Abstract
This study assesses the stability of magnetic resonance (MR) liver fat measurements against changes in T2* due to the presence of iron, which is a confound for accurate quantification. The liver T2* was experimentally shortened by intravenous infusion of a super paramagnetic iron oxide (SPIO) contrast agent. Low flip angle multi-echo gradient echo sequences were performed before, during, and after infusion. The liver fat fraction (FF) was calculated in co-localized regions-of-interest using T2* models that assumed no decay, monoexponential decay and biexponential decay. Results show that, when T2* was neglected, there was a strong underestimation of the computed FF and with monoexponential decay there was a weak overestimation of FF. Curve-fitting using the biexponential decay was found to be problematic. The overestimation of FF may be due to remaining deficiencies in the model, although is unlikely to be important for clinical diagnosis of steatosis.
INTRODUCTION
Fat quantification by magnetic resonance (MR) imaging typically refers to measurement of the fat fraction (FF), which is the ratio of signal from fat protons to the signal from all protons. Clinically the FF allows quantification of abnormal retention of fat within a cell (steatosis). The FF from MR measurements should estimate the actual proportion of fat and water protons, however there are several confounds to the accuracy. These include T1 relaxation, T2* relaxation and fat spectral complexity. T2* is particularly relevant for liver imaging since steatosis is frequently accompanied by iron accumulation, which causes T2* signal decay. While quantification methods incorporating T2* decay have been described for quantifying liver fat, it has not yet been established that these give reliable measurements in patients. The present study describes novel methodology for using a superparamagnetic iron oxide (SPIO) contrast agent as an in vivo mimic of iron overload. Under experimental perturbation of the T2* using SPIO, the consistency of the FF measurements can provide empirical verification of fat quantification methods intended to correct for T2* decay.
The deleterious effects of T2* on accuracy have long been recognized [1,2] but are often neglected or considered small. However a recent clinical study found that T2* shortening due to the presence of iron represents a limitation for the evaluation of fatty liver [3]. The “dual echo” method used in that study neglects T2* and attributes all changes in the signal during time interval ΔTE to interference between water and fat, caused by differences in chemical shift.
Letting w and f represent the water and fat signals, respectively, the dual echo method acquired two images with w and f in-phase, IP = |w + f|, and out-of-phase, OP = |w − f|. Under these conditions the fat fraction can be estimated from Eq 1.
| [1] |
Clearly T2* can be a source of error in this model, since the signal on the later echo is always systematically lowered by T2* decay. The consequences of neglecting T2* have been demonstrated previously in numerical simulations [4] and in theoretical calculations [5]. Rewriting Eq 1 in terms of FFtrue = f/(w + f) and κ = ΔTE/T2 * ≪ 1 the following is obtained.
| [2] |
From this it can be seen that FF is a function of both FFtrue and T2*. Since 1/T2 * is proportional to iron concentration, the dual echo FF typically results in underestimation of FFtrue in patients with liver iron. This illustrates how neglecting a key physical property (T2*) can introduce systematic error and create a spurious correlation between unrelated parameters (FF and T2*).
To improve upon the dual echo method, a more realistic model of the MR signal must be developed that includes parameters to express the decay with echo time. Several approaches have been considered previously. The most simple approach is to assume a two-signal model, water and fat, with an identical T2* for both. However it is known that fat has a broad spectrum compared to water so it is more realistic to assign a shorter T2* to fat [6]. Models with separate T2* terms for water and fat have been used and these confirm a short apparent T2* of fat [5,7] although this approach can fail at low FF since it becomes difficult to estimate the T2*. In any case the short T2* is an artifact of approximating fat as a single peak, whereas in reality it is a superposition of multiple peaks with different resonant frequencies [8,9,10,5,11]. The relative proportions of the peaks are similar for different fats and oils, therefore it is reasonable to assume as prior knowledge a standardized approximation of the fat spectrum. Whether it is necessary to include separate T2* terms for each component is unclear at the present time. Differences in T2* are difficult to measure reliably, since discrepancies between the assumed and the true fat spectrum will manifest as T2*. Also, since T2s are generally long (see Table I) it may be expected that differences in 1/T2 * = 1/T2 + 1/T2′ are small. Recent studies that have assumed a standardized fat spectrum with the same T2* for all components have reported good fitting of empirical data in phantoms [5,11] and excellent agreement between imaging and spectroscopy in patients [12,13].
Table I.
Chemical shifts (δ), peak areas (a) and T2 values in fatty liver taken from Ref [24] and [27]. Some values for the fat T2s are missing due to the difficulty in measuring peaks close to the water peak in vivo and there is also large variability in the ranges of the T2s.
| Chemical Shift (ppm) | Peak Area | T2 at 1.5T (ms) | T2 at 3.0T (ms) |
|---|---|---|---|
| 5.3 | 0.047 | – | – |
| 4.2 | 0.039 | – | – |
| 2.75 | 0.006 | – | 51 |
| 2.1 | 0.120 | 39.2 | 52 |
| 1.3 | 0.700 | 75.5 | 62 |
| 0.9 | 0.088 | 45.0 | 83 |
| 4.7 (water) | – | 35.2 | 23 |
The motivation of the present study is to assess how the model described above performs in correcting T2* with the application to liver fat quantification. Imaging was performed in patients before, during, and after intravenous infusion of a SPIO contrast agent used to experimentally manipulate T2*. Since the fat in the liver should remain constant during the infusion (over 30 min), any systematic changes in FF can be attributed to errors in the modeling. This methodology follows on from previous work [5,14,15].
