Abstract
Thrombopoietin (TPO) and its receptor (Mpl) have long been associated with megakaryocyte proliferation, differentiation, and platelet formation. However, studies have also shown that the extracellular domain of Mpl (Mpl-EC) interacts with human (h) NUDC, a protein previously characterized as a human homolog of a fungal nuclear migration protein. This study was undertaken to further delineate the putative binding domain on the Mpl receptor. Using the yeast two-hybrid system assay and co-immunoprecipitation, we identified that within the Mpl-EC domain 1 (Mpl-EC-D1), amino acids 102–251 were strongly involved in ligand binding. We subsequently expressed five subdomains within this region with T7 phage display. Enzyme-linked immunosorbent binding assays identified a short stretch of peptide located between residues 206 and 251 as the minimum binding domain for both TPO and hNUDC. A series of sequential Ala replacement mutations in the region were subsequently used to identify the specific residues most involved in ligand binding. Our results point to two hydrophobic residues, Leu228 and Leu230, as having substantial effects on hNUDC binding. For TPO binding, mutations in residues Asp235 and Leu239 had the largest effect on binding efficacy. In addition, deletion of the conservative motif WGSWS reduced binding capacity for hNUDC but not for TPO. These separate binding sites on the Mpl receptor for TPO and hNUDC raise interesting implications for the cytokine-receptor interactions.
Keywords: Amino Acid, Cytokine, Mutant, Protein-Protein Interactions, Receptor Structure-Function, Mutagenesis, Protein Binding, TPO, Thrombopoietin Receptor (Mpl), hNUDC
Introduction
TPO2 has long been studied as the primary cytokine regulator of megakaryocyte development. Its activity is mediated by the thrombopoietin receptor (Mpl), a member of the cytokine receptor superfamily (1–4). Genetic disruption of either cytokine or receptor drastically interferes with platelet production, indicating the major role TPO and Mpl play in the activation of megakaryopoiesis and platelet production (5–7). However, despite the platelet volume decrease, the megakaryocytes and platelets that are produced remain morphologically normal and are adequate to prevent severe hemophilia in murine experiments (8). This observation leaves open the possibility for other cytokines and receptors to have an overlapping biological potency. One such candidate we have investigated as an additional ligand for Mpl is known as hNUDC. Despite having no detectable sequence similarity with TPO, hNUDC has been shown to have similar megakaryopoietic effects (9, 10). TPO and hNUDC also exhibited a communal signal transduction cascade in several megakaryocytic cells expressing Mpl (11, 12).
Human NUDC was initially identified and cloned as a nuclear migration protein based on the similarity of its C terminus to that of fungal NUDC from Aspergillus nidulans (13). Numerous studies have implicated hNUDC as an endogenous factor involved in cell mitotic spindle formation, cell proliferation, cell cytokinesis, and nuclear migration in a wide variety of experimental animal cells (14–16). Although earlier studies have suggested the mechanisms by which hNUDC exerts its endogenous effects, evidence is now emerging that hNUDC also plays a critical role in human hematopoietic cells (10, 12, 17, 18). Over the past several years, progress has been made in linking the biological effects of hNUDC to Mpl (11, 12, 19).
Molecular modeling of Mpl tertiary structure shows that it is most related to the EPO receptor. Mpl is composed of a large extracellular domain (Mpl-EC), a single membrane-spanning region, and a short cytoplasmic tail (20, 21). The Mpl-EC is the principal ligand-binding region consisting of two domains, Mpl-EC-D1 and Mpl-EC-D2. Two conserved disulfide bridges are found in the N-terminal side of both domains, and WXXWS motifs are located at the C-terminal regions of both Mpl-EC-D1 and Mpl-EC-D2 (22, 23). These features of Mpl-EC are also conserved in other members of the class 1 cytokine receptor family, including the granulocyte-macrophage colony-stimulating factor receptor, the granulocyte colony-stimulating factor receptor, and the interleukin family of receptors (22, 23). Despite sharing these common structural motifs, functionally significant variations are seen in the extracellular domains of cytokine receptor superfamily members. The topographical study of the structure of Mpl therefore remains of considerable value.
In this study we map the extracellular domain of Mpl using the yeast two-hybrid system and co-immunoprecipitation. Furthermore, we combine site-specific mutagenesis and phage display as a method for the comparative analysis of single amino acid variations in the Mpl-EC domain on both TPO and hNUDC binding specificities.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
The two-hybrid system and mouse monoclonal antibody against the T7 tail fiber were purchased from Clontech. Rabbit polyclonal anti-c-Mpl was purchased from Millipore (Bedford, MA). Mouse monoclonal anti-TPO was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A monoclonal antibody against hNUDC was prepared in our laboratory by standard techniques from mice immunized with bacterially expressed recombinant hNUDC (9). Mouse monoclonal anti-His tag was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). A T7Select® cloning kit was obtained from Novagen (Madison, WI). A MutanBEST kit was obtained from TaKaRa Biotechnology (Dalian, China). The JetPEITM transfection kit was purchased from PolyPlus Transfection Co. (Illkirch, France).
Construction of the Yeast Two-hybrid Expression Vectors
Corresponding to amino acid number, different portions of Mpl were obtained via PCR with the following primers: 5′-CGGAATTCGATGTCTCCTTGCTGGCATCA-3′ and 5′-CCGCTCGAGGATCCAGGCGGTCTCGGTGGC-3′ for aa 1–466; 5′-CGGAATTCGATGTCTCCTTGCTGGCATCA-3′ and 5′-CCGCTCGAGAGTCACAGGGAGGGACCAGG-3′ for aa 1–251; 5′-CGGAATTCAACTGTGGACCTGCCTGGAGAT-3′ and 5′-CCGCTCGAGGATCCAGGCGGTCTCGGTGGC-3′ for aa 251–466; 5′-CGGAATTCGATGTCTCCTTGCTGGCATCA-3′ and 5′-CCGCTCGAGACCCATGGCCTTGATGATACT-3′ for aa 1–117; 5′-CGGAATTCGACAGTGTAGGCCTGCCGG-3′ and 5′-CCGCTCGAGAGTCACAGGGAGGGACCAGG-3′ for aa 102–251. EcoRI and XhoI (underlined) sites were added on the forward and the reverse primers, respectively. Fragments were subsequently cloned into the yeast expression vector pGBKT7-BD downstream and in-frame with the GAL4 DNA-binding domain using the EcoRI and XhoI sites to create the plasmids pGBK-Mpl(1–466), pGBK-Mpl(1–251), pGBK-Mpl(251–466), pGBK-Mpl(1–117), and pGBK-Mpl(102–251). Full-length hNUDC and TPO sequences were amplified by PCR with specific primers, as described previously (11), and inserted into pGADT7-AD downstream and in-frame with the GAL4 activation domain using EcoRI and XhoI sites to create the plasmids pGADT7-hNUDC and pGADT7-TPO, respectively. The yeast strain AH109 was transformed with pairwise combinations of each Mpl deletion construct paired with either pGADT7-hNUDC or pGADT7-TPO and allowed to grow at 30 °C for 2–4 days on selective medium agar plates lacking tryptophan and leucine. Growth assays were performed by plating individual colonies onto agar containing selective medium lacking tryptophan, leucine, histidine, and adenine. In addition to the growth assay, the interactions between pairs of proteins were also assessed for β-galactosidase activity using a filter assay with X-gal as substrate.
Generation of NIH 3T3 Cells Expressing Mpl-EC Deletion Constructs
The construction of the pMpl-EGFP, phNUDC-DsRed, and pTPO-DsRed vectors used in this study have been described previously (11). A 78-bp oligonucleotide duplex encoding an Mpl signal peptide was amplified from the pMpl-EGFP vector with the primers 5′-ATGCCCTCCTGGGCCCTCTTC-3′ and 5′-TTGGCTGCTGACTTGGGC-3′. The transmembrane domain following the intracellular sequence (residues 467–609) was amplified by PCR with specific primers 5′-TCCTTGGTGACCGCTCTGCATCTA-3′ and 5′-TCAAGGCTGCTGCCAATAGCTTAG-3′. The Mpl-EC regions corresponding to amino acids 1–466, 1–251, 251–466, 1–117, and 102–251 were amplified via PCR. The primers used for the amplification of these fragments are largely identical to those indicated above. However, the 5′ EcoRI sites were replaced by a tag complementary to the 3′ end of the Mpl signal peptide sequence, and the 3′ XhoI sites were replaced by a tag complementary to the 5′ end of the Mpl transmembrane sequence. Each Mpl-EC sequence was mixed and annealed with both the signal peptide and the transmembrane plus intracellular sequences. This was then amplified in a fourth PCR using the primers 5′-GCGGAATTCATGCCCTCCTGGGCCCTCTTC-3′ and 5′-CCGCTCGAGTCAAGGCTGCTGCCAATAGCTTAG-3′. The final PCR products were cut with EcoRI and XhoI restriction digests and ligated into pEGFP-N1 upstream and in-frame with EGFP. The resulting expression vectors pMpl(1–466)-EGFP, pMpl(1–251)-EGFP, pMpl(251–466)-EGFP, pMpl(1–117)-EGFP, and pMpl(102–251)-EGFP were co-transfected into NIH 3T3 cells with either phNUD-DsREd or pTPO-DsRed using JetPEITM transfection kits (PolyPlus Transfection Co.) according to the methods described previously (11).
