---|---|
| +/+ | +/− | −/− | +/+ | +/− | −/− | |
| Cystathionine (μm) | ND (6) | ND (6) | 494 ± 47 (6)c | ND (6) | ND (6) | ND (6) |
| Homocystine (μm) | ND (6) | ND (6) | 34.9 ± 11.3 (6)c | ND (6) | ND (6) | 55.5 ± 14.9 (6)c |
| Methionine (μm) | 105 ± 21 (6) | 127 ± 21 (6) | 157 ± 24 (6)b | 117 ± 9 (6) | 139 ± 15 (6) | 2,640 ± 317 (6)c |
| Taurine (μm) | 498 ± 88 (6) | 559 ± 167 (6) | 190 ± 60 (6)c | 617 ± 242 (6) | 668 ± 241 (6) | 165 ± 60 (6)b |
| Total homocysteine (μm) | 5.9 ± 1.2 (8) | 7.8 ± 2.8 (19)a | 145 ± 46 (19)b | 6.1 ± 2.4 (10) | 17.5 ± 9.5 (13)c | 209 ± 37 (9)c |
a p < 0.05 versus +/+ samples in the t test.
b p < 0.01 versus +/+ samples in the t test.
c p < 0.001 versus +/+ samples in the t test.
Low Cyst(e)ine Diet-induced Acute Muscular Atrophy in Cth−/− Mice
We previously reported that a sufficient supply of cyst(e)ine in diet is essential for the survival of C3H/HeJ-Cbs−/− mice (Cbs−/− on a C3H/HeJ background); C3H/HeJ-Cbs−/− mice could not survive with a 0.16% cystine-containing KR diet but could survive with a cystine-supplemented KR (KR+Cys) diet that contains 0.37% cystine, the dose in the standard diet (16). When fed KR from 3 weeks of age, Cth−/− mice promptly stopped growing and started to lose weight daily, in marked contrast to WT and Cth+/− mice, which developed similar to those fed the standard diet (Fig. 2A). After 1 week, Cth−/− mice showed severe paralysis of the lower extremities (supplemental Video S1) and severe atrophy in abdominal regions (Fig. 2B) as well as proximal skeletal muscles, including trapezius and rectus femoris muscles (Fig. 2C) with no sign of fasciculation. Thereafter, Cth−/− mice began to display paralysis of the upper extremities and became unable to move, drink, or eat, eventually dying after ∼2 weeks (Fig. 2A). Supplementing the KR diet with cystine (namely, the KR+Cys diet) mostly restored body weight (Fig. 2D) and cancelled the onset of all such phenotypes.
FIGURE 2.
Low cyst(e)ine diet-induced acute muscular atrophy in Cth−/− mice. A, body weight changes in WT (blue circles), Cth+/− (green triangles), and Cth−/− (red diamonds) females that were fed the KR diet after weaning at 3 weeks of age. All Cth−/− mice died after ∼2 weeks. B and C, the appearance (B) and a radiograph (C) of WT (left) and Cth−/− (right) females fed the KR diet for 1 week from 3 weeks of age. Severe atrophy was observed in abdominal regions (B) as well as in proximal muscles such as trapezius and rectus femoris muscles (arrow and arrowhead in C, respectively). Bars, 1 cm. D, body weight changes with the KR+Cys diet. E, hematoxylin/eosin-stained transverse cross-sections of rectus femoris muscles isolated from a WT or Cth−/− male fed the KR diet for 1 week from 3 weeks of age. Diffuse muscular atrophy was observed in Cth−/− mice. Bars, 50 μm. F, myofiber cross-sectional areas (μm2) of rectus femoris muscles from WT or Cth−/− males before (3 weeks of age) and after (4 weeks of age) 1 week on the KR diet. G, serum levels of creatinine after consumption of the standard (Std) or KR diet for 1 week from 3 weeks of age. H and I, body weight changes after a change from the standard diet to KR diet at 6 (H) and 12 (I) weeks of age. After the change, both 6- and 12-week-old Cth−/− lost weight daily and died within ∼4 weeks. J and K, the daily body weight change (J) and food intake (K) in WT or Cth−/− males after the change to the KR diet at 8 weeks of age. Representative pictures are shown in B, C, and E. Values are mean ± S.D. (n = 5 for each genotype in A, D, H, and I; 6 in F; 10 in G; and 8 in J and K). *, p < 0.05; ‡, p < 0.01; and †, p < 0.001 in the one-way ANOVA in F and G; and ‡, p < 0.01 in the t test in J. The asterisks for p values are abbreviated in A, H, and I.
