Abstract
Conjugates of bilirubin were studied in normal bile of man and rat, and in bile of liver patients. In general human bile was obtained by duodenal intubation. In addition T-tube bile was examined in patients operated on for mechanical obstruction. The bile pigment compositions of duodenal and T-tube bile were similar in two patients where comparison was possible. Obstruction of the bile duct in rats was used as an animal model for obstructive jaundice.
Diazotized ethyl anthranilate was used for determination of total conjugated bile pigment and for thin-layer chromatography (t.l.c.) analysis of the derived azopigments. The available t.l.c. procedures are versatile and allow rapid and quantitative analysis. A variety of conjugated azopigments can be distinguished.
With chloroform, negligible amounts of unconjugated bilirubin are extracted from bile of man. Therefore, the percentage of monoconjugated bile pigments present in the initial bile sample can be calculated from the percentage of azodipyrrole found after diazotization.
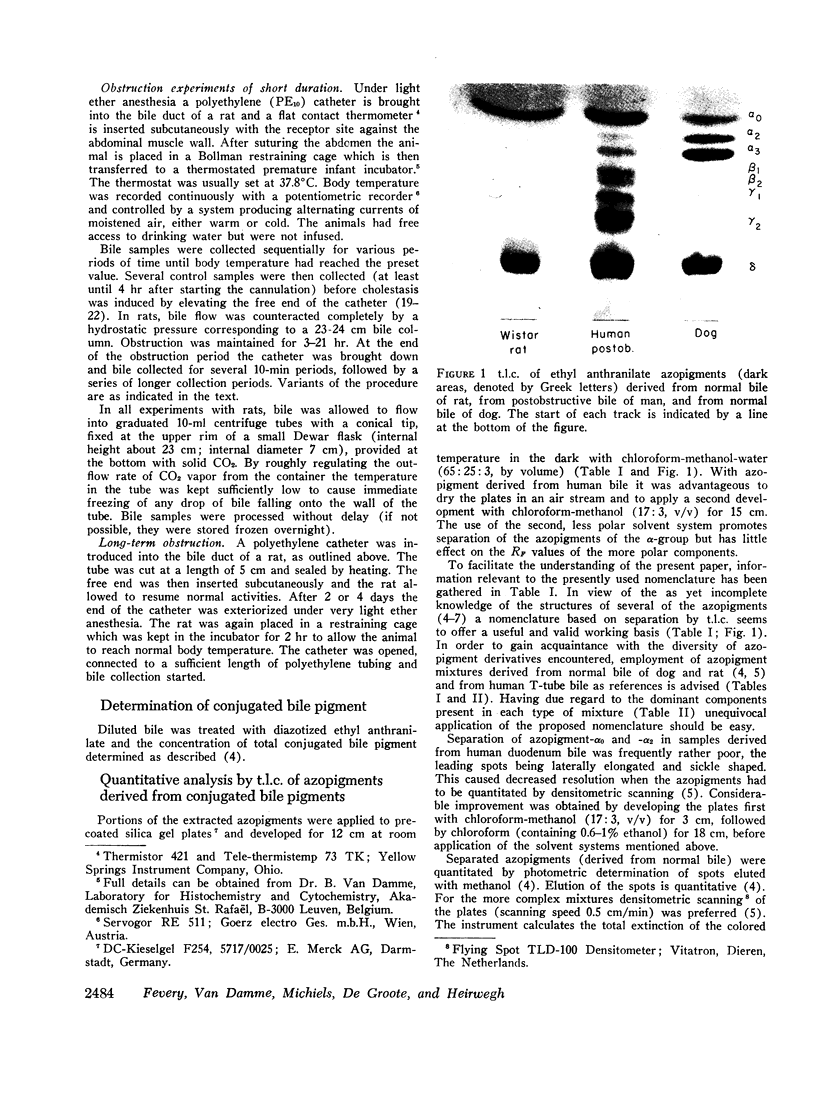
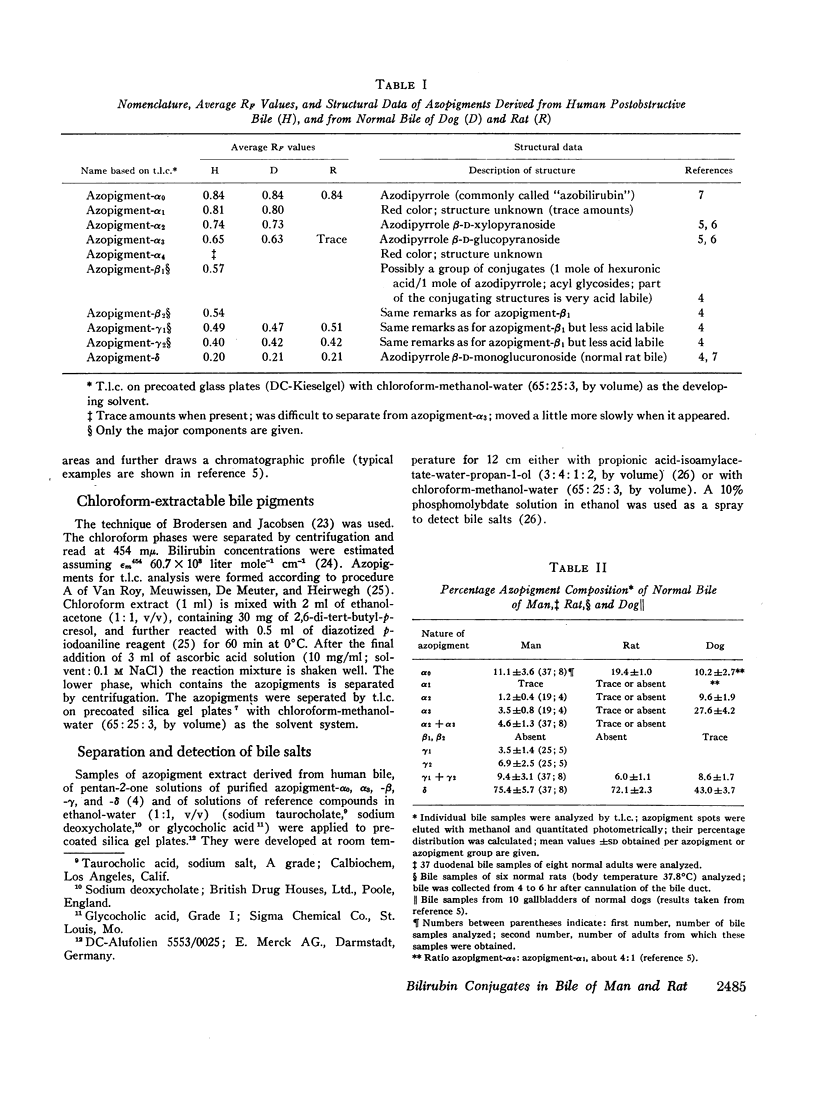
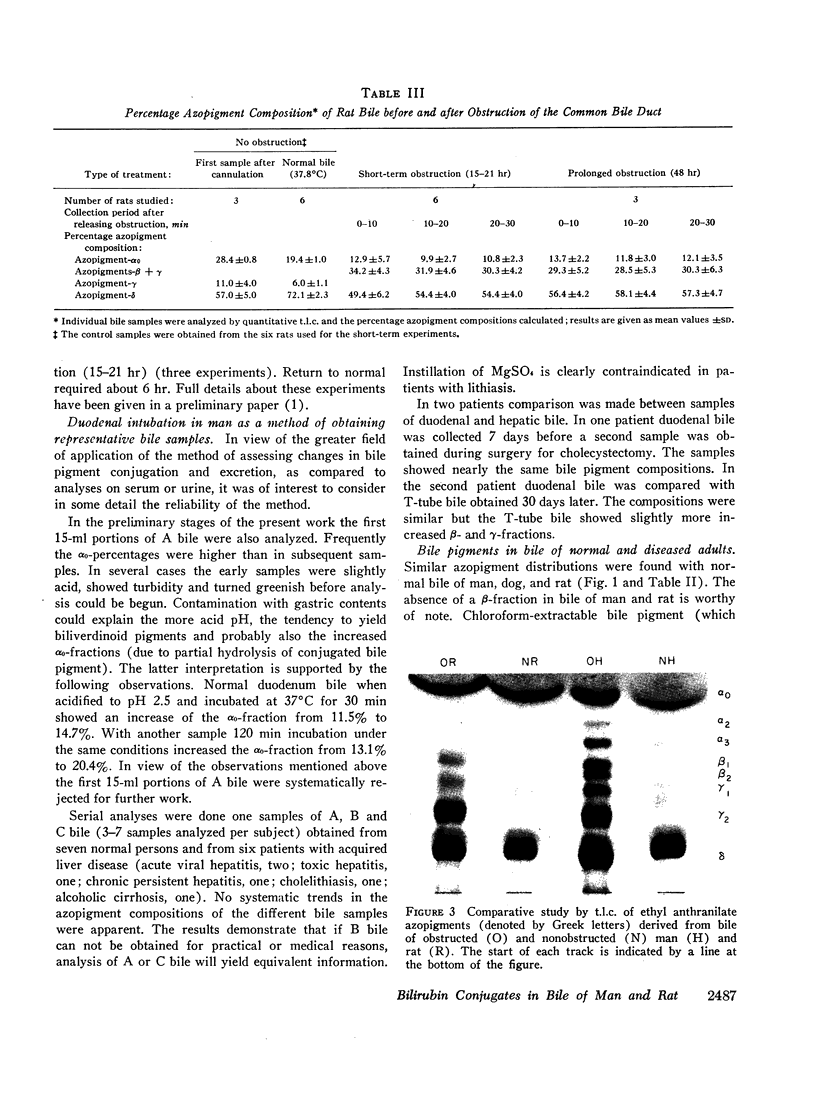