Co-immunoprecipitation
The NIH 3T3 cells co-transfected with the Mpl-EC deletion variants and either phNUDC-DsRed or pTPO-DsRed were lysed in an IP buffer (1% Triton X-100, 50 mm Tris·Cl, pH 7.4, 300 mm NaCl, 5 mm EDTA) containing protease inhibitors. A high speed centrifugation step removed cell debris from both lysates and cell culture media. Equal amounts of cell extracts and culture media (200 μl) were immunoprecipitated by addition of 5 μg of mouse monoclonal antibody raised against either hNUDC (anti-hNUDC mAb) or TPO (anti-TPO mAb) and incubated with 0.1% bovine serum albumin overnight at 4 °C. Protein A-Sepharose beads (Amersham Biosciences) were added and incubated for 2 h at 4 °C with mixing. Immune complexes were resolved by SDS-PAGE. Proteins were transferred onto polyvinylidene difluoride membranes and Western blotted with polyclonal antibody against Mpl (anti-Mpl pAb). Horseradish peroxidase-conjugated secondary antibody and 3,3′,5,5′-tetramethylbenzidene (Amersham Biosciences) were used to develop the Western blots.
Protein Purification of hNUDC and TPO
PCR was used to amplify DNA fragments encoding the N-terminal domain of TPO (residues 1–156) using the forward and reverse primers 5′-CCGGAATTCGACCATGGAGCTGACTGAATTGCTCCTCGTG-3′ and 5′-CCGCTCGAGCTAGTGATGGTGATGGTGATGGAGCAGGTGTTGGAAGCTCAG-3′. The amplification product incorporated a hexahistidyl tag (His tag, double underlined) following a TAG termination codon (boldface) downstream from coding region. To ensure high expression levels of TPO fusion proteins, we routinely added a Kozak sequence GACCATGG (italic), at the beginning of the coding region. The underlined nucleotides denote the EcoRI and XhoI sites, respectively. An amplified fragment was digested with the appropriate restriction enzymes and cloned into a similarly digested pcDNA3.1 (Clontech) resulting in a plasmid designated pcDNA-TPO-His. In a similar manner, an expression construct for hNUDC was created using the plasmids pcDNA-TPO-His and pPICZaA-hNUDC-His (10) as templates. The TPO signal peptide (TS) consisting of 21 TPO amino acid residues was fused to the 5′ terminus of the hNUDC-His fusion protein in pCDNA 3.1, giving the plasmid designated pcDNA-TS-hNUDC-His.
Dihydrofolate reductase negative cells (CHO/dhfr− cells, CRL-9096, ATCC) were grown and maintained in Iscove's modified Dulbecco's medium (Invitrogen) supplemented with 0.1 mm hypoxanthine, 0.016 mm thymidine, 100 nm methotrexate, and 10% dialyzed fetal bovine serum. All cell cultures in this study were performed at 37 °C in a 5% CO2 humidified atmosphere. CHO/dhfr− cells were co-transfected with 5 μg of pSV2-dhfr, a plasmid expressing the dihydrofolate reductase gene, with either pcDNA3.1-TPO-His (5 μg) or pcDNA3.1-TS-hNUDC-His using CaCl2 transfection kits (Beyotime, China) according to the manufacturer's protocol. Following transfection, CHO/dhfr− cells were cultured in nonselective medium for 3 days. Stably transfected clones expressing either TPO or hNUDC were selected for their ability to grow in 10% dialyzed fetal bovine serum, 500 μg/ml G418 Iscove's modified Dulbecco's medium lacking hypoxanthine and thymidine. After 2 weeks, hygromycin-resistant cells were subjected to increasing levels of methotrexate at concentrations of 0.2, 0.8, 1.0, 4.0, and 10 μm. The supernatant from methotrexate-resistant cell cultures were confirmed by Western blot assay.
The medium containing the expression proteins were subjected to nickel affinity chromatography (Clontech) to purify hNUDC and TPO and subsequently washed with 3 column volumes of 300 mm NaCl, 20 mm sodium phosphate, pH 8.0. The protein was eluted with the same buffer containing 300 mm imidazole.
Construction of the Phage Display Mpl Subdomains
Five subdomains corresponding to different portions of Mpl-EC-D1 were amplified by PCR using the following primers: 5′-CCGGAATTCGGACAGTGTAGGCCTGCCGG-3′ and CCCAAGCTTTGTGGCAATCAGCTGTATGACCGT-3′ for aa 102–164; 5′-CCGGAATTCTGAAACCTGCTGCCCTGCTCTG-3′ and 5′-CCCAAGCTTAGTCACAGGGAGGGACCAGG-3 for aa 165–251; 5′-CCGGAATTCTGAAACCTGCTGCCCTGCTCTG-3′ and 5′-CCCAAGCTTAGCTTCTCTACTTGGGGAGGT-3′ for aa 165–205; 5′-CCGGAATTCTCTGGACCAGTCTCCATGTGCT-3′ and 5′-CCCAAGCTTCTGCAGCCAGTAGGAGTTGCC-3′ for aa 180–229; 5′-CCGGAATTCTTCAGCTCTGACAGCAGAGGGT-3′ and 5′-CCCAAGCTTAGTCACAGGGAGGGACCAGGA-3′ for aa 206–251. The DNA fragments were digested with EcoRI and HindIII (underlined) and ligated into a pTZ57R/T vector (Fermentas, Burlington, Canada). DNA inserts were confirmed by DNA sequence analysis. Depending on the size of the aa residue, the inserts were cloned into the corresponding sites of the T7Select 415-1b (<50 aa) or T7Select10-3b (>50 aa) phage arms to create the constructs pT7Select10-Mpl(102–164), pT7Select10-Mpl(165–251), pT7Select415-Mpl(165–205), pT7Select415-Mpl(180–229), and pT7Select415-Mpl(206–251). Phage packaging, amplification, and titration were performed according to the manufacturer's instructions.
Alanine Scanning Mutagenesis
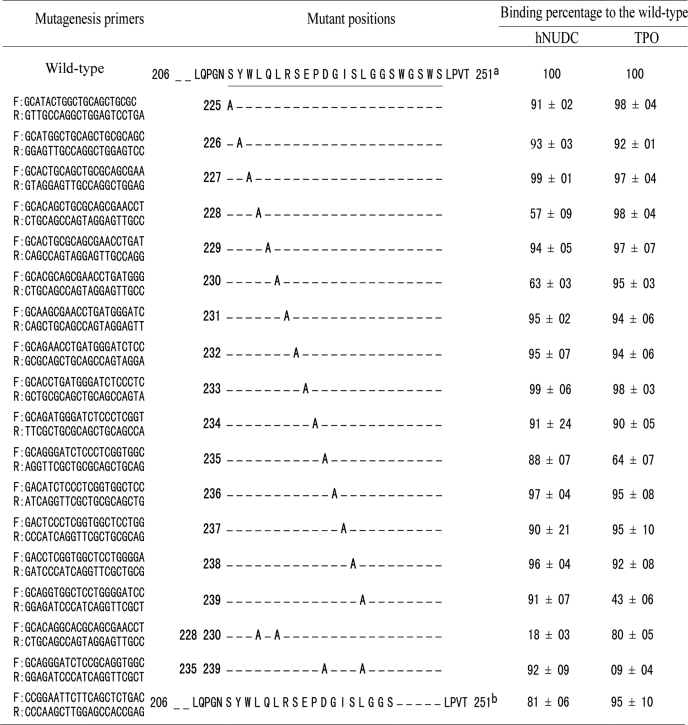
Point mutations in the ligand-binding sites on Mpl-EC-D1 were generated by standard PCR-based site-directed mutagenesis using a TaKaRa MutanBEST kit according to the manufacturer's instructions. The cDNA encoding for aa 206–251 of Mpl-EC-D1 (wild type) was subcloned into pTZ57R/T vector to obtain a pTZ57R/T-Mpl-(206–251) plasmid suitable for the mutagenesis reactions. Either single or double residues were converted to Ala using PCR with pTZ57R/T-Mpl(206–251) as a template. The mutagenic primers are summarized in Table 1. Once the mutations were confirmed by DNA sequence, the inserts were extracted by E.Z.N.A.gel extraction kit (Omega Bio-Tek, Guangzhou, China). Subsequently, they were all subcloned in T7Select 415-1b vector to allow comparison with phage display expression between mutant and wild-type receptors. Each phage clone was further verified by PCR with primers flanking the insertion site and sequencing of the insert.
