Abstract
Occlusion or blockage of silicone shunts utilized in the treatment of hydrocephalus is a major challenge that is currently addressed by multiple shunt replacements. Shunt occlusion is caused by adhesion and proliferation of reactive cells, such as glial and vascular cells, into the lumen of the catheter and on valve components. This in vitro study describes how the adhesive behavior of four human cell types on poly (dimethylsiloxane) (PDMS) surfaces can be suppressed by functionalization with trypsin, a proteolytic enzyme. The covalently conjugated trypsin retained its proteolytic activity and acted in a dose-dependent manner. Trypsin-modified PDMS surfaces supported significantly lower adhesion of normal human astrocytes, human microglia, human dermal fibroblasts and human umbilical vein endothelial cells compared to unmodified PDMS surfaces (p < 0.0001). Immunofluorescence imaging of cellular fibronectin and quantitative adsorption experiments with serum components indicated that the PDMS surfaces immobilized with trypsin inhibited surface remodeling by all cell types and resisted protein adsorption. The impact of this work lies in the recognition that the well-known proteolytic characteristics of trypsin can be harnessed by covalent surface immobilization to suppress cell adhesion and protein adsorption.
Keywords: hydrocephalus, cell adhesion, trypsin, silicone, surface modification
1. Introduction
Hydrocephalus is characterized by the accumulation of cerebrospinal fluid within the cranial cavity of the brain.1 The state of the art treatment for this disease involves implanting a silicone catheter into the ventricular system of the central nervous system (CNS) and redirecting the flow of cerebrospinal fluid (CSF) to another site of the body such as the peritoneal cavity or the right atrium of the heart.2 Although this shunting technology has been in existence for nearly 50 years,3 recent reports indicate that approximately 40% of all CSF shunting procedures conducted in the United States have resulted in shunt failure.4 In fact, the total cost of all treatments related to shunt procedures together with shunt revisions was estimated at $100 million per year.5 In addition, retrospective analyses of the hydrocephalus patient records of 94 children between the years 1993 and 2003 concluded that about 30% of the cases were diagnosed with shunt blockage, making it the most common complication in the treatment of hydrocephalus.6 Shunt blockage, also known as shunt occlusion, has been reported to occur via multiple mechanisms7–10 involving several cell types such as astrocytes,10 macrophages,7, 11 fibroblasts,7 and blood components.7 The only current solution for the shunt occlusion problem is a shunt revision, whereby the old shunt is replaced by a new one, often with several complications.10
An essential aspect in preventing multiple shunt revisions in hydrocephalus patients is to diminish shunt occlusion. Shunt occlusion is facilitated by the adhesion and proliferation of reactive cells on to the implant surface and hence, reducing cell adhesion is vital in preventing shunt revisions. A primary mode of cell adhesion on biomaterials is via integrin binding to secreted extracellular matrix (ECM) proteins such as fibronectin, vitronectin, collagen and laminin,12–14 i.e. surface remodeling. In addition, cell adhesion on shunt surfaces can also occur via the adsorption of serum components present in the CSF such as fibrin, fibrinogen, IgG and albumin.7, 15 A potential approach to inhibit cell adhesion would therefore be to modify the surface of the silicone shunts with molecules such as trypsin, hyaluronic acid or heparin. These molecules are either known to sever bonds between adhered cells and the substrate or prevent protein adsorption, which eventually inhibits cell adhesion on biomaterial surfaces.16–18 Surface modification of PDMS with biologically active molecules can be performed via methods such as adsorption or covalent linkage.18, 19 However, covalent immobilization of anti-adhesive molecules is of specific interest not only because of the ability to incorporate bioactive functionality but also to provide a measure of reliability and reproducibility from the standpoint of mass-production.
Previous efforts to reduce neural cell adhesion via covalent immobilization of heparin and hyaluronic acid on PDMS surfaces have been unsuccessful.19 Trypsin is a proteolytic enzyme that is well known for its ability to cleave proteins. It is used extensively in soluble form for detachment of cells in cell culture applications 16 and in bound form for research in bioreactors and proteomics.20–22 Indeed, immobilized trypsin has been shown to break peptides almost 6600-fold faster compared to trypsin in solution,23 possibly due to the high local concentration of immobilized enzyme.21, 24 However, to our knowledge, trypsin-modified surfaces have not been utilized in detachment of whole cells especially on biomaterials such as silicones. In addition, there are no current bioactive surface coatings for silicone shunts used to treat hydrocephalus, which could potentially reduce cell growth and protein adsorption and thereby reduce shunt blockage. In the present study, trypsin was immobilized on PDMS surfaces to facilitate detachment of reactive human cells that have been previously reported to cause shunt and valve occlusion in hydrocephalus patients. The immobilized trypsin on PDMS surfaces inhibited adhesion of four reactive human cell types via cleavage of peptide bonds formed between the surface of the substrate and the adhered cells. In addition, the adsorption of serum components was also inhibited by immobilized trypsin confirming the bioactive nature of the surface. As a result, we hypothesize that immobilized trypsin may reduce the likelihood of shunt occlusion and failure in the treatment of hydrocephalus.
2. Materials and Methods
2.1 Materials
Normal human astrocytes (NHA), neonatal human dermal fibroblasts (NHDF), human umbilical vein endothelial cells (HUVECs), advanced DMEM/F12K medium, HEPES buffered saline solution, trypsin neutralizing solution (TNS), trypsin-EDTA (0.25%), astrocyte basal medium (ABM) with AGM SingleQuot, fibroblast growth medium (FGM-2) along with FGM SingleQuots and endothelial cell growth medium (EGM) with EGM-2 SingleQuots were purchased from Lonza (Walkersville, MD). Human microglia was purchased from Clonexpress (Gaithersburg, MD). Macrophage-colony stimulating factor (m-CSF) was purchased from Peprotech Inc. (Rocky Hill, NJ). Ethanol (200 proof), 2-propanone (acetone), T-25 culture flasks, were purchased from Fisher Scientific (Fair Lawn, NJ). Fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, CA). Bovine serum albumin (BSA) was purchased from Polysciences Inc. (Warrington, PA). 3-Mercaptopropyl trimethoxysiloxane was obtained from Gelest (Morrisville, PA). Triton X-100, Dimethyl sulfoxide (DMSO), triethylamine, ethyl acetate, 4',6-diamidino-2-phenylindole (DAPI) and the cellular fibronectin antibody produced in mouse was obtained from Sigma (St Louis, MO). 24-well polystyrene plates were obtained from Becton Dickinson (Franklin lakes, NJ). Phosphate-buffered saline (PBS; 1×, without calcium or magnesium) was purchased from Mediatech (Herndon, VA). Secondary antibodies, namely anti-mouse IgG (FITC-labeled) was obtained from Vector Labs (Burlingame, CA). FITC-conjugated bovine serum albumin (BSA) and trypsin (2.5%) without EDTA was purchased from Invitrogen Corporation (Carlsbad, CA). The BCA protein assay kit, 16% formalin, and the coupling molecule N-γ-maleimidobutyryloxy succinimide ester (GMBS) were purchased from Pierce Biotechnology (Rockford, IL). Silicone elastomer (Sylgard 184) and the curing agent were obtained from Dow Corning (Midland, MI). A round handle brass punch (1.5 cm diameter) was obtained from McMaster-Carr (Elmhurst, IL).
