Abstract
Introduction
Previously, we have shown in a cross-comparison study that multifunctional photopolymerized semi-interpenetrating network (sIPN) system is an effective donor site treatment in a swine model. The advantages of sIPN include spray-on application, in-situ photopolymerization, and ability to cover large contoured areas. sIPN has also been shown to be an effective delivery vehicle for keratinocyte growth factor, dexamethasone, bupivacaine, and silver sulfadiazine in vitro. Our aim for this study was to show that these products delivered to the wound bed with sIPN would not change the wound healing characteristics compared to the control site through qualitative clinical evaluation and to compare the rate and quality of donor site healing through histologic evaluation.
Methods
Eight Yucatan swine of 40lbs each were randomly divided into four groups of two pigs prior to surgery. Each animal had 5.6 % total body surface area of skin harvested from two different dorsal regions, with one at 22/1000th inch and the other at 30/1000th inch setting on the dermatome. Each test site on each animal was then sequentially dressed with 50 cm2 of Xeroform gauze, sIPN, sIPN loaded with 0.5% bupivacaine, or sIPN loaded with 1% silver sulfadiazine. sIPN with or without soluble drugs were applied as liquid then photopolymerized in situ to form an elastic covering. Each of the test areas was separated by 50 cm2 of autograft which was used to divide the test areas. Wound assessment and euthanasia occurred at days 7, 9, 14, and 21 days. A full thickness biopsy was taken from each of the study areas for histological analysis.
Results
By 14 days, all areas show complete epidermal coverage histologically. The 30/1000th inch site revealed a thicker, more irregular dermis compared to the 22/1000th site. Evaluation of the day 21 sites revealed equal thinning and flattening of the new epidermis. No site showed full restoration of the rete ridges. No signs of infection were seen in clinical or histological evaluations of any treatment.
Conclusion
The addition of bupivacaine and silver sulfadiazine to sIPN does not show any alterations in wound healing of a donor site in a swine model when compared with sIPN without loaded drugs and a standard control dressing. This efficacy may be coupled with established localized sIPN drug delivery profiles and allow further studies to evaluate the efficacy of these drugs to promote healing, eradicate and prevent infection, and manage pain.
Introduction
Split-thickness autograft donor site wounds provide major clinical challenges such as infection, pain, and long healing times. To facilitate healing in donor site wounds, dressing treatments should ideally meet four primary requirements; the removal of non-viable or necrotic tissue, eradication and prevention of microbial infiltrate, exudate absorbance, and regrowth of healthy epidermis and dermis1, 2. Many patients have a greater level of pain from their donor sites than from the grafted burn wound and donor site infection is the most common reason for delayed healing of a donor site. There are many commonly used donor site dressings on the market today including Xeroform™ (Covidien Inc., Mansfield, MA, USA). Another experimental dressing treatment hypothesized to facilitate the key features of quality treatment is an in situ photopolymerized semi-interpenetrating network (sIPN). Each of these treatments varies in their approach to promoting healing. For example, Xeroform™ is composed of absorbent gauze coated with 3% Bismuth Tribromophenate to promote exudate absorption and eradicate bacterial infiltrate. sIPN is an in situ photopolymerizable hydrogel composed of poly(ethylene glycol) diacrylate (PEGdA) and chemically modified gelatin acting as a biodegradable tissue scaffold. Key properties of sIPN include absorbance, tissue adhesiveness, contour conformation, biocompatibility, elasticity, modifiability for cell-material interactions, and the ability to function as a drug delivery matrix3-8. Recent data has suggested that sIPN is an effective donor site treatment in an animal model9. Additional data also reveals in vitro sIPN can release a therapeutic dose of bupivacaine for up to six hours10. Bacteriostatic concentrations of silver sulfadiazine can also be released from in vitro sIPN for up to 24 hours10. Bactericidal concentrations we achieved against both S. aureus and methicillin resistant S. aureus10.
In this study, we sought to identify whether additions of soluble bupivacaine and silver sulfadiazine to the sIPN formulation would create any alterations in donor site healing compared to a Xeroform™ control treatment in a porcine partial thickness wound model. Such a treatment may meet all the requirements of an effective donor site dressing by providing an adjunct to pain control, diminishing the bacterial burden, and effectively absorbing wound exudate. Throughout the course of healing, critical cell types active in epithelialization, extracellular matrix remodeling, and inflammation were qualitatively and quantitatively analyzed as key indicators of healing.