TABLE 1.
Mutagenesis of residues in the Mpl binding domain by phage display
The effect on the binding of TPO or hNUDC by phage displayed mutant peptides as assayed using ELISA. Results shown represent the mean ± S.D., n = 6 replicates.
a Positions for mutagenesis in pT7Select-Mpl(206–251) are underlined.
b The WGSWS residues are deleted in pT7Select-Mpl(206–251).
Binding of Phages to Immobilized hNUDC or TPO
Protein-charged ELISA plates were coated with 200 nm hNUDC-His or TPO-His overnight at 4 °C. Plates were blocked by the addition of phosphate-buffered saline with 5% nonfat milk for 1 h at room temperature. After five washes with phosphate-buffered saline, phage samples (1 × 1010 pfu/well) in phosphate-buffered saline buffer were added to hNUDC-His- or TPO-His-coated wells and incubated for an additional 1 h at 37 °C. Following incubation, unbound phages were removed by washing the wells three times with phosphate-buffered saline containing 0.05% Tween 20, and the amount of retained phages was determined with phage-specific antibodies, anti-T7 tail fiber monoclonal antibody (anti-T7 fiber mAb) diluted 1:2000, followed by 1 h of incubation with a 1:1000 dilution of horseradish peroxidase-labeled anti-mouse secondary antibody. Development of the plates was done by adding 3,3′,5,5′-tetramethylbenzidene, and the color change was quantified at 450 nm on a microplate spectrophotometer. Null phage, which did not carry any insert, was used as background control.
Competitive Inhibition ELISA
Phage expressing the Mpl-binding domain and its derivative mutants (1 × 109 pfu/well) were mixed with serial dilutions of increasing TPO-His concentrations ranging from 0 to 500 nm. These phage mixtures were then added to the hNUDC-His-coated microwells. Following incubation, microtiter plates were washed thoroughly, and bound phage were detected with anti-T7 tail fiber mAb as described above for phage ELISA. IC50 values were calculated using the Statistical Package for the Social Sciences software (SPSS Statistics 17).
Molecular Modeling
A model of Mpl-EC-D1 was constructed using the crystal structure of the extracellular domain of EPO receptor (EPO-binding protein (EBP)) as a template (PDB accession number 1ERN). The sequences of Mpl-EC-D1 (NCBI Reference Sequence accession number NP-005364) and EBP (GenBankTM accession number AAA52403) were aligned based on conserved amino acids, including the Cys-Cys disulfide bonds and the WXXWS boxes according to the guidelines described by Deane et al. (23) for class 1 cytokine receptors. The Mpl-EC-D1 molecular model, both A–G and A′–G′ β-strains, was initially developed according to the corresponding domain of EBP using the Swiss-Model server (24). The molecular complex of Mpl-EC-D1 and TPO (PDB accession number 1V7M) was generated by submitting the relevant protein structures to the program Cluspro 2.0 (25). The ligand-receptor complex was manually assembled into the most complementary orientation using PyMOL version 0.99 (DeLano Scientific).
RESULTS
Characterization of the Binding Domains of Mpl with TPO or hNUDC in Yeast Two-hybrid System
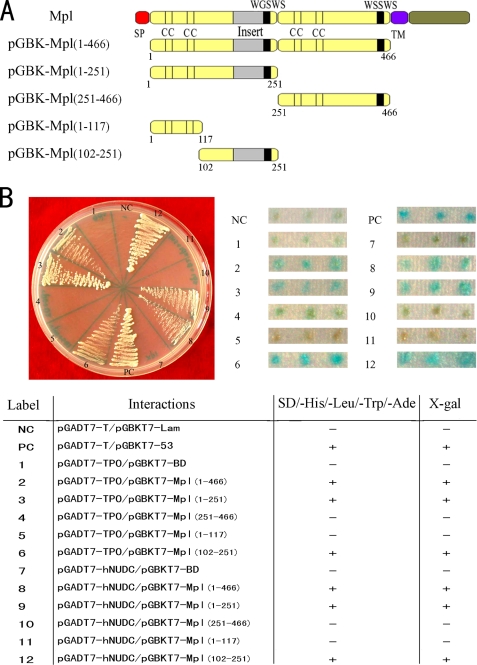
The first step toward mapping the Mpl-EC binding regions for both TPO and hNUDC was to examine the protein-protein interactions in a yeast two-hybrid system. A series of Mpl-EC deletion constructs were created in pGBKT7-BD (Fig. 1A). TPO and hNUDC constructs were created in pGADT7-AD. When co-expressed, the presence of interactions between the receptor and cytokine constructs will result in the transcription of reporter genes that code for the amino acid nutritional markers adenine and histidine. Two deletions, Mpl(1–117) and Mpl(251–466), failed to induce yeast cell growth. The other deletions Mpl(1–466), Mpl(1–251), and Mpl(102–251) induced growth of the yeast cells in a manner comparable with the positive control (Fig. 1B, left panel). These results were corroborated by the galactosidase assays. As indicated by the blue coloration, galactosidase activity in yeast cells with the Mpl(1–466), Mpl(1–251), and Mpl(102–251) constructs was comparable with the positive control (Fig. 1B, right panel). In contrast yeast containing the Mpl(1–117) and Mpl(251–466) constructs showed no galactosidase activity. These results indicate that the Mpl-EC region encompassing aa 102–251 is sufficient for some level of interaction with hNUDC and TPO.
FIGURE 1.
Mpl deletions interact with TPO and hNUDC in the two-hybrid system. A, schematic representation of the five Mpl-EC deletion constructs in a yeast two-hybrid system. Numbers refer to amino acid positions that have been cloned into pGBKT7-BD. SP, signal peptide; TM, transmembrane region. B, assay for the interactions of hNUDC or TPO with Mpl deletions. AH109 yeast colonies co-expressing Mpl deletion constructs with pGADT7-hNUDC or pGADT7-TPO were plated in selective media plates lacking leucine, tryptophan, histidine, and adenine (left panel). Mpl deletions co-transformed with pGADT7-hNUDC or pGADT7-TPO were also tested for β-galactosidase activity using a filter assay and X-gal as a substrate (right panel). Three independent triple transformants are shown. The pGBKT7–53/pGADT7-T and the pGBKT7-Lam/pGADT7-T plasmids were used as positive (PC) and negative controls (NC), respectively. − indicates no detectable growth and no galactosidase activity of co-transformants on selective medium. + indicates growth after 3–4 days and the presence of galactosidase activity in an overlay plate assay. Results shown are representative of those obtained in three independent experiments.
