Abstract
A GABAA receptor (GABAAR) α1 subunit mutation, A322D (AD), causes an autosomal dominant form of juvenile myoclonic epilepsy (ADJME). Previous studies demonstrated that the mutation caused α1(AD) subunit misfolding and rapid degradation, reducing its total and surface expression substantially. Here, we determined the effects of the residual α1(AD) subunit expression on wild type GABAAR expression to determine whether the AD mutation conferred a dominant negative effect. We found that although the α1(AD) subunit did not substitute for wild type α1 subunits on the cell surface, it reduced the surface expression of α1β2γ2 and α3β2γ2 receptors by associating with the wild type subunits within the endoplasmic reticulum and preventing them from trafficking to the cell surface. The α1(AD) subunit reduced surface expression of α3β2γ2 receptors by a greater amount than α1β2γ2 receptors, thus altering cell surface GABAAR composition. When transfected into cultured cortical neurons, the α1(AD) subunit altered the time course of miniature inhibitory postsynaptic current kinetics and reduced miniature inhibitory postsynaptic current amplitudes. These findings demonstrated that, in addition to causing a heterozygous loss of function of α1(AD) subunits, this epilepsy mutation also elicited a modest dominant negative effect that likely shapes the epilepsy phenotype.
Keywords: Confocal Microscopy, Fluorescence Resonance Energy Transfer (FRET), GABA Receptors, Genetic Diseases, Membrane Trafficking, Neuron, Protein Assembly, Electrophysiology, Epilepsy, Flow Cytometry
Introduction
GABAARs2 are ligand-gated ion channels that provide the major source of inhibitory control to the mammalian central nervous system. Each GABAAR is a pentamer whose five subunits arise from seven subunit families that contain multiple subtype isoforms. Neurons preferentially express GABAARs composed of distinct combinations of subunit isoforms in different brain regions at well defined times in development (1–3). At maturity, the most prevalent GABAAR throughout the brain consists of two α1 subunits, two β2 subunits, and one γ2 subunit in a β2-α1-β2-α1-γ2 assembly (4–6). To date, 13 autosomal dominant mutations in GABAAR subunit genes have been associated with different epilepsy syndromes (7).
The missense AD mutation in the GABAAR α1 subunit gene (GABRA1) causes ADJME (8), a monogenic form of a common epilepsy syndrome that begins at a distinct developmental time point (adolescence) and confers myoclonic, generalized tonic-clonic, and absence seizures as well as neuropsychiatric co-morbidities (9). We demonstrated previously that the AD mutation, which substitutes a negatively charged aspartate for a neutral alanine within the M3 transmembrane domain, causes the α1(AD) subunit to misfold with altered topology (10). Cells rapidly degrade the misfolded α1(AD) subunit through both proteasome- and lysosome-mediated processes (10, 11). Therefore, the α1(AD) subunit is expressed at substantially lower levels than the wild type α1 subunit. GABAARs that do incorporate the residual, nondegraded α1(AD) subunits exhibit substantially altered electrophysiological properties (8, 12, 13). Therefore, a major consequence of heterozygous expression of the AD mutation is a heterozygous loss of functional α1 subunits.
However, it is unlikely that α1 subunit haploinsufficiency alone explains the entire ADJME phenotype. Heterozygous Gabra1 knock-out mice do not exhibit spontaneous behavioral seizures. Moreover, a recently discovered GABRA1 frameshift mutation that causes complete elimination of the mutant α1 subunit protein causes a much milder form of epilepsy than juvenile myoclonic epilepsy (14, 15). These findings suggest that despite being misfolded and substantially degraded, the residual α1(AD) subunit likely has other pathophysiological actions that lead to a more severe phenotype than would be caused by haploinsufficiency alone. Recently, an extremely small dominant negative effect caused by another epilepsy-associated GABAAR subunit mutation, γ2(R43Q), proved to be critical for causing the epilepsy phenotype (16–19). Therefore, we set out to determine whether the α1(AD) subunit altered wild type GABAAR expression, physiology, or cellular viability.
MATERIALS AND METHODS
Cell Culture
HEK293T cells were purchased from American Type Culture Collection and were cultured at 37 °C in 5% CO2, 95% air in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) with 10% fetal bovine serum (FBS, Invitrogen) and 100 IU/ml streptomycin and penicillin (Invitrogen). Cells were replated twice weekly.
We adapted our neuron culture protocol from one described previously (20). Briefly, we dissected cerebral cortices from the brains of embryonic day 18 (E18) Sprague-Dawley rat pups. After trypsin digestion and trituration, we plated 6.7 × 105 neurons in 35-mm dishes on polyornithine-coated coverslips. The neurons were initially cultured in DMEM containing 80 μm glutamine, 8% FBS, and 8% F-12 nutrient mixture (Invitrogen). After 3 days, we added cytosine arabinoside to a final concentration of 1 μm to control glial proliferation (Sigma). On the following day, we exchanged the media with Neurobasal media that contained B27 supplement (Invitrogen). One-half volumes of Neurobasal media were exchanged three times/week.
Expression Vectors and Transfection
The pcDNA3.1 plasmids containing cDNAs that encode human α1, α1(AD), β2S, and γ2S GABAAR receptor subunits were described previously (10, 13, 21). The DNA encoding the α3 subunit was obtained from Origin Technologies, Inc. It was subcloned into the pcDNA3.1 vector and modified to match the coding sequences found in the GenBankTM and Swiss Protein Databases. The cDNA encoding hemagglutinin (HA) epitope-tagged transforming growth factor α (TGFHA) protein was generously contributed by Dr. Robert Coffey, Vanderbilt University. The pmaxGFP vector was from Lonza. The wild type and dominant negative K44A dynamin1 plasmids were a kind gift from Dr. Pietro De Camilli (Yale University). We purchased the pLVX-IRES-ZsGreen1 bicistronic vector from Clontech.
In this study, we numbered amino acids starting from the initiation methionine in the signal peptide. Using standard molecular biology techniques, we deleted the codon that encodes leucine 31 (leucine 4 as numbered from the putative N terminus of the mature peptide) from the human α1 subunit cDNA (α1h) to make a cDNA encoding the rat α1 subunit (α1r). We also introduced the nucleotides encoding the hemagglutinin (HA) epitope tag (YPYDVPDYA) between the codons encoding the 31st and 32nd amino acids (fourth and fifth amino acids as numbered from the putative N terminus of the mature peptide) of the α1h subunit to form the α1HA subunit. Similarly, we inserted the HA epitope in the homologous positions of the β2 and γ2 sequences to form the β2HA and γ2HA subunits. We made the AD mutation in the α1HA and α1r subunit cDNA using the QuikChange site-directed mutagenesis kit (Stratagene). To make constructs for use in neuronal transfections, we inserted the cDNA encoding the human α1, α1(AD), α1HA, and α1(AD)HA subunits into the pLVX-IRES-ZsGreen1 vector.
We transfected HEK293T cells in 6-cm dishes with 3 μg of total cDNA using the FuGENE 6 transfection reagent (Roche Diagnostics, 3 μl/μg DNA). The amount of cDNA encoding each subunit is listed in the figure legends. If the total GABAAR subunit cDNA was less than 3 μg, we added sufficient empty pcDNA3.1 vector so that the total DNA transfected equaled 3 μg. Neurons were transfected 10 days after plating (DIV10) using a calcium phosphate method as described elsewhere (22).
Antibodies
The mouse monoclonal anti-α1 subunit antibody N95/35 was from the University of California, Davis/National Institutes of Health NeuroMab Facility. The mouse monoclonal anti-human GABAAR α1 (α1h) subunit antibody (BD24), the rabbit polyclonal anti-rat α1 subunit antibody (α1r, 06-868, lot 026K4848), the rabbit polyclonal anti-β2 subunit antibody (AB5561, lot LV1512412), and the mouse monoclonal anti-neuronal nuclei (NeuN) antibody (clone A60) were from Millipore. The rabbit polyclonal anti-GABAAR α3 subunit antibody (AGA-003, lot AN-02) was from Alamone. The unconjugated and Alexa 647- (A647) and Alexa 555 (A555)-conjugated mouse monoclonal anti-HA antibodies (HA-555, HA-647) were from Covance (clone 16B12). The rabbit polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, AB948, lot 708812) and mouse monoclonal anti-sodium potassium ATPase α1 subunit (ATPase, AB7671) were from Abcam. For the flow cytometry experiments, we directly conjugated the mouse anti-α1h antibody to the A647 fluorphore (α1h-647) using a commercial monoclonal antibody labeling reagent (Invitrogen).
Biotinylation and Western Blot Assays
The biotinylation and Western blot assays have been described previously (13, 23). Briefly, live cells were washed in PBS containing 1 mm CaCl2 and 0.5 mm MgCl2 (PBS-CM) and incubated with the membrane-impermeable, amine-reactive biotinylation reagent, NHS-SS-biotin (3 mm, Thermo), for 15 min at 4 °C. The biotinylation reaction was quenched by washing the cells three times with PBS-CM containing 100 mm glycine.
We lysed the biotinylated cells in modified radioimmunoassay solution (RIPA, 20 mm Tris, pH 7.4, 1% Triton X-100, 250 mm NaCl, 0.5% deoxycholate, 0.1% SDS) that also contained 1 pellet of protease inhibitor per 10 ml (Roche Diagnostics). The lysates were centrifuged at 10,000 × g for 30 min, and the protein concentration was measured using a bicinchoninic acid-based assay (Thermo). To determine total GABAAR subunit expression, we applied the unpurified lysates directly to a 10% SDS-polyacrylamide gel. To measure surface protein, we incubated equal masses of biotinylated lysates with immobilized neutravidin beads (Thermo) overnight. The neutravidin beads were washed with RIPA buffer, and the biotinylated protein was liberated by incubation with Laemmli sample buffer and fractionated on a 10% SDS-polyacrylamide gel. We confirmed that the proteins of interest did not nonspecifically adhere to the neutravidin beads by also incubating lysates from nonbiotinylated cells with the beads before applying them to SDS-PAGE (data not shown). In each experiment, we confirmed that the biotinylation reagent did not modify intracellular proteins by measuring GAPDH immunoreactivity in the neutravidin-purified lysates (data not shown).
After SDS-PAGE, we electrotransferred the proteins to nitrocellulose membranes. For the Western blots using the anti-α1, -α1h, -α3, -β2, -ATPase, and -GAPDH antibodies, we blocked the membranes with Tris-buffered saline containing 0.1% Tween (TTBS) and 5% nonfat milk. We blocked the Western blots using the anti-HA antibody with PBS containing 0.1% Tween and 0.5% bovine serum albumin (BSA, Sigma).
After incubation with the primary antibody, the immunoblots were incubated with a horseradish peroxidase-coupled goat anti-mouse or anti-rabbit secondary antibody (Jackson ImmunoResearch, 1:5000 dilution) and then visualized with a chemiluminescent detection system (Amersham Biosciences) using a digital imager (Alpha Innotech). The integrated intensities of the Western blot bands were calculated using the ImageJ software (National Institutes of Health). We confirmed that the integrated intensities of all the bands remained in the linear range of detection by loading three different masses of protein on the gels and performing a linear regression through the origin of the integrated intensity versus protein mass. The integrated intensities of all proteins were normalized to the loading control (ATPase).
Flow Cytometry and Fluorescent Resonance Energy Transfer (FRET)
Flow cytometry and FRET were performed as described previously (24, 25). Briefly, transfected cells were detached from the plastic plates by incubating them with trypsin for 1 min at room temperature, a process that does not cause degradation of surface GABAAR protein (24). All subsequent steps were performed at 0 °C. We stopped the trypsinization by adding 5 ml of FACS buffer (PBS containing 2% FBS and 0.05% sodium azide). The cells were pelleted and placed into 96-well plates. To measure surface protein expression, the cells were stained without fixation, and to measure total protein, the cells were treated with a commercial fixation/permeabilization reagent (BD Biosciences) before staining. The cells were stained with the anti-α1h-647 (1:100), -α1r (1:50), -α3 (1:50), and HA-555 or HA-647 (1:100) antibodies for 1 h. The cells stained with the anti-α1r and -α3 antibodies were then stained with A647-conjugated goat anti-rabbit antibody (1:250, Invitrogen). In each experiment, we confirmed that these dilutions represented saturating concentrations of the respective antibodies by also staining negative control cells and wild type GABAAR cells with a 2-fold higher concentration of antibody and verifying that the fluorescence did not substantially change (data not shown). After staining, the cells were fixed with 2% paraformaldehyde in PBS.