Materials and Methods
Surgical Methods
Approximately 40 lbs. Yucatan pigs (Sinclair Research Center Inc., Columbia, MO, USA) were used for this study in accordance with University of Wisconsin-Madison Research Animal Resource Center protocol M1653. This is the same procedure as described in our previous study9. Anesthesia was induced with Telazol® (4.4 mg/kg IM) and xylazine (2.2 mg/kg IM), then maintained with Isoflurane while monitoring pulse oxygenation and heart rate. Pig backs were shaved and prepped with Betadine®. Subcutaneous injections of 1:100,000 epinephrine and 0.9% saline solution were given over the entire surgical region to assist with hemostasis. A thin layer of mineral oil was applied immediately prior to wounding. Two dorsal wounds were produced using a Zimmer® electric dermatome set at 0.022″ and 0.030″ settings covering 5.4% (330 cm2) total body area wound. Epinephrine solution was applied to wound bed with non-adherent gauze for hemostasis. Dressing treatments included sIPN, sIPN loaded with soluble 0.5% bupivacaine, sIPN loaded with soluble 1% silver sulfadiazine, and Xeroform™ (Covidien Inc., Mansfield, MA, USA) were applied over epidermal autograft separated regions as shown in Figure 1. Doses were based on in vitro sIPN drug release profiles established previously10. Bupivacaine and silver sulfadiazine loading densities were established from commonly used doses. Autografts separated treatments to prevent overlap of the dressing and facilitate the identification of each treatment type. Starting materials for sIPN preparation, silver sulfadiazine, and bupivacaine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Unpolymerized sIPN solutions were prepared at 20% gelatin (porcine type A bloom 300) and 30% PEGdA (MW 575 Da) in water at 40°C in H2O. 0.045% 2,2-dimethoxy-2-phenylacetophenone, dissolved in PEGdA, was added to this solution within an hour prior to treatment. With the drug loaded sIPN, either 0.5% bupivacaine or 1% silver sulfadiazine was dissolved or suspended in this solution respectively. Liquid sIPN solution was applied to the test area of the wound in a drop wise fashion while under simultaneous UV light exposure (Clearstone Technologies CF1000 with 365nm LED head) until the wound was covered with an initial solidified film. Then, additional sIPN solution is added and polymerized to thicken the dressing. This process takes approximately one minute to cover the wound. Xeroform™ and autografts were immobilized with surgical staples. Treatments were overlaid with burn gauze, and wrapped in Coban™ (3M, St. Paul, MN, USA) and tape. Animals were treated with 0.01 mg/kg IM buprenorphine twice daily for three days then given carprofen 2.2 mg/kg PO for three additional days. These medications were used for pain control after our invasive procedure as part of the usual University of Wisconsin Animal Care Facility standard of care. Pigs were housed independently with unrestricted movement in cages at the UW-Madison Medical Sciences Center and fed daily with no diet restrictions per our standard protocols for animal research. Secondary dressings were removed for observation (i.e., for incident of infection) on post operative day 10 for studies beyond 10 days. No additional primary and secondary dressings were reapplied thereafter. On the day of euthanasia, pigs were injected with 0.2mL/kg IV Beuthanasia-D.
Figure 1.
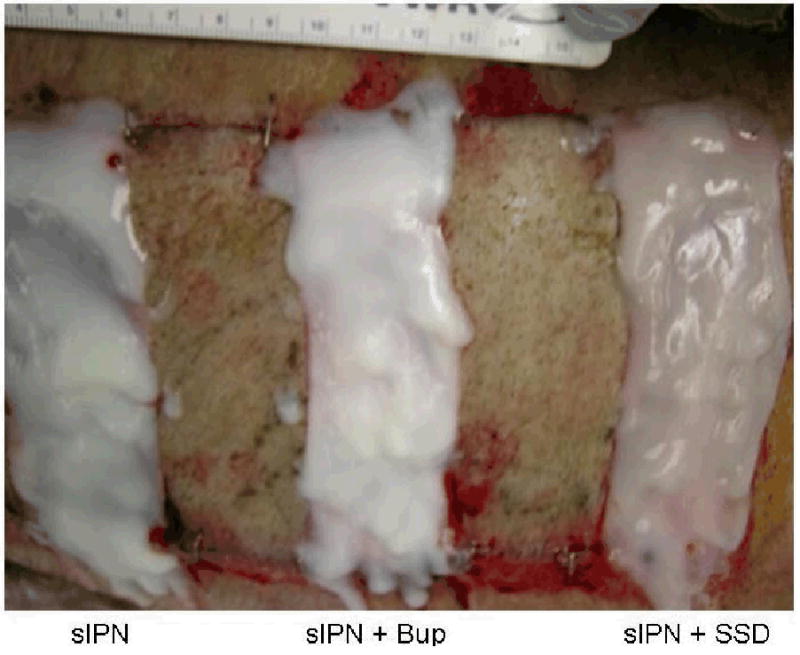
Example of wound treatment arrangement on pig wounds. Wounds cut at 0.022 in or 0.030 in dermatome cut depth settings were treated with semi-interpenetrating network (sIPN) containing no drug (left), sIPN with 0.5% bupivacaine (Bup) (middle), sIPN with 1% silver sulfadiazine (SSD) (right), and Xeroform™ (not pictured). Alternating split thickness autografts were applied between each treatment to provide physical separation between experimental wound sites.