Histological examination of rectus femoris muscles from KR-fed Cth−/− mice revealed diffuse (rather than grouped) myofiber atrophy (Fig. 2E); the myofiber cross-sectional area of the muscles from KR-fed Cth−/− mice was 60% of that of muscles from KR-fed WT (Fig. 2F). Serum biochemistry revealed that the creatinine concentration was significantly lower in KR-fed Cth−/− mice than KR-fed WT or standard diet-fed Cth−/− mice (Fig. 2G), although activity levels of creatine kinase and lactate dehydrogenase were not increased (supplemental Fig. S3, A and B); lactate dehydrogenase activity was rather lower in KR-fed Cth−/− mice than in standard diet-fed Cth−/− mice. When compared with levels in KR-fed WT or standard diet-fed Cth−/− mice, serum levels of blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase were elevated; the total bilirubin level was unaltered; and levels of albumin and triglyceride were decreased in KR-fed Cth−/− mice (supplemental Fig. S3, C–I). The histology of other major organs, including the brain, liver, and kidney, did not exhibit any obvious abnormalities in KR-fed Cth−/− mice (data not shown). In addition, the examination of transverse spinal sections (near the 4th cervical and 4th lumber vertebrae, which may be involved in the contraction of trapezius and rectus femoris muscles, respectively) did not show any specific difference between WT and Cth−/− mice that were fed the KR diet for 2 weeks (supplemental Fig. S4, A and B). KR did not increase the amount of serum homocysteine in Cth−/− mice (supplemental Fig. S5), indicating that the phenotypes were not caused by further accumulation of homocysteine.
When fed KR from 6 and 12 weeks of age, Cth−/− females gradually lost weight and died within 4–5 weeks (Fig. 2, H and I). When fed KR from 8 weeks of age, Cth−/− males lost 15.4% of their body weight within 1 week, whereas WT males gained 6.8% (Fig. 2J), although their daily food intake was not altered significantly (Fig. 2K). Cth−/− mice went lame after 2 weeks on the KR diet (supplemental Video S2) probably due to paralysis of the lower extremities and then became unable to move, eat, or drink and died. Such paralysis was not apparent in C3H/HeJ-Cbs−/− males that were similarly fed KR (supplemental Video S3), whereas they also displayed severe weight loss (16).
Amino Acid Response and Autophagy in Skeletal Muscles of KR-fed Cth−/− Mice
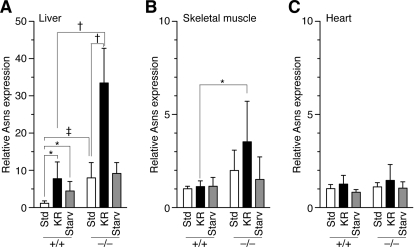
Impaired trans-sulfuration, especially in combination with dietary Cys restriction, may induce an amino acid (starvation) response signal transduction pathway (33) in KR-fed Cth−/− mice. Expression of the asparagine synthetase gene (Asns), a representative of the genes regulated by ATF4 (activating transcription factor 4)-mediated amino acid response (33, 34), was examined in livers, skeletal muscles, and hearts. In WT livers, 1 week on the KR diet and 2 days of starvation significantly increased Asns expression to similar levels (Fig. 3A). In Cth−/− livers, comparable levels of Asns induction were observed even with the standard diet; the levels after feeding on KR were much higher (33.5-fold) (Fig. 3A). Significant increases in Asns expression were observed in skeletal muscles but not hearts (composed of cardiac muscles, another type of striated muscles) of KR-fed Cth−/− mice (Fig. 3, B and C).
FIGURE 3.
Increased expression of asparagine synthetase gene in the liver and muscle of KR-fed Cth−/− mice. The expression of asparagine synthetase (Asns) in A, livers, B, skeletal muscles, and C, hearts of 9-week-old WT and Cth−/− males that were fed the standard (Std) diet, the KR diet (for 1 week) or starved (Starv) for 2 days, was analyzed by RT-PCR and normalized to that of housekeeping hypoxanthine guanine phosphoribosyl transferase 1 (Hprt1). The Asns/Hprt1 expression ratios in the standard diet-fed WT were set at 1. Values are mean ± S.D. (n = 6). *, p < 0.05; ‡, p < 0.01; and †, p < 0.001 in the t test.
Autophagy is required to maintain muscle mass, but its excessive activation leads to myopathic muscle loss (35). Femur skeletal muscle sections were stained with the autophagosomal marker LC3 (microtubule-associated protein 1 light chain 3), p62/sequestosome-1, which is degraded by the autophagy-lysosome system and accumulated upon its collapse, and sarcolemmal dystrophin (Fig. 4). There was no obvious LC3 staining in WT or Cth−/− on the standard diet, but after 1 week on the KR diet, LC3-positive punctation was observed in the peripheral regions of both WT and Cth−/− myofibers (Fig. 4, arrowheads). After 3 weeks on the KR diet, LC3 was diffusely increased within some Cth−/− myofibers (Fig. 4, arrows); similar expression patterns were observed with a different anti-LC3 antibody (data not shown), and the diffuse p62 expression was observed in some of the LC3-negative atrophic Cth−/− myofibers (Fig. 4, asterisks). Muscle inactivation of mTOR (mammalian target of rapamycin), a key regulator of another amino acid response pathway, is shown to reduce muscle dystrophin content (36); however, the sarcolemmal distribution of dystrophin was preserved even in p62-positive atrophic myofibers of the KR-fed Cth−/− mice (Fig. 4). TUNEL-positive apoptotic cells were not observed in femur skeletal muscle sections of KR-fed Cth−/− mice (data not shown).
FIGURE 4.