For each sample, we analyzed 10,000 cells on an LSR II digital flow cytometer (BD Biosciences). We identified viable cells based upon their forward and side scatter properties (data not shown) that were originally determined from staining with the membrane-impermeable dye, 7-aminoactinomycin D (Invitrogen) (24, 25). The A647 fluorophore was excited by a 635 nm laser and detected with a 675/20 bandpass filter, and the A555 fluorophore was excited with a 535 nm laser and detected with a 575/26 bandpass filter. GFP was excited by a 488-nm laser and detected using a 530/30 bandpass filter. In each of the samples, we measured the mean GFP, A555, and A647 fluorescence in viable cells using the FlowJo 7.2 software (Treestar) and subtracted the mean fluorescence obtained from negative control cells.
We measured the FRET between the samples stained with A555-coupled antibody (donor) and a A647-coupled antibody (acceptor) by exciting the A555 fluorophore with the 535-nm laser and detecting the FRET to the A647 fluorophore using 670/20 bandpass filter. To eliminate the fluorescence resulting from direct 535 nm stimulation to the A555 and A647 fluorophores from the FRET signal, we performed spectral compensation using singly stained samples and the FlowJo 7.2 software (Treestar). We then subtracted the mean FRET fluorescence from the samples stained with only the A647 fluorophore from those stained with both the A555 and A647 fluorophores to calculate the specific mean FRET fluorescence.
Confocal Microscopy and Image Analysis
Microscopic analyses were performed using the coverslips on which the neurons were originally plated. On DIV17, 7 days after transfection, we washed the neurons three times in PBS and then fixed them in 4% paraformaldehyde, 4% sucrose in PBS for 15 min at room temperature. We washed the neurons three times with PBS and then incubated them with 0.1% Triton X-100 in PBS for 5 min before washing three more times with PBS. We blocked nonspecific binding by incubating the fixed neurons with 0.5% BSA in PBS for 1 h at room temperature. We stained the neurons with either anti-NeuN (1:200) or anti-α1h antibody (1:1000) diluted in 0.2% BSA in PBS overnight at 4 °C. The following day, we washed the neurons five times with PBS and then incubated them for 1 h with Alexa 546-conjugated goat anti-mouse antibody diluted in PBS containing 0.2% BSA. We washed them with PBS five more times and counterstained them with 4′,6-diamidino-2-phenylindole (DAPI).
Confocal imaging was performed through the use of the Vanderbilt University Medical Center Cell Imaging Shared Resource. We visualized the neurons with an Olympus FV1000 confocal microscope using a 60×/1.45 numerical aperture plan-apochromat objective. We used the recommended Olympus laser and filter combinations to separately detect DAPI, ZsGreen1, and A546 fluorescence and used singly stained specimens to verify that these fluorophores did not spectrally overlap. We adjusted the pinhole of all channels to obtain 3-μm sections, and we obtained a single section at the level of the nucleus. In each experiment, we adjusted the laser intensity and detector sensitivity to utilize the full linear range of detection, and we used the same settings for all the samples acquired during the experiment. Images were obtained with 12-bit, 1024 × 1024 pixel resolution.
To determine the percentage of cells in the culture that consisted of neurons, we stained the cells with an antibody against NeuN. In three different regions of the slide, we determined the fraction of cells stained with DAPI that were also stained with NeuN. To determine the percentage of neurons transfected, we calculated the fraction of neurons stained with NeuN that also expressed ZsGreen1.
To determine the expression of recombinant α1h and α1(AD)h subunits, we visually identified several ZsGreen1-expressing neurons from each culture. We then acquired confocal images of each neuron. We analyzed the images off line using the ImageJ software. We determined the mean fluorescence of a 5-pixel wide line drawn either through the nucleus (for ZsGreen1 fluorescence) or though the soma excluding the nucleus (for α1h fluorescence). We normalized the fluorescence of each neuron to the mean fluorescence of the neurons transfected with wild type α1h that were imaged on the same day.
Electrophysiology
We recorded mIPSCs on pyramidal neurons on DIV17, 7 days after transfection. The coverslips that contained the neurons were placed in 35-mm plastic culture dishes and covered with external recording solution that contained the following (in mm): NaCl (142), HEPES (10), KCl (8), CaCl2 (1), glucose (10), and MgCl2 (6), pH 7.4. We added 1 μm tetrodotoxin, 40 μm d-(−)-2-amino-5-phosphonovaleric acid, 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione, and 1 μm CGP52432 to the external solution to block action potentials and NMDA, AMPA, and GABAB currents. The intrapipette solution contained the following (in mm): KCl (153), EGTA (5), HEPES (10), MgCl2 (1), and Mg-ATP (2), pH 7.3. Recording electrodes were pulled from borosilicate capillary glass (Fisher) on a micropipette electrode puller (Sutter) and typically had a resistance of ∼2 megohms.
We visually identified transfected pyramidally shaped neurons by ZsGreen1 fluorescence using an inverted fluorescence microscope (Nikon). After making a gigaohm seal with the soma, we broke through the membrane to establish electrical contact with the cytoplasm. We voltage-clamped the neurons at −60 mV using an Axopatch 200B amplifier and using the pClamp software (Molecular Devices). We recorded more than 100 mIPSC events from each neuron. Each mIPSC recording was analyzed off line by an investigator who was blinded to the transfection conditions. The mIPSCs were chosen manually using the Mini Analysis software (Synaptosoft). The detection threshold was set at 15 pA. The inter-mIPSC intervals, mIPSC amplitude, rise time, and decay time constants were recorded.
Data Analysis
Expression levels of the GABAAR subunits were normalized to those obtained from the wild type condition and were reported as means ± S.E. Statistical significance was determined using the Student's paired or unpaired t test or paired or unpaired analysis of variance test, as appropriate.
To simplify the presentation of our data, which determined the effects of co-transfecting different masses of α1(AD) subunit cDNA on wild type GABAAR expression, we fit the expression levels to the sigmoidal concentration-response equation as follows: Y = 100%/(1 + (X/IC50)Hill slope), where Y is the expression level of the GABAAR subunit normalized to that obtained in the absence of the α1(AD) subunit, and X is the mass of α1(AD) subunit cDNA. We must emphasize that although we connected our data points with the fit concentration dependence equation, we are not asserting that the α1(AD) subunit necessarily acted by binding competitively with wild type GABAARs. Therefore, we made statistical comparisons between the mean expression levels instead of the fit IC50 and Hill slope values.
The mIPSC inter-mIPSC intervals, rise times, decay time constants, and peak amplitudes were compared using both a nonparametric analysis of variance and Kolmogorov-Smirnov tests. For the Kolmogorov-Smirnov test, the Bonferroni correction was applied to assess statistical significance among the three transfection conditions.
RESULTS
Heterozygous Cells Express Greater Amounts of Total α1 Subunit Protein than Hemizygous Cells
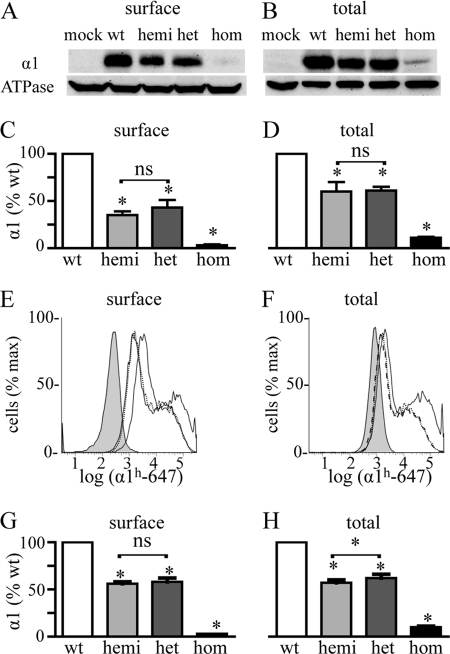
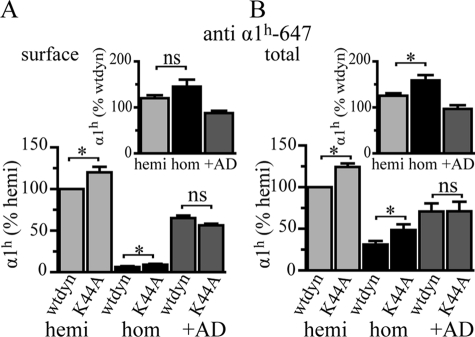
As a first step to determine whether expression of the α1(AD) subunit altered expression of α1β2γ2 receptors, we co-transfected HEK293T cells with 0.250 μg of β2 and γ2 subunit cDNA and either 0.250 μg of α1 subunit (wild type (WT)), 0.125 μg of α1 subunit (hemizygous), 0.125 μg of α1 subunit plus 0.125 μg α1(AD) subunit (heterozygous), or 0.250 μg of α1(AD) subunit (homozygous) cDNA. We use the terms hemizygous, heterozygous, and homozygous solely as a convenient naming convention to convey the ratio of masses of wild type to mutant cDNAs transfected. We used biotinylation assays and Western blots to determine the amount of surface and total α1 subunit expression in each of these transfection conditions (Fig. 1, A–D). As described previously, both surface and total heterozygous and homozygous α1 subunit expression differed substantially from each other and from wild type α1 subunit expression. Here, we found that both surface and total hemizygous α1 subunit expression was also substantially reduced compared with wild type expression. However, there was no significant difference between hemizygous and heterozygous α1 subunit expression.
FIGURE 1.
Effect of heterozygous α1(AD) subunit expression on surface and total α1 subunit expression. We transfected HEK293T cells with empty plasmid (negative control) or 0.250 μg of β2 and γ2 subunit cDNA and either 0.250 μg of wild type α1 (wt), 0.125 μg α1 (hemizygous (hemi)), 0.125 μg each of α1 and α1(AD) (heterozygous (het)), or 0.250 μg of α1(AD) (homozygous (hom)) cDNA. We biotinylated surface proteins and performed Western blots to determine the surface (A and C) and total (B and D) GABAAR α1 subunit expression relative to that of the ATPase α1 subunit (loading control). Surface and total hemizygous and heterozygous α1 subunit expression was greater than that of homozygous expression and was significantly reduced relative to wild type expression (*), but there was no significant (ns) difference between them (n = 5). We also performed flow cytometry assays (E–H) as a second method to quantify the surface and total α1 subunit for cells transfected with negative control (filled histogram), wild type (solid line), hemizygous (dotted line), heterozygous (dashed line), or homozygous (data not shown) GABAAR. There was no significant difference between hemizygous and heterozygous receptors in surface α1 subunit expression (n = 7), but total heterozygous α1 subunit expression was larger than that of hemizygous α1 subunit expression (n = 6, p = 0.03).
We next used flow cytometry as a second technique to measure surface and total α1 subunit expression (Fig. 1, E–H). Previous studies demonstrated that flow cytometry can measure total and surface GABAAR subunit expression with high precision and thus could potentially detect differences between hemizygous and heterozygous expression that could not be detected with Western blot assays (24). We found that flow cytometry did quantify α1 subunit expression with smaller standard deviations than Western blot experiments and was able to detect that heterozygous cells expressed 9% more total α1 subunit than hemizygous cells (p < 0.05). However, the flow cytometry experiments did not detect a difference between heterozygous and hemizygous cells in surface α1 subunit expression.
Wild Type α1 Subunit Accounted for 92–96% of the Surface α1 Subunit with Heterozygous Expression
The experiments in Fig. 1 demonstrated that compared with hemizygous cells, heterozygous cells possessed increased amounts of total α1 subunit protein but did not have significantly different amounts of surface α1 subunit protein. We thought that the increase in total α1 subunit protein expression in the heterozygous cells likely resulted from the residual, nondegraded α1(AD) subunit in intracellular compartments and that the lack of a significant change in surface expression resulted because the residual α1(AD) subunit did not efficiently traffic to the cell surface. Therefore, we next determined the fraction of surface α1 subunit with heterozygous expression that consisted of wild type or mutant α1 subunit.
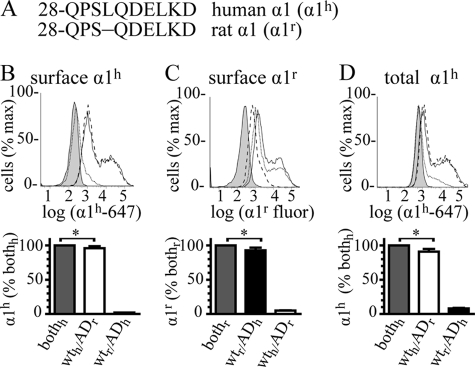
Previously, we used α1 and α1(AD) subunits fused to different fluorescent proteins and estimated that 5–25% of surface α1 subunit with heterozygous expression consisted of the α1(AD) subunit (21). Here, we used flow cytometry with two naturally occurring α1 subunits to obtain a more precise estimate of the amount of wild type and mutant α1 subunit on the cell surface in heterozygous expression. Although fluorescent protein- and epitope-tagged GABAAR subunits incorporate into pentamers to form functional receptors, the addition of the fused fluorescent protein or epitope tag alters post-translational processing and surface expression of the GABAAR subunits (10, 21). Therefore, we differentiated between wild type and mutant α1 subunits by transfecting cells using natural α1 and α1(AD) subunits from two different species (human and rat) and then detecting them individually with species-selective antibodies. The mature human α1 (α1h) and rat α1 (α1r) subunit proteins differ by only one amino acid; human α1 subunits possess an additional leucine at position 31 (position 4 as numbered from the putative N terminus of the mature peptide) that is absent in the rat sequence (Fig. 2A). Two commercially available antibodies, mouse anti-α1h and rabbit anti-α1r, bind specifically to their respective subunits.