2.2 Trypsin immobilization on PDMS substrates
A mixture of silicone elastomer and curing agent (10:1 ratio) was poured into a petri dish, degassed, and allowed to cure for 60 minutes in an oven at 65 °C to prepare PDMS substrates with 3–5 mm thickness prior to trypsin immobilization. To prevent hydrophobic recovery, the cured PDMS was cut into thin slabs and subjected to a short-chain oligomers extraction procedure following the method of Vickers et al.25 Briefly, the PDMS substrates were first immersed in 200 mL of triethylamine for 2 h with constant stirring. The triethylamine was replaced after 1 h. The PDMS substrates were then removed and placed in a beaker containing 200 mL of ethyl acetate and fresh ethyl acetate was added after one hour of stirring at room temperature. The PDMS substrates were then placed in a solution containing 200 mL of acetone for 2 h and fresh acetone was added after one hour. The PDMS substrates were finally dried at 65 °C in an oven for 4 hours. They were then punched to fit into individual wells of a 24 well plate using a 1.5 cm diameter round handle brass punch.
The surface immobilization of trypsin was carried out on the extracted PDMS surfaces using a three-step process described previously.26 Prior to surface modification, a 4% (v/v) solution of 3-mercaptopropyl trimethoxysiloxane in ethanol was prepared under an inert N2 atmosphere in a glove bag. The coupling molecule GMBS, was stored as a stock solution containing 50 mg GMBS in 0.5 mL DMSO and a working solution of 0.28 % (v/v) GMBS was prepared by dissolution in ethanol. The punched PDMS substrates were exposed to oxygen plasma (100 mW with 8% oxygen for 30 s) in a PX-250 plasma chamber (March Instruments, Concord, CA). Immediately after plasma exposure, the first step of surface functionalization was carried out in which, the PDMS substrates were covered with the silane solution and allowed to react at room temperature for 30 min. Unreacted silane was removed by rinsing with ethanol. In the second step, the glass surfaces were covered with the GMBS solution in ethanol and allowed to react for 15 min prior to flushing with ethanol and then with PBS.
For experiments with immobilized trypsin, the stock solution (0.25% or 2.5 mg/mL) was diluted with PBS to the selected concentration or used directly. About 1 mL of this trypsin working solution was cast onto the PDMS substrates modified with GMBS and silane. After a reaction time of 30 min, unbound trypsin was removed by rinsing with PBS.
2.3 Immobilized trypsin surface density quantification
Following the immobilization steps described above, four different casting solution concentrations of trypsin were prepared, cast onto the PDMS substrates, and allowed to react for 30 minutes. After 30 minutes of binding, the supernatant containing the unbound protein was collected and the concentration of unbound trypsin remaining in solution was determined using the BCA Protein Assay Kit. A two-step approach was used to obtain an estimate of the immobilized protein surface density and binding efficiency on both extracted and non-extracted PDMS substrates. In the first step, a calibration curve was created by mixing 0.1 mL of known solution concentrations of trypsin concentrations with 2 mL of kit reagents A and B which were pre-mixed in a 24-well plate in the ratio of 50:1 (v/v) as per the manufacturer’s instructions. The 24-well plate was then incubated at 37 °C for 30 minutes and absorbance was measured at 562 nm using a Bio-Tek HT (Winooski, VT) fluorescence micro-plate reader. Once the calibration curves were obtained from known trypsin concentrations in solution, the sample surfaces on both non-extracted and extracted PDMS substrates were subjected to the 3 step surface modification protocol as described above. Subsequent to the protein binding step, the unbound trypsin solution was collected and mixed with the BCA assay kit reagents followed by incubation at 37 °C for 30 min. Following incubation, the absorbance value at 562 nm was measured and this value was matched to the appropriate concentration in mg/mL from the calibration curve obtained in the first step. The surface densities on non-extracted and extracted PDMS substrates and binding efficiency were calculated using this concentration data.
2.4 Cell culture
Human astrocytes were cultured in T-25 flasks at 5000 cells/cm2 in AGM supplemented with growth factors and cytokines (AGM SingleQuots) per the vendor’s instructions. Human dermal fibroblasts were maintained in T-25 flasks at 3500 cells/cm2 in FGM-2 supplemented with FGM SingleQuots. HUVECs were seeded at 2500 cells/cm2 and maintained in EGM supplemented with EGM-2 SingleQuots. Human microglia were grown in 50:50 DMEM/F12K medium supplemented with 5% FBS, 10 ng/mL m-CSF and 1% penicillin/streptomycin. All cells were maintained at 37 °C, 5% CO2, humidified 95% air, and harvested prior to experiments by first washing with HEPES and removed from the T-25 flask surface using trypsin/EDTA and subsequently neutralized by the addition of TNS. Following TNS neutralization, the cell suspensions were centrifuged at 220 × g for 5 min and re-suspended in supplemented respective media at desired seeding concentration into the 24-well plates containing PDMS substrates.
2.5 Dose response of immobilized trypsin on human astrocytes
Trypsin was immobilized at four casting solution concentrations, 0.25, 0.5, 1.0 and 2.5 mg/mL, using the aforementioned surface immobilization technique. Human astrocytes were seeded at 2.5 × 104 cells/mL onto the extracted and trypsin-modified PDMS substrates and allowed to grow for 24 hours at 37 °C. After one day of culture, the surfaces were washed gently with HEPES solution, and the cells were removed using 1mL trypsin-EDTA and subsequently neutralized with TNS. The cells suspension was centrifuged at 220 × g for 5 min at 4 °C and resuspended in 1 mL of supplemented AGM. The number of viable adherent cells on each sample was identified by the trypan blue exclusion test and counted using a hemacytometer.
2.6 Cell adhesion assay
Trypsin was immobilized at the highest casting solution concentration (2.5 mg/mL) and all the four human cell types were seeded onto the trypsin immobilized and PDMS control substrates with 1 mL of suspensions with a concentration of 2.5 × 104 cells/mL. The substrate area in all cases was 1.9 cm2, yielding a seeding density of 1.3 × 104 cells/cm2. The cells were cultured for 7 days at 37 °C and fresh medium was added every 3 days. The seeding density value specified above was chosen to ensure that no cell type would reach confluence prior to the end of the 7-day experimental period. The cells were cultured for 7 days at 37 °C and fresh medium was added every 3 days. Following 7 days of culture, the cells were washed, removed and neutralized using the aforementioned protocol and viable adherent cells were counted via the trypan blue exclusion test using a hemacytometer. Since certain cell types reached confluence following 8–10 days of culture on PDMS controls, experiments were limited to 7 days to obtain an accurate comparison of cellular adhesion between the samples and controls.
2.7 Immunofluorescence
Human astrocytes, fibroblasts, endothelial cells and microglia were seeded on the trypsin-modified surfaces and PDMS controls at 2.5 × 104 cells/mL. The cells were cultured to 40–60% confluence on PDMS controls and fixed using 4% formalin solution for 15–20 minutes. Following fixation, the cellular monolayer was permeabilized using 0.1% Triton X-100 with 2% BSA in PBS for 10 minutes. The cells were rinsed with PBS and incubated for 30 min at 37 °C with blocking buffer (2% BSA) to prevent non-specific binding. The cells were washed twice with PBS and subsequently incubated with the primary antibody, monoclonal anti-fibronectin cellular antibody produced in mouse for 1 hour at 37 °C. The primary antibody solution was aspirated and the surfaces were rinsed with PBS followed by incubation with FITC anti-mouse IgG (1:100) and DAPI (0.1µg/mL) in PBS for 1 hour at 37 °C. The surfaces were washed with PBS twice and imaged using a Nikon Eclipse TE-2000 inverted microscope.
2.8 Serum protein adsorption
To estimate serum adsorption, trypsin-modified surfaces and PDMS controls were covered with 0.3 mL (100 µg/mL) of FITC-conjugated BSA solution overnight at 37 °C. Following incubation, the surfaces were washed thrice with PBS and the fluorescence images were analyzed using Nikon NIS Elements Advanced Research software. Fluorescence intensities were averaged over 6 image fields on a 4× objective for each of three independent experiments for both trypsin-immobilized and PDMS control surfaces and reported as mean FITC brightness ± standard error of mean.