Specific Co-immunoprecipitation of Mpl with TPO or hNUDC in Mammalian Cell
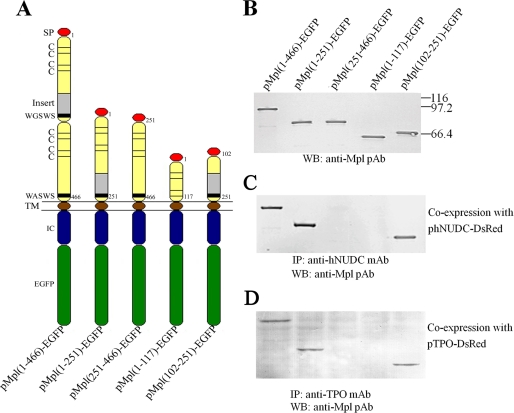
Co-immunoprecipitation experiments were performed in the NIH 3T3 cell line to investigate the specific binding interactions between Mpl-EC deletions with TPO or hNUDC. In an effort to facilitate cytokine secretion, all of the deletion constructs of Mpl were designed to contain a signal peptide, the transmembrane domain and the intracellular domain (Fig. 2A). These cDNA fragments were subcloned in the mammalian expression vector pEGFP-N1 upstream and in-frame with EGFP coding sequences. NIH 3T3 cells were stably transfected with these plasmids, and the subsequent cell lysates were Western blotted with anti-Mpl pAb (Fig. 2B). The molecular masses of the receptor portions were in the range of 111 kDa for Mpl(1–466)-EGFP, 84 kDa for Mpl(1–251)-EGFP, 85 kDa for Mpl(251–466)-EGFP, 71 kDa for Mpl(1–117)-E-GFP, and 66 kDa for Mpl(102–251)-EGFP. These values were all in line with calculated molecular weights (Fig. 2B). The NIH 3T3 cells stably expressing Mpl-EC deletion constructs were co-transfected with expression plasmids encoding either hNUDC or TPO for a period of 48 h. As reported previously, hNUDC is secreted outside the cell when co-expressed with Mpl. Similarly TPO is released into the culture medium as a composite cytokine. The mixtures of cell lysates and culture medium were subjected to, depending on the cytokine, immunoprecipitation with anti-hNUDC or anti-TPO mAb. The strongest interactions were observed with Mpl(1–466)-EGFP, Mpl(1–251)-EGFP, and Mpl(102–251)-EGFP co-precipitated hNUDC as detected by anti-Mpl pAb Western blot (Fig. 2C). Similar results were observed when co-expressing TPO together with Mpl-EC deletions (Fig. 2D). In contrast, co-expression of Mpl(1–117)-EGFP or Mpl(251–466)-EGFP with either TPO or hNUDC did not result in interactions. These results reinforce that the region encompassing aa 102–251 within the Mpl-EC-D1 is sufficient for interactions with both hNUDC and TPO.
FIGURE 2.
Co-immunoprecipitation of Mpl deletions with TPO or hNUDC in NIH 3T3. A, schematic representation of the EGFP-tagged Mpl deletion constructs tested in the co-immunoprecipitation assay. Numbers refer to amino acid positions that have been cloned into pEGFP-N1. The signal peptide, transmembrane, intracellular domains, and green fluorescent protein are abbreviated as SP, TM, IC, and EGFP respectively. B, Western blot (WB) analysis of total lysates. An equal amount of proteins (5 μg) from extracts of NIH 3T3 cell stably transfected with Mpl deletion constructs were loaded onto a 12% SDS-PAGE. Western blot analysis of expressed Mpl deletions was performed with anti-Mpl polyclonal antibody (pAb). C, co-immunoprecipitate of Mpl deletions with hNUDC. NIH 3T3 cells stably transfected with different EGFP-tagged Mpl deletions were co-transfected with phNUDC-DsRed. Culture media and cell lysates were immunoprecipitated (IP) with the anti-hNUDC mAb. Western blot analysis of the immunoprecipitates was performed with anti-Mpl polyclonal antibody. D, co-immunoprecipitate of Mpl deletions with TPO. NIH 3T3 cells stably transfected with different EGFP-tagged Mpl deletions were co-transfected with pTPO-DsRed. Culture media and cell lysates were immunoprecipitated with the anti-TPO mAb. Western blot analysis of the immunoprecipitates was performed with anti-Mpl pAb.
Purification of Secreted TPO-His and hNUDC-His
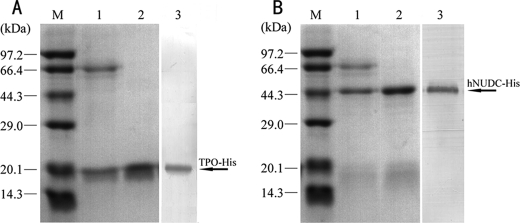
Western blot experiments indicate that the CHO/dhfr− cell line does not endogenously produce Mpl. Therefore, there remains a high abundance of expressed hNUDC in CHO/dhfr− cell lysates (data not shown). We wanted to investigate the influence that a TPO signal peptide might have on increasing the efficiency of hNUDC secretion. For this reason, we constructed an expression vector encoding full-length hNUDC downstream of TPO secretion signal peptide. We expressed both the N-terminal 157 aa of TPO and full-length of hNUDC as His fusion proteins in the pcDNA3.1 vector to allow for affinity purification. Stably transfected CHO/dhfr− cells were generated and selected for high secretion levels of TPO-His or TS-hNUDC-His. Very high cell densities were obtained when cells were grown in serum-free medium containing methotrexate. This was accompanied by high concentrations of hNUDC-His or TPO-His. Both hNUDC-His and TPO-His were the main protein components, and SDS-PAGE revealed that protein purity was already greater than 50% at this stage (Fig. 3, A and B). Purification of TPO-His and hNUDC-His was performed using single step nickel chromatography of the CHO/dhfr− cell culture. Size shifts of around 18 kDa for TPO-His and 45 kDa for TS-hNUDC-His were observed, and these smaller sizes corresponded to the loss of the TPO signal peptide. Coomassie Blue staining and Western blotting with an anti-His antibody showed that the purity of these preparations was greater than 90% (Fig. 3, A and B).
FIGURE 3.
Purification of recombinant fusion proteins. A, expression of TPO-His. Lane M, molecular mass protein markers; lane 1, culture supernatant from CHO/dhfr− expressing TPO-His; lane 2, fraction containing purified TPO-His eluted from nickel-charged affinity chromatography; lane 3, Western blot analysis to detect expression of TPO-His with an anti-His mAb. B, expression of hNUDC-His. Lane M, molecular mass protein markers; lane 1, culture supernatant from CHO/dhfr− expressing hNUDC-His; lane 2, fraction containing purified hNUDC-His eluted from nickel-charged affinity chromatography; lane 3, Western blot analysis to detect expression of hNUDC-His with an anti-His mAb. The arrows indicate the positions of the TPO-His and hNUDC-His fusion proteins.
T7 Phage Clones Expressing Mpl-EC Deletions
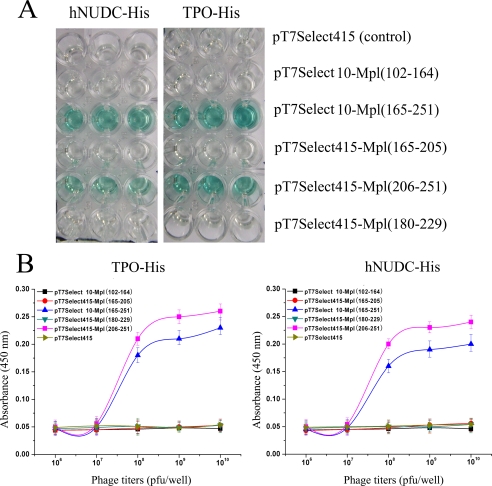
Yeast two-hybrid system and co-immunoprecipitation experiments have demonstrated that TPO and hNUDC bind with similar affinity to the 102–251-amino acid region of Mpl-EC-D1. We next sought to further delineate the minimal ligand-binding domain using the T7 phage display system. With this in mind, two larger DNA fragments encoding 102–164 and 165–251 of Mpl-EC-D1 were placed into T7Select10-3b phage arms. These were designated T7Select10-Mpl(102–164) and T7Select10-Mpl(165–251), respectively. In addition, three slightly smaller overlapping DNA fragments encoding for the residues 165–205, 180–229 and 206–251 were individually inserted into a T7Select415-1b phage arms, designated T7Select415-Mpl(165–205), T7Select415-Mpl(180–229), and T7Select415-Mpl(206–251). Equal amounts of phage expressing Mpl-EC-D1 subdomains (109 pfu/well) were then added to the hNUDC-His- or TPO-His-coated plates, respectively. The ability of these phage constructs to bind TPO or hNUDC was measured using ELISA. As showed in Fig. 4A, T7Select415-Mpl(165–251) and T7Select415-Mpl(206–251) bind to immobilized hNUDC or TPO, whereas T7Select10-Mpl(165–205), T7Select415-Mpl(102–156), and T7Select415-Mpl(180–229) did not. This suggests that the Mpl-EC-D1 subdomain containing amino acids 206–251 is involved in specific recognition by both TPO and hNUDC.
FIGURE 4.