FIGURE 2.
Fraction of wild type α1 and mutant α1(AD) subunits in heterozygous expression. We depicted the N-terminal amino acids of the mature human (α1h) and rat (α1r) α1 subunits (A) with the amino acids numbered starting at the N termini of the signal peptides. The Gln-28 residues are located at the putative N termini of the mature peptides. The full-length mature α1h and α1r sequences are identical except that the rat sequence lacks leucine 31 (leucine 4 as numbered from the putative N terminus of the mature peptide). We transfected HEK293T cells with mock vector (negative control, filled histograms) or β2 and γ2 subunits (0.250 μg) and heterozygous α1 subunits in which the sequences of the wild type and mutant subunits were both human or both rat (bothh, bothr, solid lines and gray bars). We also transfected cells with heterozygous receptors in which the sequence of the wild type subunit was human and the mutant subunit was rat (wth/ADr, dashed lines, white bars), and the sequence of the wild type subunit was rat and the mutant subunit was human (wtr/ADh, dotted lines, black bars). We stained surface receptors with either the anti-α1h (B) or α1r antibody (C) and total receptors with the anti-α1h antibody (D). We determined the fraction of surface heterozygous subunits composed of wild type subunit by dividing α1h mean fluorescence in WTh/ADr, by the α1h mean fluorescence in bothh cells (B, 96 ± 3%). We obtained a similar result when we divided α1r staining in WTr/ADh cells by that in bothr cells (C, 92 ± 2%). Similarly, we determined the fraction of mutant subunit in surface heterozygous receptors by dividing α1(AD)h staining in WTr/ADh cells by that in bothh cells (B, 2 ± 0.1%) and dividing α1(AD)r staining in WTh/ADr cells by bothr cells (C, 5 ± 0.4%). In total, heterozygous subunits, the wild type α1h subunit included 91 ± 4% heterozygous expression and the mutant α1h subunit included 8 ± 4% heterozygous expression. In both surface and total heterozygous expression, the contribution of the wild type subunit was modestly but significantly (*, p < 0.05) less than 100%.
Initial flow cytometry experiments demonstrated that the species-selective antibodies were very specific for the corresponding species of α1 subunit (supplemental Fig. 1). Moreover, the anti-α1h-647 antibody demonstrated remarkable species specificity such that compared with cells transfected with 0.250 μg of β2 and γ2 subunits and only 0.125 μg of the α1h subunit, cells transfected with a 16-fold excess (2 μg) of α1r subunit exhibited 0 ± 0% the surface fluorescence and 2 ± 0.2% of the total fluorescence (data not shown). Because the anti-α1r antibody could not be directly coupled to a fluorophore, its detection required use of a fluorescent secondary antibody, which produced high levels of background staining in permeabilized cells. Therefore, the anti-α1r antibody was only used for surface staining.
We transfected HEK293T cells with 0.250 μg of β2 and γ2 subunit cDNA and heterozygous α1 subunit consisting of either 0.125 μg of cDNA each of α1h and α1(AD)h subunits (both human, bothh), α1r and α1(AD)r subunits (both rat, bothr), α1h and α1(AD)r subunits (WTh/ADr), or α1r and α1(AD)h subunits (WTr/ADh). We stained surface protein with the anti-α1h subunit antibody, quantified the fluorescence with flow cytometry, and divided the WTh/ADr and WTr/ADh fluorescence by that obtained from cells transfected with both human. These data demonstrated that with heterozygous expression, wild type α1h subunits and mutant α1(AD)h subunits included 96 ± 3 and 2 ± 0.1% of the surface α1 subunit expression, respectively (Fig. 2B). These results were concordant with the complementary experiment in which we stained surface protein with the anti-α1r antibody and found that the wild type α1r subunits and mutant α1(AD)r subunits included 92 ± 2 and 5 ± 0.4% of the surface α1 subunit expression (Fig. 2C). In permeabilized cells, wild type α1h subunits and mutant α1(AD)h subunits included 91 ± 4 and 8 ± 4% of total α1 subunit (Fig. 2D). Therefore, the preponderance (92–96%) of α1 subunits on the surface of heterozygous cells was wild type α1 subunits.
Our data also demonstrated that in both surface and total heterozygous expression, the contribution of the wild type subunit was modestly, but significantly (p < 0.05), less than 100%. This result suggests that the mutant α1(AD) subunit could cause a dominant negative effect either by substituting for wild type α1 subunit or by reducing wild type α1 subunit surface expression.
Finally, we compared the surface expression of wild type α1h or α1r protein in heterozygous cells (WTh/ADr or WTr/ADh) with the corresponding hemizygous receptors (hemizygous α1h or α1r). The presence of the mutant subunit caused small but, in the case of the α1r protein, statistically significant reductions in surface wild type α1 subunit protein expression (8 ± 2%, p = 0.009, not shown). This result also suggested that the α1(AD) subunit could reduce wild type α1 subunit surface expression.
α1(AD) Subunit Did Not Substitute for Wild Type α1 Subunits on the Cell Surface
Experiments in Fig. 2 demonstrated that when heterozygously expressed in HEK293T cells, mutant α1(AD) subunits included 8% of the total α1 subunits and 2–5% of the surface α1 subunits. However, the fraction of mutant α1(AD) subunits expressed in neurons from ADJME patients is unknown. If the neurons of patients expressed a greater fraction of total α1(AD) subunit than HEK293T cells, would more α1(AD) subunit be expressed on the cell surface and confer a dominant negative effect by substituting mutant α1(AD) subunits for wild type α1 subunits?
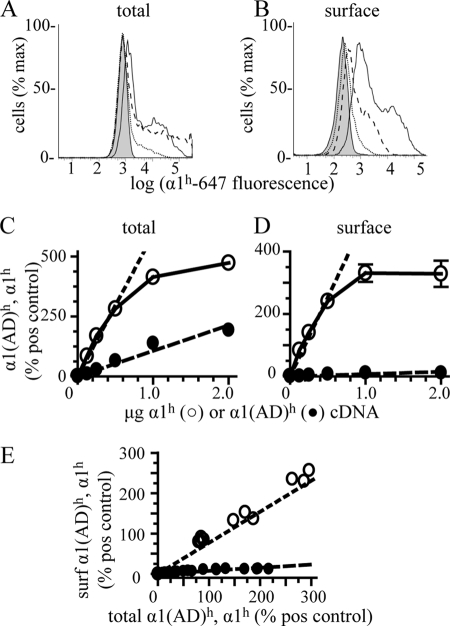
In a heterologous expression system, we can increase the amount of protein expression by increasing the mass of cDNA transfected. Therefore, we transfected cells with β2 and γ2 subunit cDNA (0.250 μg) and hemizygous (0.125 μg) α1r subunits and a range (0- 2 μg) of wild type α1h or α1(AD)h α1 cDNA. We quantified the amount of relative total and surface α1h and α1(AD)h subunit expression by flow cytometry and normalized all the values to the total or surface expression cells transfected with hemizygous α1h (“positive control,” α1hβ2γ2 0.125, 0.250, and 0.250 μg).
As expected, increasing the masses of wild type α1h and α1(AD)h subunit cDNA increased the amount of total α1h and α1(AD)h subunit expression (Fig. 3, A and C). The total α1(AD)h subunit expression increased linearly with the mass of cDNA from 0 to 2 μg, and the total α1h subunit expression increased linearly from 0 to 0.5 μg of cDNA before saturating. Because the α1(AD) subunit was degraded, total α1(AD)h subunit expression was smaller than total α1h subunit expression.
FIGURE 3.
Effect of increasing mutant α1(AD) subunit cDNA on surface and total α1(AD) subunit protein expression. We transfected HEK293T cells with empty vector (negative control, shaded histograms) or 0.250 μg of β2 and γ2S subunits and 0.125 μg of α1h subunit cDNA (hemizygous positive control, solid line histogram) or 0.250 μg of β2 and γ2S subunits, 0.125 μg of α1r subunit, and increasing amounts of either α1(AD)h or α1h subunit cDNA. We measured the total (A and C) and surface (B and D) α1(AD)h and α1h subunit fluorescence by flow cytometry. A and B, dotted lines depict the fluorescence of cells transfected with 0.125 μg of α1(AD)h subunits, and the dashed lines depict the fluorescence of cells transfected with 2 μg of α1(AD)h subunits. C and D, we depicted the α1(AD)h (●) and α1h (○) fluorescence normalized to positive control for each mass of α1(AD)h or α1h subunit cDNA that we transfected. When measuring total α1(AD)h and α1h expression (C), increasing both α1(AD)h and α1h subunit cDNA caused linear increases in α1(AD)h and α1h subunit protein expression until α1h subunit expression saturated when it was greater than 300% that of positive (pos) control. When measuring surface α1(AD)h and α1h expression (D), increasing α1h subunit cDNA caused proportional increases in α1h subunit expression, but increasing α1(AD)h subunit cDNA resulted in very little change in surface α1(AD)h subunit expression. E, we replotted the surface (surf) α1(AD)h and α1h subunit expression as a function of total expression. Both surface α1(AD)h and α1h subunit expression increased linearly with total expression (r2 = 0.82 and 0.95, respectively, n ≥ 3). However, there was an 8-fold greater increased surface α1h subunit expression than α1(AD)h subunit expression for equal increases in total expression. To determine whether the presence of the wild type α1r subunit facilitated the surface trafficking of α1(AD)h subunits, we repeated experiments in the absence of the α1r subunit and found no significant difference in the surface expression of the α1(AD)h subunit in the absence of the α1r subunit (n = 3, data not shown).
Increasing the mass of α1h subunit cDNA increased the amount of surface α1h subunit expression. In contrast, increasing the mass of α1(AD)h subunit cDNA caused little change in surface α1(AD)h expression (Fig. 3, B and D). Replotting the surface α1h and α1(AD)h expression versus total α1h and α1(AD)h expression (Fig. 3E) demonstrated that at the same amount of total α1h and α1(AD)h subunit expression there was 8-fold more α1h subunit on the cell surface. Repeating these studies using cells lacking the hemizygous α1r subunit produced identical results (data not shown). These data demonstrated that in addition to reducing total α1(AD) expression, the AD mutation also strongly inhibited the surface expression of receptors containing the α1(AD) subunit. Therefore, even if neurons from ADJME patients expressed larger relative amounts of α1(AD) subunits than HEK293T cells, the mutant subunits would not produce a dominant negative effect by substituting for wild type subunits on the cell surface.
α1(AD) Subunit Reduced Surface Expression of the Wild Type α1 Subunit
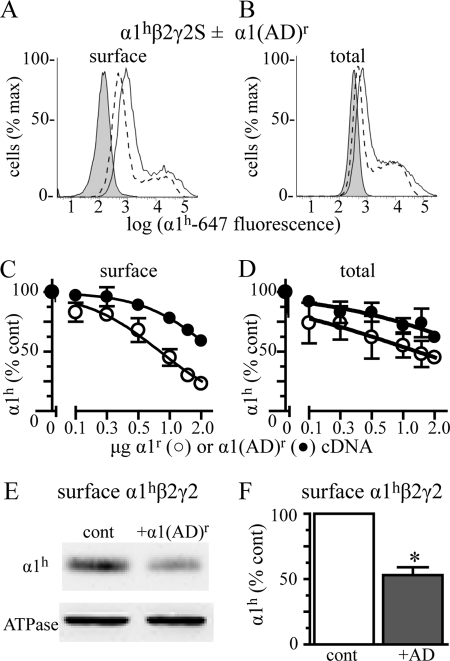
Experiments in Fig. 3 demonstrated that α1(AD) subunits did not cause a dominant negative effect by substituting for wild type α1 subunits on the cell surface, and experiments in Fig. 2 suggested that compared with hemizygous expression the heterozygous expression reduced the wild type α1 subunit on the cell surface. Therefore, we next determined the effect transfecting different amounts of α1(AD)r subunit on wild type α1h subunit expression.