2.9 Statistical Analysis
Statistical analysis of the data was performed by carrying out Tukey’s tests with one-way ANOVA using Kaleidagraph software. The data was considered statistically significant only if the p value was ≤ 0.001. All experiments were performed in triplicates and the statistics were reported as mean ± standard error of mean.
3. Results
3.1 Surface density analysis of immobilized trypsin
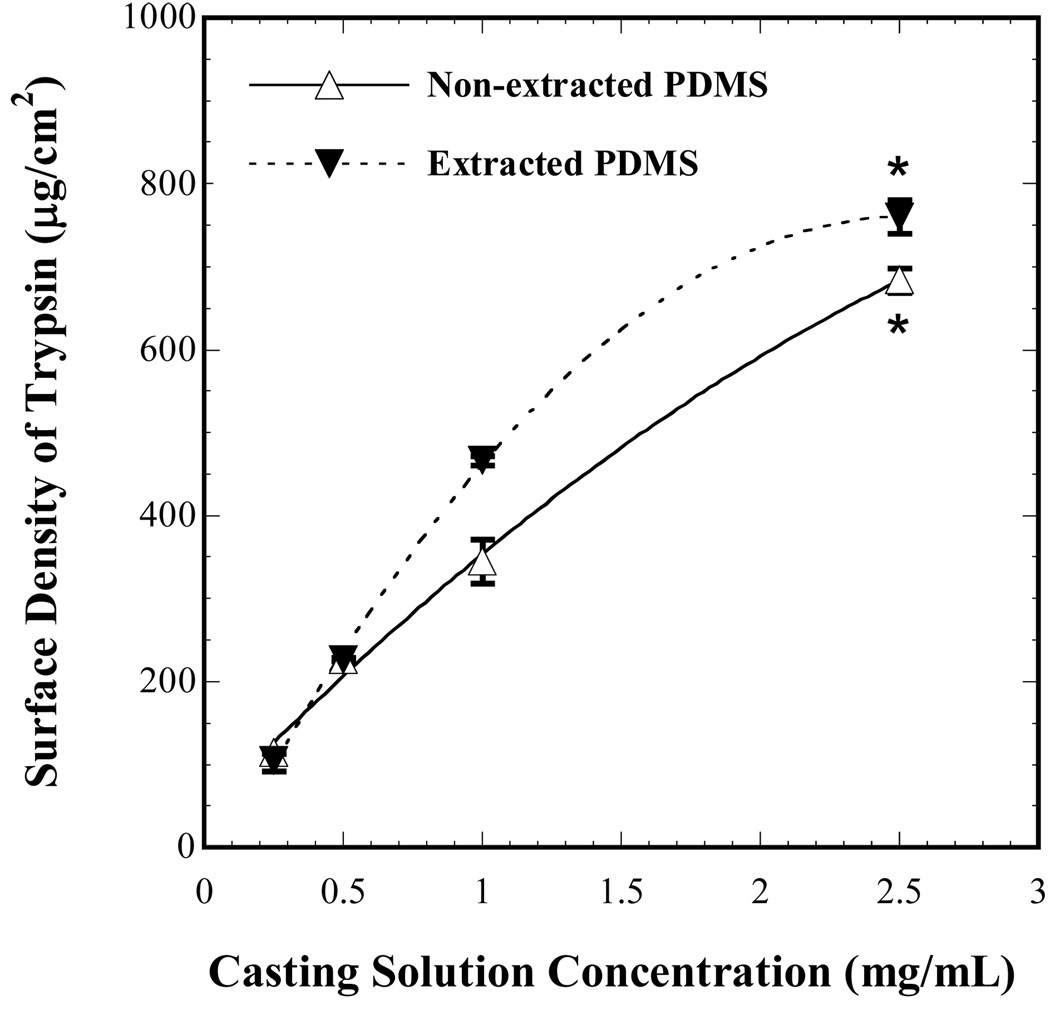
The surface immobilization technique used in the present study allows for covalent attachment of trypsin onto PDMS substrates. Estimates of surface composition of trypsin on non-extracted and extracted PDMS substrates were obtained by measuring the solution concentrations of the unbound protein in the last step of surface modification using a total protein assay. In the concentration ranges examined for trypsin immobilization on both non-extracted and extracted PDMS, the surface density increased monotonically with the casting solution concentration as shown in Fig. 1. In addition, the binding efficiency was found to be higher at lower casting solution concentrations (Table 1) for both non-extracted and extracted PDMS. As the casting solution concentrations were increased, the binding efficiencies decreased on both non-extracted and extracted PDMS substrates indicating that the binding sites on the surface were approaching saturation. The surface density and binding efficiencies on both non-extracted and extracted PDMS were comparable to each other at 2.5 mg/mL trypsin casting solution concentration (p = 0.03). Although the binding efficiencies were comparable, the extracted PDMS had a slightly higher value compared to its non-extracted counterpart. Consequently, the extracted PDMS at a surface density of 760 µg/cm2 was utilized for the cell adhesion, matrix production and protein adsorption studies.
Figure 1.
Surface density of trypsin immobilized on extracted and non-extracted PDMS substrates as a function of casting solution concentration. Each point represents the average from three independent experiments and error bars denote standard errors. The dashed and solid curves represent polynomial fits to the data points. At a casting solution concentration of 2.5 mg/mL for both extracted and non-extracted PDMS surfaces, the surface densities were not statistically significant indicated by (*) (p > 0.001).
Table 1.
Calculated binding efficiencies of trypsin on extracted and non-extracted PDMS as a function of casting solution concentration.