THEORY
The water and fat signals are modeled as a function of echo time (TE) as in Eq 3 and 4
| [3] |
| [4] |
were w/f are the proton densities of water/fat and represent their decays. Separate terms for and are included (biexponential model), although it may be reasonable to assume they are equal (monoexponential model). Prior knowledge of chemical shifts of water and fat is contained in the terms α (TE) = exp (2 π iω δwater TE) and β(TE) = Σaj exp(2π iω δj TE), and specific values are given in Table I. The Larmor frequency ω is 42.576 × 106 B0 Hz. Note that for the purposes of this work, the effect of T1 relaxation is assumed negligible, which can be accomplished by using a long repetition time (TR) and low flip angle.
The amplitude of the signal |s(TE)| = |w(TE) + f(TE)| is used to avoid having to model B0 field inhomogeneities. This introduces ambiguity between fat and water but is considered an acceptable trade-off in the present study, since fat in the liver is almost always the minority component. Also |s(TE)| is rewritten in terms of FF and η = w + f.
| [5] |
The dual echo method makes the simplification and neglects all fat peaks except the CH2 peak at 1.3 ppm. Then two TEs are chosen such that α(TE)/β (TE) is equal to ±1, which correspond to IP and OP images, |w + f| and |w − f|.
METHODS
There are two main parts to the study. The first part tests the feasibility of using a biexponential decay model. For this part, 16-echo data were acquired in 54 patients. Models assuming no decay, monoexponential decay, and biexponential decay were fitted to the data and the accuracy of the FF was assessed by comparison with spectroscopy. In 4 patients the effect of SPIO on FF was examined. Based on the results of the first part of the study, the biexponential model was not used in the second part. The second part of the study statistically analyses the stability of the FF against changes in T2* brought about by SPIO in 110 patients. 6-echo data were analyzed using the monoexponential model and 2-echo data were analyzed using the dual echo method. The dual echo method was included to see the worst case of neglecting T2*. Correlations were measured between the FF and the concentration of SPIO.
Study Design and Patients
This was a cross-sectional clinical study, approved by our Institutional Review Board (IRB) and compliant with Health Insurance Portability and Accountability Act (HIPAA). In total 110 adult and pediatric patients were recruited (80 men, 30 women; age 41.4 ± 18.1, range 10 – 88). Written informed consent was obtained from all adult patients. The parent(s) of all patients <18 years of age provided written informed consent for their children. The breakdown of the study population was as follows: research imaging in patients with biopsy-proven nonalcoholic fatty liver disease (n=43) and with biopsy-proven hepatitis C (n=21); clinical imaging in patients with (n=29) or without (n=17) chronic liver disease. Among the 29 patients with chronic liver disease, the etiology of liver disease was hepatitis C (n=17), hepatitis B (n=5), non-alcoholic fatty liver disease (n=5), cryptogenic cirrhosis (n=5), primary biliary cirrhosis (n=1), and primary sclerosing cholangitis (n=1).
MR Imaging
Each patient was scanned supine with an 8-element torso phased-array coil centered over the liver, after at least two hours fasting, either at 1.5T or 3T (details below). Respiratory bellows were applied to monitor breathing. Patients were instructed on breath-holding during the image acquisition. A dielectric pad was placed between coil and the abdominal wall (3.0 T only). A multiple echo spoiled gradient echo sequence was used with a bipolar echo train of 6 or 16 echos. The field of view was adjusted to individual body habitus and imaging matrices ranged from 192×192 to 256×256. Other imaging parameters were 7–12 slices, 8 mm slice thickness, intersection gap 0 mm and one signal average. Several dummy excitations were performed to attain steady state of the magnetization prior to data acquisition. Field-specific scanning parameters are described below.
At 1.5T 37 patients were scanned (Siemens Symphony, Siemens Medical Systems, Erlangen, Germany) with a maximum gradient 30 mT m−1. Six full echoes were acquired in a single TR with TE = ΔTE = 2.3 ms. The TR was 99–150 ms, flip angle 10° and bandwidth 500 Hz/pixel.
At 3.0T 73 patients were scanned (Signa HDx, GE Healthcare, Milwaukee, WI) with a maximum gradient 40 mT m−1. Six fractional echos (factor 0.8) were acquired in a single TR with TE = ΔTE = 1.15 ms. The TR was 125–325 ms, flip angle 10° and bandwidth 142 to 166 kHz. Of these patients, 54 were also imaged using 16 echos with initial TE = ΔTE = 1.0, 1.15 or 1.2 ms.
A SPIO contrast agent (Feridex, Bayer Healthcare, Wayne, NJ) was prepared as a suspension diluted in 100 ml of 5% dextrose and infused intravenously through a 5 micron filter over 30 min (iron dose 10 μmol/kg of body weight) at a rate of 2–4 ml/min. The choice of imaging protocols used was based on patient willingness, scanner availability and the clinical schedule. Whenever possible, imaging was performed before, during and after the SPIO infusion (median time interval 3 min; range 1–19 min). Otherwise imaging was performed only before and after the SPIO infusion (median time interval 120 min; range 28–301 min).
MR Spectroscopy
The same 54 patients who were imaged using the 3.0T 16-echo protocol were scanned prior to the SPIO infusion using Stimulated Echo Acquisition Mode (STEAM) spectroscopy. Five spectra were acquired in a single breath-hold at TE = 10, 15, 20, 25 and 30 ms to permit correction of T2 decay. The mixing time was 5 ms and the TR was 3500 ms. One dummy excitation was performed and all the saturation pulses (spatial, water and fat) were disabled. Analysis was performed in jMRUI v.3.1.
Image Analysis
After acquisition, all images were transferred to a workstation (AGFA Impax, Agfa HealthCare, Belgium) A radiology research fellow (16 months experience in MR) selected a representative transverse slice of the liver on each patient. Three regions of interest (ROIs) of approximately 300–400 mm2 were drawn in the right hepatic lobe avoiding focal liver lesions, artifacts, and biliary or vascular structures. The ROIs were automatically propagated to co-localized multi-echo images obtained during and after contrast infusion. The registration of the ROI between separate breath-hold series was visually checked and adjusted as needed. The mean signal intensity within the registered ROI was recorded.