Histological Observation and Measurement
The same procedure was applied as in our previous study9. At day 7, 9, 14, and 21, multiple tissue biopsies were taken from unwounded tissue, and each wounded tissue at both cut depths after euthanasia. The unwounded skin was found to be 0.102 inches thick with little variation in our animals. This is the same thickness as human skin in areas where donor sites are commonly taken. Tissues were fixed in 10% buffered formalin or embedded in Optimal Cutting Temperature compound (Tissue-Tek, Torrance, CA) and snap frozen in cold isopentane. Biopsies were embedded in formalin or cryosectioned at 10μm and stained with hematoxylin and eosin (UW-Madison Research Animal Resources Center). To account for animal to animal variability along with variability within the healing wound, ten regions of each of the five biopsy areas were observed; half from the epidermis and half from the dermis. This left us with twenty points of data from each study area for each day studied. Viewing regions were evenly spaced across the length of the biopsy. Only the healing wounds were observed as we did not biopsy the autograft areas as this was already accomplished in our previous study9. Individual cell types were identified by morphology after staining.
For epidermal cell counts and measurements, data was collected for analysis of keratinocyte density (normalized to stratum spinosum area as an observable histological feature since it is consistently the only region containing viable, non-basal keratinocytes), stratum spinosum thickness, stratum corneum thickness, and melanocyte density (normalized to stratum basale length). Keratinocytes are predominately found within the stratum spinosum and are an element of epithelialization. The cell counts were compared between the test groups, not state that a given number implies a healed wound. The density of cells was evaluated to know how the density changes over time. There are very few other cells within this layer to confound the data.
For dermal cell counts and measurements, the viewing regions were selected to focus on the interfaces between the epidermis and remodeling region in the healing areas and between the epidermis and dermis in the unwounded sites. Data was collected for PMN density, macrophage density, lymphocyte density, remodeling tissue thickness, and fibroblast density (all densities normalized to area of viewing region). Thicknesses, lengths, and areas were all calculated using NIH ImageJ image processing and analysis software (National Institutes of Health, Washington DC, USA).
Statistical Analysis
Two pigs were utilized for each time period. Data from the twenty points at each time point, treatment type, and cut depth were analyzed as a pooled mean ± standard error. Data was compared using student's t-tests. Statistical significance is noted in text. A lack of statistical significance is noted where p>0.05.
Results and Discussion
General Observations
Throughout the time course of this study, we found the sIPN tightly adhered to the wound bed that biopsies and photos were taken prior to the removal. On day 21, the sIPN was removed more easily. It is possible that the sIPN is absorbing serum and clotting factors into the matrix yet not absorbing the erythrocytes as the sIPN never turned red. Increasing the cut depth to 0.030″ was important and it shows that we are now to the deepest layers of the dermis based on the fact that we found increased inflammation, slowed healing, and increased remodeling compared to the 0.022″ cut depth and as described in our previous work9.