Comparative specificities of TPO and hNUDC binding by recombinant T7 phage displaying Mpl deletions. A, binding capacity of T7 phage displaying different subdomains of Mpl-EC for hNUDC or TPO was assessed by ELISA. Wells in a 96-well plate were coated with 200 nm purified hNUDC-His or TPO-His followed by addition of recombinant phages as indicated. After washing, binding was evaluated with anti-T7 tail fiber mAb. B, comparison of different titration of phage binding to hNUDC and TPO. A fixed amount of hNUDC-His or TPO-His coated on plates was blocked and incubated with increasing concentrations of T7 phage expressing Mpl-EC subdomains. The titers were determined by ELISA using anti-T7 tail fiber mAb. The error bars denote the standard deviation of n = 6 replicates (experiments were performed in triplicate in two assays).
To evaluate cytokine binding receptor in a more quantitative way, the recombinant phages displaying the different regions of Mpl-EC-D1 to either hNUDC or TPO were evaluated by phage titrations. Within the range of phage titers from 1 × 107 to 1 × 1010 pfu/well, binding of T7Select10-Mpl(165–251) and T7Select415-Mpl(206–251) phages to hNUDC or TPO increased in a concentration-dependent manner (Fig. 4B). However, T7Select415-Mpl(165–205), T7Select415-Mpl(102–164), and T7Select415-Mpl(180–229) phages displayed no binding affinity for hNUDC or TPO regardless of concentration (Fig. 4B). Notably, ligand binding affinity for T7Select10-Mpl(165–251) was somewhat lower than that for T7Select415-Mpl(206–251). This result may perhaps be explained by lower DNA copy numbers per phage in a T7Select10-3b system compared with that of T7Select415-1.
Mutagenesis of Mpl-binding Sequence in Phage Display
To characterize the residues critical to the binding interaction, we carried out Ala scanning within the 206–251 region of Mpl-EC-D1. Site-directed mutagenesis was used to generate sequential substitutions of each amino acid for Ala in T7Select415 phage constructs. Each of the resulting mutant peptides was then tested for its ability to bind either hNUDC or TPO relative to wild-type pT7Select415-Mpl(206–251). As shown in Table 1, the majority of mutant variants displayed a weak reduction in binding ability (<10%) when compared with the wild type. In contrast, the L228A and L230A mutants resulted in a more marked reduction in the hNUDC bound. Compared with the wild type, there was a decrease of 37 and 43%, respectively (Table 1). Two other Ala mutations D235A and L239A revealed decreases in TPO binding capability of 36 and 57%, respectively (Table 1).
To investigate if double substitutions lead to a further decrease of ligand binding, we generated the following two sets of mutations: L228A/L230A and D235A/L239A. Assay revealed that the binding ability of the double mutants was essentially compromised. In comparison with wild type, the level of hNUDC bound to the L228A/L230A mutant was only 18% of wild type, and the level of TPO bound to the D235A/L239A mutant was only 9% of wild type (Table 1). We also investigated the influence the conserved WGSWS motif would have on hNUDC or TPO binding. An additional deletion experiment was conducted by removal of the WGSWS amino acids in the 206–251 region of Mpl-EC-D1. As illustrated in Table 1, this motif has no apparent effect on TPO binding but reduced the level of hNUDC binding by about 19% compared with wild type.
Homology Modeling
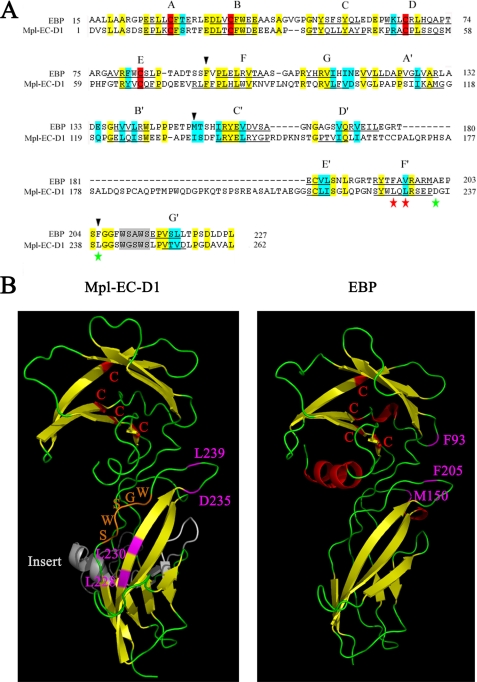
An Mpl-EC-D1 model was built based on the alignment of its sequence with that of EBP, which has a resolved crystallographic structure (26). The overall amino acid alignment revealed a low identity score between Mpl-EC-D1 and EBP (28%). Nevertheless, the general location of certain structurally important residues is conserved between Mpl-EC-D1 and EBP protein structures. These include the conservation of two Cys pairs, several hydrophobic and polar residues, as well as the WXXWS motif (Fig. 5A). Based on the predicted model, Mpl-EC-D1 is made up of two fibronectin type III (FnIII)-like domains. Each contains seven antiparallel β-strands, denoted as A–G and A′–G′, respectively (Fig. 5, A and B). It is also characterized by a large insert sequence (Ala172–Gly213) located with the second FnIII-like domain flanked by the D′ and F′ β-strands (Fig. 5, A and B). This insertion was not homologous to any other known structure associated with cytokine receptors and therefore was considered to most likely be unrelated to ligand binding and functioning (23). The WGSWS sequence located in the C-terminal region of the second FnIII-like domain of Mpl runs roughly antiparallel to the F′ β-strand (Fig. 5B). Furthermore, the two disulfide bridges between the four conserved Cys in the crystal structure are present in our model. The residues associated with hNUDC binding, Leu228 and Leu230, are located in the F′ β-strand. The Asp235 and Leu239 residues involved in TPO interactions are situated in the loop region between the adjacent F′ and G′ β-strands (Fig. 5B). In comparison, the critical determinants of EPO binding, residues Phe93, Met150, and Phe205, are located at the E–F, B′–C, and F′–G′ loops of EBP, respectively (27, 28). Interestingly, the Phe205 residue located in the F′–G′ loop of EBP is situated in the analogous position to Leu239 in Mpl (Fig. 5B).
FIGURE 5.
Alignment of the Mpl-EC-D1 used in this study in comparison with EBP. A, primary amino acid sequence alignment. Identical residues are shown in yellow, and homologous residues are in blue. The β-strands of EBP (underlined) were determined by crystal structure (26). The Mpl-EC-D1 β-strands (underlined) were putatively determined from our modeling. The individual strands are lettered A–G and A′–G′, respectively. The conserved WXXWS regions are showed in gray. Amino acid positions denoted by red and green stars indicate sites important for hNUDC and TPO binding, respectively. Black triangles indicate the sites for EPO binding. B, proposed model of Mpl-EC-D1 in comparison with the known structure of EBP. Loops are represented as thin green coils and β-strands as wide yellow ribbons. Four Cys residues are shown in red. The residues corresponding to an extra insert are shown in white. The residues important for hNUDC binding are labeled in purple. The residues significant for TPO or EPO binding are labeled in pink. The WXXWS motif is indicated in orange.
Homology Modeling of Deletion Variants
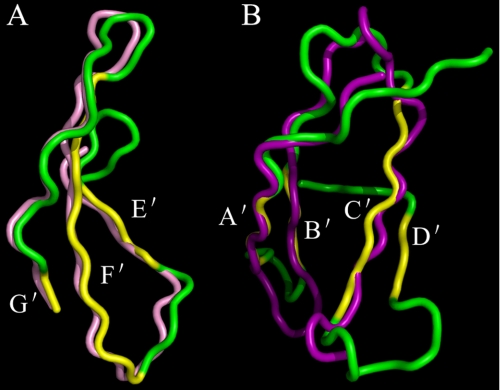
The Swiss-Model server was used to construct models to allow us to further rationalize the tertiary structures of the residues 102–164, 165–251, 165–205, 205–251, and 180–229 from Mpl-EC-D1. Our phage display binding analysis revealed the residues 206–251 to be most involved with affinity binding to hNUDC and TPO (Fig. 4). When this smaller stretch of amino acids was modeled individually, it was shown that the partial construct closely matched the model in Mpl-EC-D1. This suggests a similar domain arrangement in the Mpl-EC-D1 and in the deletion. The best superimpositions include E′, F′, and F′ β-strands together with the turn linking loops of E′–F′ and F′–G′ (Fig. 6A). A structural similarity search revealed a model of the deletion domain 102–164 lacking C-terminal residues 151–164 topologically similar to that of Mpl-EC-D1 except for shifts in turn of the A′–B′ and B′–C′ loops (Fig. 6B). Unfortunately, the software was unable to model the 165–205, 180–229, and 165–251 subdomain structures because of the poor identity of the primary sequences. According to our model, these three deletion constructs all contain residues that are extraneous when compared with other comparable cytokine receptors (23). Although it might be possible that such insertions would disrupt the predicated structural conformations, it should be noted that residues 165–205 are unlikely to induce profound structural alterations, because this deletion displays a binding ability with both TPO and hNUDC in phage display (Fig. 4).