Fat Fraction Computation
Nonlinear optimization was used to estimate the unknown parameters in Eq 5 (η, FF, ) by minimizing the sum of squares error between the model and the data points at different echo times. The linear term η was estimated directly using separable least squares [16], as in other spectroscopic fitting techniques [17,18,19]. The MATLAB (The Mathworks, Natick, MA) function lsqnonlin was used (Levenberg-Marquardt, TolFun=eps, TolX=eps, MaxIter=1000) with initial estimates FF = 0.1 and T2* = 20 ms. A lower bound on T2* of 5 ms was employed to prevent the algorithm from approaching T2* → 0. In our experience in over 2000 patients, SPIO administration rarely causes the T2* of liver to decrease below 6 ms, although in actual clinical cases of iron overload the T2* may be below this value.
Statistical Analyses
The overall purpose of the analysis was to examine how FF estimation is affected by SPIO concentration ([SPIO]). Results from the monoexponential model were analyzed and also the dual echo method to demonstrate the worst-case of neglecting T2* decay.
The entire data set comprises FF and T2* in 110 patients at 3 ROIs in the liver, measured at several time-points before and after SPIO infusion. In some cases additional time-points were obtained during the infusion. The exact time, the infusion rate and the [SPIO] at each observation are not known, however we may assume that [SPIO] increases monotonically in observation number. To obtain a proxy for [SPIO], we use the relation R2 *= R2 + r[SPIO] where r is the relaxivity of the contrast agent [20]. Hypothesizing that FF depends on [SPIO] leads to an errors-in-variables model given by Eq 6 and 7
| [6] |
| [7] |
where aj and bj are patient-specific constants (random effects), c is the dependence of FF on [SPIO] (a random effect), and u and v are error terms. We use the average R2* over the 3 ROIs as the best estimate of the R2* at each observation. The overall method follows [21]:
We model Eq 6 by using the “pool adjacent violators” algorithm to obtain isotonized and smoothed estimates of R2* as a function of the observation number for each patient. Each patient’s estimates were shifted so that the first observation corresponds to 0. This is the proxy for SPIO, or r[SPIO], used in subsequent analyses. It is essentially just the ΔR2* (change in R2*) caused by the contrast agent.
We model Eq 7 using a linear mixed effects model. Observed FFs are regressed on r[SPIO] from step 1, with nested patient and ROI-specific (random) intercepts and fixed slope. The slope and confidence interval describe the “total average deflection” in the FF.
To examine individual trends, we fit a linear mixed effects model, similar to step 2, but with nested patient and ROI-specific slopes as well as intercepts. (Note the average of the individual slopes is similar to the fixed slope in step 2.) Finally, we examine the relationship between the slopes and intercepts by centering r[SPIO] values by patient.
RESULTS
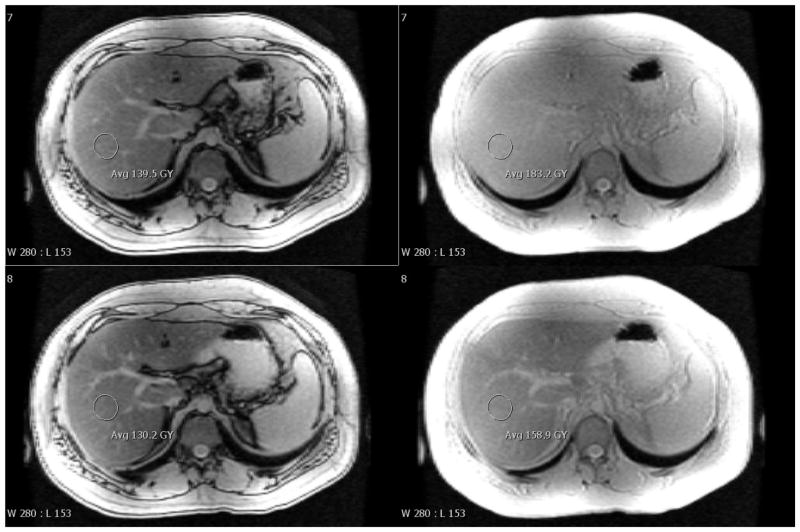
Figure 1 shows representative images of the liver acquired by the multi-echo gradient echo sequence at 3.0T. The top row shows OP and IP images (at TE 1.15 and 2.3 ms) obtained pre-SPIO and the bottom row shows the corresponding images 1 hour later post-SPIO. The annotation shows the approximate size and location of ROIs used for analysis. Note the T2* decay over this TE range is small but the effect on the dual echo FF is not negligible: 11.9% to 9.0% for pre- and post-SPIO data, as calculated by Eq 1. With the monoexponential model, the R2*s were estimated to be 0.047 and 0.149 ms−1 and the FFs 16.9% and 17.8%, respectively. The gold-standard result from spectroscopy is FF 17.2%.
Figure 1.
Representative gradient echo images of the liver at 3.0T using TR 200 ms and flip angle 10°. (A) shows pre-SPIO at TE 1.15 and 2.3 ms and (B) shows 1 hour post-SPIO at the same TEs. Annotations give the mean signal intensity inside an ROI and the window/level settings are identical. The calculated dual echo FFs are 11.9% and 9.0% for the pre- and post-SPIO images. The corresponding R2*s are 0.047 and 0.149 ms−1 and FFs are 16.9 and 17.8% (monoexponential). The gold-standard spectroscopy FF (pre-SPIO) is 17.2%.
