The discovery of primitive cardiac stem-like cells with the ability for self-renewal and the capacity for differentiation into all cardiac cell lineages has completely changed the prevailing view of the heart as a static, terminally differentiated organ 1. Like virtually all somatic tissues, the adult heart harbors a pool of stem/progenitor cells responsible for the maintenance of organ cell homeostasis. In this new concept, cardiac stem cells take central stage in the maintenance of the cellular and functional integrity of the heart throughout a lifetime. Perturbation of the cardiac stem cell pool by premature senescence or cellular injury may thus result in inadequate replenishment of functionally competent cardiac cells and ultimately lead to the development of organ failure 2. Hence, an understanding of the mechanisms regulating this cardiac stem cell pool is pivotal towards the investigation of cardiac injury and dysfunction, and may offer opportunities to promote myocardial repair and regeneration following tissue injury.
The obvious conceptual and therapeutic significance of cardiovascular stem cell biology has sparked an enormous interest in the study of resident cardiac stem/progenitor cells. Since the first identification of endogenous cardiac stem/progenitor in adult hearts six years ago by Hierlihy et al 3, several independent laboratories have confirmed the existence of resident cardiac stem-like cells and characterized their cardiomyogenic potential in vitro and in vivo in mammalian as well as human hearts (for review 4). The urgent need for a convenient methodology to identify and isolate putative cardiac stem cells prompted early studies to focus on their phenotypic characterization. As a cell’s “stemness” is not linked to a single specific biological marker, combinations of different stem cell associated cell surface markers including c-kit (a tyrosine kinase receptor that binds to steel factor) 1 and Sca-1 (stem cell antigen-1) 5 as well as stem cell associated properties have been used for their isolation. Other, cell surface marker-independent cell isolation strategies have employed methodologies in which cardiac tissue fragments generate cellular tissue aggregates, or so called cardiospheres, that are enriched with stem-like cells 6 or have taken advantage of the capacity of stem cells to efflux vital dyes such as Hoechst 33342 3,7-9. This unique Hoechst-efflux phenomenon is mediated by the ABC-transporters Mdr1 and Abcg2 and has become a highly useful primary purification technique for the isolation of putative stem cells, or so-called side population (SP) cells (for review 10). Although individual laboratories have reported significant discrepancies regarding the expression pattern of above mentioned markers in their putative cardiac stem cell population, each of these markers has been identified in cardiac cells with inherent cardiomyogenic potential; thereby suggesting that the phenotypic differences reported among laboratories may root in methodological variations or reflect the identification of cells at phenotypically different stages of development(Table 1).
Table 1.
Current Summary of Resident Cardiac Stem/Progenitor Cells
| Major surface markers | Proliferation and cardiac differentiation capacity | Other notes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| c-kit | Sca1 | CD45 | CD34 | CD31 | Abcb1/Mdr1 | Abcg2/Bcrp1 | |||
| SP | - | + | - | - | +/- | ND | Yes | ||
| c-kit | + | + | - | - | - | +/- | ND | Yes | |
| Sca1 | - | + | - | - | + | ND | + | Yes | |
| Cardiosphere | + | +/-* | - | + | + | - | ND | Yes | Also CD105+, CD90+, CD 133- |
| Isl1 | - | ND | - | - | - | ND | -* | Yes | * via dye efflux capacity |
SP: side population; ND: not determined.
While extensive work has focused on phenotypic characterization of cardiac stem cells, little is known about the factors regulating cardiac stem cell survival and function. In the heart, lifelong exposure to cardiotoxins, reactive oxygen species (ROS) and metabolic stress take their toll on the aging myocardium, leading to premature cardiac cell death and senescence 11. In this harsh environment, effective defense systems are essential, particularly for stem cells that are required to continuously proliferate and replenish dying cells. A common defense mechanism adopted by stem cells is their high expression of ABC membrane transporters 12. These proteins are characterized by expression of an ATP-binding cassette region that hydrolyses ATP to support energy–dependent substrate exportation (e.g. cytotoxins, DNA-binding dyes) against steep concentration gradients from the intra- to the extracellular space. As mentioned above, this property defines the molecular basis of the side population (SP) phenotype in stem cells. SP cells isolated from myocardium qualify as cardiac stem/progenitor cells as they are clonogenic and multipotent in vitro and give raise to cardiomyocytes and coronary vessels in vivo 3,7-9. Similar to SP cells from other tissues, cardiac SP cells express the ATP-binding cassette subfamily G member 2 (Abcg2), also known as the breast cancer resistant protein (Bcrp1) 7. Recent evidence from hematopoietic stem cells indicates that Abcg2 is involved in promoting cell survival and protection from environmental stressors, particularly under hypoxic conditions 13.