We transfected HEK293T cells with β2 and γ2 subunits (0.250 μg), hemizygous (0.125 μg) human α1h subunits, and a range (0–2 μg) of rat α1(AD)r cDNAs and measured surface and total α1h expression by flow cytometry using the anti-α1h subunit antibody (Fig. 4). For comparison, we also determined the effect of wild type α1r subunit on α1hβ2γ2 expression. Because the wild type α1r subunits are not detected by the α1h subunit antibody, they reduce α1h subunit expression by substitution rather than α1(AD)r subunit-mediated inhibition of expression.
FIGURE 4.
Effect of α1(AD) subunit expression on surface and total α1β2γ2 expression. We transfected HEK293T cells with empty vector (negative control, shaded histograms) or 0.250 μg of β2 and γ2S subunits and 0.125 μg α1h subunit cDNA (hemizygous) and with varying masses (0–2 μg) of α1r or α1(AD)r cDNA. We quantified the surface (A and C) and total (B and D) α1h subunit expression using flow cytometry with anti-α1h subunit antibodies. A and B, we depicted hemizygous fluorescence as a solid line and hemizygous + 2 μg of α1(AD)r fluorescence as a dashed line. C and D, we plotted α1(AD)h (●) and α1h (○) subunit fluorescence normalized to that of hemizygous subunit fluorescence versus the masses of α1(AD)h or α1h cDNA that was transfected. Increasing the masses of α1(AD)r subunit cDNA reduced surface and total α1h subunit expression. We also determined the effect 2 μg of α1(AD)r subunit cDNA co-transfection on surface α1hβ2γ2 receptor expression using biotinylation assays with Western blot (E and F). We stained the Western blots with the anti-α1h subunit antibody and quantified surface α1h subunit relative to that of the Na+/K+-ATPase α subunit (loading control (cont)). The addition of 2 μg of α1(AD)r subunit cDNA (+AD) reduced surface expression of α1hβ2γ2 receptors by 46 ± 6% (n = 4; p = 0.005). The α1(AD)r subunit did not alter the surface expression of the endogenous ATPase α subunit (97 ± 10%, n = 5).
The α1(AD)r subunit caused a concentration-dependent reduction in surface and total α1h expression (Fig. 4, A–D). As expected, the α1(AD)r subunit caused smaller reductions in α1h subunit expression than the wild type α1r subunit. Nonetheless, the α1(AD)r-mediated reduction of α1h subunit expression demonstrated that nondegraded α1(AD) subunit was not inert and caused a small but potentially clinically significant dominant negative effect by reducing wild type α1 subunit expression.
To confirm that transfection of the α1(AD)r subunit did not reduce α1hβ2γ2 receptor expression simply by inhibiting the transcriptional or translational capacity of the cells, we compared the effects of transfecting 2 μg of α1(AD)r or pmaxGFP on α1β2γ2 expression. Cells transfected with pmaxGFP demonstrated strong GFP fluorescence (data not shown). However, unlike the α1(AD)r subunit, pmaxGFP did not alter surface or total α1β2γ2 receptor expression (supplemental Fig. 2, A and B).
We used biotinylation assays with Western blotting as a second method to determine the effect of the α1(AD) subunit on surface wild type α1h subunit expression. We transfected HEK293T cells with β2 and γ2 subunits (0.250 μg) and hemizygous α1h subunits with or without 2 μg of α1(AD)r subunit cDNA. We performed biotinylation assays with Western blots and detected the α1h subunit with the anti-α1h subunit antibody. Similar to the flow cytometry studies, we found that the α1(AD)r subunit reduced the surface expression of the wild type α1h subunit (Fig. 4, E and F).
Because the α1(AD) subunit is grossly misfolded, it could potentially be a toxic protein and reduce α1β2γ2 expression by nonspecifically decreasing cellular viability. During flow cytometry analyses, we gated viable cells based on their forward and side scatter properties that corresponded to the population of viable cells that excluded the membrane-impermeable dye, 7-aminoactinomycin D (24). We found that transfection of 2 μg of α1(AD) subunit cDNA did not alter the percentage of “viable cells” (62 ± 3%) when compared with cells transfected in the absence of the α1(AD) subunit (60 ± 3%, n = 5, p = 0.710; data not shown).
To determine whether the α1(AD) subunit inhibited surface expression of α1β2γ2 receptors by nonspecifically preventing surface trafficking of proteins processed through the secretory pathway, we measured the effect of the α1(AD) subunit on the surface expression of two membrane proteins not thought to interact with GABAARs. Using biotinylation assays and Western blots, we found that transfection of 2 μg of α1(AD) subunit did not alter the surface expression of the endogenous HEK293T cell Na+/K+-ATPase α1 subunit (Fig. 4E; 97 ± 10%, n = 5, p = 0.776). Next, we determined the effect of the α1(AD)r subunit on surface expression of recombinantly expressed TGFHA protein. We transfected cells with α1β2γ2 receptors, TGFHA, and with or without 2 μg of α1(AD)r cDNA. We quantified expression of TGFHA by flow cytometry with the anti-HA-647 antibody and found that the α1(AD) subunit did not reduce expression of TGFHA (116 ± 10%, see supplemental Fig. 3). These data demonstrated that the dominant negative effect conferred by α1(AD) subunit did not result simply by nonspecifically inhibiting protein expression through the secretory pathway.
α1(AD) Subunit Reduced α1 Subunit Expression in a Dynamin-independent Manner
Bradley et al. (11) demonstrated that total homozygous α1(AD)β2γ2 expression increased by co-expression of dominant negative dynamin a mutant protein that inhibits clathrin-mediated endocytosis. These data suggested that in addition to causing degradation of the α1(AD) subunit by ER-associated degradation, the AD mutation also increased endocytosis of α1(AD)β2γ2 receptors from the cell surface.
Here, we determined whether the α1(AD) subunit conferred its dominant negative effect by increasing α1β2γ2 receptor endocytosis through a dynamin-dependent mechanism. We co-transfected HEK293T cells with β2 and γ2 subunits and either hemizygous α1h, homozygous α1(AD)h, or hemizygous α1h subunits that also contained 2 μg of α1(AD)r cDNA (+AD). In addition, we co-transfected cells with 0.375 μg of cDNA expressing either wild type dynamin or a dominant negative mutant dynamin (K44A) that inhibits endocytosis.
We measured the amount of surface and total human α1h or α1(AD)h subunit expression by flow cytometry. In agreement with Bradley et al. (11), we demonstrated that co-expression of K44A dynamin increased expression of total homozygous GABAARs to a greater extent than “hemizygous” GABAARs (Fig. 5B). In addition, we demonstrated that K44A dynamin increased surface expression of homozygous receptors to a greater extent than that of hemizygous receptors, although this latter result was not statistically significant (Fig. 5A). Although the K44A dynamin did cause a greater effect on homozygous than hemizygous expression, the magnitude of the expression of homozygous receptors was still very small, and thus we do not think that enhanced endocytosis of homozygous α1(AD)β2γ2 makes an appreciable contribution to ADJME pathology. More importantly for the goals of this study, K44A dynamin did not increase the total or surface expression of wild type α1h subunits when it was co-expressed with α1(AD)r subunits, and thus the mutant α1(AD) subunit did not produce the dominant negative effect by augmenting dynamin-mediated endocytosis. Interestingly, not only did the K44A dynamin fail to increase expression of the +α1(AD)r subunit samples to a greater extent than the hemizygous subunit samples, it did not cause any increase in α1h subunit expression, suggesting that the α1(AD) subunit may actually inhibit dynamin-mediated endocytosis.
FIGURE 5.
Inhibiting dynamin-mediated endocytosis on the dominant effect of the α1(AD) subunit. We transfected HEK293T cells with 0.250 μg of β2 and γ2S subunit cDNA and either 0.125 μg of human wild type α1h subunit (hemi, light gray), 0.250 μg of human α1(AD)h subunit (hom, black), or 0.125 μg of human wild type α1h subunit and 2 μg α1(AD)r subunit (+AD, dark gray). In addition, we also co-transfected the cells with either 0.375 μg of wild type (wtdyn) or dominant negative (K44A) dynamin. We measured surface and total human α1 subunit expression by flow cytometry and normalized each value to the expression of the hemizygous samples that were co-transfected with wild type dynamin. In the insets, we plotted the percentage change in α1h and α1(AD)h expression because of the K44A dynamin. The K44A dynamin increased the total and surface α1 subunit expression in the hemizygous and homozygous conditions (p < 0.05), but not alter the dominant effect in the +α1(AD)r condition (n = 5). The K44A dynamin significantly increased total homozygous α1(AD)h expression to a greater extent than hemizygous α1h expression (p < 0.01). ns = p ≥ 0.05; *, p < 0.05.
α1(AD) Subunit Associated with Wild Type GABAAR Subunits
Experiments in Fig. 5 demonstrated that the α1(AD) subunit did not confer its dominant negative effect by increasing dynamin-mediated GABAAR endocytosis. Previous studies demonstrated that nondegraded α1(AD) subunits were retained in the ER (21, 26). Could the α1(AD) subunit confer its dominant negative effect by associating with wild type GABAAR subunits and entrapping them in the endoplasmic reticulum (ER)? Bradley et al. (11) reported that FLAG epitope-tagged α1(AD) subunits co-immunoprecipitated with γ2-GFP subunit fusion proteins and thus demonstrated that the mutant α1(AD) protein could associate with and potentially entrap wild type GABAAR subunits (11). We extended these studies and used FRET to determine whether α1(AD) subunits specifically associated with wild type GABAARs. FRET measures the nonradiative transfer of energy between two fluorophores that are situated within at least 10 nm of one another and can be performed in situ in cells using flow cytometry (25, 27).
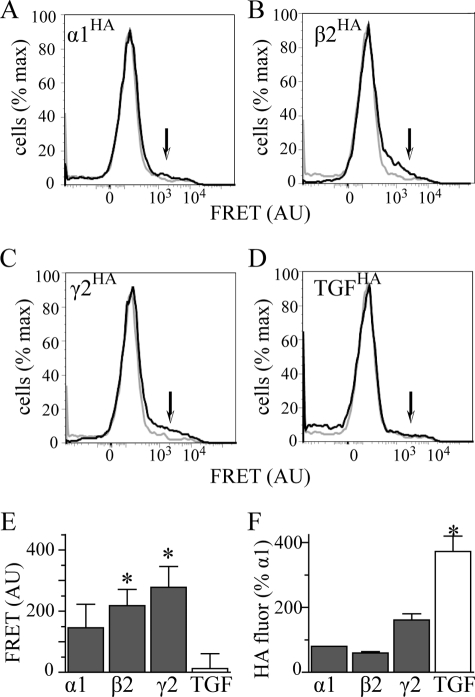
We transfected HEK293T cells with α1HA β2γ2 (Fig. 6A), α1rβ2HAγ2 (Fig. 6B), or α1rβ2γ2HA (Fig. 6C) receptors. In addition, we co-transfected cells with α1rβ2γ2 receptors and TGFHA protein (Fig. 6D). Staining the HA-tagged protein with an A555-conjugated antibody served as the FRET donor. In each of the conditions, we also co-transfected 2 μg of α1(AD)h subunits that we stained with the A647-conjugated anti-α1h antibody to serve as the FRET acceptor.
FIGURE 6.
Interactions of the α1(AD) subunit with wild type GABAAR subunits. We transfected HEK293T cells with 2 μg of α1(AD)h subunit and 0.125:0.250:0.250 μg ratios of either α1HAβ2γ2 (A), α1rβ2HAγ2 (B), or α1rβ2γ2HA subunits to form receptors in which either the α1, β2, or γ2 subunit is tagged with the HA epitope. In addition, to determine whether the α1(AD)h subunit interacted nonspecifically with the TGFHA protein, we transfected cells with 2 μg of α1(AD)h subunit, a 0.125:0.250:0.250 μg ratio of α1rβ2γ2 subunits, and 0.250 μg of TGFHA (D). We permeabilized the cells and stained them with either the anti-α1h-647 antibody (FRET acceptor), the antiHA-555 antibody (FRET donor), or both anti-α1h-647 and antiHA-555 antibodies. A–D are flow cytometry histograms of FRET fluorescence. The gray line plots the histogram for cells stained with only the α1h-647 acceptor antibody, and the black line plots the histogram for cells stained with both HA-555 donor and α1h-647 acceptor antibodies. The arrows point to the regions of the histograms where one would find specific FRET fluorescence. We quantified the specific FRET fluorescence and plotted it in E (n ≥ 5). Samples transfected with α1β2HAγ2 and α1β2γ2HA receptors possessed substantial FRET fluorescence that significantly differed compared with samples transfected with TGFHA protein. This difference in FRET fluorescence did not result from reduced HA fluorescence from the TGFHA protein because TGFHA possessed substantially more HA florescence than HA-tagged GABAAR subunits (F). AU, arbitrary units.