Bi-exponential Fitting
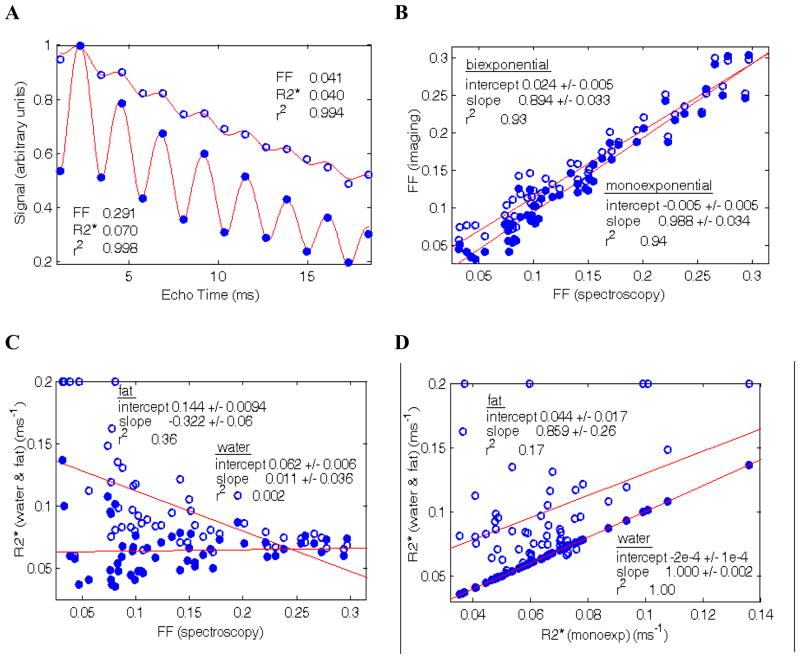
Figure 2(A) shows typical curve-fitting results for the monoexponential model. Note that there is little improvement in the quality of fit when using the biexponential model, since the r2 values are already close to unity. (B) shows the correlation between FF from imaging and from spectroscopy. The monoexponential model strongly agrees with the spectroscopy results, while the biexponential model shows a correlation with non-zero intercept and non-unit slope. For comparison, the dual echo method gave an intercept −0.023 and slope 0.824 with r2 = 0.91. (C) shows how the estimated R2* parameters correlate with FF. A few points with low FF reached the upper bound for R2* fitting of 0.2 ms−1 and cannot be considered reliable; as noted in Introduction, the fat R2* is difficult to estimate at low FF. (D) shows the correlation between water and fat R2*s with the monoexponential R2*. The water and monoexponential R2* values are in complete agreement while there is considerable scatter in the fat R2* data especially at low FF. The mean values for the parameters are given in Table II.
Figure 2.
(A) Plot of signal versus TE for monoexponential fitting in two patients (low and high fat fraction). The signals are normalized to the IP data point. The quality of fit is excellent and indistinguishable from the biexponential model (not shown). (B) Correlation between FF from monoexponential (solid circles) and biexponential (open circles) fitting and spectroscopy. The monoexponential estimate shows better agreement with spectroscopy. (C) Plot of R2* for water (solid circles) and fat (open circles) versus spectroscopy FF. Data points at low FF exhibit large scatter, particularly R2* for fat since the fat signal is low. (D) Plot of R2* for water and fat versus monoexponential R2*. The monoexponential and water R2* are in almost perfect agreement; there is a correlation with the fat R2* although large scatter in the data.
Table II.
Mean fat fractions and T2*s for the monoexponential and biexponential signal models described in Theory using the 3.0T 16-echo data. Note that data from patients with FF < 0.1 were fitted using the monoexponential model only, since the biexponential fitting was unreliable.
| FF (mono) (%) | R2* (mono) (ms−1) | FF (biexp) (%) | R2*w (biexp) (ms−1) | R2*f (biexp) (ms−1) | |
|---|---|---|---|---|---|
| 21 patients with FF<0.1 | 0.067 ± 0.027 | 0.064 ± 0.028 | – | – | – |
| 33 patients with FF>0.1 | 0.175 ± 0.063 | 0.063 ± 0.011 | 0.187 ± 0.058 | 0.064 ± 0.011 | 0.081 ± 0.016 |
Excluding the FF < 0.1 data from (C) yields somewhat different regression results. In particular, a weak correlation is observed between FF and the water R2*. This could be due to modeling error; e.g. if the prior knowledge of fat peaks at 4.2 and 5.3 ppm were too small, then the water peak would appear to broaden as FF increases. Alternatively there may be a physical explanation. A mechanism that has been hypothesized in bone marrow is that the bulk susceptibility difference causes fat to disturb the magnetic field in the water and hence shorten the T2* [22].
Biexponential Fitting with SPIO
As described in Methods, some patients were imaged at several time-points during the infusion and some were imaged using the 16-echo protocol. Of the patients who were in both categories, 4 also had a FF that was greater than 0.1 on spectroscopy. These data were curve-fitted using both the monoexponential and biexponential models to examine the effect of SPIO on FF during the infusion.