| Trypsin Casting Solution Concentration (mg/mL) |
Binding Efficiency (%) | |
|---|---|---|
| Non-extracted PDMS |
Extracted PDMS | |
| 0.25 | 87 ± 1 | 80 ± 12 |
| 0.5 | 85 ± 1 | 86 ± 1 |
| 1.0 | 66 ± 8 | 89 ± 1 |
| 2.5 | 52 ± 2 | 58 ± 3 |
3.2 Dose response of immobilized trypsin on human astrocytes
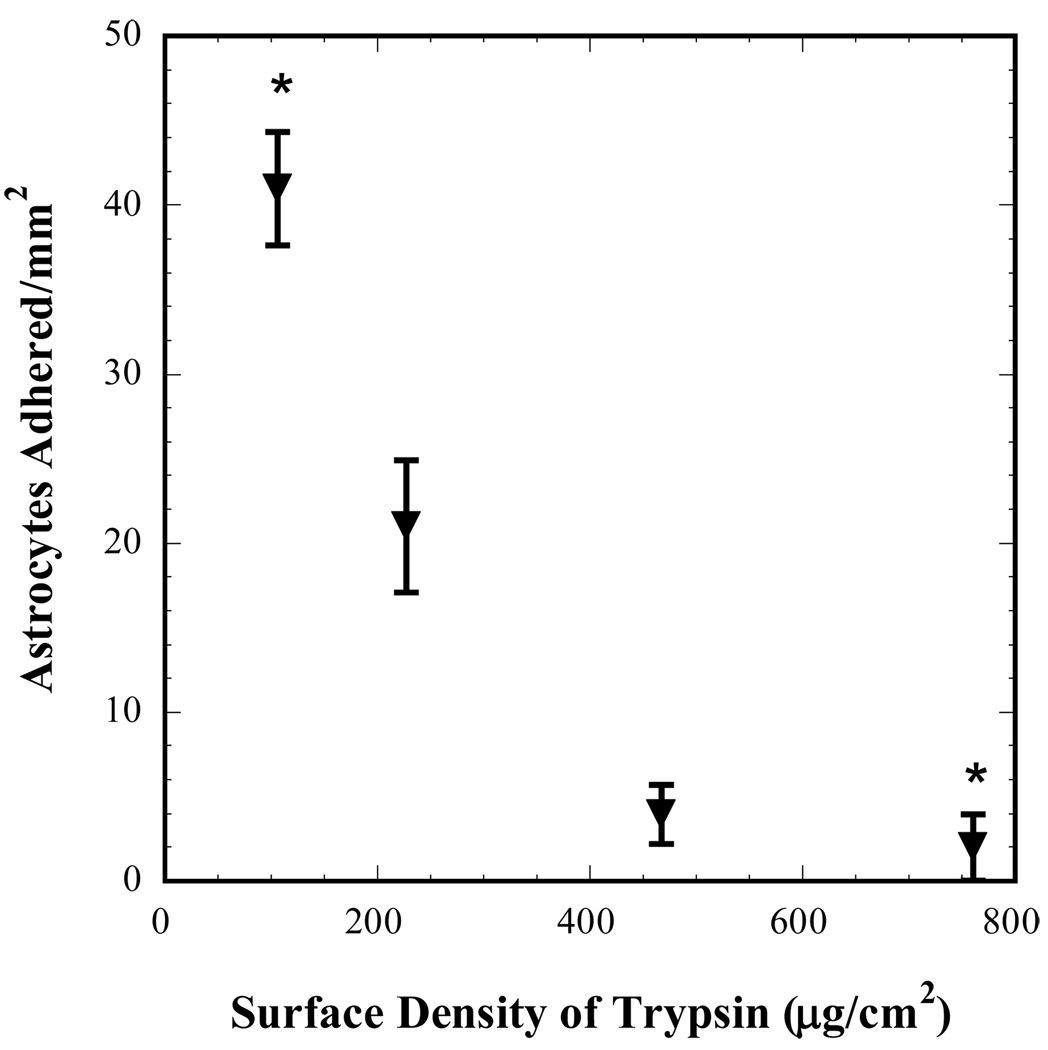
Trypsin was immobilized at surface densities of 106, 226, 466 and 760 µg/cm2 on extracted PDMS substrates and astrocyte attachment on these surfaces after 24 hours of incubation at 37 °C is shown in Fig. 2. As expected, this study indicated that the cell attachment was sensitive to the surface density of trypsin with lower surface densities of trypsin allowing significantly higher adhesion of astrocytes compared to that at higher surface densities (p = 0.0005). Consequently, the casting solution concentration of 2.5 mg/mL that corresponded to the surface density 760 µg/cm2 was used for all subsequent experiments. In addition, since the number of human astrocytes that adhered on this surface was very low (close to zero), higher concentrations of immobilized trypsin were not used for the subsequent experiments.
Figure 2.
Human astrocyte adhesion after 24 h of incubation on trypsin-modified PDMS as a function of trypsin surface density. Each data point denotes a distinct experiment and * denotes statistically significant difference with p = 0.0005 as determined by Tukey’s test with one-way ANOVA.
3.3 Cell adhesion
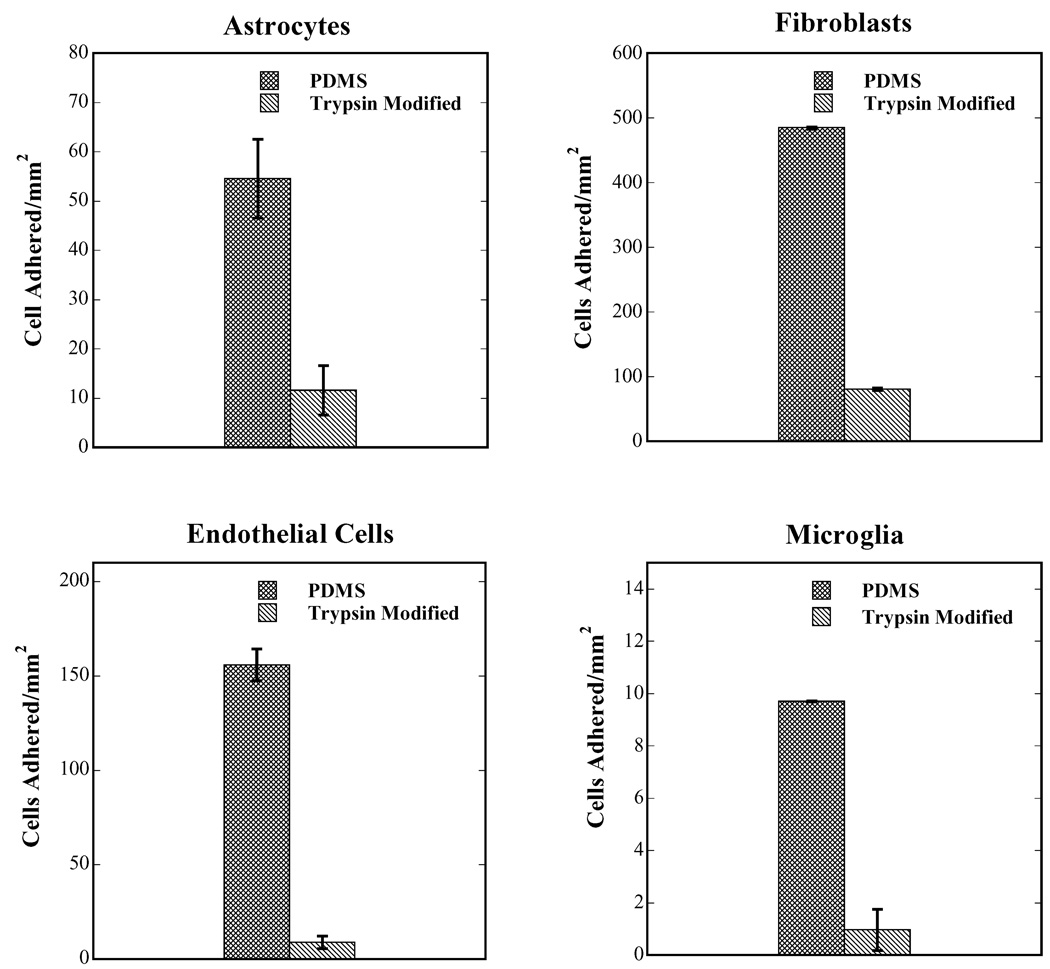
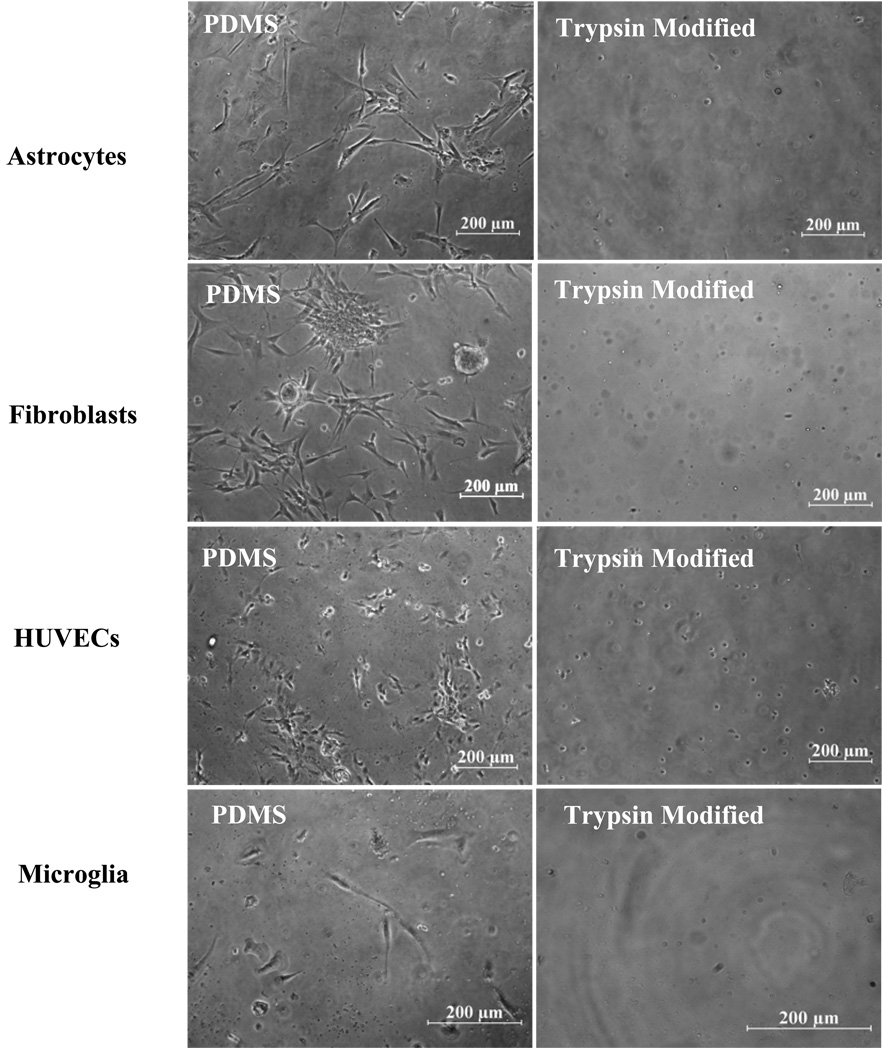
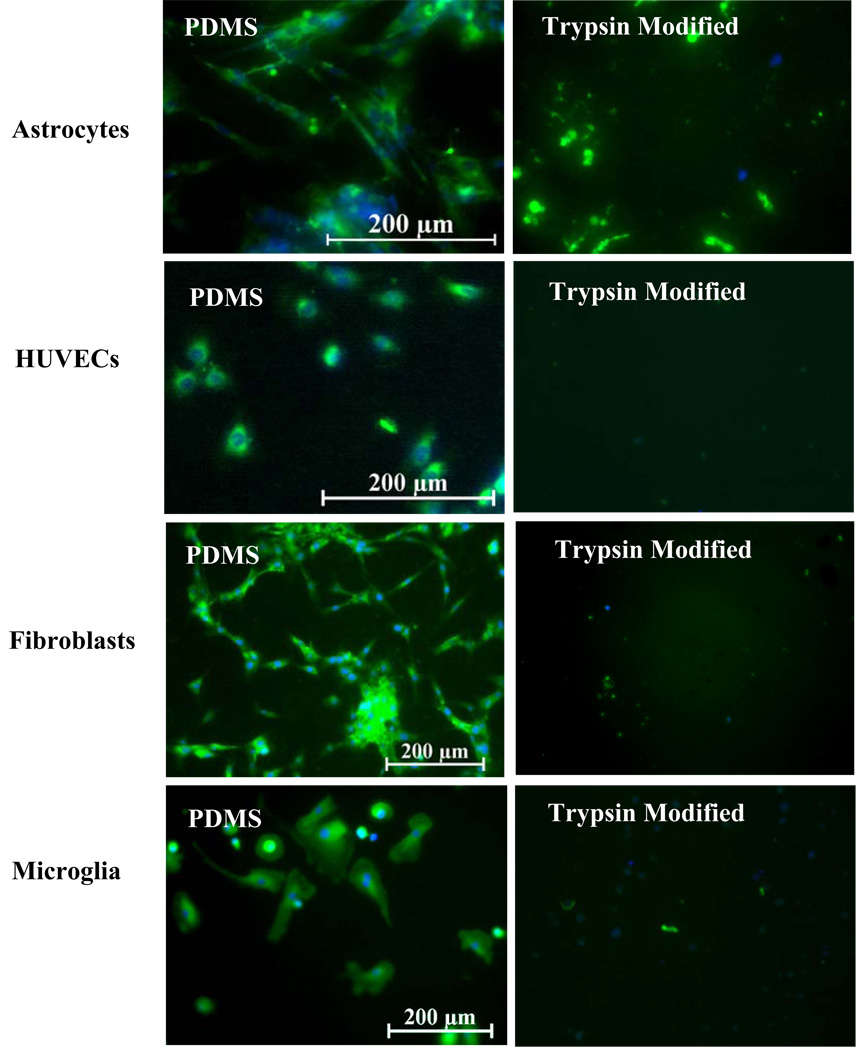
Fig. 3 shows a plot of cell adhesion per unit area for human astrocytes, fibroblasts, endothelial cells and microglia following 7 days of culture in vitro on PDMS controls (unmodified and non-extracted) compared to that of trypsin-modified PDMS substrates. The adhesion of cells on unmodified PDMS surfaces is slow initially (relative to tissue culture polystyrene), but increases over time. As shown in Fig. 3, the adhesion levels of the four cell types were markedly different with microglia having the lowest adhesion and fibroblasts the highest. Since the data presented in Fig. 3 is a plot of the total number of cells adhered/mm2 vs. surface type, the data can be viewed as an unequivocal estimate of cell growth, since cell adhesion to the silicone substrate is essential for survival of cells in a long-term culture. The trypsin-modified surfaces significantly inhibited astrocyte, fibroblast, endothelial cell and microglial adhesion compared to unmodified PDMS