Normal bile from man and rat yields similar azopigment patterns. The dominant component is azopigment-δ (azodipyrrole β-D-monoglucuronoside). Small amounts of azopigments with complex conjugating structures (γ-azopigments) are present in both cases. Human bile further yields small amounts of azopigments containing xylose or glucose (called azopigments-α2 and -α3, respectively). Monoconjugated bilirubin (estimated from the percentage of azodipyrrole) amounts of 22% of total bile pigments in human bile and to 39% in murine bile. In both, the bulk of bile pigment is bilirubin diglucuronoside.
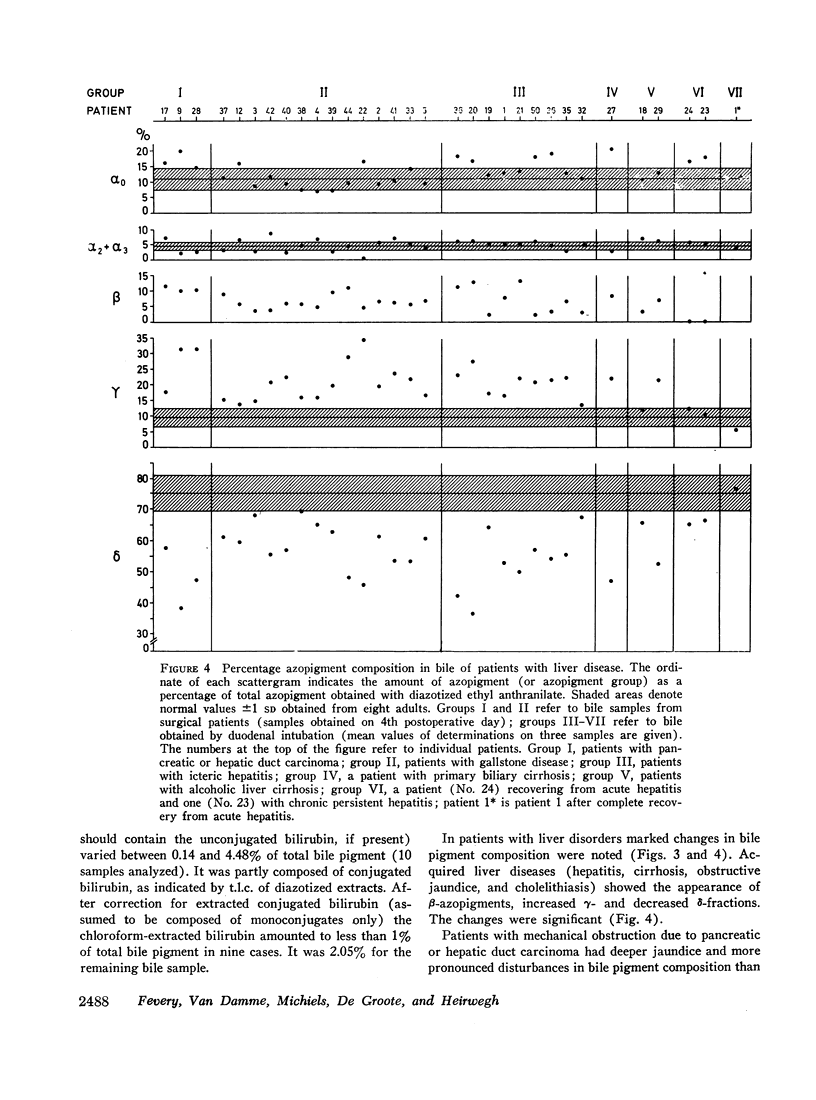
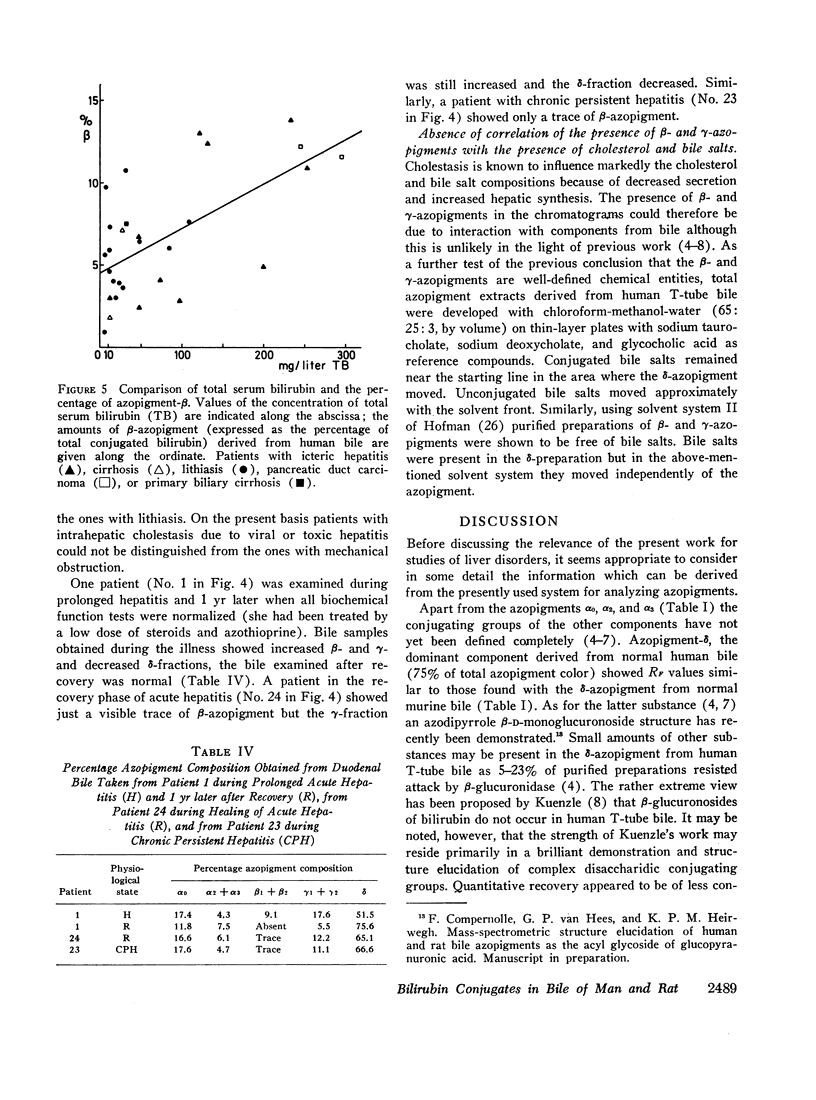
From bile of patients with acquired liver diseases a new azopigment group (β-azopigment) was derived. The γ-azopigment group was increased; the δ-azopigment group (containing azodipyrrole β-D-monoglucuronoside) was decreased. No differentiation was possible between intra- and extrahepatic cholestasis. The percentage of β-azopigment showed a positive correlation with serum bilirubin concentration (r = 0.6).
Recovery of the diseases was accompanied by normalization of the azopigment patterns.