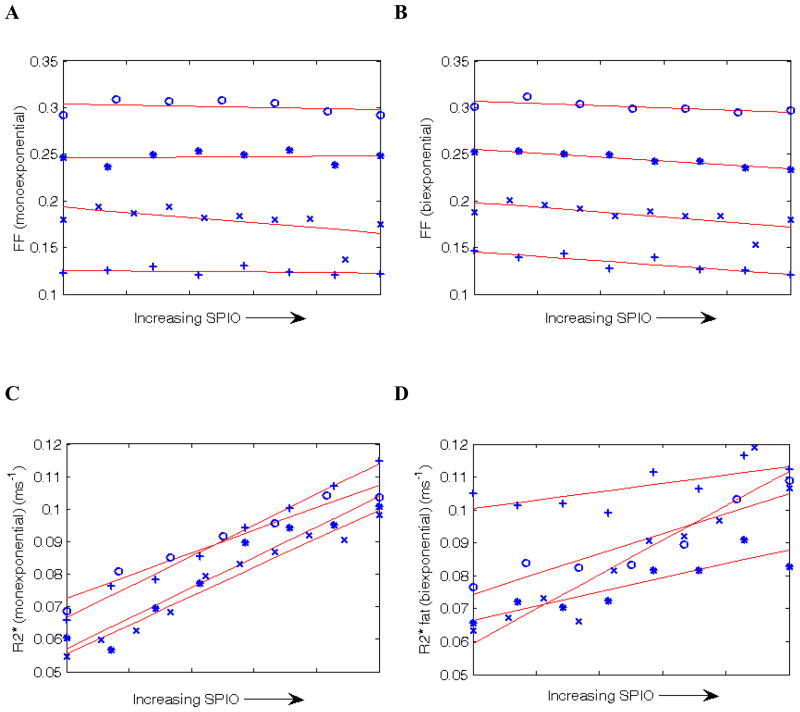
Figures 3(A) and (B) show FF estimated by the monoexponential and biexponential models. For the monoexponential model there is no clear dependence of FF on [SPIO], whereas for the biexponential model there appears to be a negative correlation. (C) shows the monoexponential R2* confirming that the R2* increases with contrast agent. (D) shows the fat R2* from the biexponential model, also confirming an increase in R2*. (Note the water R2* is virtually identical to the monoexponential R2* and is not shown.)
Figure 3.
(A) Plot of FF estimated by the monoexponential model as SPIO was administered. Of the 4 patients, 3 show a small negative trend and 1 shows a small positive trend. (B) shows FF estimated by the biexponential model. In all 4 patients the trend is negative and is more negative than the corresponding data in B. (C) and (D) show the corresponding changes in the monoexponential R2* and the biexponential fat R2*. These both increase with [SPIO], as expected. (Note the biexponential water R2* is almost identical to the monoexponential R2* and is not shown.)
There are several empirical observations to be made from Figures 2 and 3. (i) The quality of fit (r2 values) in Figure 2A indicates >99% of the signal variation is explained by the monoexponential model. (ii) The biexponential model is less accurate than the monoexponential model when compared to an independent gold-standard (spectroscopy). (iii) Curve-fitting with the biexponential model is numerically unstable even with 16 echos, since there is no reliable way to estimate the fat R2* at low FF. (iv) The FF estimated by the biexponential model seems to correlate (negatively) with R2* whereas the FF from the monoexponential model is relatively constant.
Taken together these observations suggest the biexponential model is not currently suitable for estimating FF in clinical patients. The additional rigidity of constraining fat and water to have the same R2* (and accounting for the reasonably well-known fat spectrum) appears to be a necessary simplification. Including fat R2* as a free parameter may result in overfitting of the data, whereby small errors from discrepancies in the prior knowledge and/or the neglect of J-coupling are erroneously attributed to the fat T2* term. The positive intercept in Figure 2B (overestimation of FF) supports this interpretation, since it means the fat signal extrapolated to a TE of zero is too high. Consequently the rate of decay of fat must have been overstated. The fitted values in Table II confirm the fat T2* is estimated as being shorter than the water T2*.
Statistical Analyses
Figure 4 shows the measured R2* in all patients and the estimated proxy for SPIO, r[SPIO] or ΔR2*, obtained from step 1.
Figure 4.
(A) Plot of the estimated R2* in liver following SPIO infusion versus the observation number. (B) shows the estimate of r[SPIO], or ΔR2*, from step 1 in Statistical Analyses, versus the observation number. The expected increase is due to the accumulation of SPIO.
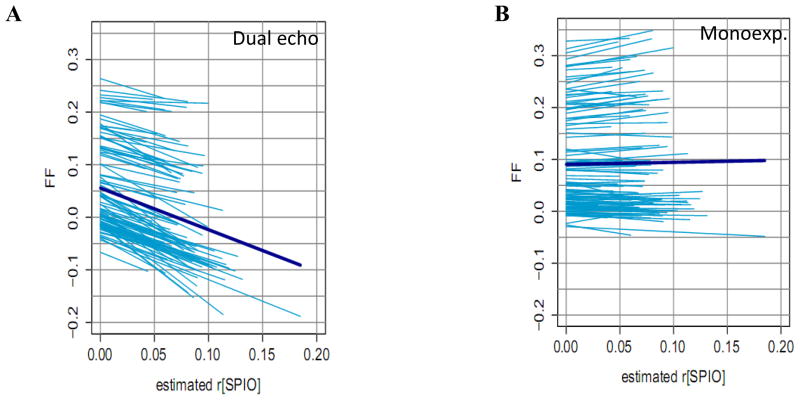
Figure 5 shows whether the FF estimated by the dual echo and monoexponential model is robust against experimentally induced changes in iron (caused by an infusion of SPIO). The key results are listed in Table III. As expected from Eq 2, the dual echo FF correlates strongly and significantly with [SPIO] and is captured by the “total average deflection” plotted as a bold line. The more physically accurate monoexponential model exhibits a weaker correlation, although also statistically significant. The total average deflection is small, however it is discernable that the lower FF data tend to trend downwards while the higher FF trend upwards.
Figure 5.
Fanplots of the measured FF versus r[SPIO] for (A) the dual echo and (B) the monoexponential model. The bold line represents the overall trend, given by the “total average deflection” of step 2 of Statistical Analyses starting from the mean FF. The strongly negative slope for the dual echo method indicates severe underestimation of FF as R2* increases. The monoexponential model exhibits a much weaker trend, indicating relatively stable FF measurements against R2* (see Table III).
Table III.