substrates (p < 0.0001). Bright field micrographs of these surfaces are shown in Fig. 4 clearly showing the effect of immobilized trypsin on all cell types compared to unmodified PDMS controls. Seeding the cells at higher densities, specifically 4–6 × 104 cells/cm2, did not affect the efficiency of trypsin-action and very few cells adhered (< 10 cells/mm2) on the trypsin-modified surfaces after 7 days of culture (results not shown).
Figure 3.
Cell adhesion following 7 days of culture in vitro as a function of surface type. The growth of all four human cell types was inhibited by the immobilization of trypsin on PDMS substrates compared to unmodified and non-extracted PDMS. Cellular adhesion of human astrocytes, fibroblasts, endothelial cells, and microglia was significantly lower on trypsin-modified PDMS surfaces when compared with uncoated PDMS (p < 0.0001 for all cell-types).
Figure 4.
Micrographs showing the inhibitory effect of trypsin-modified surfaces on cellular adhesion after 7 days of culture of all cell types, in vitro.
3.4 Extracellular matrix production
Mammalian cells are known to adhere to synthetic surfaces via integrin binding to the secreted ECM proteins by cells on surfaces such as fibronectin, laminin and collagen.12, 14, 16, 27, 28 Since the trypsin-modified surfaces inhibited cell growth of all cell types examined in the previous section, determining the mode of inhibition of cell adhesion from these surfaces was essential. All four-cell types were therefore grown to 40–60% confluence (an arbitrary reference point) on unmodified PDMS substrates and trypsin-modified surfaces and then stained for cellular fibronectin. Fig. 5 shows the production of cellular fibronectin of all cell types on unmodified PDMS substrates compared to trypsin-modified surfaces. All unmodified PDMS surfaces were observed to bind sufficient numbers of cells that were anti-fibronectin positive with an intact cell membrane. In addition, the cell nuclei were undamaged as seen by the DAPI counter-stain (blue) in Fig. 5. In contrast, the trypsin-modified surfaces consisted of random blotches of cellular fibronectin with a few isolated spots of DAPI, which was representative of either fragmented fibronectin due to trypsin-induced proteolysis and/or ruptured cell membranes. Either or both of these effects would lead to the detachment of cells from the trypsin-modified PDMS.
Figure 5.
Cellular fibronectin production (green) by all cell types was inhibited by trypsin-modified surfaces. Blue denotes DAPI nuclear staining.