FIGURE 6.
Stereo view of superimpositions between the deletion domains and Mpl-EC-D1. A, superimposition of Mpl-EC-D1 and deletion domain, focusing on the residues 206–251. A structural similarity search revealed the residues in the 206–251 deletion model are colored in pink. The corresponding part of the Mpl-EC-D1 is shown in green for the loop coils and yellow for the β-strands. The locations of E′, F′, and G′ β-strands are noted. B, superimposition of Mpl-EC-D1 and deletion domain, focusing on the residues 102–164. A structural similarity search revealed the residues in the 102–164 deletion model are colored in purple. The corresponding part of the Mpl-EC-D1 is shown in green for the loop coils and yellow for the β-strands. The locations of A′, B′, C′, and D′ β-strands are noted.
Predicting the Mpl-EC-D1 and TPO Complex
In view of the current lack of x-ray crystallographic or NMR structural information on hNUDC, it is difficult to predict how exactly this ligand interacts with Mpl. We are currently carrying out mutagenic and structural analyses of hNUDC in our laboratory. Homology modeling and deeper experimental exploration of the hNUDC to Mpl complex will occur in the near future. Nonetheless, information from the above studies together with the site-directed mutagenesis of TPO (29, 30) allowed us to model Mpl-TPO interactions. Specifically, we used molecular modeling programs to generate the homodimer Mpl complexed to TPO using the structure of the EPO-EBP complex as a template (PDB accession number 1CN4). The crystal structure of TPO (PDB accession number 1V7M) shows that it shares many characteristics with EPO, such as a four-helix bundle in the N-terminal domain (31).
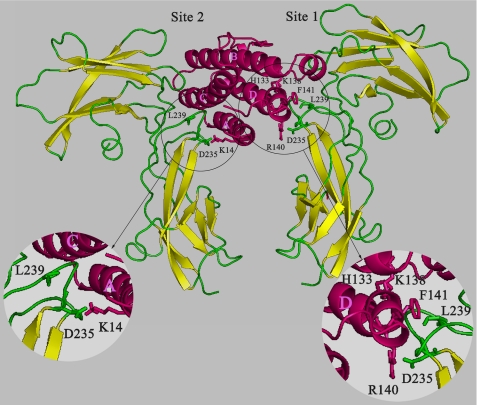
EPO is an asymmetric monomeric molecule and employs two different interfaces to bind to the two monomers of the EBP homodimer. There is a high affinity site (Kd <1 nm) and a low affinity site (Kd <1 mm) (32). Given the importance of Asp235 and Leu239 in Mpl-EC-D1 involved in TPO binding, we concentrated on these two residues for assembling the ligand-receptor interfaces in the most complementary orientation. TPO contains a number of residues that were thought to be involved in receptor binding, including His133, Lys14, Lys138, Phe141, and Arg140 (29, 30). By monitoring the movement of the individual residues, we identified Leu239 located in F′–G′ loop of MPl-EC-D1 in close contact with the Phe141 side chain from αD-helix of TPO on site 1. Asp235 within the F′–G′ loop of Mpl-EC-D1 establishes two salt bridges with Arg140 from αD-helix of TPO on site 1 as well as Lys14 from αA-helix of TPO on site 2 (Fig. 7). Two other important receptor-binding residues, His133 and Lys138, are not oriented toward the receptor based on our TPO-Mpl complex model (Fig. 7). Consistent with our model, solving the structure of TPO with the antagonistic anti-TPO monoclonal antibodies showed that these two residues indeed point to inner faces (30).
FIGURE 7.
Schematic representation of the complex between TPO and Mpl-EC-D1. Structure of TPO is shown in pink. Ribbon model of Mpl-EC-D1 is shown in yellow for β-strands and green for loops. Side chains contributing to interaction residues are shown as sticks. Binding site residues in TPO and Mpl-EC-D1 are colored pink and green, respectively (circles). The residues 172–213 in Mpl-EC-D1 are omitted for clarity.
Competitive Inhibition between hNUDC and TPO
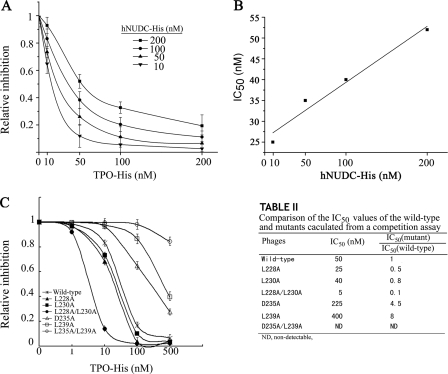
The results of the mutagenesis experiments and the crystal structure modeling suggest that the TPO- and hNUDC-binding sites on Mpl are either very close or overlapping. To verify this, we used a competition binding assay to determine whether hNUDC and TPO have inhibitory effects on the affinity of the other cytokine to the Mpl-EC-D1 region from the residues 206 to 251. pT7Select415-Mpl(206–251) phage (1 × 109 pfu/well) was pretreated with different concentrations of TPO-His and added to microwells coated with increasing concentrations of hNUDC-His ranging from 10 to 200 nm (Fig. 8A). The competitive interaction between hNUDC and TPO increased proportionally to the hNUDC concentration, yielding a linear plot for IC50 (Fig. 8B). These results suggest that hNUDC and TPO with greater competition bind to the amino acid region from 206 to 251.
FIGURE 8.
Competitive binding of two ligands for Mpl. A, TPO inhibits binding to immobilized hNUDC. Increasing amounts of TPO were added together with pT7Select415-Mpl(206–251) to wells of ELISA plates that were coated with different concentrations of hNUDC as indicated. Bound phages were determined using anti-T7 tail fiber mAb and detected at 450 nm. The error bars denote the standard deviation of n = 6 replicates (experiments were performed in triplicate in two assays). B, relationship between IC50 and concentration-response curves of hNUDC shown in A. Data from six replicates were pooled to generate the linearity plots. C, left, competition of wild-type pT7Select415-Mpl(206–251) and its derivative mutants with TPO for binding to immobilized hNUDC. Increasing amounts of TPO were mixed individually with wild-type pT7Select415-Mpl(206–251) and mutants L228A, L230A, L228A/L230A, D235A, L239A, or D235A/L239A (1 × 109 pfu/well) before incubation in hNUDC-coated ELISA plates. Bound phages were detected using anti-T7 tail fiber mAb. Right, IC50 values for wild type and each mutant calculated from a competition assay are summarized. The error bars denote the standard deviation of n = 6 replicates (experiments were performed in triplicate in two assays).
In parallel, we investigated if this inhibition could be reduced if the binding sites for one of the cytokines were mutated. The wild-type and mutant phages were pretreated with increasing concentrations of TPO-His as competitor and added to microwells that were coated with 200 nm hNUDC, respectively (Fig. 8C). As explored earlier in this paper, the residues Asp235 and Leu239 are critical binding sites for TPO. We would expect that either single and or double mutants of D235A and L239A would demonstrate a partial or complete disruption of TPO competition for hNUDC to Mpl binding. The results are the most striking for the D235A/L239A, and TPO lost its ability to inhibit hNUDC binding and resulted in nondetectable IC50 values (Fig. 8C, right). D235A or L239A mutants also resulted in dramatic decreases in the ability of TPO to inhibit hNUDC binding (Fig. 8C, right). In these mutants, nearly 4.5–8-fold higher concentrations of TPO-His were needed when compared with wild type. Importantly, D235A displayed an IC50 of 56% lower value when compared with L239A, indicating that the Leu239 residue is more specific to the binding of TPO. Competition binding analyses were also performed on both the single and double mutations of L228A and L230A. The IC50 values of these mutants are all significantly lower than the IC50 value for the wild type (Fig. 8C, right). These results suggest that Leu228 and Leu230 are both involved in hNUDC binding. However, L228A displayed an IC50 value 63% lower than L230A, indicating that Leu228 is more specific for hNUDC binding than Leu230.
DISCUSSION
We have previously reported on the overlapping biological function of hNUDC and TPO as mediated through the receptor Mpl (9–12, 19). This drove our direction of inquiry to investigate the binding mechanism associated with the cytokine-receptor interactions. In this study, our approach was to use a stepwise application of different molecular techniques to determine the minimal binding domain within Mpl for these ligands. This would allow us to characterize and compare the binding sites for hNUDC and TPO in the Mpl-EC.