Accumulation of LC3 and p62 in the myofibers of KR-fed Cth−/− mice. Eight-week-old WT and Cth−/− females were fed without or with KR for 1 or 3 weeks, and femur skeletal muscle sections were stained with DAPI, and anti-LC3, dystrophin, or p62 antibodies. LC3-positive punctation appeared in the peripheral regions of both WT and Cth−/− myofibers after 1 week on the KR diet (arrowheads), and LC3 was diffusely increased within some Cth−/− myofibers after 3 weeks of KR feeding (arrows). Diffuse p62 expression was observed in some Cth−/− myofibers after 3 weeks (asterisks). The structure of dystrophin was preserved even in p62-positive myofibers of KR-fed Cth−/− mice. Bars, 25 μm.
Increased Sensitivity to Oxidative Injury in Cth−/− Mice
Liver is the major locus for CBS/CTH expression (15, 21). Isolated hepatocytes were cultured in cystine- and/or Met-free medium. Cth−/− hepatocytes showed greater sensitivity to cystine depletion compared with WT or Cth+/− hepatocytes; most of them died after 3 days of culture (Fig. 5A). In contrast, the sensitivity to Met depletion did not differ among Cth genotypes for up to 3 days (Fig. 5B). In a 5-day culture, Met supplementation did not rescue cystine-depleted Cth−/− hepatocytes (Fig. 5C). Cth−/− hepatocytes showed greater sensitivity to paraquat, the producer of reactive oxygen species (37); the deleterious effects were concentration- and time-dependent (Fig. 5D, left and right, respectively). To evaluate the hepatic ability to neutralize such cell-damaging reactive oxygen species, the total antioxidant activities (to reduce Cu2+ to Cu+) were measured in WT, Cth+/−, and Cth−/− liver extracts; the activities were 0.496 ± 0.054, 0.506 ± 0.043, and 0.427 ± 0.013 (arbitrary) units per μg protein (n = 5, 7, and 6), respectively. The level of activity was significantly lower in Cth−/− than in WT and Cth+/− (p < 0.05 and p < 0.01, respectively, in the one-way ANOVA).
FIGURE 5.
Increased sensitivity of Cth−/− hepatocytes to cystine depletion and oxidative injury. A and B, hepatocytes isolated from 8-week-old WT (circles), Cth+/− (triangles), and Cth−/− (diamonds) males, were cultured in A, cystine- or B, Met-free DMEM supplemented with 10% dialyzed FBS. Cell survivability was evaluated by measuring cellular ATP levels after the indicated hours of incubation. Survivability at 0 h was set as 100%. C, WT (circles in left panel) and Cth−/− (diamonds in right panel) hepatocytes were cultured for 5 days in 0.2 mm Met-containing (closed symbols) or Met-free (open symbols) medium containing varied concentrations of cystine and 10% dialyzed FBS. Met supplementation did not rescue cystine-depleted hepatocytes in the absence of CTH. D, WT and Cth−/− hepatocytes were treated with paraquat. Concentration- (in a 1-day incubation) and time-dependent (at 100 μm) inhibitory effects on cell survival are shown on the left and right, respectively. Values are mean ± S.D. (n = 4); *, p < 0.05; ‡, p < 0.01; and †, p < 0.001 versus +/+ samples (in A, B, and D) or +Met samples (in C) in the t test.
When 9-week-old WT and Cth−/− males were intraperitoneally injected with paraquat (50 mg/kg body weight), Cth−/− mice showed more sensitivity than WT in the Kaplan-Meier survival analysis (Fig. 6A). One week on the KR diet increased the sensitivity in both WT and Cth−/− males, although the sensitivity was still higher in Cth−/− mice than in WT mice (Fig. 6B). Levels of total glutathione (GSH and oxidized glutathione) in liver extracts were significantly lower in Cth−/− mice than in WT, whereas the levels in femur skeletal muscle, kidney, heart, and brain extracts did not differ significantly between WT and Cth−/− mice (Fig. 6C). However, after 1 week on the KR diet, total glutathione levels in skeletal muscle, liver, heart, and brain became significantly lower in Cth−/− mice than in WT mice, which was most prominent in skeletal muscle as well as liver (Fig. 6C). GSH/total glutathione ratios did not differ with Cth deletion or KR feeding in any tissues tested (Fig. 6D). These results suggest that a glutathione deficiency due to low cyst(e)ine diets may influence the systemic resistance to oxidative injury.
FIGURE 6.
Increased sensitivity of Cth−/− mice to oxidative injury from paraquat and the impact of 1 week on the KR diet on sensitivity and tissue glutathione contents. A, Kaplan-Meier survival analysis after the intraperitoneal injection of paraquat (50 mg/kg) into 9-week-old WT (n = 17) and Cth−/− (n = 15) males. Health status was monitored every 8 h for a week. Cth−/− mice showed higher sensitivity to paraquat than WT (p = 0.038). B, the same as in A except that WT and Cth−/− males (n = 15 each) were fed KR for a week before the injection. KR increased the sensitivity to paraquat in both WT and Cth−/− mice (p = 0.017 and 0.049, respectively). When fed KR for a week, Cth−/− mice still showed greater sensitivity to paraquat than WT (p = 0.031). C, total glutathione (GSH+oxidized glutathione) levels. D, percentages of GSH/total glutathione in skeletal muscle, liver, kidney, heart, and brain extracts in 3-week-old WT (open bars) and Cth−/− (filled bars) females. After 1 week on the KR diet, total glutathione levels decreased in most tissues of WT and Cth−/− and especially Cth−/− mice. Values are mean ± S.D. (n = 5); *, p < 0.05; ‡, p < 0.01; and †, p < 0.001 in the one-way ANOVA. Std, standard.