3.5 Serum protein adsorption
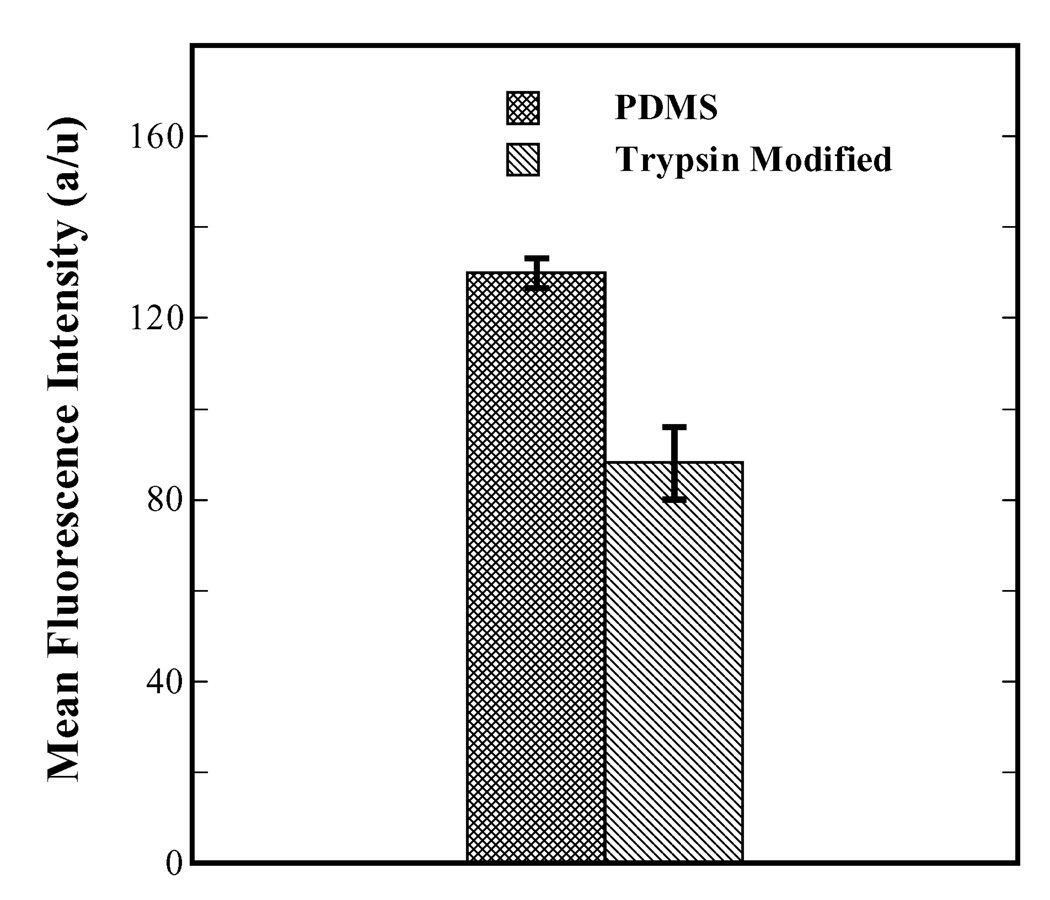
Adsorption of serum proteins on biomaterial surfaces also results in cell adhesion and has been documented extensively.29–34 Fig. 6 shows a plot of mean fluorescence intensity of FITC-conjugated BSA plotted against surface type, which serves as a direct measure of magnitude of protein adsorption on unmodified PDMS and trypsin-modified surfaces. The highly hydrophobic nature of native unmodified PDMS makes it unfavorable for protein adsorption compared to slightly hydrophobic materials such as polystyrene (result not shown). Still, unmodified PDMS does allow appreciable amount of serum protein adsorption compared to trypsin-modified surfaces as shown in Fig. 6. The mean fluorescence intensity of adsorbed FITC-BSA on unmodified PDMS was significantly higher than the trypsin-modified PDMS (p < 0.0001) showing that the latter resiliently inhibited serum protein adsorption.
Figure 6.
Adsorption of serum proteins on unmodified PDMS compared to that on trypsin-modified PDMS as indicated by the intensity of FITC-conjugated BSA. Trypsin-modified PDMS surfaces inhibited serum protein adsorption significantly compared to unmodified PDMS (p < 0.0001).
4. Discussion
Silicone catheters used in redirecting CSF flow in the treatment of hydrocephalus have been reported to fail via multiple mechanisms that cause shunt occlusion.7–10 These failure modes include astroglial cell growth into proximal and distal catheter,35 dysfunction of valve components due to blood elements, cellular debris, or infiltration of fibroblasts.7 Reactive cells such as astrocytes, macrophages, fibroblasts and blood cells are primarily responsible for ventricular catheter obstruction.7, 36 As a result, the current study incorporates studying adhesion characteristics of neural cells such as astrocytes and microglia on PDMS and trypsin-modified PDMS. PDMS was chosen on the basis of its chemical similarity to the clinically implanted silicone catheters. The disruption of the blood brain barrier7 during the time of shunt catheter insertion results in the infiltration of endothelial cells from the capillaries and astrocytes from glia-limitans.12, 37 It is also likely that other vascular cells along with blood components permeate towards the shunt surface following the blood brain barrier rupture. Furthermore, fibroblast in-growth in the rear end of the catheter, i.e. the peritoneum, has been observed previously.7 Moreover, transcranial implants such as shunt catheters have been previously shown to elicit exacerbated astrogliosis characterized by higher inflammation (microglial reactivity), reactive astrocyte proliferation and meningeal fibroblast infiltration due to their chronic contact with the meningeal tissue.37 Hence, in the current work, the adhesion of endothelial cells and fibroblasts were investigated in addition to that of astrocytes and microglia. While other cell types such as the choroid plexus cells and ependymal cells have been previously implicated in shunt occlusion, the current study only involves cells that proliferate extensively and/or are reactive in nature to implanted biomaterials in the CNS. Furthermore, ependymal cells are not known to be reactive8 and hence the current work did not include studying this cell population.
In this work, to verify if hydrophobic recovery of PDMS surfaces38 affects the efficiency of trypsin immobilization, both extracted and non-extracted PDMS were utilized and the amount of trypsin bound to the surface was quantified using the BCA kit. The surface density of trypsin was augmented with the increase in casting solution concentration on both extracted and non-extracted PDMS as seen in Fig. 1. The maximum value of surface density for both the extracted and non-extracted PDMS were similar statistically (p > 0.001) indicating that surface rearrangements were unlikely possibly due to the fact that both extracted and non-extracted PDMS surfaces were exposed to the silane solution within 2 minutes of oxygen plasma treatment. This fact is in accordance with prior work by Lee et al.39 where the authors suggested that surface rearrangements on non-extracted PDMS surfaces are observable only after about one day of exposure to ambient air. Although the hydrophobic recovery of non-extracted PDMS surfaces was found to be negligible, the magnitude of trypsin surface density was nevertheless higher on extracted PDMS compared to the non-extracted ones. This higher trypsin surface density may possibly be due to higher density of Si-OH groups on extracted PDMS after plasma treatment, which is in accordance with results reported by Vickers et al.25 The observed higher surface density, along with the known ability of extracted PDMS to retain its hydrophilicity for prolonged periods following plasma treatment39 makes the extraction process attractive from the standpoint of consistency in mass production. Hence, extracted PDMS surfaces were used as substrates for trypsin immobilization for all the experiments reported in this article.