Summary of the slopes, standard error and p-value for the results in Figure 5.
| Model | Slope (ms−1) | Deg. Freedom | p-value |
|---|---|---|---|
| Dual echo | −0.793 ± 0.013 | 1361 | 0 |
| Monoexp. | 0.040 ± 0.010 | 1361 | 9.9 × 10−5 |
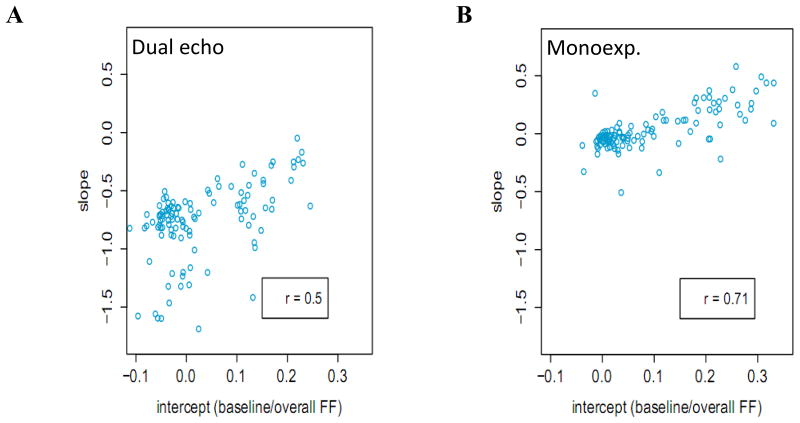
Figure 6 plots the per-patient slope versus intercept for the data in Figure 5. These plots indicate that, for the dual echo method, FF always decreases when there is R2* decay but lower FFs decrease more than higher FFs. For the monoexponential model, the FF decreases when FF is low but increases when FF is high. In other words, in the presence of strong R2* decay, the FF tends to be underestimated when the FF is low and overestimated when the FF is high. The observed trends are statistically significant in both plots.
Figure 6.
Plot of the slopes from Figure 5 versus the intercepts. (A) shows that the effect of R2* on the dual echo method is to decrease the estimated FF, although in patients with low fat the decrease is greater. (B) shows that the effect of R2* on the monoexponential model is to decrease the estimated FF in patients with low fat but to increase the estimated FF in patients with high fat.
DISCUSSION
This study uses a novel in vivo mimic of hepatic iron overload, where pathologic T2* shortening is induced experimentally using an iron-based contrast agent. This methodology makes it possible to test whether fat quantification techniques adequately account for T2* decay.
To summarize the main results of the study, the dual echo method (which neglects T2* decay) exhibits a strong negative correlation between FF and SPIO concentration and hence underestimates fat content as T2* decreases, as expected from Eq 2. The more physically realistic monoexponential model (based on Eq 5), which includes correction for T2* decay as well as prior knowledge of the fat spectrum, exhibits a much weaker positive correlation between FF and SPIO concentration. Further analysis indicates that the weak trend is a consequence of cancellation between larger under- and overestimation errors at low and high FF, respectively. The use of a biexponential model with separate water and fat T2*s does not appear to be feasible at the current time due to underestimation of the fat T2* (i.e. it appears to decay too fast).
The slight positive trend in Figure 5B indicates that, using a monoexponential model, the error in FF is approximately 0.040 × ΔR2*. To provide a point of reference, the ΔR2* between healthy liver tissue and severe iron overload of around 0.3 ms−1 would give a fat fraction error ΔFF of around 1.2%. However the trends in the individual patients in Figure 6 can be more than 10 times this average value (i.e. ± 0.5 × ΔR2*) and also correlate significantly with the FF. In the presence of strong T2* decay, low FFs tend to be underestimated and high FFs tend to be overestimated. This suggests there is some systematic error or physical property that has not been correctly modeled that could be improved in future models.
In terms of clinical practice, if the monoexponential FF at a native R2* (of say, 0.05 ms−1) is used as a gold-standard (which is supported by comparisons with spectroscopy), then iron-induced errors in the measured FF are at worst ±0.5 × (R2* − 0.05). Thus, if the patient has an actual R2* of 0.15 ms−1 due to the presence of iron, then the most pessimistic error in the patient’s FF is ±5% although usually the error will be much smaller (e.g. see Figure 1). By way of comparison, the dual echo method already underestimates FF at the native R2* of liver [12] and may underestimate by 10% in the worst case. The underestimation is partly due to R2* and partly due to neglect of minority components in the fat spectrum, which make up 30% of the fat signal (Table I).
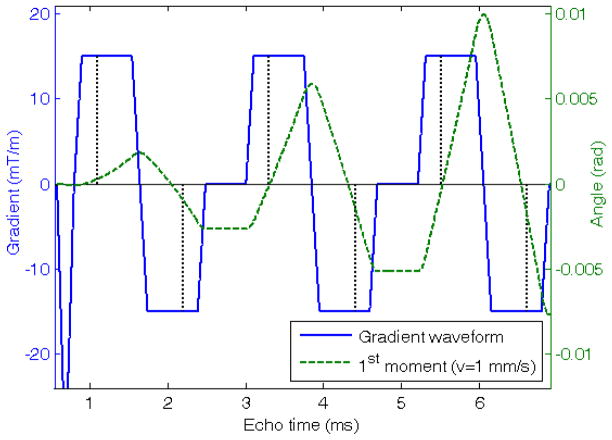
In addition to T2* there are various other potential sources of error in fat quantification. These include: (a) T1 relaxation, (b) noise bias, (c) B0 inhomogeneity, (d) prior knowledge of the chemical shifts, (e) diffusion and (f) flow. The latter two are associated with gradient echo-trains. (a) The T1s of all components are shortened by SPIO, so T1 weighting would affect the earlier time-points more. Since T1 weighting tends to elevate FF the infusion of SPIO would lead to a negative trend in FF, which is opposite to what is observed. (b) Noise rectification has been reported to give a large positive error in FF using certain methods [23]. However numerical simulations and analysis of simple cases show there is slight underestimation of FF with the methods used in this study (see Appendix). Therefore a decrease in signal to noise ratio (SNR) due to SPIO would give a negative trend. (c) By using the magnitude of the signal, difficulties from B0 inhomogeneity are avoided (at a cost of introducing water/fat ambiguity). (d) The fat spectrum was recently studied in liver and found to be highly consistent between patients [24]. Although differences undoubtedly exist between individual patients, these differences might be expected to produce random errors in the estimated FF rather than a consistent trend. (e) The diffusion of spins leads to signal dephasing under the action of applied gradients. We calculate the b-value to be <0.02 s/mm2 per bipolar pair in Figure 7, which is negligible. (f) To assess flow effects, we assume a sinusoidal flow velocity of ~1 mm/s and calculate the phase change in the moving spins due to the gradients [25]. At all times this is <0.01 radians, see Figure 7, which is negligible. Thus, overall, we conclude that none of the effects described above were a serious source of error in this study.