To determine whether or not there were FRET interactions between α1(AD)h subunits and wild type GABAAR subunits or TGFHA proteins, we excited the FRET donor near its absorption maximum and detected the emission from the FRET acceptor at its emission maximum. In Fig. 6, A–D, we plotted flow cytometry histograms that depict the intensity of emission in the FRET channel on the abscissa and the number of cells having that intensity on the ordinate. The gray plots in Fig. 6, A–D, depict the data for cells stained with only the FRET acceptor and demonstrate the lack of nonspecific FRET signal when the donor was not present. Similar experiments demonstrated even smaller nonspecific FRET signals when the donor but not the acceptor was present (data not shown). The black plots in Fig. 6, A–D, depict the data for cells stained with both the FRET acceptor and donor. FRET signals are specific when there is greater FRET signal in the doubly stained (Fig. 6, A–D, black) than in the singly stained (gray) samples. The regions of the histograms where one finds specific FRET signals are indicated by arrows in Fig. 6, A–D.
We quantified the specific FRET signals as the difference between the mean FRET emissions of the doubly and singly stained samples. Specific FRET data are summarized in Fig. 6E (n ≥ 5). Both β2HA and γ2HA subunits, but not the negative control protein TGFHA, demonstrated specific FRET with α1(AD)h subunits. Although the α1HA also appeared to demonstrate a specific FRET interaction with α1(AD)h, this value was not statistically significant (p = 0.128). The lack of FRET interactions between α1(AD)h subunits and TGFHA protein did not result from decreased donor fluorescence. As demonstrated in Fig. 6F, detection of TGFHA protein had substantially greater HA-555 florescence than that of the GABAAR subunits.
These results demonstrated that the α1(AD) subunit specifically associated with other GABAAR subunits. Based on the prior subcellular localization studies (21, 26), this association must occur in the ER. Because GABAARs containing α1(AD) subunits are strongly inhibited from trafficking to the cell surface (Fig. 3), this result suggests that the α1(AD) subunit reduced GABAAR surface expression by associating with and entrapping GABAAR in the ER.
α1(AD) Subunit Reduced Surface Expression of α3 Subunit-containing GABAARs
Neurons predominantly express α2 and α3 subunits early in development and then express α1 subunits later in development. Adult homozygous Gabra1 knock-out mice express increased amounts of α3 subunits, which partially compensate for the lack of α1 subunits (28–31). Therefore, we determined the effect of α1(AD) subunits on surface expression of α3 subunit-containing GABAARs.
We transfected HEK293T cells with 0.250 μg of α3, β2, and γ2 subunit cDNA and varying amounts (0–2 μg) of either α1 or α1(AD) subunits. As in the experiments in Fig. 4, we included the wild type α1 subunit to serve as a basis for comparison between α3 subunit substitution by the wild type α1 subunit and α1(AD)-mediated inhibition of expression. To emulate the GABAAR expression in ADJME patients who have two wild type GABRA3 genes but only one wild type GABRA1 gene, we transfected 0.250 μg of α3 subunit cDNA instead of 0.125 μg as we did for the hemizygous α1β2γ2 conditions. We performed flow cytometry experiments and stained for surface α3 subunit expression.
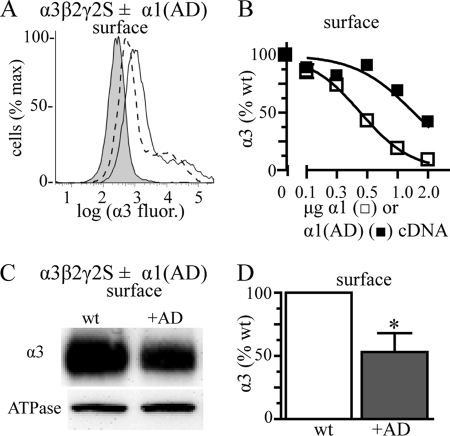
Co-transfecting increasing amounts of α1(AD) subunit cDNA caused a concentration-dependent reduction in surface α3 subunit expression (Fig. 7, A and B). We confirmed the inhibition of α3 subunit expression using biotinylation assays and Western blots. We transfected cells with α3, β2, and γ2 subunits with or without 2 μg of α1(AD) subunit cDNA. We performed biotinylation assays and Western blots and found that α1(AD) subunits significantly reduced expression of surface α3 subunits (Fig. 7, C and D). As described in Fig. 4, we confirmed that the α1(AD) subunit did not inhibit α3β2γ2 receptor expression by competing for transcriptional and translational machinery by measuring the effect of pmaxGFP on α3β2γ2 receptor expression (supplemental Fig. 2C).
FIGURE 7.
Effect of the α1(AD) subunit on wild type α3β2γ2 GABAAR. We transfected HEK293T cells with empty vector (negative control, shaded histogram) or 0.250 μg of α3, β2, and γ2S subunits without any additional subunit (wild type) or with varying masses (0.125–2 μg) of mutant α1(AD) or wild type (wt) α1 subunit cDNA. We quantified the surface (A and B) α3 subunit expression using flow cytometry. A, we presented a sample flow cytometry histogram with wild type fluorescence as a solid line and wild type + 2 μg of α1(AD) fluorescence as a dashed line. B, we quantified the surface α3 subunit fluorescence in the presence of the different amounts of α1(AD) (■, n ≥ 4) or wild type α1 (□, n = 3) subunit cDNA and normalized the α3 subunit fluorescence to that of wild type cells. Increasing the mass of α1(AD) and α1 subunit cDNA reduced surface α3 subunit expression. We also determined the effect of 2 μg of α1(AD) subunit cDNA co-transfection on surface α3β2γ2 receptor expression using biotinylation assays with Western blot (C and D). We stained the Western blots with the anti-α3 subunit antibody and quantified surface α3 subunit relative to that of the Na+/K+-ATPase α subunit (loading control). The presence of the α1(AD) subunit reduced surface expression of α3β2γ2 receptors by 47 ± 15% (n = 6; p = 0.024). *, p < 0.05.
α1(AD) Subunits Reduced Surface Expression of α3 Subunit-containing GABAARs to a Greater Extent than α1 Subunit-containing GABAARs
Surprisingly, when we compared the effects of α1(AD) subunits on surface expression of α1 (Fig. 4) and α3 (Fig. 7) subunits, we found that α1(AD) subunits reduced α3 subunit expression to a greater extent than α1 subunit expression (Fig. 8A). We next determined whether α1(AD) subunits also reduced surface expression of partnering β2 and γ2HA subunits to a greater extent when co-expressed with α3 subunits than with α1 subunits.
FIGURE 8.
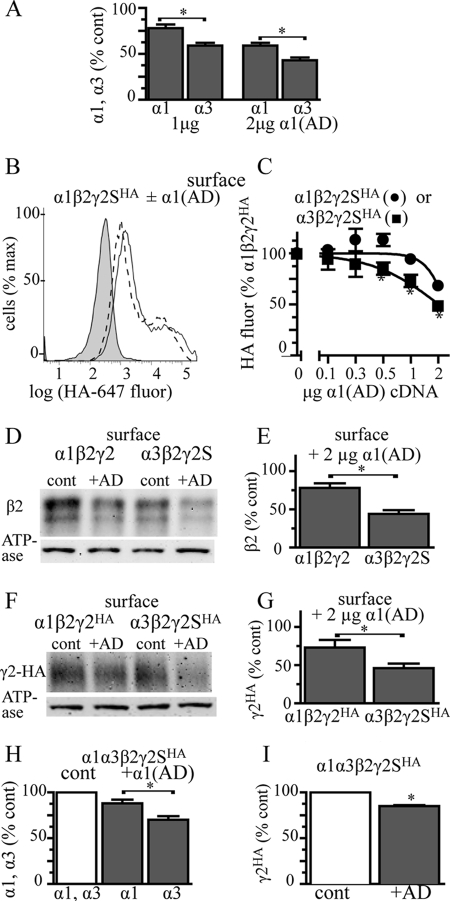
Effect of the α1(AD) subunit on surface β2 and γ2 subunit expression in α1β2γ2 and α3β2γ2 receptors. A, we replotted the data from Figs. 4 and 7 depicting the effect of 1.0 and 2.0 μg of α1(AD)r subunit cDNA on surface α1 and α3 subunit expression. The α1(AD)r subunit reduced surface α3 subunit expression to a greater extent than α1 subunit expression (p < 0.002). B and C, we transfected HEK293T cells with empty vector (negative control (cont)) or 0.250 μg of β2 and γ2SHA cDNA and either 0.125 μg of α1 subunit (hemizygous α1) or 0.250 α3 subunit (WT α3). We co-transfected different masses (0–2 μg) of mutant α1(AD) subunit cDNA. We quantified the surface γ2SHA subunit expression using an anti-HA antibody and flow cytometry. B presents a sample flow cytometry histogram that plots the γ2HA fluorescence versus the number of cells. Negative control fluorescence is shaded; fluorescence from α1β2γ2HA receptors in the absence of the α1(AD) subunit is a solid line, and fluorescence from α1β2γ2HA receptors + 2 μg of α1(AD) cDNA is a dashed line. C, we quantified the surface γ2HA expression for α1β2γ2HA (●) and α3β2γ2HA (■) receptors for each amount of α1(AD) subunit cDNA. The γ2HA subunit fluorescence was normalized to the γ2HA fluorescence in the α1β2γ2 receptors in the absence of α1(AD) subunit. In the absence of the α1(AD) subunit, there was no significant difference in γ2HA expression between the α1β2γ2HA and α3β2γ2HA receptors (99 ± 5%, p = 0.895). Addition of the α1(AD) subunit caused concentration-dependent reductions in γ2HA subunit that were greater in α3β2γ2HA than α1β2γ2HA receptors (* p < 0.05, n ≥ 6). Biotinylation and Western blot assays (d–G) demonstrated that transfecting 2 μg of α1(AD) subunit cDNA reduced surface β2 subunit expression to a greater extent in α3β2γ2 receptors (56 ± 5%) than α1β2γ2 receptors (22 ± 6%, n = 5, p = 0.002) and also reduced γ2HA subunit expression by a greater extent in α3β2γ2HA receptors (54 ± 6%) than α1β2γ2HA receptors (27 ± 10%, n = 5, p = 0.049). Finally, we transfected HEK293T cells with α1h, α3, β2, and γ2HA subunits in a 0.125:0.250:0.250:0.250 ratio and with or without 1.0 μg of α1(AD)r subunit. We determined the amount of surface α1h, α3, and γ2HA subunit expression by flow cytometry. The α1(AD)r subunit reduced α3 subunit expression (30 ± 4%) to a greater extent than α1h subunit expression (12 ± 4%, p = 0.010, n = 5, H), and it reduced γ2HA expression by 15 ± 1% (p = 0.001, n = 5, I).
We co-transfected HEK293T cells with β2 and γ2HA subunits (0.250 μg), either hemizygous α1 (0.125 μg) or wild type α3 subunits, and a range (0–2 μg) of α1(AD) subunit cDNA. We measured surface γ2HA subunit expression by flow cytometry and normalized the HA fluorescence to that obtained from hemizygous α1β2γ2HA receptors (Fig. 8, B and C). This experiment demonstrated that in the absence of α1(AD) subunits, there was no significant difference in surface γ2HA subunit expression between α1β2γ2HA and α3β2γ2HA receptors (99 ± 5%). This result indicated that under these transfection conditions, cells express similar amounts of α1β2γ2HA and α3β2γ2HA receptors.
The addition of α1(AD) subunit reduced α3β2γ2HA receptor expression to a greater extent than α1β2γ2HA receptor expression. Interestingly, when cells expressing α1β2γ2 receptors were transfected with 0.250 and 0.500 μg of α1(A322D) subunits, there were small increases in γ2HA subunit expression, a result that may suggest that in a small fraction of receptors the α1(AD) subunit changes the composition of the GABAAR pentamer to one that contains more than one γ2HA subunit.
We performed biotinylation assays and Western blots as a second method to determine the effect of α1(AD) subunits on the surface expression of α3β2γ2 and α1β2γ2 receptors. We transfected HEK293T cells with α1β2γ2, α3β2γ2, α1β2γ2HA, or α3β2γ2HA subunits and either with or without 2 μg of α1(AD) subunit cDNA. We performed biotinylation assays and Western blots to quantify the relative amounts of surface β2 and γ2HA subunits (Fig. 8, D–G). These experiments demonstrated that α1(AD) subunits reduced expression of surface β2 and γ2HA subunits to a greater extent when co-expressed with α3 subunits than with α1 subunits.