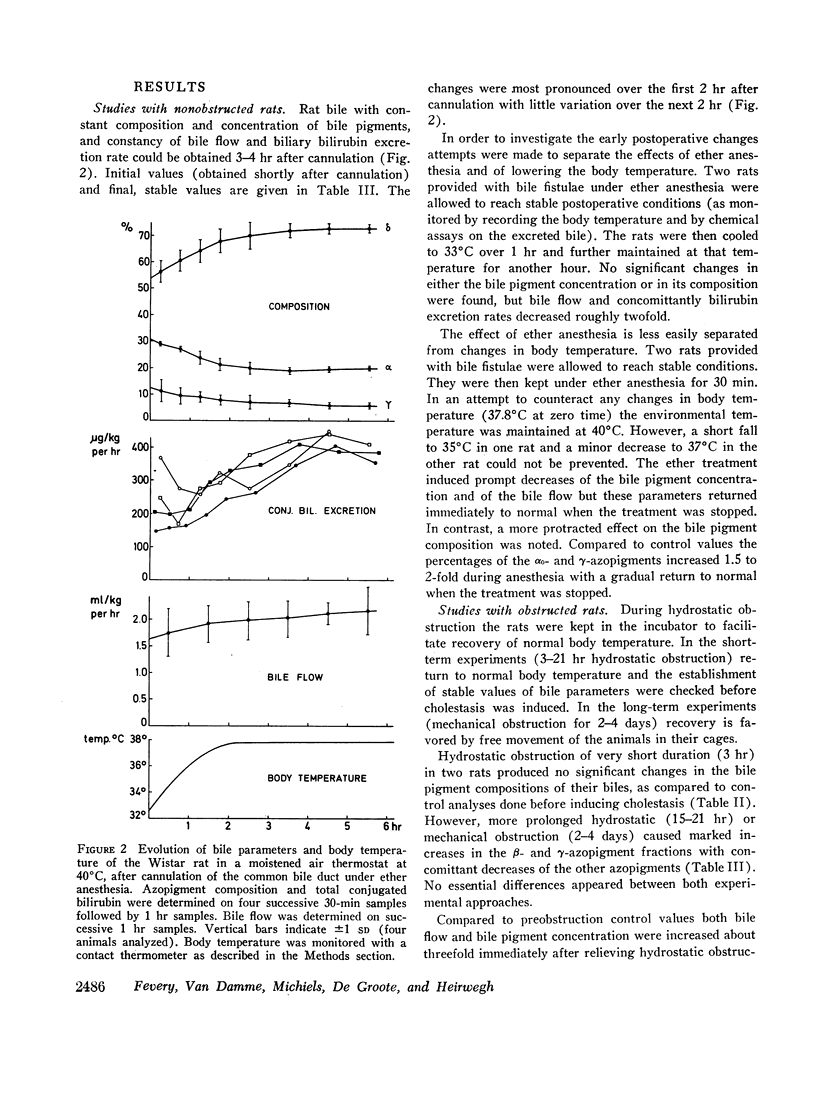
In rats, hydrostatic or mechanical obstruction induced increases in β- and γ-azopigments and a decrease in δ-azopigment similar to the changes observed in bile of liver patients. Complete normalization was obtained 6 hr after relieving the hydrostatic obstruction (duration 15-21 hr). In contrast, with man after surgery for extrahepatic obstruction, T-tube bile was not normalized when the T-tube was withdrawn (10 days after operation).
Hydrostatic obstruction in rats provides an easy model when postobstructive bile pigment composition and parameters have to be investigated.