Figure 7.
Plot of gradient waveforms (solid line) and calculated first moment (dashed line) for spins with a velocity of 1 mm/s. The center of the echos (dotted vertical lines) do not occur at the center of the trapezoids due to using a fractional echo to reduce echo times. Note in general the first moment is not nulled on any echo but the accrued angle is small <0.01 radians.
In recent studies, there has been agreement reported between carefully designed MR imaging experiments and an independent gold-standard using spectroscopy [12,13]. Agreement between imaging and spectroscopy should be expected since MR essentially measures the same quantity. However imaging techniques use different modeling assumptions, e.g. see Refs [12,26], and the gold-standard spectroscopy is also vulnerable to confounds – namely T1, T2 and J-coupling, which interferes with the T2 correction of fat components [27]. In future studies, it is important that comparable methodologies are used and that the model of the MR signal takes into account the confounding effects. Histology is also often used as the independent measure of the fat content and correlation is generally reported [3,28,29,30], but the precise relationship between histology measure (based on the percentage of fat-containing hepatocytes [3]) and MR fat fraction (based on the percentage of protons attached to fat molecules) has not yet been established.
Acknowledgments
The authors thank GE Healthcare for research support and Jean Brittain, Michael Carl, Ann Shimikawa, Atsushi Takahasi and Huanzhou Yu for technical help. Research grants were also provided by Berlex (Bayer) and from NIH grant R01 (#DK075128). The study was ancillary (#AS009) to the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). The NASH CRN is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (UCSD #1U01DK061734, Data Coordinating Center #5U01DK061730) and the National Institute of Child Health and Human Development (NICHD).
APPENDIX
Noise bias transforms the IP and OP images to (IP2 + κσ2)½ and (OP2 + κσ2)½, respectively, where σ2 is the noise variance and κ is a scalar that depends on the image reconstruction method. In the present study, either the method of Ref [31] (κ = 1) or a homodyne sum-of-squares ( ) were used. Substituting the transformed IP and OP into Eq 1 and making a two term Taylor expansion (requires IP2, OP2 ≫ κσ2) yields the following expression.
For FFtrue < 0.5 the second term on the right-hand side is positive and so FF < FFtrue, which shows the estimated FF is always an underestimate. The magnitude of the error is typically small: when the SNR in the IP image = 10 and FFtrue = 0.1, the error is −0.001κ. Numerical simulations confirm a similar effect when the IP and OP images are curve-fitted to Eq 5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buxton RB, Wismer GL, Brady TJ, Rosen BR. Quantitative Proton Chemical-Shift Imaging. Magn Reson Med. 1986;3:881. doi: 10.1002/mrm.1910030609. [DOI] [PubMed] [Google Scholar]
- 2.Levenson H, Greensite F, Hoefs J, Friloux L, Applegate G, Silva E, Kanel G, Buxton R. Fatty infiltration of the liver: quantification with phase-contrast MR imaging at 1.5 T vs biopsy. American Journal of Roentgenology. 1991;156:307. doi: 10.2214/ajr.156.2.1898804. [DOI] [PubMed] [Google Scholar]
- 3.Westphalen ACA, Qayyum A, Yeh BM, Merriman RB, Lee JA, Lamba A, Lu Y, Coakley FV. Liver Fat: Effect of Hepatic Iron Deposition on Evaluation with Opposed-Phase MR Imaging. Radiology. 2007;242:450. doi: 10.1148/radiol.2422052024. [DOI] [PubMed] [Google Scholar]
- 4.Hussain HK, Chenevert TL, Londy FJ, Gulani V, Swanson SD, McKenna BJ, Appelman HD, Adusumilli S, Greenson JK, Conjeevaram HS. Hepatic Fat Fraction: MR Imaging for Quantitative Measurement and Display—Early Experience. Radiology. 2005;237:1048. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 5.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magnetic Resonance Imaging. 2008;26:347. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glover GH. Multipoint Dixon Technique for Water and Fat Proton and Susceptibility Imaging. J Magn Reson Imag. 1991;1:521. doi: 10.1002/jmri.1880010504. [DOI] [PubMed] [Google Scholar]
- 7.O’Regan DP, Callaghan MF, Arridge M, Fitzpatrick J, Naoumova RP, Hajnal JV, Schmitz SA. Liver Fat Content and T2*: Simultaneous Measurement by Using Breath-hold Multiecho MR Imaging at 3.0 T—Feasibility. Radiology. 2008;247:550. doi: 10.1148/radiol.2472070880. [DOI] [PubMed] [Google Scholar]
- 8.Brix G, Heiland S, Bellemann ME, Kock T, Lorenz WJ. MR Imaging of Fat-Containing Tissues: Valuation of Quantitative Imaging Techniques in Comparison with Localized Proton Spectroscopy. Magnetic Resonance Imaging. 1993;11:977. doi: 10.1016/0730-725x(93)90217-2. [DOI] [PubMed] [Google Scholar]
- 9.Wehrli FW, Ma J, Hopkins JA, Song HK. Measurement of R2* in the Presence of Multiple Spectral Components Using Reference Spectrum Deconvolution. Journal of Magnetic Resonance. 1998;131:61. doi: 10.1006/jmre.1997.1327. [DOI] [PubMed] [Google Scholar]