Unlike the transfected heterologous cells used in the previous experiments that expressed either α1 or α3 subunits, neurons simultaneously express an endogenous mixture of GABAAR subunits, including α1, α3, and other α subunit subtypes. Therefore, we next determined whether or not α1(AD) subunits would reduce surface expression of α3 subunits to a greater extent than α1 subunits in HEK293T cells simultaneously expressing both α1 and α3 subunit cDNA.
We transfected HEK293T cells with α1h, α3, β2, and γ2HA subunits (0.125:0.250:0.250:0.250 μg of cDNA ratio) and with or without 1 μg of α1(AD)r subunit cDNA. We quantified the relative surface expression of α1h, α3, and γ2HA subunits using flow cytometry (Fig. 8, H and I). We found that α1(AD)r subunits reduced expression of surface α3 subunits (30 ± 4%) to a greater extent than α1h subunits (12 ± 4%, p = 0.010). α1(AD)r subunits reduced expression of surface γ2HA subunits by a similar amount as it reduced surface α1h subunit expression, 15 ± 1%. These results indicated that in cells expressing both α1 and α3 subunits, α1(AD) subunits produced a modest reduction in total surface GABAAR expression and conferred a greater reduction of α3β2γ2HA than α1β2γ2HA GABAARs. This would result in an alteration of the composition of surface GABAARs to those with a greater fraction of α1 than α3 subunit-containing receptors.
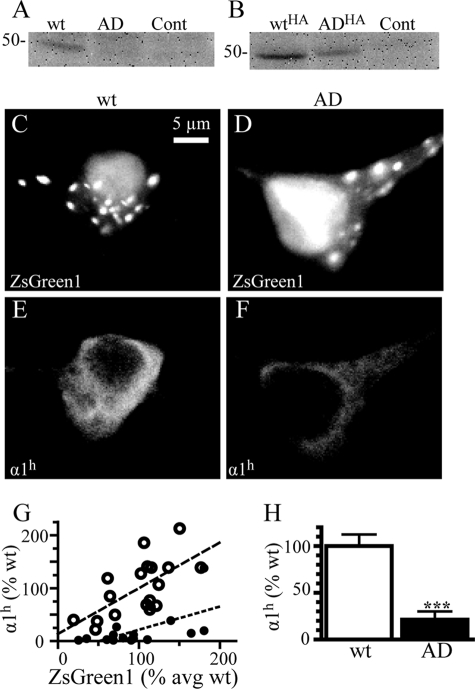
AD Mutation Reduced α1(AD) Subunit Expression in Neurons
The previous experiments determined the effect of α1(AD) subunits on GABAARs overexpressed in heterologous cells. Here, we determined the effect of α1(AD) subunits on endogenous GABAARs in cultured neurons. To enable the nondestructive identification of transfected neurons without the use of large fluorescent fusion proteins, we inserted our cDNAs of interest into a commercial plasmid upstream of an internal ribosome entry site, which was followed by the cDNA encoding the fluorescent coral protein, ZsGreen1. We made five constructs by inserting either nothing (control), α1h, α1(AD)h, α1HA, or α1(AD)HA subunits. Because these constructs expressed the cDNA of interest and the fluorescent protein as separate peptides, the fluorescent protein did not interfere with α1 subunit trafficking, post-translational modification, or function.
We obtained cortical neurons from E18 rats and established neuron-enriched cultures. We transfected neurons at DIV10 and analyzed them at DIV17, the time point at which the cultured neurons develop mature GABAergic synapses. We first characterized the composition of our neuronal cultures at DIV17. We measured the number of DAPI-positive nuclei that also stained with the neuron-specific marker NeuN. We found that 89 ± 5% of the cells were neurons (n = 3) and that the neuronal transfection efficiency was 4 ± 0.4% (data not shown). In these characterization experiments, no non-neuronal cells were transfected. However, in subsequent experiments, we did identify cells rarely with a non-neuronal morphology that expressed ZsGreen1; these non-neuronal cells were not included in the analyses.
We next determined the effect of the AD mutation on expression of α1(AD) subunits in neurons. We transfected neurons with control-, α1h-, α1(AD)h-, α1HA-, or α1(AD)HA-IRES-ZsGreen1 constructs. We performed Western blots on neuronal lysates and stained them with either anti-α1h or anti-HA antibodies. Visual inspection demonstrated that the AD mutation substantially reduced both α1(AD)h and α1(AD)HA subunit expression (Fig. 9, A and B). Because of weak signal, we could not quantify α1(AD)h subunit expression on Western blot. Quantification of α1HA and α1(AD)HA subunit expression revealed that the AD mutation reduced α1(AD)HA subunit expression by 39 ± 9%, a result consistent with what we and others reported for epitope-tagged α1(AD) subunits expressed in HEK293T cells (10, 11) and a much lower level of reduction than we typically observe using untagged α1 subunits (Fig. 1).
FIGURE 9.
Effect of the AD mutation on α1(AD) subunit expression in cultured cortical neurons. We transfected DIV10 cultured cortical neurons with either control-IRES-ZsGreen1, α1h-IRES-ZsGreen1 (wt), α1(AD)h-IRES-ZsGreen1 (AD), α1HA-IRES-ZsGreen1 (WTHA), or α1(AD)HA-IRES-ZsGreen1 (ADHA). On DIV17, we performed Western blots of neuronal lysates and stained them with either the anti-α1h antibody (A, n = 4) or the anti-HA antibody (B, n = 5). Visual inspection demonstrated that the mutation substantially reduced both α1(AD)h and α1(AD)HA expression. Because of weak signal intensity, the untagged human α1(AD)h subunits in neuronal lysates could not be quantified. However, quantification of the gels stained with the anti-HA antibodies demonstrated that α1(AD)HA subunit expression was reduced by 39 ± 9% compared with wild type α1HA expression (p = 0.010). Next, in four separate experiments, we transfected DIV10 neurons with control-IRES-ZsGreen1, α1h-IRES-ZsGreen1 (wt), or α1(AD)h-IRES-ZsGreen1 (AD) and performed immunofluorescence studies on DIV17 to identify transfected neurons by ZsGreen1 fluorescence in the green channel (C and D) and recombinant α1h and anti-α1(AD)h subunit immunofluorescence in the red channel (E and F). Visual inspection demonstrated that the ZsGreen1 fluorescence localized primarily within the nuclei and in small puncta within the cytoplasm and that recombinant α1h and α1(AD)h subunits expressed diffusely throughout the soma and the dendrites. There was no significant difference in ZsGreen1 fluorescence among the control (103 ± 4%), α1h- (100 ± 9%) and α1(AD)h (99 ± 12%)-transfected cells The anti-α1h antibody specifically labeled recombinant α1h protein with the neurons transfected with control vector (n = 16) having 2 ± 1% the A546 fluorescence as those transfected with α1h (data not shown). The α1h immunofluorescence intensity correlated with the ZsGreen1 intensity for both the samples transfected with α1h (○, p = 0.003, r2 = 0.42) and α1(AD)h (●, p = 0.017, r2 = 0.35) (G). Compared with neurons transfected with α1h (n = 18), the neurons transfected with the α1(AD)h subunit possessed 21 ± 9% of the α1h immunoreactivity (H, n = 16, p < 0.001).
We used immunofluorescence studies to determine the effect of the mutation on untagged human α1(AD)h subunit expression in neurons. We transfected neurons with control-, α1h-, or α1(AD)h-IRES-ZsGreen1 plasmids and analyzed total expression on DIV17. We fixed, permeabilized, and stained the neurons with anti-α1h subunit antibody to quantify total recombinant α1h and α1(AD)h subunit expression. We identified transfected neurons by ZsGreen1 fluorescence (Fig. 9, C and D). Compared with wild type α1h subunit-transfected neurons, there was no significant difference in ZsGreen1 fluorescence in control (103 ± 4%) or α1(AD)h subunit (99 ± 12%)-transfected neurons.
The anti-α1h antibody specifically labeled recombinant α1h subunit protein. Neurons transfected with control vector (n = 16) possessed 2 ± 1% of the fluorescence of those transfected with α1h subunits (data not shown). Both α1h and α1(AD)h subunits were expressed diffusely throughout the cytoplasm and dendrites and did not form any definite inclusions (Fig. 9, E and F). The intensity of ZsGreen1 fluorescence correlated with the intensity of both α1h and α1(AD)h subunit immunofluorescence (Fig. 9G). The AD mutation reduced α1(AD)h subunit expression to 21 ± 9% that of the α1h subunit (Fig. 9H).
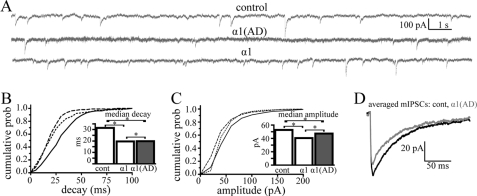
Expression of the α1(AD) Subunits Altered Current Time Course and Reduced Peak Amplitude of mIPSCs
We determined whether expression of α1 and α1(AD) subunits altered mIPSC. We transfected neurons in five separate experiments with control-, α1-, or α1(AD)-IRES-ZsGreen1 constructs. On DIV17, we recorded more than 100 mIPSCs from pyramidally shaped ZsGreen1-positive neurons (Fig. 10A). We quantified the inter-mIPSC interval, rise time, decay time, and peak current amplitudes.
FIGURE 10.
Effect of the α1(AD) subunit on mIPSCs in cultured cortical neurons. In five separate experiments, we transfected DIV10 cultured cortical neurons with either control-IRES-ZsGreen1 (control), α1-IRES-ZsGreen1 (wild type, α1), or α1(AD)-IRES-ZsGreen1 (α1(AD)). On DIV17, we identified transfected neurons by ZsGreen1 fluorescence and recorded >100 mIPSCs events from each. A, we showed sample mIPSC current traces. Transfection of both the α1 and α1(AD) subunit altered mIPSC current kinetics (B and D). B, we plotted a histogram that depicts the mIPSC decay time constants on the abscissa and the cumulative probability (prob) of an mIPSC having that decay time on the ordinate. Neurons transfected with control vector (solid line) had the biggest decay time constants followed by those transfected with α1(AD) (dotted line) and α1 (dashed line, p < 0.001). In the inset, we plotted the median mIPSC decay times. C, we plotted a histogram depicting mIPSC peak amplitudes on the abscissa and cumulative probability of a mIPSC having that peak amplitude on the ordinate. Neurons transfected with control subunit possessed larger mIPSC amplitudes than α1(AD)-transfected neurons, which possessed larger amplitudes than those transfected with α1 subunit (p < 0.001). The median mIPSC amplitudes are plotted in the inset. D, we displayed averaged (>100 events) mIPSC traces for wild type (black) and α1(AD)-transfected neurons (gray) demonstrating both the accelerated decay and reduced mIPSC amplitudes in neurons transfected with the α1(AD) subunit. *, p < 0.05.
Transfection of wild type α1 subunits, but not α1(AD) subunits, caused a very small but statistically significant reduction in the inter-mIPSC interval compared with control transfection (median mIPSC interval reduced from 1.5 to 1.4 s, p = 0.015, data not shown). The α1 and α1(AD) subunit conditions did not significantly differ from each other.
In contrast to the very modest effects of α1 subunit transfection on inter-mIPSC intervals, both α1 and α1(AD) subunits substantially altered mIPSC current kinetics, a result consistent with altered GABAAR composition. Compared with neurons transfected with control vector, neurons transfected with either α1 or α1(AD) subunits possessed reduced mIPSC rise (data not shown) and decay times (Fig. 10, B and D, p < 0.001). Moreover, transfection of the α1 subunit reduced mIPSC decay time to a greater extent than the α1(AD) subunit (p < 0.001). The change in the GABAAR mIPSC kinetics suggested a shift in receptor composition from non-α1 to α1 subunit-containing receptors, a change that could result if overexpressed wild type α1 subunits replaced non-α1 subunit-containing GABAARs in synapses and if overexpressed mutant α1(AD) subunits prevented surface trafficking of non-α1 subunit-containing GABAARs to a greater extent than α1 subunit-containing GABAARs. GABA-evoked currents from recombinant GABAARs expressed in HEK293T cells revealed that of the four α subunit isoforms found in synaptic GABAARs in the cerebrum (α1–3 and α5), only GABAARs expressing the α3 subunits possessed slower deactivation rates than α1 subunit-containing GABAARs (32). Therefore, it is likely that α1(AD) subunits selectively inhibited expression of α3 subunit-containing GABAARs.