In this issue of Circulation Research, Martin et al. address for the first time the cytoprotective role of Abcg2 in skeletal muscle cells.14 Using a murine myocardial cryoinjury model, these authors demonstrate that resident Abcg2-expressing cardiac SP cells substantially increase in numbers following injury. Interestingly, this increase in cardiac SP cells was accompanied by increased Abcg2 transcript expression in individual SP cells. In extension to their previous work, which defined the molecular signature of cardiac SP cells 7, their present work defines a common SP cell signature by comparing the transcriptomes of murine cardiac SP cells with the transcriptomes of SP cells isolated from other murine tissues. This analysis identifies cell cycle regulatory genes, cytoprotective factors as well as oxidative stress pathways as the common molecular program of SP cells. In order to determine whether Abcg2 expression is the mediator of this specific cytoprotective gene program, C2C12 myoblasts were transfected to express Abcg2. Expression of Abcg2 in C2C12 myoblasts resulted in upregulation of cytoprotective and oxidative stress pathways, similar to as seen in the transcriptome analysis of SP cell populations. Importantly, Abcg2 expression induced low levels of oxidative stress as indicated by the ratio of reduced to oxidized glutathione (GSH/GSSG). This oxidative priming was associated with higher levels of the antioxidant enzyme α-glutathione reductase. The beneficial effect of such ROS “preconditioning” was further tested in mouse embryonic fibroblasts (MEFs) expressing Abcg2. Upon exposure to hydrogen peroxide, Abcg2-expressing MEFs displayed a significant survival benefit when compared to wildtype MEFs. Finally, the authors decipher the molecular regulation of the Abcg2 gene and unveil HIF-2α as a potent transcriptional regulator of Abcg2.
At present, the specific role of ABC transporters in cardiac progenitor cells remains incompletely understood. The fact, however, that ABC transporters are inherent components of stem cells implies a regulative function for these proteins that goes beyond their traditional role in conferring the SP phenotype. This study by Martin et al. furthers our understanding of the cell regulatory function of the ABC transporter Abcg2 by providing mechanistic insight into its transcriptional regulation and its pro-survival functions. The finding that Abcg2 is a downstream target of the redox-sensitive transcription factor HIF-2α implies a specific role of Abcg2 in the induction of adaptive processes in response to hypoxia. In line with these findings, Krishnamurthy et al. demonstrated upregulation of Abcg2 in bone marrow SP cells exposed to hypoxia 13. This upregulation of Abcg2 and improved cell survival, however, involved another member of the hypoxia-inducible transcription factor family, HIF-1α. Future investigations tailored to dissect the regulation of Abcg2 by either HIF-1α or HIF-2α are certainly warranted.
One important issue to note is the demonstration that Abcg2 expression itself promoted low levels of oxidative stress that “prime” the cell to activate its antioxidant defense system and thereby protects against the burden of oxidative stress caused by prolonged deleterious hypoxia (Figure 1). This “auto-preconditioning” mediated by Abcg2 supports the concept of the dual role of ROS. Although high levels of ROS are toxic, low concentrations may serve as second messengers that stimulate pro-survival pathways via different signaling kinases (such as MAP kinases or PI3 kinase/Akt) and/or regulation of transcription factors 15. The pathways directly involved in the pro-survival effects of Abcg2, however, remain to be established.
Figure 1.