Cystine Is an Essential Amino Acid in Cth−/− Mice
We examined whether dietary cystine is essential for Cth−/− mice using a protein-free amino acid preparation (16). When fed the Met-free, cystine-containing (1×, 0.37%) diet from 3 weeks of age, WT and Cth-mutants did not grow at all and died after 8–9 weeks (Fig. 7A), which indicates that Met is indeed an essential amino acid. With a normal amount of Met (0.44%) in the diet, WT mice grew normally, even in the total absence of cystine (Fig. 7B, left). In contrast, dietary cystine was essential for Cth−/− mice; normal body weight increase was impaired in Cth−/− mice with the diet containing less than 0.222% (0.6×) cystine (Fig. 7B, right).
FIGURE 7.
Cth−/− mice require dietary cystine as an essential amino acid. A, after weaning at 3 weeks of age, WT (circles; n = 4), Cth+/− (triangles; n = 9), and Cth−/− (diamonds; n = 4) males were fed a protein-free amino acid diet that contained cystine (0.37%: an amount equivalent to the standard diet) but not Met. No developmental body weight increase was observed in any of the mice up until their death after 8–9 weeks. B, WT (left) and Cth−/− (right) males were fed a protein-free amino acid diet that contained Met (0.44%: an amount equivalent to the standard diet) and an amount of cystine (open circles, 0× (0%); open triangles, 0.23× (0.0851%); open diamonds, 0.45× (0.1665%); filled circles, 0.6× (0.222%); filled triangles, 0.8× (0.296%); and filled diamonds, 1× (0.37%)). Cth−/− but not WT mice required cystine as an essential amino acid. Values are mean ± S.D. (n = 5); the asterisks for p values are abbreviated in B (right panel).
DISCUSSION
Cystathioninuria was first described in 1959 by Harris et al. (19), who discovered abnormal secretion of cystathionine in the urine of a 64-year-old, mentally retarded woman. Several lines of evidence indicate that cystathioninuria/cystathioninemia is caused by a deficiency of CTH; for example, 1) CTH activity was markedly reduced in the liver extracts or the cultured lymphoid cell lines of patients with cystathioninuria (38, 39); 2) systemic administration of propargylglycine (an irreversible CTH inhibitor) to rats induced cystathioninemia (40, 41); and 3) multiple Cth mutations were found in unrelated probands with cystathioninuria (42, 43). Here, we provide the first genetic evidence that the deletion of CTH alone causes cystathioninuria and cystathioninemia.
Cth−/− mice developed normally in general; they were free of several severe phenotypes that Cbs−/− mice exhibit, including juvenile lethality, hepatic dysfunction/steatosis, and abnormal lipid metabolism (15, 16). This result is consistent with previous studies that regard cystathioninemia as an apparently benign biochemical anomaly with no visible clinical symptoms (10, 44). Yang et al. (20) recently reported that their Cth−/− mice are also grossly normal except for hypertension, a phenotype not observed in our Cth−/− (supplemental Fig. S1B); this discrepancy may be related to the difference of genetic background (C57BL/6J × 129SvEv mixed versus C57BL/6J (our mice)). However, we revealed several (occasionally severe) phenotypes in Cth−/− mice, some of which can be observed in buried CTH-deficient patients. First, Cth−/− mice required dietary cyst(e)ine as an essential amino acid to protect against acute lethal muscular atrophy. This atrophy was rapidly induced by feeding Cth−/− mice with the low cyst(e)ine diet (Fig. 2, A, H, and I) independently of homocysteine accumulation (supplemental Fig. S5) and had a myogenic (not neurogenic) nature based on several criteria: 1) diffuse rather than grouped atrophy of myofibers was observed (Fig. 2E); 2) the main focus was the proximal (not the distal) skeletal muscle (Fig. 2C and supplemental Video S1); 3) fasciculation was not observed; 4) histology of spinal transverse sections did not reveal any abnormality (supplemental Fig. S4), even in anterior horn cells that are severely impaired in amyotrophic lateral sclerosis-type neurogenic muscular atrophy; 5) serum creatinine levels were decreased (Fig. 2G), whereas blood urea nitrogen levels were elevated (supplemental Fig. S4C) in KR-fed Cth−/− mice compared with standard diet-fed Cth−/− mice; and 6) formation of LC3-positive autophagosomes was observed in myofibers of KR-fed Cth−/− mice (Fig. 4).