In addition to altering mIPSC kinetics, the α1 and α1(AD) subunits also reduced mIPSC peak amplitudes (Fig. 10, C and D, p < 0.001). The effect of α1(AD) subunits on mIPSC peak amplitudes was consistent with its reduction of wild type GABAAR surface expression (Figs. 4, 7, and 8). The effect of α1 subunits on peak mIPSC amplitudes was unexpected because studies in heterologous cells demonstrated that α1β2γ2 and α3β2γ2 GABAARs possess similar peak current amplitudes. Different possible mechanisms could explain this unexpected finding. First, the exact composition of endogenous synaptic GABAARs is unknown. Although it is assumed that endogenous GABAARs at DIV17 consist of a mixture of α1β2γ2 and α3β2γ2 receptors, it is possible that endogenous GABAARs have a composition that demonstrates higher peak current amplitudes than the α1 subunit-containing GABAARs that replace them. Second, it is also possible that overexpression of wild type α1 subunits could reduce mIPSC peak amplitudes by increasing the tonic, persistently active, currents of the neurons, which shunt synaptic currents (33). Although tonic currents are mediated by δ subunit-containing GABAARs that typically co-assemble with either α4 or α6 subunits (34, 35), recent results demonstrated that they can also co-assemble with α1 subunits (36).
DISCUSSION
We and others reported that the AD mutation caused a loss of α1(AD) subunit expression and function (8, 10–13, 21, 26). Although the heterozygous loss of α1 subunits causes neuronal hyperexcitability, α1 subunit haploinsufficiency alone does not cause the full ADJME phenotype (14, 15, 28, 37). The main finding of this study is that residual, nondegraded α1(AD) subunits conferred a dominant effect that reduced GABAAR expression more than would result from haploinsufficiency alone. Unexpectedly, the α1(AD) subunit reduced expression of α3β2γ2 receptors to a greater extent than α1β2γ2 receptors. Although these effects were small, they likely contribute to the cortical excitability produced by the heterozygous reduction in functional α1 subunit expression and thus may shape the epilepsy phenotype.
α1(AD) Subunit Reduced α1β2γ2 Expression by Entrapping Wild Type GABAA Receptor Subunits within the ER
The disruption of wild type gene function by a mutant gene product can be accomplished by several mechanisms. A mutation may cause a nonspecific effect if it produces a toxic gene product that enhances cell death (25, 38) or represses protein synthesis through the unfolded protein response (39, 40). In addition, mutations in multimeric ion channels such as GABAARs can cause specific effects by associating with wild type subunits and either forming dysfunctional ion channels on the cell surface, causing increased endocytosis, or preventing surface trafficking of the wild type protein complex.
Our study demonstrated that despite being grossly misfolded (10), the α1(AD) protein did not decrease HEK293T cell viability. In addition, overexpression of the α1(AD) subunit did not cause visible neuronal injury or death, alter neuronal physiology, or change neuronal membrane properties. In addition, the α1(AD) subunit did not nonspecifically reduce expression of transmembrane proteins unrelated to GABAARs (Fig. 4). Our experiments also showed that even with substantial overexpression, the α1(AD) subunit did not produce a dominant negative effect by substituting for wild type GABAAR on the cell surface (Fig. 2) or increasing dynamin-mediated endocytosis of wild type α1β2γ2 receptors (Fig. 5).
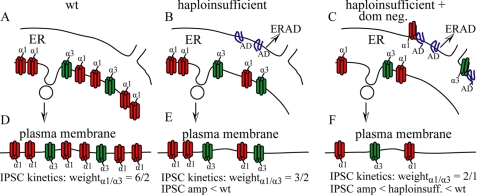
The α1(AD) subunit did cause a dominant negative effect by producing specific, concentration-dependent reductions in expression of surface wild type GABAARs (Figs. 4, 7, and 8). Because previous studies demonstrated that essentially all residual α1(AD) subunits were localized to the ER (21, 26), and because our experiments (Fig. 6) as well as those of Bradley et al. (11) showed that α1(AD) subunits specifically associated with wild type GABAAR subunits, we conclude that the α1(AD) subunit reduced expression of surface wild type GABAAR subunits by entrapping them within the ER. Moreover, our data provided direct evidence that the α1(AD) subunit reduced total as well as surface GABAAR expression (Fig. 4B), a result consistent with degradation of entrapped GABAAR subunits via ER-associated degradation (Fig. 11).
FIGURE 11.
Model of the effects of the α1(AD) subunit on surface GABAAR expression and isoform composition. This figure depicts our model of how the α1(AD) subunit reduces wild type surface GABAAR expression and alters their composition. GABAARs assemble in the ER (A–C) and traffic to the plasma membrane (d--F). At maturity, wild type neurons (wt, A and D) predominantly express α1β2γ2 receptors (red) but also express non-α1-containing GABAAR (depicted here as α3 GABAAR, green), and thus we depicted a 6:2 ratio of α1β2γ2 to α3β2γ2 receptors. In a purely haploinsufficient model (B and E), all the misfolded α1(AD) subunit (blue) is degraded without it interacting with wild type GABAAR. Therefore, haploinsufficient neurons express reduced α1β2γ2 receptors and unchanged non-α1-containing GABAAR. The time course of the IPSC current kinetics would result from the currents contributed from α1 and α3 subunit containing receptors weighted by their abundance on the neuron surface. Therefore, as depicted here, the α1/α3 weighting would decrease from 6/2 in wild type neurons to 3/2 in α1 haploinsufficient neurons thus producing IPSCs that more resembled α3 subunit containing GABAAR in the haploinsufficient neurons compared with wild type neurons. Our data are more consistent with a haploinsufficient plus dominant negative model (C and F). In this model, neurons degrade some α1(AD) subunit but also retain some misfolded α1(AD) subunit (blue) in the ER, which can associate with and retain a greater fraction of α3β2γ2 than α1β2γ2 receptors. This is shown here as α1(AD) retaining ⅓ α1 subunit containing receptors and ½ α3 subunit containing receptors. The retention of wild type GABAAR expression in the haploinsufficient plus dominant negative case reduces IPSC peak amplitudes to a greater extent than in the α1 haploinsufficient neurons. In addition, the selective retention of α3 subunit containing receptors increases the α1/α3 weighting from 3/2 to 2/1 thus producing IPSC current kinetics that are more similar to α1 subunit containing GABAAR than in the α1 haploinsufficient condition.
α1(AD) Subunit Reduced α3 Subunit Expression to a Greater Extent than α1 Subunit Expression
Homozygous Gabra1 knock-out mice increase the protein expression of other α subunits, including the α3 subunit (28–30). Because increased synaptic expression of α3 subunits compensates for absent α1 subunits, we tested if α1(AD) subunits also reduced expression of α3 subunit-containing GABAARs. Surprisingly, we found that α1(AD) subunits reduced the surface expression of α3β2γ2 receptors to a greater extent than α1β2γ2 receptors in HEK293T cells (Figs. 7 and 8). We also found that expression of α1(AD) subunits in cultured cortical neurons altered the time course of endogenous GABAergic mIPSCs, a result that suggested that non-α1 subunit-containing GABAARs were replaced by α1 subunit-containing GABAARs (Fig. 10). In ADJME patients, the extent to which α1(AD) subunits would preferentially inhibit expression of α3 subunit-containing receptors would depend on the amount of α1(AD) subunits expressed in endogenous conditions.
Different mechanisms could explain why α1(AD) subunits reduced α3β2γ2 receptors to a greater degree than α1β2γ2 receptors. A simple explanation would hold that α1(AD) subunits associate with α3 subunits with higher affinity than with α1 subunits and thus entrap a greater percentage of α3 subunits in the ER. Although functional GABAARs that traffic to the cell surface require a defined subunit stoichiometry and assembly order without two adjacent α subunits (α-β-α-γ-β) (4–6, 41), it is possible that nonfunctional α1/α3 oligomers could form, be sequestered in the ER, and undergo ER-associated degradation.
α1(AD) subunits could also selectively reduce expression of surface α3β2γ2 receptors by sequestering β2 and γ2 subunits. If α1 and α1(AD) subunits have the same affinity for β2/γ2 subunits, and α3 subunits have a lower affinity for β2/γ2 subunits, the presence of α1(AD) subunits would deprive α3 subunits of their necessary assembly partners to a greater extent than α1 subunits. Previous studies identified domains within the extracellular region of α1 subunits that are responsible for its oligomerization with β2 and γ2 subunits (42–44). The β2 subunit binding domains in the α1 and α3 subunits are identical except for a single conserved substitution. However, the γ2 subunit binding domains have four differences, including nonconserved substitutions of a threonine to a lysine and an arginine to a proline. It would be of interest in future studies to determine whether γ2 subunits oligomerize with α3 subunits with lower affinity than with α1 subunits and if either of these two amino acids explains the difference.
Differences in oligomerization affinities between the α1 and α3 subunits with β2 or γ2 subunits would explain the differential effects of the α1(AD) subunit only if β2 or γ2 subunit expression was not in excess. However, our data in Fig. 3 demonstrated that at a fixed concentration of β2 and γ2 subunits, a 4-fold increase in α1 subunit cDNA produced corresponding increases in α1β2γ2 subunit expression, evidence that β2 and γ2 subunits were in excess. A mechanism that could explain a greater effect of α1(AD) subunits on α3β2γ2 receptors than α1β2γ2 receptors in conditions of excess β2 and γ2 subunits would be that α3β2γ2 receptors traffic to the cell surface at slower rates than α1β2γ2 receptors. In this case, α3β2γ2 receptors would be retained in the ER longer than α1β2γ2 receptors, which would provide them with more opportunity to incorporate an α1(AD) subunit and be targeted for degradation.
Although our experiments focused on determining the effect of α1(AD) subunits on α1β2γ2 and α3β2γ2 receptors, interactions of α1(AD) subunits with other GABAAR subunits may make even more important contributions to the epilepsy phenotype. Future studies will be needed to determine the effects of α1(AD) subunits on expression of other GABAAR isoforms.
Model of the Dominant Effect
We depicted our model for the effect of α1(AD) subunits on α1β2γ2 and α3β2γ2 receptors in Fig. 11. In this model, wild type neurons express both α1β2γ2 and α3β2γ2 receptors in the ER (Fig. 11A) and on the neuron surface (Fig. 11D). Because mature cortical neurons express more α1 than α3 subunits (1–3, 45), α1β2γ2 and α3β2γ2 receptors are shown here in a 6:2 ratio. The amplitude and time course of GABAergic currents would result from a weighted average of α1β2γ2 and α3β2γ2 currents. In a haploinsufficient model, the α1(AD) subunits are degraded and do not associate with wild type receptors. Therefore, in a completely haploinsufficient case, neurons express reduced α1β2γ2 receptors but the same amount of α3β2γ2 receptors in the ER (Fig. 11B) and the neuron surface (Fig. 11E). Therefore, in a haploinsufficient model, the reduction in α1β2γ2 but not α3β2γ2 expression would result in a greater proportion of α3β2γ2 receptors on the surface than found in wild type neurons (shown here as a 3:2 ratio). Therefore, the time course of GABAergic currents, as a weighted sum of the α1β2γ2 and α3β2γ2 receptors, would be more similar to that of α3β2γ2 receptors in haploinsufficient than wild type neurons. Because there would be fewer GABAAR on the cell surface, inhibitory postsynaptic current peak amplitudes would be smaller in haploinsufficient than wild type neurons.
Rather than a haploinsufficient model, our data presented here suggests a haploinsufficient plus dominant negative model (Fig. 11, C and F). In this model, neurons express α1(AD) subunit in the ER. Some α1(AD) subunits degrade, but some α1(AD) subunits oligomerize with α1 and α3 subunit-containing GABAARs (Fig. 11C). Retained α1(AD) subunits reduce a greater fraction of α3β2γ2 receptors than α1β2γ2 receptors, and thus the ratio of α1 to α3 subunit-containing receptors is shown here as a 2:1 ratio (Fig. 11F). The time course of the GABAergic currents is thus more similar to that of α1β2γ2 receptors in a haploinsufficient plus dominant negative model than in the pure haploinsufficient model. Because both α1β2γ2 and α3β2γ2 receptor expression is reduced, peak current amplitudes are reduced relative to wild type and haploinsufficient currents.
Implications of Dominant Negative Effects for ADJME and Other Dominant Epilepsy Syndromes
Although the amount of endogenous α1(AD) subunit expressed in neurons of ADJME patients is unknown, our data demonstrated that neurons degrade recombinant α1(AD) subunit with high efficiency (79%, Fig. 9). Therefore, assuming that the endogenous α1(AD) subunit in ADJME patients also expresses at 21% of wild type α1 subunit and that the α1(AD) subunit in neurons cause similar sigmoidal reductions in surface wild type α1 and α3 subunit expression as in HEK293T cells (Figs. 4, 7, and 8), we expect that heterozygous α1(AD) subunit expression in ADJME patients would reduce surface α1 and α3 subunit expression by an additional 4 and 5%, respectively, more than hemizygous α1 subunit expression.