The surface modification technique utilized in this study allowed us to covalently immobilize trypsin on PDMS surfaces. Prior work26 by our group utilized the same surface modification technique to enhance cellular adhesion and outgrowth. As a result, the mitigated cellular adhesion achieved on trypsin-modified surfaces in the current study could be attributed to the bioactivity of the surface rather than a toxic insult induced by the reagents mentioned in the surface modification technique. All the four cell types examined in this work failed to grow on trypsin-modified surfaces compared to PDMS controls (Figs. 3 and 4). High surface densities of tethered trypsin significantly reduced the cells’ ability to form adhesive bonds in a dose dependent manner (Fig. 2). However, at lower surface densities (105 µg/cm2) appreciable numbers of cells were present on the surface (~40 cells/mm2) likely due to the presence of large amounts of adsorbable protein in the serum, media and the growth supplements. Recent work by Patel et al.19 suggested coating highly hydrophobic materials (contact angle around 110°) onto silicones to inhibit neural cell adhesion and growth. However, silicones such as PDMS used in shunt catheters are also hydrophobic (contact angle ~ 108°)40 but are capable of supporting cell adhesion given a sufficient amount of time as indicated by our cell adhesion experiments in Fig. 3. This observation is important in the context of surgical catheter insertion in hydrocephalus patients because the blood-brain barrier, which is disrupted during the insertion process typically, takes 2–3 weeks to heal.41 This period of time is sufficiently long for the infiltrating cells to settle, deposit their own ECM proteins, and then proliferate. In addition, due to the large amounts of serum present in cerebrospinal fluid (CSF),7 the cells and other living organisms such as bacteria that enter the brain during the insertion of catheter can colonize, proliferate and cause occlusion and/or infections.42 On the other hand, several studies have also reported protein/cell adhesion resistant properties by coating hydrophilic materials such as polyethylene glycol (PEG) on biomaterials.51, 52, 53 However, some reports have highlighted diminished PEG activity in the presence of protein aggregates49(which is the case in vivo) or during long-term use50 making PEGylation not ideal for the current application. Similarly, few studies report protein resistant agents called kosmotropes that create a barrier to proteins when present in solution.54, 55 However, to our knowledge, there are no reports showing tethered kosmotropic agents to biomaterials as a means to mitigate whole mammalian cell adhesion.
Serum protein adsorption on biomaterial surfaces can stimulate macrophages and monocytes to produce growth factors such as fibroblast growth factor (FGF) and other pro-inflammatory cytokines that regulate astrocyte proliferation.43, 44 Furthermore, PDMS can elicit a chronic inflammatory response if serum proteins such as fibrin are adsorbed on the surface even though it is considered relatively inert.45, 46 Serum protein adsorption is also known to augment the adhesion of staphylococcus aureus on silicone implants.47 These observations highlight the need to target protein adsorption on shunt surfaces in addition to preventing cell adhesion. We investigated protein adsorption via two modes namely, secretion of ECM protein (fibronectin) by cells (Fig. 5) and serum adsorption from solution (Fig. 6). The ability of the trypsin-immobilized surfaces to disrupt surface remodeling by cells, shown visually in Fig. 5, suggests that fibronectin produced by the cells as a precursor to adhesion was broken into fragments by the immobilized trypsin. Another possibility is the proteolysis of integrins present on the cells along with the rupture of the linkage between any adsorbed fibronectin and the integrins due to the immobilized trypsin. Such an effect was reported by Brown et al.48 where breakdown of α5β1 and αvβ3 integrins was observed in an investigation of the effect of trypsin in solution on human endothelial cells. With respect to serum protein adsorption, since the CSF in the brain is sporadically in motion (pulsatile flow),1 the concentration of adsorbed serum on the surface of the silicone may be much lower, in vivo than the concentration of 100 µg/mL examined in the present work. Nevertheless, the adsorption of such high concentrations of serum did not affect the efficiency of trypsin activity where, the trypsin-modified surfaces significantly inhibited serum protein adsorption compared to unmodified PDMS (p < 0.0001). It should also be noted that the adsorption of serum proteins might have been very effective on trypsin-modified surfaces initially. However, any such proteins that adsorb could then be digested due to trypsin-action (suggested by broken fragments of cellular fibronectin in the fluorescence micrographs of Fig. 5). Hence, it is hypothesized that the mitigation of serum adsorption may be due to the degradation of initially adsorbed serum proteins on trypsin-modified surfaces, which was observed as diminished FITC-BSA intensity compared to PDMS controls (Fig. 6).
A limitation of the present work is the inability to assess or predict how these trypsin-immobilized silicone surfaces will perform in vivo. Such an assessment would require knowledge of the numbers and types of cells that come in contact with implanted silicone shunts as a function of time post-implantation and also take into account variations inherent in surgical implantation. It is worth noting that the surface density of trypsin utilized in the present work, 760 µg/cm2, was not an optimal surface density, but rather a sufficiently high density to prevent cell adhesion and protein adsorption at selected conditions. It may be possible to obtain higher surface densities on shunt surfaces. Furthermore, another parameter relevant to in vivo studies but not in vitro studies is the total available surface area for functionalization, which would be related to the inner diameter of the shunt and its length.
Another related limitation of the current work is with respect to the active timeline of the immobilized trypsin on the silicone surfaces. Ideally, 2–3 weeks of trypsin activity is desirable (due to the healing time for the blood brain barrier i.e. 2–3 weeks). The present work demonstrates activity over a 7-day period. However, given dose-dependent activity of trypsin shown in Fig. 2, increasing the surface density of trypsin will almost certainly result in retained activity over longer time periods. As discussed above, this higher surface density can be increased directly (by using higher casting solution concentrations during the surface modification step), but also by increasing the overall surface area available, and potentially by incorporating branched spacer molecules that could provide multiple binding sites for trypsin for individual tether points on the surface.56
5. Conclusion
This paper illustrates the role of covalently conjugated trypsin on PDMS substrates in the mitigation of reactive human cell adhesion via a simple three-step dip coating method. Plain silicone (uncoated PDMS) surfaces were shown to support significant growth of all the four cell types compared to trypsin-modified silicone surfaces (p < 0.0001). Trypsin-modified PDMS inhibited protein adsorption via two modes, namely secretion of fibronectin and serum adsorption compared to unmodified PDMS. While our results indicate the efficacy of immobilized trypsin in preventing cell adhesion and protein adsorption, further in vivo studies are required to establish if these surfaces can indeed curtail shunt occlusion in hydrocephalus patients.
Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health through grant R44 NS047952. We also thank Dr. Rebecca Carrier for access to the plate reader.