Our yeast two-hybrid and co-immunoprecipitation results demonstrated that the amino acid residues 102–251 located in the C terminus of Mpl-EC-D1 are sufficient for affinity binding of both hNUDC and TPO (Figs. 1 and 2). This result is in keeping with the findings of several other independent approaches in the literature (33–35). It is also consistent with the fact that the Mpl-EC-D2 is not involved in ligand binding but is instead needed for receptor activation (36). With what we believe to be a novel use of a T7 phage display system, we were able to further refine our knowledge of the Mpl-EC-D1 and characterize the 45-amino acid long peptide necessary for both hNUDC and TPO binding (Fig. 4).
Subsequently, we subjected the Mpl-EC-D1 to Ala scanning mutagenesis in an effort to identify the amino acid residues most involved with binding. The two residues Leu228 and Leu230 in the putative binding domain of Mpl-EC-D1 were found to most affect hNUDC binding. In addition a double mutant at these two sites resulted in a further decrease in binding affinity. TPO binding was most affected by the mutation of residues D235A and L239A. Likewise, a double mutation at these sites resulted in a synergistic decrease in TPO binding (Table 1).
We were also able to demonstrate that TPO can compete with hNUDC for binding to the wild-type pT7Select415-Mpl(206–251) phage in a concentration-dependent manner (Fig. 8, A and B). This suggests that occupancy at one ligand site may either occlude the other binding site or alter it in an allosteric manner. Furthermore, our competition assay of four mutated residues supports the conclusion that hNUDC and TPO bind to separate sites (Fig. 8C, right). The double mutants obviously had the most impact on the competition assay. However, the ability of each of the single mutants to reduce the level of inhibition was not equal. The more potent competing sites were the two amino acids, Leu230 and Asp235 (Fig. 8C, right). It is possible that these two residues may be either shared or closely associated contact sites for the two cytokines on the receptor. It is not overly surprising that the distinct binding sites of TPO and hNUDC are in topologically similar areas (Fig. 5B). One would expect that because both cytokines should result in similar biological activity, the conformational changes they elicit in Mpl would most likely be similar. However, TPO plays more of a role in the early stages of megakaryocyte proliferation, whereas hNUDC associates in late megakaryocyte differentiation (10, 12). The presence of distinct binding sites on Mpl-EC-D1 for hNUDC and TPO may provide a part of the mechanism for the differences in biological function of two cytokines.
Furthermore, deletion of WGSWS residues near the C terminus of Mpl-EC-D1 reduced binding with hNUDC by around 19% (Table 1), suggesting that this motif may be involved in the interaction between hNUDC and Mpl. Although early evidence indicates that the WXXWS box may be directly involved in the interaction between the ligand and the receptor, more recent studies have suggested that its role is in stabilizing the conformation of the cytokine-binding domain to facilitate efficient transport of the receptors through the secretory pathway (37). Previous studies have demonstrated that co-expression of hNUDC and Mpl in NIH 3T3 cells induced rapid internalization, and hNUDC was released into the culture medium in an Mpl-dependent manner (14). This study supports the notion that WGSWS residues participating in the interaction between Mpl and hNUDC may play a role in stabilizing the conformation of the receptor during cytokine binding. This in turn allows for more efficient transport of hNUDC through the secretary pathway. The details of this role in the overall cellular process might be further addressed.
In recent years, crystal structure data have greatly advanced our understanding of the cytokine receptor superfamily and their ligand interactions. In lieu of a crystal structure for Mpl-EC-D1, we used the extracellular domain of the EPO receptor as a structural template. This modeling revealed a type I cytokine receptor fold in Mpl-EC-D1 similar to that seen in members of the cytokine receptor superfamily (Fig. 5). When placed into this model, the two pairs of residues Leu228/Leu230 and Asp235/Leu239 are shown to be distributed over the F′–G′ domain of Mp-EC-D1. Across members of the cytokine receptor superfamily, there are many instances where residues on the F′–G′ loop are critical to ligand binding. For example, a mutational analysis of granulocyte-colony stimulating factor receptor indicates that a residue that contributes to ligand binding, Arg288, lies within the predicted F′–G′ loop (38). Similarly, mutagenesis has identified an Arg280 residue in the F′–G′ loop of granulocyte-macrophage colony-simulating factor receptor critical for granulocyte-macrophage colony stimulating factor binding (39). Finally, the two residues Glu277 and Glu278 thought to be involved in the interleukin-6 binding loop of interleukin-6 receptor are also found in the F′–G′ region (40). An alignment of the F′–G′ domain of the several class 1 cytokine receptors highlights a couple of common features (Fig. 9). As with all class 1 cytokine receptors, the WXXWS motif is present. In addition, the positions at which the hydrophobic residues Leu228 and Leu230 lie on hNUDC are found to be conserved as hydrophobic in the other cytokine receptors. This finding suggests that the mechanism in which hNUDC binds to Mpl may have corollaries in all class 1 cytokine receptors.
FIGURE 9.
Alignment of the F′–G′ loop regions containing ligand-binding residues from Mpl with those of other cytokine receptors. Alignments are adapted from Cosman (22) and Deane et al. (23). Residues within the F′ and G′ strands implicated in ligand binding are in italic. Identical residues are indicated by a triangle and homologous residues are indicated by stars. The conserved WXXWS sequences are in gray.
The combination of site-directed mutagenesis and molecular modeling has led to a first pass characterization of the Mpl-TPO ternary complex formation. The interaction of Mpl-EC-D1 with TPO is likely very similar to the interaction between EPO and EBP (32). In the Mpl-EC-D1, Leu239 in the F′–G′ loop coincided with Phe205 of EBP to generate the binding surface with Phe141 of TPO through a hydrophobic interaction on site 1. The side chain of Asp235 donated by the F′–G′ loop of Mpl-EC-D1 forms salt bridges with the side chains of Arg140 and Lys14 of TPO on both sites 1 and 2 (Fig. 7). This residue may play a key role in the receptor dimerization or in stability of the TPO-Mpl complex. However, confirmation of this speculation will require more extensive experimental investigation.
An unexpected difference between the Mpl-EC-D1 and EBP was the disparate role the E–F and B′–C′ loops play in ligand binding. In EBP, Phe93 and Met150 located in the E–F and in B′–C′ loops, respectively, are found to be important for EPO binding (27, 28). Our deletion mutants containing either the E–F or B′–C loop regions within the Mpl-EC-D1 did not show any interaction with TPO (Figs. 1 and 2). The simplest explanations would be that the residues located in E–F and B′–C′ loops would be involved in low affinity binding or involved in an obligatory intermediate step for maintaining the correct orientation of the ligand-receptor complexes. However, the scanning deletion technique is more structurally disruptive than scanning point mutagenesis. At present, it cannot be excluded that the presence of other key residues, even in the Mpl-EC-D2, may have important implications for receptor and ligand interactions. Moreover, because the F′–G′ domain is unlikely to be involved in dimerization, it may be that additional contacts between the receptor monomers are formed utilizing other residues (41). The contact between these residues would form a secondary interaction following ligand binding and could significantly contribute to the binding affinity of the complex. Further biochemical experiments are currently underway to more accurately map the binding interface of the of Mpl extracellular domain with its natural partner molecules.
In summary, the experiments revealed that cytokine binding to the Mpl-EC-D1 involves amino acid residues 206–251. Mutational analysis of individual candidate amino acids and competition assays suggest several candidate residues in the F′–G′ domain as potential sites of interaction with hNUDC or TPO. Additionally the WGSWS motif was determined to be important for hNUDC binding. This is an important step in understanding the relationship between receptor structure and the mechanisms behind receptor activation. Further functional analysis will be required to completely map the interaction of Mpl with its ligands using cell-based bioassays.
Acknowledgments
We are most grateful for the comments and criticisms from Duane Wang, Ohio State University College of Medicine, Columbus. We also thank our laboratory colleagues for their technical assistance.
This work was supported by National Natural Science Foundation of China Grant 30971069, National High-Tech Research and Development Program of China Grant 2007AA21009, and Guang Dong National High-Tech Research and Development Program 2007B030702002.