Could such a seemingly small additional reduction in surface α1 and α3 subunit expression alter the epilepsy phenotype? Both empirical and theoretical systems do suggest that seizures are threshold events and that even small changes in GABAAR availability can transition an organism into a seizure-prone state. For example, even though the GABAAR antagonists, bicuculline and pentylenetetrazole, inhibit GABA-evoked currents with typical sigmoidal concentration-response curves (46, 47), they produce seizures in experimental animals at specific doses with very small variances (48, 49). These findings suggest that a specific threshold of functional GABAARs is required to prevent an organism from experiencing a seizure. Moreover, recent computational models of neural networks predicted that very small changes in ion channel function can produce seizure-like activities (50).
Small changes in GABAAR expression and composition may shape the phenotypes of other autosomal dominant GABAAR subunit mutations that are associated with epilepsy. The best studied mutation is the γ2(R43Q) mutation associated with childhood absence epilepsy and febrile seizures (51). The γ2(R43Q) subunit produces smaller changes in GABAAR expression and composition than the α1(AD) subunit. Although the R43Q mutation does not reduce the total expression of the γ2(R43Q) subunit, it disrupts its ability to directly oligomerize with β2 subunits (52). The inability of γ2(R43Q) subunits to oligomerize may explain why they do not produce a dominant negative effect on α1, α3, or β2 subunit expression or alter mIPSC amplitude or current kinetics (17, 18). The γ2(R43Q) subunit does reduce surface trafficking of the α5 subunit-containing GABAARs and thereby reduces tonic GABAAR currents (17). Although the effects of the γ2(R43Q) subunit are small, they do shape the epilepsy phenotype because the heterozygous γ2(R43Q) subunit knock-in mice but not heterozygous γ2 subunit knock-out mice experience seizures (16, 19, 53).
Therefore, we think that the small dominant negative effects of the α1(AD) subunit could confer an epilepsy phenotype that is different from the one conferred by α1 subunit haploinsufficiency alone. This hypothesis needs to be directly tested by comparing heterozygous α1 knock-out with α1(AD) knock-in genetically modified mice.
Supplementary Material
Acknowledgments
We thank Dr. Bruce Carter (Vanderbilt University) for helpful comments concerning this manuscript. Flow cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The Vanderbilt University Medical Center Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center Grant P30 CA68485 and the Vanderbilt Digestive Disease Research Center Grant DK058404. Flow cytometry was supported in part by National Institutes of Health Vanderbilt CTSA Grant 5UL1 RR024975-03 from NCRR.
This work was supported, in whole or in part, by National Institutes of Health Grants NS055979 (to M. J. G.), NS33300 (to R. L. M.), and NS51590 (to R. L. M.) from the USPHS.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- GABAAR
- GABA receptor type A
- mIPSC
- miniature inhibitory postsynaptic current
- ER
- endoplasmic reticulum
- ADJME
- autosomal dominant form of juvenile myoclonic epilepsy
- DIV
- days in vitro.
REFERENCES
- 1.Brooks-Kayal A. R., Jin H., Price M., Dichter M. A. (1998) J. Neurochem. 70, 1017–1028 [DOI] [PubMed] [Google Scholar]
- 2.Laurie D. J., Wisden W., Seeburg P. H. (1992) J. Neurosci. 12, 4151–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. (2000) Neuroscience 101, 815–850 [DOI] [PubMed] [Google Scholar]
- 4.Tretter V., Ehya N., Fuchs K., Sieghart W. (1997) J. Neurosci. 17, 2728–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann S. W., Baur R., Sigel E. (2001) J. Biol. Chem. 276, 36275–36280 [DOI] [PubMed] [Google Scholar]
- 6.Baumann S. W., Baur R., Sigel E. (2002) J. Biol. Chem. 277, 46020–46025 [DOI] [PubMed] [Google Scholar]
- 7.Macdonald R. L., Kang J. Q., Gallagher M. J. (2010) J. Physiol. 588, 1861–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cossette P., Liu L., Brisebois K., Dong H., Lortie A., Vanasse M., Saint-Hilaire J. M., Carmant L., Verner A., Lu W. Y., Wang Y. T., Rouleau G. A. (2002) Nat. Genet. 31, 184–189 [DOI] [PubMed] [Google Scholar]
- 9.Alfradique I., Vasconcelos M. M. (2007) Arq Neuropsiquiatr. 65, 1266–1271 [DOI] [PubMed] [Google Scholar]
- 10.Gallagher M. J., Ding L., Maheshwari A., Macdonald R. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12999–13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley C. A., Taghibiglou C., Collingridge G. L., Wang Y. T. (2008) J. Biol. Chem. 283, 22043–22050 [DOI] [PubMed] [Google Scholar]
- 12.Fisher J. L. (2004) Neuropharmacology 46, 629–637 [DOI] [PubMed] [Google Scholar]
- 13.Gallagher M. J., Song L., Arain F., Macdonald R. L. (2004) J. Neurosci. 24, 5570–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J. Q., Shen W., Macdonald R. L. (2009) J. Neurosci. 29, 2833–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maljevic S., Krampfl K., Cobilanschi J., Tilgen N., Beyer S., Weber Y. G., Schlesinger F., Ursu D., Melzer W., Cossette P., Bufler J., Lerche H., Heils A. (2006) Ann. Neurol. 59, 983–987 [DOI] [PubMed] [Google Scholar]
- 16.Berry R. B., Chandra D., Diaz-Granados J. L., Homanics G. E., Matthews D. B. (2009) Neurosci. Lett. 455, 84–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eugène E., Depienne C., Baulac S., Baulac M., Fritschy J. M., Le Guern E., Miles R., Poncer J. C. (2007) J. Neurosci. 27, 14108–14116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frugier G., Coussen F., Giraud M. F., Odessa M. F., Emerit M. B., Boué-Grabot E., Garret M. (2007) J. Biol. Chem. 282, 3819–3828 [DOI] [PubMed] [Google Scholar]
- 19.Tan H. O., Reid C. A., Single F. N., Davies P. J., Chiu C., Murphy S., Clarke A. L., Dibbens L., Krestel H., Mulley J. C., Jones M. V., Seeburg P. H., Sakmann B., Berkovic S. F., Sprengel R., Petrou S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 17536–17541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeiger S. L., Musiek E. S., Zanoni G., Vidari G., Morrow J. D., Milne G. J., McLaughlin B. (2009) Free Radic. Biol. Med. 47, 1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallagher M. J., Shen W., Song L., Macdonald R. L. (2005) J. Biol. Chem. 280, 37995–38004 [DOI] [PubMed] [Google Scholar]
- 22.Jiang M., Chen G. (2006) Nat. Protoc. 1, 695–700 [DOI] [PubMed] [Google Scholar]
- 23.Saliba R. S., Pangalos M., Moss S. J. (2008) J. Biol. Chem. 283, 18538–18544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo W. Y., Botzolakis E. J., Tang X., Macdonald R. L. (2008) J. Biol. Chem. 283, 29740–29752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J., Matthies D. S., Botzolakis E. J., Macdonald R. L., Blakely R. D., Hedera P. (2008) J. Neurosci. 28, 13938–13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krampfl K., Maljevic S., Cossette P., Ziegler E., Rouleau G. A., Lerche H., Bufler J. (2005) Eur. J. Neurosci. 22, 10–20 [DOI] [PubMed] [Google Scholar]
- 27.Kumar A., Kremer K. N., Sims O. L., Hedin K. E. (2009) Methods Enzymol. 460, 379–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kralic J. E., Korpi E. R., O'Buckley T. K., Homanics G. E., Morrow A. L. (2002) J. Pharmacol. Exp. Ther. 302, 1037–1045 [DOI] [PubMed] [Google Scholar]
- 29.Kralic J. E., Sidler C., Parpan F., Homanics G. E., Morrow A. L., Fritschy J. M. (2006) J. Comp. Neurol. 495, 408–421 [DOI] [PubMed] [Google Scholar]
- 30.Ogris W., Lehner R., Fuchs K., Furtmüller B., Höger H., Homanics G. E., Sieghart W. (2006) J. Neurochem. 96, 136–147 [DOI] [PubMed] [Google Scholar]
- 31.Bosman L. W., Heinen K., Spijker S., Brussaard A. B. (2005) J. Neurophysiol. 94, 338–346 [DOI] [PubMed] [Google Scholar]
- 32.Picton A. J., Fisher J. L. (2007) Brain Res. 1165, 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brickley S. G., Cull-Candy S. G., Farrant M. (1996) J. Physiol. 497, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones A., Korpi E. R., McKernan R. M., Pelz R., Nusser Z., Mäkelä R., Mellor J. R., Pollard S., Bahn S., Stephenson F. A., Randall A. D., Sieghart W., Somogyi P., Smith A. J., Wisden W. (1997) J. Neurosci. 17, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sur C., Farrar S. J., Kerby J., Whiting P. J., Atack J. R., McKernan R. M. (1999) Mol. Pharmacol. 56, 110–115 [DOI] [PubMed] [Google Scholar]
- 36.Glykys J., Peng Z., Chandra D., Homanics G. E., Houser C. R., Mody I. (2007) Nat. Neurosci. 10, 40–48 [DOI] [PubMed] [Google Scholar]
- 37.Kralic J. E., O'Buckley T. K., Khisti R. T., Hodge C. W., Homanics G. E., Morrow A. L. (2002) Neuropharmacology 43, 685–694 [DOI] [PubMed] [Google Scholar]
- 38.Szegezdi E., Logue S. E., Gorman A. M., Samali A. (2006) EMBO Rep. 7, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Page K. M., Heblich F., Davies A., Butcher A. J., Leroy J., Bertaso F., Pratt W. S., Dolphin A. C. (2004) J. Neurosci. 24, 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Page K. M., Heblich F., Margas W., Pratt W. S., Nieto-Rostro M., Chaggar K., Sandhu K., Davies A., Dolphin A. C. (2010) J. Biol. Chem. 285, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly C. N., Krishek B. J., McDonald B. J., Smart T. G., Moss S. J. (1996) J. Biol. Chem. 271, 89–96 [DOI] [PubMed] [Google Scholar]
- 42.Klausberger T., Sarto I., Ehya N., Fuchs K., Furtmuller R., Mayer B., Huck S., Sieghart W. (2001) J. Neurosci 21, 9124–9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bollan K., King D., Robertson L. A., Brown K., Taylor P. M., Moss S. J., Connolly C. N. (2003) J. Biol. Chem. 278, 4747–4755 [DOI] [PubMed] [Google Scholar]
- 44.Bollan K., Robertson L. A., Tang H., Connolly C. N. (2003) Biochem. Soc. Trans. 31, 875–879 [DOI] [PubMed] [Google Scholar]
- 45.Poulter M. O., Barker J. L., O'Carroll A. M., Lolait S. J., Mahan L. C. (1992) J. Neurosci. 12, 2888–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Xue F., Chang Y. (2008) Mol. Pharmacol. 74, 941–951 [DOI] [PubMed] [Google Scholar]
- 47.Huang R. Q., Bell-Horner C. L., Dibas M. I., Covey D. F., Drewe J. A., Dillon G. H. (2001) J. Pharmacol. Exp. Ther. 298, 986–995 [PubMed] [Google Scholar]
- 48.Vlainiæ J., Periciæ D. (2009) Neuropharmacology 56, 1124–1130 [DOI] [PubMed] [Google Scholar]
- 49.Hentschke M., Wiemann M., Hentschke S., Kurth I., Hermans-Borgmeyer I., Seidenbecher T., Jentsch T. J., Gal A., Hübner C. A. (2006) Mol. Cell. Biol. 26, 182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas E. A., Reid C. A., Berkovic S. F., Petrou S. (2009) Arch. Neurol. 66, 1225–1232 [DOI] [PubMed] [Google Scholar]
- 51.Wallace R. H., Marini C., Petrou S., Harkin L. A., Bowser D. N., Panchal R. G., Williams D. A., Sutherland G. R., Mulley J. C., Scheffer I. E., Berkovic S. F. (2001) Nat. Genet. 28, 49–52 [DOI] [PubMed] [Google Scholar]
- 52.Hales T. G., Tang H., Bollan K. A., Johnson S. J., King D. P., McDonald N. A., Cheng A., Connolly C. N. (2005) Mol. Cell. Neurosci. 29, 120–127 [DOI] [PubMed] [Google Scholar]
- 53.Chiu C., Reid C. A., Tan H. O., Davies P. J., Single F. N., Koukoulas I., Berkovic S. F., Tan S. S., Sprengel R., Jones M. V., Petrou S. (2008) Ann. Neurol. 64, 284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.