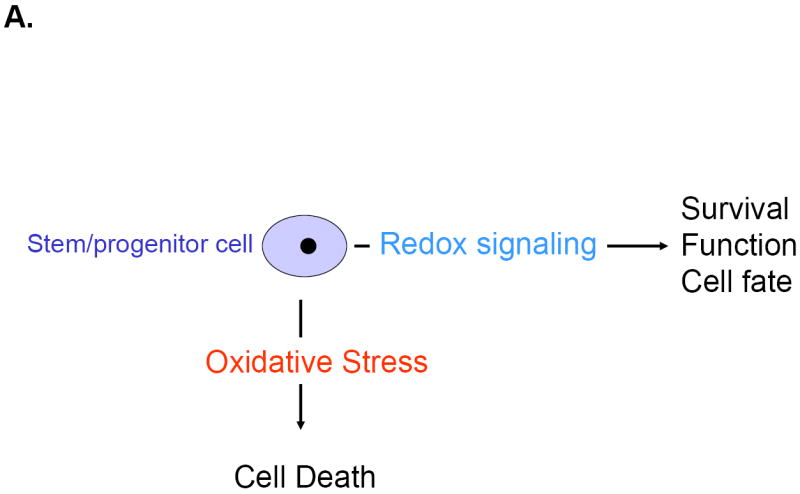
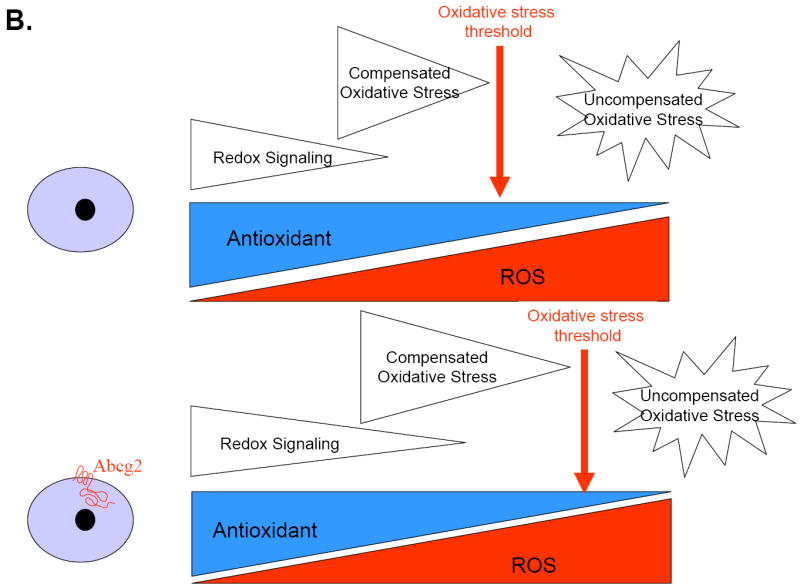
The net balance between ROS and antioxidant capacity determines cellular redox state. Emerging evidence suggests that redox signaling and oxidative stress play pivotal roles in regulating the fate, function and survival of stem cells (A). As suggested by the findings of Martin et al, Abcg2 expression may lead to a shift in the threshold of uncompensated oxidative stress via activation of redox signaling and thereby protect cardiac progenitor cells against injury (B).
Mouquet and colleagues recently showed that cardiac SP cells are responsive to myocardial injury. In a mouse model of permanent coronary ligation, a sharp decline in cardiac SP cells was observed 24 hours after myocardial infarction (MI), due to the continuous loss of myocardial cells caused by ischemic necrosis. Cardiac SP cells were subsequently reconstituted to baseline levels within 7 days after MI through both proliferation of resident cardiac SP cells as well as homing of bone marrow derived SP cells 16. The in vivo findings reported by Martin et al contrast to some degree with previous observations.14 In their myocardial cryoinjury model, no decline of cardiac SP cells occurred. Instead, they report an increase in total cardiac SP cell numbers by day 3 post-cryoinjury that was sustained through day 7. These dynamic differences of the cardiac SP cell response to myocardial injury reported by the two laboratories are most likely attributable to the different injury models used. Permanent artery ligation results in hypoxia/anoxia with concomitant activation of cellular adhesion molecules and cytokine expression and the generation of a sizeable area of infarction with continuous cell death. The pathophysiology of cryoinjury differs from coronary ligation, in that it results in acute cell death at the moment of freezing without concomitant ischemia and therefore may have limited clinical correlate or relevance 17.