- TPO
- thrombopoietin
- h
- human
- Mpl-EC-D1
- the extracellular domain 1 of thrombopoietin receptor (Mpl)
- EPO
- erythropoietin
- EBP
- EPO-binding protein
- FnIII
- fibronectin type III
- ELISA
- enzyme-linked immunosorbent binding assays
- mAb
- monoclonal antibody
- PDB
- Protein Data Bank
- CHO
- Chinese hamster ovary
- aa
- amino acid
- EGFP
- enhanced green fluorescent protein
- pfu
- plaque-forming unit
- X-gal
- 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
REFERENCES
- 1.Alexander W. S., Begley C. G. (1998) Cytokines Cell Mol. Ther. 4, 25–34 [PubMed] [Google Scholar]
- 2.Kaushansky K., Drachman J. G. (2002) Oncogene 21, 3359–3367 [DOI] [PubMed] [Google Scholar]
- 3.Kaushansky K. (2003) Ann. N.Y. Acad. Sci. 996, 39–43 [DOI] [PubMed] [Google Scholar]
- 4.Murone M., Carpenter D. A., de Sauvage F. J. (1998) Stem Cells 16, 1–6 [DOI] [PubMed] [Google Scholar]
- 5.Gurney A. L., Carver-Moore K., de Sauvage F. J., Moore M. W. (1994) Science 265, 1445–1447 [DOI] [PubMed] [Google Scholar]
- 6.de Sauvage F. J., Carver-Moore K., Luoh S. M., Ryan A., Dowd M., Eaton D. L., Moore M. W. (1996) J. Exp. Med. 183, 651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander W. S., Roberts A. W., Nicola N. A., Li R., Metcalf D. (1996) Blood 87, 2162–2170 [PubMed] [Google Scholar]
- 8.Bunting S., Widmer R., Lipari T., Rangell L., Steinmetz H., Carver-Moore K., Moore M. W., Keller G. A., de Sauvage F. J. (1997) Blood 90, 3423–3429 [PubMed] [Google Scholar]
- 9.Pan R. M., Yang Y., Wei M. X., Yu X. B., Ge Y. C., Xu P. (2005) J. Cell. Biochem. 96, 741–750 [DOI] [PubMed] [Google Scholar]
- 10.Wei M. X., Yang Y., Ge Y. C., Xu P. (2006) J. Cell. Biochem. 98, 429–439 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y. P., Tang Y. S., Chen X. S., Xu P. (2007) Exp. Cell Res. 313, 3210–3221 [DOI] [PubMed] [Google Scholar]
- 12.Tang Y. S., Zhang Y. P., Xu P. (2008) Leukemia 22, 1018–1025 [DOI] [PubMed] [Google Scholar]
- 13.Morris S. M., Anaya P., Xiang X., Morris N. R., May G. S., Yu-Lee L. Y. (1997) Mol. Endocrinol. 11, 229–236 [DOI] [PubMed] [Google Scholar]
- 14.Zhang M. Y., Huang N. N., Clawson G. A., Osmani S. A., Pan W., Xin P., Razzaque M. S., Miller B. A. (2002) Exp. Cell Res. 273, 73–84 [DOI] [PubMed] [Google Scholar]
- 15.Aumais J. P., Williams S. N., Luo W., Nishino M., Caldwell K. A., Caldwell G. A., Lin S. H., Yu-Lee L. Y. (2003) J. Cell Sci. 116, 1991–2003 [DOI] [PubMed] [Google Scholar]
- 16.Aumais J. P., Tunstead J. R., McNeil R. S., Schaar B. T., McConnell S. K., Lin S. H., Clark G. D., Yu-Lee L. Y. (2001) J. Neurosci. 21, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller B. A., Zhang M. Y., Gocke C. D., De Souza C., Osmani A. H., Lynch C., Davies J., Bell L., Osmani S. A. (1999) Exp. Hematol. 27, 742–750 [DOI] [PubMed] [Google Scholar]
- 18.Gocke C. D., Reaman G. H., Stine C., Zhang M. Y., Osmani S. A., Miller B. A. (2000) Leuk. Lymphoma 39, 447–454 [DOI] [PubMed] [Google Scholar]
- 19.Pang S. F., Li X. K., Zhang Q., Yang F., Xu P. (2009) Exp. Cell Res. 315, 3563–3573 [DOI] [PubMed] [Google Scholar]
- 20.Vainchenker W., Methia N., Debili N., Titeux M., Wendling F. (1995) Thromb. Haemost. 74, 526–528 [PubMed] [Google Scholar]
- 21.Alexander W. S., Dunn A. R. (1995) Oncogene 10, 795–803 [PubMed] [Google Scholar]
- 22.Cosman D. (1993) Cytokine 5, 95–106 [DOI] [PubMed] [Google Scholar]
- 23.Deane C. M., Kroemer R. T., Richards W. G. (1997) J. Mol. Graph. Model 15, 170–178 [DOI] [PubMed] [Google Scholar]
- 24.Guex N., Peitsch M. C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 25.Comeau S. R., Kozakov D., Brenke R., Shen Y., Beglov D., Vajda S. (2007) Proteins 69, 781–785 [DOI] [PubMed] [Google Scholar]
- 26.Livnah O., Stura E. A., Middleton S. A., Johnson D. L., Jolliffe L. K., Wilson I. A. (1999) Science 283, 987–990 [DOI] [PubMed] [Google Scholar]
- 27.Middleton S. A., Johnson D. L., Jin R., McMahon F. J., Collins A., Tullai J., Gruninger R. H., Jolliffe L. K., Mulcahy L. S. (1996) J. Biol. Chem. 271, 14045–14054 [DOI] [PubMed] [Google Scholar]
- 28.Middleton S. A., Barbone F. P., Johnson D. L., Thurmond R. L., You Y., McMahon F. J., Jin R., Livnah O., Tullai J., Farrell F. X., Goldsmith M. A., Wilson I. A., Jolliffe L. K. (1999) J. Biol. Chem. 274, 14163–14169 [DOI] [PubMed] [Google Scholar]
- 29.Jagerschmidt A., Fleury V., Anger-Leroy M., Thomas C., Agnel M., O'brien D. P. (1998) Biochem. J. 333, 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce K. H., Jr., Potts B. J., Presta L. G., Bald L. N., Fendly B. M., Wells J. A. (1997) J. Biol. Chem. 272, 20595–20602 [DOI] [PubMed] [Google Scholar]
- 31.Feese M. D., Tamada T., Kato Y., Maeda Y., Hirose M., Matsukura Y., Shigematsu H., Muto T., Matsumoto A., Watarai H., Ogami K., Tahara T., Kato T., Miyazaki H., Kuroki R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 1816–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed R. S., Reid S. W., Li C., Cheetham J. C., Aoki K. H., Liu B., Zhan H., Osslund T. D., Chirino A. J., Zhang J., Finer-Moore J., Elliott S., Sitney K., Katz B. A., Matthews D. J., Wendoloski J. J., Egrie J., Stroud R. M. (1998) Nature 395, 511–516 [DOI] [PubMed] [Google Scholar]
- 33.Sabath D. F., Kaushansky K., Broudy V. C. (1999) Blood 94, 365–367 [PubMed] [Google Scholar]
- 34.Luoh S. M., Stefanich E., Solar G., Steinmetz H., Lipari T., Pestina T. I., Jackson C. W., de Sauvage F. J. (2000) Mol. Cell. Biol. 20, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabath D. F., Lofton-Day C., Lin N., Lok S., Kaushansky K., Broudy V. C. (2002) Biochim. Biophys. Acta 1574, 383–386 [DOI] [PubMed] [Google Scholar]
- 36.Otto K. G., Broudy V. C., Lin N. L., Parganas E., Luthi J. N., Drachman J. G., Ihle J. N., Blau C. A. (2001) Blood 98, 2077–2083 [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner J. W., Wells C. A., Chen C. M., Waters M. J. (1994) J. Biol. Chem. 269, 29094–29101 [PubMed] [Google Scholar]
- 38.Layton J. E., Iaria J., Smith D. K., Treutlein H. R. (1997) J. Biol. Chem. 272, 29735–29741 [DOI] [PubMed] [Google Scholar]
- 39.Rajotte D., Cadieux C., Haman A., Wilkes B. C., Clark S. C., Hercus T., Woodcock J. A., Lopez A., Hoang T. (1997) J. Exp. Med. 185, 1939–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yawata H., Yasukawa K., Natsuka S., Murakami M., Yamasaki K., Hibi M., Taga T., Kishimoto T. (1993) EMBO J. 12, 1705–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander W. S., Metcalf D., Dunn A. R. (1995) EMBO J. 14, 5569–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]