- 10.Hilaire L, Wehrli FW, Song HK. High-speed spectroscopic imaging for cancellous bone marrow R2* mapping and lipid quantification. Magnetic Resonance Imaging. 2000;18:777. doi: 10.1016/s0730-725x(00)00165-x. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magnetic Resonance in Medicine. 2008;60:1122. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, Hassanein T, Patton HM, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic Fatty Liver Disease: Diagnostic and Fat-Grading Accuracy of Low-Flip-Angle Multiecho Gradient-Recalled-Echo MR Imaging at 1.5 T. Radiology. 2009;251:67. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meisamy S, Hines CD, Hamilton G, Vigen K, Brittain JH, McKenzie CA, Yu H, Nagle SK, Zeng YG, Sirlin CB, Reeder SB. Validation of Chemical Shift Based Fat-Fraction Imaging with MR Spectroscopy. Proc Intl Soc Mag Reson Med. 2009:207. [Google Scholar]
- 14.Sugay S, Bydder M, Yokoo T, Hamilton G, Pinto N, Znamirowski R, Soumekh R, Wolfson T, Pacheco L, Sirlin CB. Fat Quantification Using SPIO as a Surrogate Marker for Iron Accumulation in the Liver. Proc Intl Soc Mag Reson Med. 2008:711. [Google Scholar]
- 15.Okuaki T, Yoshimitsu K, Zimine I, Saiki S, Van Cauteren M, Miyati T. Estimation of fat fraction considering T2* decay in liver after SPIO injection. Proc Intl Soc Mag Reson Med. 2009:4092. [Google Scholar]
- 16.Bjorck A. Numerical methods for least squares problems. Society for Industrial and Applied Mathematics; 1996. p. 351. [Google Scholar]
- 17.Vanhamme L, van den Boogaart A, Van Huffel S. Improved Method for Accurate and Efficient Quantification of MRS Data with Use of Prior Knowledge. J Magn Reson. 1997;129:35. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- 18.Reeder SB, Wen Z, Yu H, Pineda AR, Gold GE, Markl M, Pelc NJ. Multicoil Dixon chemical species separation with an iterative least squares estimation method. Magn Reson Med. 2004;51:35. doi: 10.1002/mrm.10675. [DOI] [PubMed] [Google Scholar]
- 19.Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang Z-P. Joint estimation of water/fat images and field inhomogeneity map. Magnetic Resonance in Medicine. 2008;59:571. doi: 10.1002/mrm.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McRobbie DW, Moore EA, Graves MJ, Prince MR. MRI From Picture to Proton. 2. Cambridge University Press; Cambridge: 2007. p. 165. [Google Scholar]
- 21.Anderson TW. Estimating Linear Statistical Relationships. Annals of Statistics. 1984;12:1. [Google Scholar]
- 22.Hopkins JA, Wehrli FW. Magnetic Susceptibility Measurement of Insoluble Solids by NMR: Magnetic Susceptibility of Bone. Magn Reson Med. 1997;37:494. doi: 10.1002/mrm.1910370404. [DOI] [PubMed] [Google Scholar]
- 23.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T1 and noise. Magn Reson Med. 2007;58:354. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton G, Yokoo T, Bydder M, Mwangi I, Schroeder ME, Sirlin CB, Middleton MS. In-Vivo Characterization of the Liver Fat 1H Magnetic Resonance Spectrum. Magn Reson Med. (accepted 2009) [Google Scholar]
- 25.Puhl G, Schaser KD, Vollmar B, Menger MD, Settmacher U. Noninvasive In Vivo Analysis Of The Human Hepatic Microcirculation Using Orthogonal Polarization Spectral Imaging. Transplantation. 2003;75:756. doi: 10.1097/01.TP.0000056634.18191.1A. [DOI] [PubMed] [Google Scholar]
- 26.Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point Dixon and three-point IDEAL. Magn Reson Med. 2008;59:521. doi: 10.1002/mrm.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton G, Middleton MS, Bydder M, Yokoo T, Schwimmer JB, Kono Y, Patton HM, Lavine JE, Sirlin CB. Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging. 2009;30:145. doi: 10.1002/jmri.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuchmann S, Weigel C, Albrecht L, Kirsch M, Lemke A, Lorenz G, Warzok R, Hosten N. Non-invasive quantification of hepatic fat fraction by fast 1.0, 1.5 and 3.0 T MR imaging. European Journal of Radiology. 2007;62:416. doi: 10.1016/j.ejrad.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.d’Assignies G, Ruel M, Khiat A, Lepanto L, Chagnon M, Kauffmann C, Tang A, Gaboury L, Boulanger Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. European Radiology. 2009;19:2033. doi: 10.1007/s00330-009-1351-4. [DOI] [PubMed] [Google Scholar]
- 30.Levenson H, Greensite F, Hoefs J, Friloux L, Applegate G, Silva E, Kanel G, Buxton R. Fatty infiltration of the liver: quantification with phase-contrast MR imaging at 1.5 T vs biopsy. American Journal of Roentgenology. 1991;156:307. doi: 10.2214/ajr.156.2.1898804. [DOI] [PubMed] [Google Scholar]
- 31.Bydder M, Larkman DJ, Hajnal JV. Combination of signals from array coils using image-based estimation of coil sensitivity profiles. Magn Reson Med. 2002;47:539. doi: 10.1002/mrm.10092. [DOI] [PubMed] [Google Scholar]