Hypoxia as well as members of the HIF-family promote proliferation in mesenchymal stem cells and multiple cell lines 18,19. In vivo, ischemic myocardial injury activates resident cardiac progenitor cells to re-enter the cell cycle and proliferate 16. In their myocardial cryoinjury model, Martin et al demonstrate increased cardiac SP cell numbers after injury. Importantly, this increase was associated with increased Abcg2 expression in individual SP cells. It is therefore tempting to speculate that HIF-induced Abcg2 expression regulates the proliferation state of cardiac SP cells in response to injury. Further studies that define the role of Abcg2 in cardiac progenitor cell proliferation will be important for a comprehensive characterization of the cell-regulatory effects of Abcg2 in this cell type.
The findings by Martin and colleagues also have significant clinical implications. Earlier work from these authors demonstrated that Abcg2 identifies cardiac progenitor cells in the developing and adult heart 7. Moreover, Abcg2 is expressed in human hearts and its expression is increased in ischemic and dilated cardiomyopathy 20. Given a role of Abcg2 in adaptive, pro-survival processes in response to hypoxia, the proper function of Abcg2 may be relevant not only to coronary artery patients with repetitive ischemia, but also to diabetic patients with microvascular heart disease and patients with increased wall stress and consequently greater oxygen demand. The new generation of anticancer drugs designed to inhibit Abcg2 may thus have significant cardiac side effects in this patient population.
In conclusion, Martin et al have made an important contribution towards a better understanding of the regulation and functional role of the ABC transporter, Abcg2. Although the limitation that the regulatory mechanisms and cytoprotective actions of Abcg2 were studied in skeletal myoblast cell lines and not in cardiac progenitor cell populations, these findings will help to better define the biology of cardiac progenitors and may provide the basis for future therapeutic strategies to enhance stem cell function. Further research will now have to translate these findings into cardiac progenitor cells in order to definitely establish a cytoprotective role of Abcg2 in the cardiac progenitor cell compartment.
Acknowledgments
Source of Funding The authors are supported by funding from the National Institutes of Health grants: HL073756, HL071775, HL086967, and HL088533 (RL), and the Swiss Heart Foundation and the Mach-Gaensslen Foundation Switzerland (OP).
Footnotes
Disclosure None.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 3.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–43. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 4.Sohn RL, Jain M, Liao R. Adult stem cells and heart regeneration. Expert Rev Cardiovasc Ther. 2007;5:507–17. doi: 10.1586/14779072.5.3.507. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 7.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 9.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 11.Sussman MA, Anversa P. Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- 12.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 14.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG, Garry DJ. HIF-2α transactivates Abcg2 and promotes cytoprotection in cardiac SP cells. Circ Res. 2007;102:XXX–XXX. doi: 10.1161/CIRCRESAHA.107.161729. [DOI] [PubMed] [Google Scholar]
- 15.Goswami SK, Maulik N, Das DK. Ischemia-reperfusion and cardioprotection: a delicate balance between reactive oxygen species generation and redox homeostasis. Ann Med. 2007;39:275–89. doi: 10.1080/07853890701374677. [DOI] [PubMed] [Google Scholar]
- 16.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–2. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 17.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–49. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 18.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–47. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meissner K, Heydrich B, Jedlitschky G, Meyer Zu Schwabedissen H, Mosyagin I, Dazert P, Eckel L, Vogelgesang S, Warzok RW, Bohm M, Lehmann C, Wendt M, Cascorbi I, Kroemer HK. The ATP-binding cassette transporter ABCG2 (BCRP), a marker for side population stem cells, is expressed in human heart. J Histochem Cytochem. 2006;54:215–21. doi: 10.1369/jhc.5A6750.2005. [DOI] [PubMed] [Google Scholar]