The present investigations stress the importance of the physiopathological state when studying bilirubin conjugation. Hindrance to bile secretion induced heterogeneity of bilirubin conjugates and stimulated the formation of complex structures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER-RILEY G. MEASUREMENT OF CAPACITY OF BILIARY TREE IN RATS. Am J Physiol. 1963 Dec;205:1122–1126. doi: 10.1152/ajplegacy.1963.205.6.1122. [DOI] [PubMed] [Google Scholar]
- BARBER-RILEY G. RAT BILIARY TREE DURING SHORT PERIODS OF OBSTRUCTION OF COMMON DUCT. Am J Physiol. 1963 Dec;205:1127–1131. doi: 10.1152/ajplegacy.1963.205.6.1127. [DOI] [PubMed] [Google Scholar]
- BILLING B. H., COLE P. G., LATHE G. H. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957 Apr;65(4):774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUER R. W., LEONG G. F., HOLLOWAY R. J. Mechanics of bile secretion; effect of perfusion pressure and temperature on bile flow and bile secretion pressure. Am J Physiol. 1954 Apr;177(1):103–112. doi: 10.1152/ajplegacy.1954.177.1.103. [DOI] [PubMed] [Google Scholar]
- Bouchier I. A. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. 1969 Sep;10(9):705–710. doi: 10.1136/gut.10.9.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet V. J., Bullens A. M., De Groote J. A clinical and histochemical study of cholestasis. Gut. 1970 Jun;11(6):516–523. doi: 10.1136/gut.11.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet V. J., Bullens A. M., De Groote J., Heirwegh K. P. New diazo reagent for specific staining of conjugated bilirubin in tissue sections. J Histochem Cytochem. 1968 Jun;16(6):419–427. doi: 10.1177/16.6.419. [DOI] [PubMed] [Google Scholar]
- Erlinger S. Les mecanismes de la secretion biliaire. Rev Int Hepatol. 1968;18(1):1–39. [PubMed] [Google Scholar]
- Erlinger S., Preisig R. Les mécanismes de la cholérèse. Rev Fr Etud Clin Biol. 1969 Feb;14(2):117–121. [PubMed] [Google Scholar]
- FOG J. BILIRUBIN-PURIFICATION-PURITY. Scand J Clin Lab Invest. 1964;16:49–54. doi: 10.3109/00365516409060482. [DOI] [PubMed] [Google Scholar]
- Fevery J., Van Hees G. P., Leroy P., Compernolle F., Heirwegh K. P. Excretion in dog bile of glucose and xylose conjugates of bilirubin. Biochem J. 1971 Dec;125(3):803–810. doi: 10.1042/bj1250803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischner G., Arias I. M. Recent advances in bilirubin formation, transport, metabolism and excretion. Am J Med. 1970 Nov;49:576–589. doi: 10.1016/s0002-9343(70)80126-7. [DOI] [PubMed] [Google Scholar]
- GREGORY C. H. Studies of conjugated bilirubin. III. Pigment I, a complex of conjugated and free bilirubin. J Lab Clin Med. 1963 Jun;61:917–925. [PubMed] [Google Scholar]
- Heirwegh K. P., Meuwissen J. A., Fevery J. Enzymic formation of beta-D-monoglucuronide, beta-D-monoglucoside and mixtures of beta-D-monoxyloside and beta-D-dixyloside of bilirubin by microsomal preparations from rat liver. Biochem J. 1971 Nov;125(2):28P–29P. doi: 10.1042/bj1250028pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. F., Small D. M. Detergent properties of bile salts: correlation with physiological function. Annu Rev Med. 1967;18:333–376. doi: 10.1146/annurev.me.18.020167.002001. [DOI] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R., Troxler R. F. Recent advances in bile pigment metabolism. Gastroenterology. 1969 Jan;56(1):143–169. [PubMed] [Google Scholar]
- METGE W. R., OWEN C. A., Jr, FOULK W. T., HOFFMANN H. N., 2nd BILIRUBIN GLUCURONYL TRANSFERASE ACTIVITY IN LIVER DISEASE. J Lab Clin Med. 1964 Jul;64:89–98. [PubMed] [Google Scholar]
- Ostrow J. D., Murphy N. H. Isolation and properties of conjugated bilirubin from bile. Biochem J. 1970 Nov;120(2):311–327. doi: 10.1042/bj1200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER D. Estimation of bilirubin mono- and diglucuronide in the plasma and urine of patients with nonhemolytic jaundice. J Lab Clin Med. 1959 Apr;53(4):557–562. [PubMed] [Google Scholar]
- SCHACHTER D. Nature of the glucuronide in direct-reacting bilirubin. Science. 1957 Sep 13;126(3272):507–508. doi: 10.1126/science.126.3272.507-a. [DOI] [PubMed] [Google Scholar]
- SCHOENFIELD L. J., BOLLMAN J. L. Further studies on the nature and source of the conjugated bile pigments. Proc Soc Exp Biol Med. 1963 Apr;112:929–932. doi: 10.3181/00379727-112-28213. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roy F. P., Meuwissen J. A., De Meuter F., Heirwegh K. P. Determination of bilirubin in liver homogenates and serum with diazotized p-iodoaniline. Clin Chim Acta. 1971 Jan;31(1):109–118. doi: 10.1016/0009-8981(71)90367-6. [DOI] [PubMed] [Google Scholar]
- Waldeck F. Sekretion und Rückfluss von Galle während und nach chronischer Gallenstauung bei Ratten. Pflugers Arch. 1970;320(4):300–317. doi: 10.1007/BF00588210. [DOI] [PubMed] [Google Scholar]