Epidermal healing
Epidermal healing was examined as an indicator of initial healing. Biopsies taken from wounds treated with sIPN, sIPN with 0.5% bupivacaine, sIPN with 1% silver sulfadiazine, and Xeroform™ after 7, 10, 14, and 21 days showed persistent changes in multiple features of the epidermis in a time-dependent manner (Figure 2-5). At seven days, the Xeroform group shows epidermis formation and this is not seen in any of the sIPN formulations. However, at 10 days, there is greater keratinocyte density in the sIPN formulations than in the Xeroform group. (Figure 6C) At the 0.022″ cut depth, Xeroform shows near complete epidermal coverage and only partial coverage at the 0.030″ cut depth. The 0.022″ cut depth covered with Xeroform also shows a statistically significant increase in stratum spinosum thickness and keratinocyte density compared to the sIPN covered regions. (Figure 6) There were no melanocytes identified in any of the healing regions. At 10 days, still no melanocytes appeared in any treatment. Melanocytes have dendritic processes that extend into several layers, but the cell bodies are found exclusively in the stratum basale. Previous research has normalized certain cell types to the number of epithelial cells or keratinocytes11, 12. We argue that this would be confounding since we know that keratinocyte density changes throughout healing. Furthermore, in early phases of epidermal formation, the surface area of the stratum basale is much larger due to deep hills and valleys in immature squamous epithelium. This is why we have normalized melanocytes to the length of the basal layer rather than to the number of basal keratinocytes.
Figure 2.
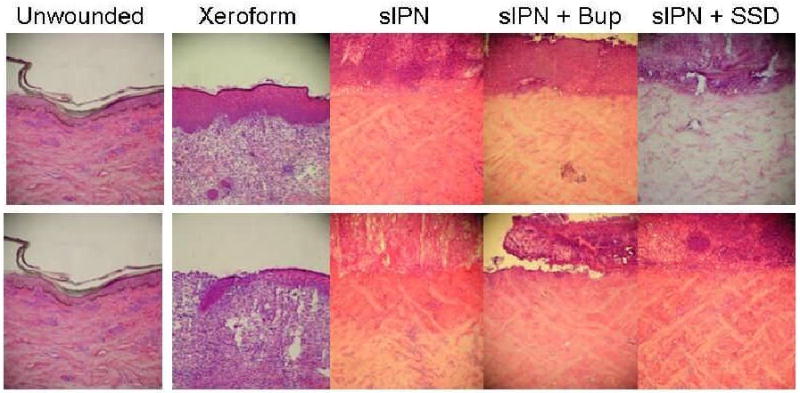
Representative histologic photomicrographs of wounded tissues 7 days after treatment. The 0.022 in dermatome cut depth wounds are shown on top, whereas 0.030 in wounds are on bottom. Tissue are aligned so that approximately the top 3rd of each photo Contains dead or necrotic tissue such as stratum corneum, fibrin clot, dead cells, or degraded extracellular matrix. (H & E stain, objective magnification ×10).
Figure 5.
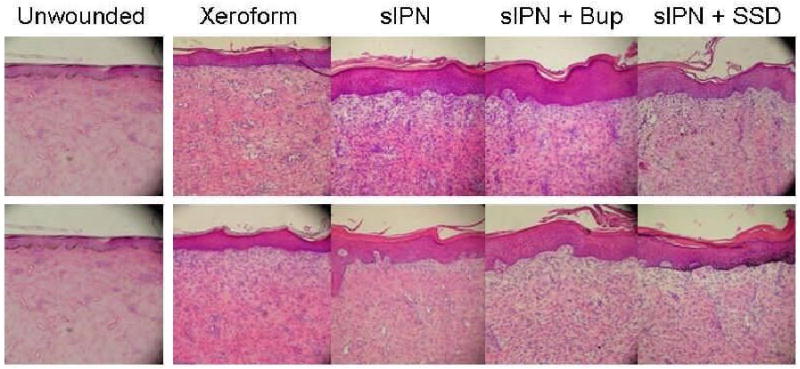
Representative histologic photomicrographs of wounded tissues 21 days after treatment. The 0.022 in dermatome cut depth wounds are shown on top, whereas 0.030 in wounds are on bottom. Tissue are aligned so that approximately the top 3rd of each photo contains dead or necrotic tissue such as stratum corneum, fibrin clot, dead cells, or degraded extracellular matrix. (H & E stain, objective magnification ×10).
Figure 6.