To our knowledge, this is the first example of diet-induced myopathy; its early onset (Fig. 2, A, H, and I) and severity (supplemental Videos S1 and S2) were exceptional. Serum levels of creatine kinase and lactate dehydrogenase activity were not elevated (supplemental Fig. S3, A and B); some types of myopathy are not associated with raised creatine kinase/lactate dehydrogenase levels (45), which may mainly involve the autophagic loss (atrophy) of myofibers rather than necrotic/apoptotic loss. Indeed, the increased Asns expression in livers and muscles (Fig. 3, A and B) suggests the activation of amino acid response, and the accumulation of p62 in some Cth−/− myofibers (Fig. 4, asterisks) reflects the accumulation of polyubiquitinated aggregates after excessive autophagy that had depleted autophagosomal components such as LC3 via the autophagy-lysosome system (46). The amino acid response in muscles is likely mediated by ATF4 rather than mTOR because Asns expression was induced (Fig. 3B), and the structure of dystrophin was intact in p62-positive (autophagy-defective) myofibers of KR-fed Cth−/− mice (Fig. 4) (33, 34, 36). TUNEL-positive apoptotic cells were not observed in muscles of KR-fed Cth−/− mice (data not shown), and therefore, this acute myopathy may reflect the rapid metabolic turnover of muscular proteins in WT and its failure in Cth−/− mice fed the low-cyst(e)ine diet. The reason for difference in sensitivity to the diet between Cth−/− and C3H/HeJ-Cbs−/− mice (supplemental Videos S2 and S3) remains unknown, and some other factor(s) such as markedly elevated levels of cystathionine in Cth−/− mice could be implicated in acute myopathy via unknown mechanisms. Remarkable hepatic Asns induction and in KR-fed Cth−/− mice (Fig. 3A) suggests that liver serves as a major source of Cys by autophagic degradation of hepatic proteins in cases of systemic Cys deficiency and that heart is protected from such degradation (Fig. 3C). Induction of Asns (Fig. 3B) and greater reduction in glutathione contents (Fig. 6C) in skeletal muscle of KR-fed Cth−/− mice suggest that skeletal muscle is an organ more vulnerable to Cys depletion than heart (Figs. 3C and 6C). Serum levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase were elevated slightly, whereas those of albumin and triglyceride were decreased in KR-fed Cth−/− mice (supplemental Fig. S3, D–F, H, and I), indicating mild hepatic failure in KR-fed Cth−/− mice. Nonetheless, this disease model may be useful for evaluating new drugs and therapies against currently incurable progressing myopathy.
The second finding is that Cth−/− mice were hyperhomocysteinemic like Cbs−/− mice, but not hypermethioninemic unlike Cbs−/− mice (Table 1). Hypermethioninemia is the most prominent biochemical feature of CBS-deficient patients (29). For this reason, and technical difficulties in measuring (total) homocysteine levels in filter-spotted blood specimens, the screening of newborns for homocysteinemia in Japan and some parts of the United States and European Union (11, 12), has detected high Met levels using Guthrie's bacterial inhibition assay (11, 12); the cut-off value for requesting another analysis was 1–2 mg/dL (67–134 μm) (47), whereas its normal range was 13–43 μm (48). Therefore, CTH-deficient patients are predicted to pass the screening even though they may suffer from hyperhomocysteinemia. An elevated plasma homocysteine level is considered an independent risk factor for cardiovascular diseases (10, 49); mild or moderate hyperhomocysteinemia (total homocysteine concentration of 15–50 μm) is found in up to 40% of patients with myocardial infarction, stroke, or venous thrombosis (50), and a 5-μm increase leads to a 30% increase in cardiovascular risk (51). In contrast to such epidemiological evidence, high plasma levels of Met but not homocysteine were correlated with atherogenesis in apolipoprotein E-deficient mice (52), and drug-inducible transgenic CBS expression in Cbs−/− mice rescued neonatal lethality without lowering serum levels of homocysteine (53). Our data is consistent with such studies in that elevated levels of homocysteine itself are not pathogenic in mice, although the threshold effect for homocysteine (54) may contribute to pathogenic phenotypes in mice; its serum levels in Cbs−/− are somewhat higher than Cth−/− mice (209 versus 145 μm; Table 1).
The third finding is the sensitivity to oxidative injury in Cth−/− mice. Both Cth−/− hepatocytes and mice displayed increased sensitivity to oxidative stress from paraquat (Figs. 5D and 6A). The sensitivity was more prominent when mice were fed the KR diet (Fig. 6B); this may be, in part, due to decreased levels of glutathione in livers (Fig. 6C) because elevated hepatic glutathione levels can act against paraquat-induced oxidative injury in mice (55). Reduced serum levels of antioxidative taurine in Cth−/− mice (Table 1) also may participate in the systemic vulnerability to oxidative injury. Decreased levels of glutathione in muscles (Fig. 6C) may accelerate myopathy in KR-fed Cth−/− mice (56). In human liver cells, approximately half of the intracellular glutathione was considered to derive from trans-sulfuration (57). Levels of CTH activity in liver and lenses were significantly lower in aged rodents than younger rodents (58–60), and inhibition of CTH by propargylglycine caused glutathione depletion in lenses and led to cataractogenesis in vitro (60). An adequate supply of cyst(e)ine may be more important for older people who accumulate oxidative damage. In contrast, the enhanced flux of Met to the trans-sulfuration pathway that associates with increased Cth expression may contribute to the longevity found in Ames dwarf mice (61). CBS is expressed highly in both human and mouse brains (15, 62), and CTH activity is 100-fold higher in the human brain than mouse brain (63). Mental retardation is commonly observed in CBS-deficient patients (10), and Cbs−/− mice displayed impaired learning in the passive avoidance test (16). Taken together, the antioxidative property of trans-sulfuration might contribute to the maintenance of the central nervous system (64) and quality of life, which will be pursued using (long lived) Cth−/− mice.