References
- 1.Schurr PH, Polkey CE. Hydrocephalus. Oxford: Oxford Medical Publications; 1993. [Google Scholar]
- 2.Pudenz RH. Surg. Neurol. 1981;15(1):15–26. doi: 10.1016/s0090-3019(81)80084-5. [DOI] [PubMed] [Google Scholar]
- 3.Scarff JE. J. Neurol. Neurosurg. Psychiatry. 1963;26(1):1–26. doi: 10.1136/jnnp.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patwardhan RV, Nanda A. Neurosurgery. 2005;56(1):139–144. doi: 10.1227/01.neu.0000146206.40375.41. [DOI] [PubMed] [Google Scholar]
- 5.Bondurant CP, Jimenez DF. Pediatr. Neurosurg. 1995;23(5):254–258. doi: 10.1159/000120968. [DOI] [PubMed] [Google Scholar]
- 6.Paran TS, Koenigs I, Fitzgerald R. Cerebrospinal. Fluid. Res. 2004;1 Suppl 1:S25. [Google Scholar]
- 7.Del Bigio MR. Neurosurgery. 1998;42(2):319–325. doi: 10.1097/00006123-199802000-00064. [DOI] [PubMed] [Google Scholar]
- 8.Delbigio MR. Glia. 1995;14(1):1–13. [Google Scholar]
- 9.Kazan S, Acikbas C, Rahat O, Tuncer R. Childs. Nerv. Syst. 2000;16(6):351–356. doi: 10.1007/s003810050530. [DOI] [PubMed] [Google Scholar]
- 10.Lazareff JA, Peacock W, Holly L, Ver Halen J, Wong A, Olmstead C. Childs. Nerv. Syst. 1998;14(6):271–275. doi: 10.1007/s003810050223. [DOI] [PubMed] [Google Scholar]
- 11.Fitch MT, Silver J. Exp. Neurol. 1997;148(2):587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- 12.Manwaring ME, Biran R, Tresco PA. Biomaterials. 2001;22(23):3155–3168. doi: 10.1016/s0142-9612(01)00068-0. [DOI] [PubMed] [Google Scholar]
- 13.Milner R, Campbell IL. J. Immunol. 2003;170(7):3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen I, Vartio T, Badley RA, Lehto VP. Nature. 1982;298(5875):660–663. doi: 10.1038/298660a0. [DOI] [PubMed] [Google Scholar]
- 15.Sevastianov VI, Tseytlina EA. J. Biomed. Mater. Res. 1984;18(9):969–978. doi: 10.1002/jbm.820180902. [DOI] [PubMed] [Google Scholar]
- 16.Creighton TE. Proteins: Structure and Molecular Properties. 2nd ed. W.H. Freeman and Company; 1984. [Google Scholar]
- 17.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Biomaterials. 2004;25(17):3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Lev EI, Assali AR, Teplisky I, Rechavia E, Hasdai D, Sela O, Shor N, Battler A, Kornowski R. Am. J. Cardiol. 2004;93(6):741–743. doi: 10.1016/j.amjcard.2003.11.067. [DOI] [PubMed] [Google Scholar]
- 19.Patel KR, Tang HY, Grever WE, Ng KYS, Xiang JM, Keep RF, Cao T, McAllister JP. Biomaterials. 2006;27(8):1519–1526. doi: 10.1016/j.biomaterials.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kang K, Kan CY, Yeung A, Liu DS. Mater. Sci. Eng. C-Biomimetic Supramol. Syst. 2006;26(4):664–669. [Google Scholar]
- 21.Krenkova J, Svec F. J. Sep. Sci. 2009;32(5–6):706–718. doi: 10.1002/jssc.200800641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temporini C, Perani E, Mancini F, Bartolini M, Calleri E, Lubda D, Felix G, Andrisano V, Massolini G. J. Chromatogr A. 2006;1120(1–2):121–131. doi: 10.1016/j.chroma.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Ma JF, Liang Z, Qiao XQ, Deng QL, Tao DY, Zhang LH, Zhang YK. Anal. Chem. 2008;80(8):2949–2956. doi: 10.1021/ac702343a. [DOI] [PubMed] [Google Scholar]
- 24.Shuler ML, Kargi F. Bioprocess Engineering Basic Concepts. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2002. [Google Scholar]
- 25.Vickers JA, Caulum MM, Henry CS. Anal. Chem. 2006;78(21):7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- 26.Achyuta AKH, Cieri R, Unger K, Murthy SK. Biotechnol. Prog. 2009;25(1):227–234. doi: 10.1002/btpr.58. [DOI] [PubMed] [Google Scholar]
- 27.Biran R, Noble MD, Tresco PA. J. Biomed. Mater. Res. 1999;46(2):150–159. doi: 10.1002/(sici)1097-4636(199908)46:2<150::aid-jbm3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Leung BK, Biran R, Underwood CJ, Tresco PA. Biomaterials. 2008;29(23):3289–3297. doi: 10.1016/j.biomaterials.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 29.Anselme K. Biomaterials. 2000;21(7):667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 30.Grinnell F, Feld MK. J. Biol. Chem. 1982;257(9):4888–4893. [PubMed] [Google Scholar]
- 31.Keselowsky BG, Bridges AW, Burns KL, Tate CC, Babensee JE, LaPlaca MC, Garcia AJ. Biomaterials. 2007;28(25):3626–3631. doi: 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JS, Lee JB, Kim J, Lim YJ. Tissue. Eng. Regen. Med. 2009;6(4–11):875–881. [Google Scholar]
- 33.Steele JG, Johnson G, Griesser HJ, Underwood PA. Biomaterials. 1997;18(23):1541–1551. doi: 10.1016/s0142-9612(97)80006-3. [DOI] [PubMed] [Google Scholar]
- 34.Vogler EA. Adv. Colloid. Interface. Sci. 1998;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 35.Sekhar LN, Moossy J, Guthkelch AN. J. Neurosurg. 1982;56(3):411–416. doi: 10.3171/jns.1982.56.3.0411. [DOI] [PubMed] [Google Scholar]
- 36.Zhong YH, Bellamkonda RV. J. R. Soc. Interface. 2008;5(26):957–975. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Biomaterials. 2004;25(12):2229–2237. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Fritz JL, Owen MJ. J. Adhes. 1995;54(1–2):33–45. [Google Scholar]
- 39.Lee JN, Park C, Whitesides GM. Anal. Chem. 2003;75(23):6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 40.Morra M, Occhiello E, Marola R, Garbassi F, Humphrey P, Johnson D. J. Colloid. Interface. Sci. 1990;137(1):11–24. [Google Scholar]
- 41.Jaeger CB, Winn SR, Tresco PA, Aebischer P. Brain. Res. 1991;551(1–2):163–170. doi: 10.1016/0006-8993(91)90929-p. [DOI] [PubMed] [Google Scholar]
- 42.Liang XM, Wang AF, Cao T, Tang HY, McAllister JP, Salley SO, Ng KYS. J. Biomed. Mater. Res. Part A. 2006;76A(3):580–588. doi: 10.1002/jbm.a.30559. [DOI] [PubMed] [Google Scholar]
- 43.Bonfield TL, Colton E, Anderson JM. J. Biomed. Mater. Res. 1989;23(6):535–548. doi: 10.1002/jbm.820230602. [DOI] [PubMed] [Google Scholar]
- 44.Bonfield TL, Colton E, Anderson JM. J. Biomed. Mater. Res. 1991;25(2):165–175. doi: 10.1002/jbm.820250204. [DOI] [PubMed] [Google Scholar]
- 45.Sergott TJ, Limoli JP, Baldwin CM, Laub DR. Plast. Reconstr. Surg. 1986;78(1):104–114. doi: 10.1097/00006534-198607000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Tang LP, Eaton JW. Am. J. Clin. Pathol. 1995;103(4):466–471. doi: 10.1093/ajcp/103.4.466. [DOI] [PubMed] [Google Scholar]
- 47.Barrett SP. J. Med. Microbiol. 1985;20(2):249–253. doi: 10.1099/00222615-20-2-249. [DOI] [PubMed] [Google Scholar]
- 48.Brown MA, Wallace CS, Anamelechi CC, Clermont E, Reichert WM, Truskey GA. Biomaterials. 2007;28(27):3928–3935. doi: 10.1016/j.biomaterials.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park K, Shim HS, Dewanjee MK, Eigler NL. J. Biomater. Sci.-Polym. Ed. 2000;11(11):1121–1134. doi: 10.1163/156856200744228. [DOI] [PubMed] [Google Scholar]
- 50.Branch DW, Wheeler BC, Brewer GJ, Leckband DE. Biomaterials. 2001;22(10):1035–1047. doi: 10.1016/s0142-9612(00)00343-4. [DOI] [PubMed] [Google Scholar]
- 51.Vladkova T. J. Appl. Polym. Sci. 2004;92(3):1486–1492. [Google Scholar]
- 52.Scott EA, Nichols MD, Cordova LH, George BJ, Jun YS, Elbert DL. Biomaterials. 2008;29(34):4481–4493. doi: 10.1016/j.biomaterials.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko YG, Kim YH, Park KD, Lee HJ, Lee WK, Park HD, Kim SH, Lee GS, Ahn DJ. Biomaterials. 2001;22(15):2115–2123. doi: 10.1016/s0142-9612(00)00400-2. [DOI] [PubMed] [Google Scholar]
- 54.Russo D. Chem. Phys. 2008;345(2–3):200–211. [Google Scholar]
- 55.Curtis RA, Ulrich J, Montaser A, Prausnitz JM, Blanch HW. Biotechnol. Bioeng. 2002;79(4):367–380. doi: 10.1002/bit.10342. [DOI] [PubMed] [Google Scholar]
- 56.Murthy SK, Olsen BD, Gleason KK. Langmuir. 2004;20(11):4774–4776. doi: 10.1021/la036102v. [DOI] [PubMed] [Google Scholar]