In summary, we analyzed Cth−/− mice as an animal model of cystathioninemia/cystathioninuria. CTH-deficient patients may pass Guthrie's method-based newborn screening for homocysteinemia; however, they may suffer from hyperhomocysteinemia and severe pathological conditions, including acute myopathy and vulnerability to oxidative injury, especially in cases of dietary Cys deficiency. The prevalent use of tandem mass spectrometry to screen newborns with dried blood spots (65, 66) is anticipated to discover currently buried innate metabolic disorders including cystathioninemia. Moreover, innate and acquired hepatic failure accompanied with decreased CTH activities may underlie unidentified myopathy.
Supplementary Material
Acknowledgments
We thank Drs. Hideaki Tomura and Fumikazu Okajima (Gunma University) for the hepatocyte culture; Drs. Hiroyuki Katoh and Hiroki Kiyohara (Gunma University) for the radiograph; and Dr. Naoto Matsuyama (Alfresa Pharma) for homocysteine measurement.
This work was supported by Grants-in-aid for Scientific Research 18590047 and 22590292 (to I. I.) and 22790219 (to N. A.), Leading Project for Biosimulation (to M. S.), and Creative Science Research 17GS0419 (to M. S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a research grant for Feasibility Study (to I. I.) from the Japan Science and Technology Agency; and research grants from Gunma University (to I. I. and N. A.).

The on-line version of this article (available at http://www.jbc.org) contains Table S1, Figs. S1–S5, and Videos S1–S3.
- CBS
- cystathionine β-synthase
- BP
- blood pressure
- CTH
- cystathionine γ-lyase
- GSH
- reduced glutathione
- WT
- wild-type
- ANOVA
- analysis of variance
- KR
- Kojin Rayon®.
REFERENCES
- 1.Finkelstein J. D. (2000) Semin. Thromb. Hemost. 26, 219–225 [DOI] [PubMed] [Google Scholar]
- 2.Stipanuk M. H. (2004) Annu. Rev. Nutr. 24, 539–577 [DOI] [PubMed] [Google Scholar]
- 3.Sturman J. A., Gaull G., Raiha N. C. (1970) Science 169, 74–76 [DOI] [PubMed] [Google Scholar]
- 4.Zlotkin S. H., Anderson G. H. (1982) Pediatr. Res. 16, 65–68 [DOI] [PubMed] [Google Scholar]
- 5.Viña J., Vento M., García-Sala F., Puertes I. R., Gascó E., Sastre J., Asensi M., Pallardó F. V. (1995) Am. J. Clin. Nutr. 61, 1067–1069 [DOI] [PubMed] [Google Scholar]
- 6.Laidlaw S. A., Kopple J. D. (1987) Am. J. Clin. Nutr. 46, 593–605 [DOI] [PubMed] [Google Scholar]
- 7.Viña J., Gimenez A., Puertes I. R., Gasco E., Viña J. R. (1992) Br. J. Nutr. 68, 421–429 [DOI] [PubMed] [Google Scholar]
- 8.Szabó C. (2007) Nat. Rev. Drug Discov. 6, 917–935 [DOI] [PubMed] [Google Scholar]
- 9.Shintani T., Iwabuchi T., Soga T., Kato Y., Yamamoto T., Takano N., Hishiki T., Ueno Y., Ikeda S., Sakuragawa T., Ishikawa K., Goda N., Kitagawa Y., Kajimura M., Matsumoto K., Suematsu M. (2009) Hepatology 49, 141–150 [DOI] [PubMed] [Google Scholar]
- 10.Mudd S. H., Levy H. L., Kraus J. P. (2001) The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D, eds) 8th Ed., pp. 2007–2056, McGraw-Hill, New York [Google Scholar]
- 11.Kaye C. I., Accurso F., La Franchi S., Lane P. A., Northrup H., Pang S., Schaefer G. B. (2006) Pediatrics 118, 1304–1312 [DOI] [PubMed] [Google Scholar]
- 12.Naughten E. R., Yap S., Mayne P. D. (1998) Eur. J. Pediatr. 157, S84–87 [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H., Jiang X., Yang F., Gaubatz J. W., Ma L., Magera M. J., Yang X., Berger P. B., Durante W., Pownall H. J., Schafer A. I. (2003) Blood 101, 3901–3907 [DOI] [PubMed] [Google Scholar]
- 15.Namekata K., Enokido Y., Ishii I., Nagai Y., Harada T., Kimura H. (2004) J. Biol. Chem. 279, 52961–52969 [DOI] [PubMed] [Google Scholar]
- 16.Akahoshi N., Kobayashi C., Ishizaki Y., Izumi T., Himi T., Suematsu M., Ishii I. (2008) Hum. Mol. Genet. 17, 1994–2005 [DOI] [PubMed] [Google Scholar]
- 17.Frimpter G. W., Haymovitz A., Horwith M. (1963) N. Engl. J. Med. 268, 333–339 [DOI] [PubMed] [Google Scholar]
- 18.Vargas J. E., Mudd S. H., Waisbren S. E., Levy H. L. (1999) Am. J. Obstet. Gynecol. 181, 753–755 [DOI] [PubMed] [Google Scholar]
- 19.Harris H., Penrose L. S., Thomas D. H. (1959) Ann. Hum. Genet. 23, 442–453 [DOI] [PubMed] [Google Scholar]
- 20.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A. K., Mu W., Zhang S., Snyder S. H., Wang R. (2008) Science 322, 587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii I., Akahoshi N., Yu X. N., Kobayashi Y., Namekata K., Komaki G., Kimura H. (2004) Biochem. J. 381, 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chui D., Oh-Eda M., Liao Y. F., Panneerselvam K., Lal A., Marek K. W., Freeze H. H., Moremen K. W., Fukuda M. N., Marth J. D. (1997) Cell 90, 157–167 [DOI] [PubMed] [Google Scholar]
- 23.Yagi T., Nada S., Watanabe N., Tamemoto H., Kohmura N., Ikawa Y., Aizawa S. (1993) Anal. Biochem. 214, 77–86 [DOI] [PubMed] [Google Scholar]
- 24.Ishii I., Friedman B., Ye X., Kawamura S., McGiffert C., Contos J. J., Kingsbury M. A., Zhang G., Brown J. H., Chun J. (2001) J. Biol. Chem. 276, 33697–33704 [DOI] [PubMed] [Google Scholar]
- 25.Akahoshi N., Izumi T., Ishizaki Y., Ishii I. (2006) Biol. Pharm. Bull. 29, 1799–1802 [DOI] [PubMed] [Google Scholar]
- 26.Mudd S. H., Finkelstein J. D., Refsum H., Ueland P. M., Malinow M. R., Lentz S. R., Jacobsen D. W., Brattström L., Wilcken B., Wilcken D. E., Blom H. J., Stabler S. P., Allen R. H., Selhub J., Rosenberg I. H. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1704–1706 [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama N., Yamaguchi M., Toyosato M., Takayama M., Mizuno K. (2001) Clin. Chem. 47, 2155–2157 [PubMed] [Google Scholar]
- 28.Melnyk S., Pogribna M., Pogribny I., Hine R. J., James S. J. (1999) J. Nutr. Biochem. 10, 490–497 [DOI] [PubMed] [Google Scholar]
- 29.Stabler S. P., Steegborn C., Wahl M. C., Oliveriusova J., Kraus J. P., Allen R. H., Wagner C., Mudd S. H. (2002) Metabolism 51, 981–988 [DOI] [PubMed] [Google Scholar]
- 30.Pitkin R. M. (2007) Am. J. Clin. Nutr. 85, 285S–288S [DOI] [PubMed] [Google Scholar]
- 31.Jacques P. F., Selhub J., Bostom A. G., Wilson P. W., Rosenberg I. H. (1999) N. Engl. J. Med. 340, 1449–1454 [DOI] [PubMed] [Google Scholar]
- 32.Boushey C. J., Beresford S. A., Omenn G. S., Motulsky A. G. (1995) JAMA 274, 1049–1057 [DOI] [PubMed] [Google Scholar]
- 33.Kilberg M. S., Shan J., Su N. (2009) Trends Endocrinol. Metab. 20, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., Ron D. (2003) Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 35.Masiero E., Agatea L., Mammucari C., Blaauw B., Loro E., Komatsu M., Metzger D., Reggiani C., Schiaffino S., Sandri M. (2009) Cell Metab. 10, 507–515 [DOI] [PubMed] [Google Scholar]
- 36.Risson V., Mazelin L., Roceri M., Sanchez H., Moncollin V., Corneloup C., Richard-Bulteau H., Vignaud A., Baas D., Defour A., Freyssenet D., Tanti J. F., Le-Marchand-Brustel Y., Ferrier B., Conjard-Duplany A., Romanino K., Bauché S., Hantaï D., Mueller M., Kozma S. C., Thomas G., Rüegg M. A., Ferry A., Pende M., Bigard X., Koulmann N., Schaeffer L., Gangloff Y. G. (2009) J. Cell Biol. 187, 859–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suntres Z. E. (2002) Toxicology 180, 65–77 [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein J. D., Mudd S. H., Irreverre F., Laster L. (1966) Proc. Natl. Acad. Sci. U.S.A. 55, 865–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bittles A. H., Carson N. A. (1974) J. Med. Genet. 11, 121–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Triguero A., Barber T., García C., Puertes I. R., Sastre J., Viña J. R. (1997) Br. J. Nutr. 78, 823–831 [DOI] [PubMed] [Google Scholar]
- 41.Awata S., Nakayama K., Kodama H. (1984) Biochem. Int. 8, 171–179 [PubMed] [Google Scholar]
- 42.Wang J., Hegele R. A. (2003) Hum. Genet. 112, 404–408 [DOI] [PubMed] [Google Scholar]
- 43.Kraus J. P., Hasek J., Kozich V., Collard R., Venezia S., Janosíková B., Wang J., Stabler S. P., Allen R. H., Jakobs C., Finn C. T., Chien Y. H., Hwu W. L., Hegele R. A., Mudd S. H. (2009) Mol. Genet. Metab. 97, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry T. L., Hardwick D. F., Hansen S., Love D. L., Israels S. (1968) N. Engl. J. Med. 278, 590–592 [DOI] [PubMed] [Google Scholar]
- 45.Nambu M., Kaneko K., Iijima T. (1996) Liver 16, 19–22 [DOI] [PubMed] [Google Scholar]
- 46.Komatsu M., Waguri S., Koike M., Sou Y. S., Ueno T., Hara T., Mizushima N., Iwata J., Ezaki J., Murata S., Hamazaki J., Nishito Y., Iemura S., Natsume T., Yanagawa T., Uwayama J., Warabi E., Yoshida H., Ishii T., Kobayashi A., Yamamoto M., Yue Z., Uchiyama Y., Kominami E., Tanaka K. (2007) Cell 131, 1149–1163 [DOI] [PubMed] [Google Scholar]
- 47.Peterschmitt M. J., Simmons J. R., Levy H. L. (1999) N. Engl. J. Med. 341, 1572–1576 [DOI] [PubMed] [Google Scholar]
- 48.Harvey, Mudd S., Braverman N., Pomper M., Tezcan K., Kronick J., Jayakar P., Garganta C., Ampola M. G., Levy H. L., McCandless S. E., Wiltse H., Stabler S. P., Allen R. H., Wagner C., Borschel M. W. (2003) Mol. Genet. Metab. 79, 6–16 [DOI] [PubMed] [Google Scholar]
- 49.Herrmann W., Knapp J. P. (2002) Clin. Lab. 48, 471–481 [PubMed] [Google Scholar]
- 50.Dayal S., Bottiglieri T., Arning E., Maeda N., Malinow M. R., Sigmund C. D., Heistad D. D., Faraci F. M., Lentz S. R. (2001) Circ. Res. 88, 1203–1209 [DOI] [PubMed] [Google Scholar]
- 51.Weiss N., Heydrick S., Zhang Y. Y., Bierl C., Cap A., Loscalzo J. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 34–41 [DOI] [PubMed] [Google Scholar]
- 52.Troen A. M., Lutgens E., Smith D. E., Rosenberg I. H., Selhub J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15089–15094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L., Chen X., Tang B., Hua X., Klein-Szanto A., Kruger W. D. (2005) Hum. Mol. Genet. 14, 2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta S., Kühnisch J., Mustafa A., Lhotak S., Schlachterman A., Slifker M. J., Klein-Szanto A., High K. A., Austin R. C., Kruger W. D. (2009) FASEB J. 23, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berruyer C., Martin F. M., Castellano R., Macone A., Malergue F., Garrido-Urbani S., Millet V., Imbert J., Duprè S., Pitari G., Naquet P., Galland F. (2004) Mol. Cell. Biol. 24, 7214–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mårtensson J., Meister A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosharov E., Cranford M. R., Banerjee R. (2000) Biochemistry 39, 13005–13011 [DOI] [PubMed] [Google Scholar]
- 58.Nakata K., Kawase M., Ogino S., Kinoshita C., Murata H., Sakaue T., Ogata K., Ohmori S. (1996) Mech. Ageing Dev. 90, 195–207 [DOI] [PubMed] [Google Scholar]
- 59.Ferrer J. V., Gascó E., Sastre J., Pallardó F. V., Asensi M., Viña J. (1990) Biochem. J. 269, 531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sastre J., Martín J. A., Gómez-Cabrera M. C., Pereda J., Borrás C., Pallardó F. V., Viña J. (2005) Free Radic. Biol. Med. 38, 575–582 [DOI] [PubMed] [Google Scholar]
- 61.Uthus E. O., Brown-Borg H. M. (2006) Mech. Ageing Dev. 127, 444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ichinohe A., Kanaumi T., Takashima S., Enokido Y., Nagai Y., Kimura H. (2005) Biochem. Biophys. Res. Commun. 338, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 63.Diwakar L., Ravindranath V. (2007) Neurochem. Int. 50, 418–426 [DOI] [PubMed] [Google Scholar]
- 64.Troen A. M. (2005) Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1140–1151 [DOI] [PubMed] [Google Scholar]
- 65.Banta-Wright S. A., Steiner R. D. (2004) J. Perinat. Neonatal. Nurs. 18, 41–58 [DOI] [PubMed] [Google Scholar]
- 66.Gan-Schreier H., Kebbewar M., Fang-Hoffmann J., Wilrich J., Abdoh G., Ben-Omran T., Shahbek N., Bener A., Al Rifai H., Al Khal A. L., Lindner M., Zschocke J., Hoffmann G. F. (2010) J. Pediatr. 156, 427–432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.