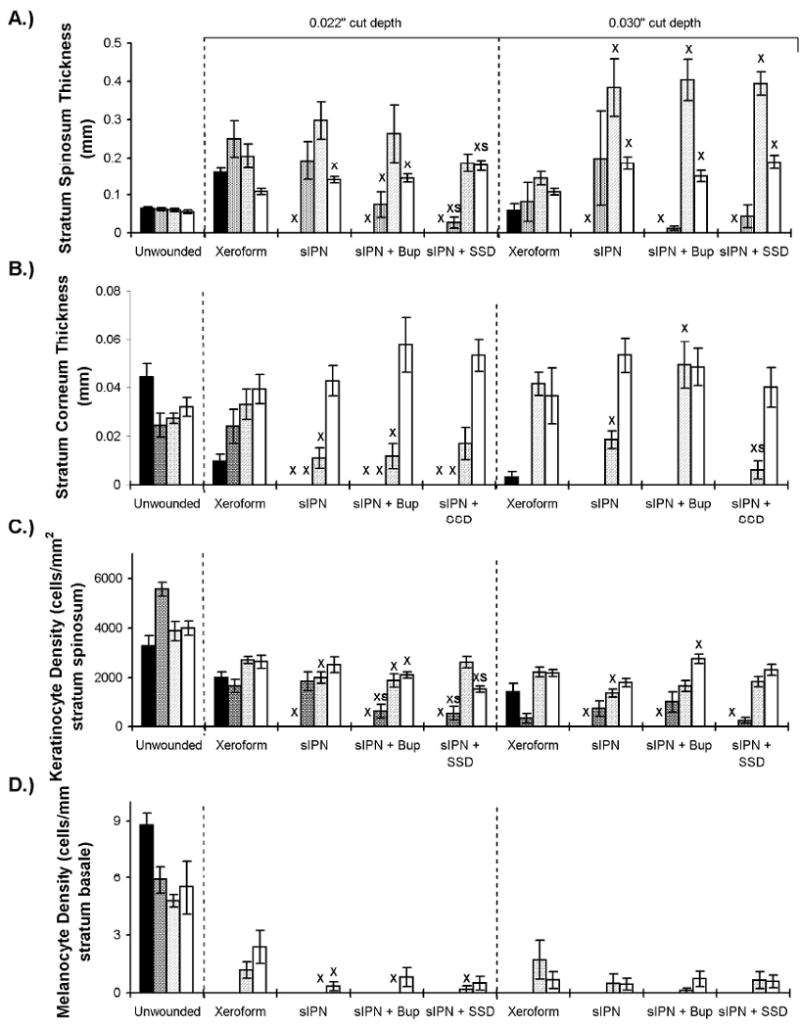
Epidermal features of wounds dressed with Xeroform™, sIPN, sIPN with 0.5% bupivacaine (Bup), and sIPN with 1% silver sulfadiazine (SSD) examined histologically at 7 (■), 9 ( ), 14 (
), 14 ( ), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Thickness of stratum spinosum(A), thickness of stratum corneum(C), viable keratinocyte density within epidermis(C), and melanocyte density(D) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Thickness of stratum spinosum(A), thickness of stratum corneum(C), viable keratinocyte density within epidermis(C), and melanocyte density(D) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
There is complete epidermal coverage under the Xeroform at both cut depths. There remains statistically thicker stratum corneum and stratum spinosum at the 0.022″ cut depth compared to the 0.030″ depth. (Figure 6) These findings are similar to that of all recently healed wounds. Longer observation time would be needed to assess resolution to normal skin architecture. The sIPN covered wounds showed near complete epidermal coverage where as the drug loaded sIPN showed only partial epidermal coverage at the 0.022″ cut depth. All areas at 0.030″ showed an increased neutrophil infiltration within the viable dermis. (Figure 7) There was complete epidermal coverage of all sIPN and Xeroform covered regions at the 0.022″ cut depth by day 14. (Figure 8) The 0.030″ cut depth yielded no significantly increased remodeling regions and increased fibroblast densities along with a more irregular dermis. A stratum corneum was produced under all treatments. (Figure 6) By day 21, the epidermis of all treatment areas and wound depths had thinned and flattened. The rete ridges were fully not restored in all treatments. (Figure 2-5) In summary, all healing areas showed a significantly lower keratinocyte and melanocyte density compared to unwounded areas at both cut depths. The stratum spinosa and stratum cornea thickness along with fibroblast density was also statistically greater for many treatment areas compared to unwounded areas. These results are variable in the sense that many different quantified cell types represent various aspects of wound healing and tissue architecture. This data shows a comprehensive view of the assessment of healing as mediated by different treatments. This quantitative histology is inherently variable when animal to animal variation is present. Healing was evaluated both by clinical definitions and end-points. The detailed analysis presented above addresses what is missing in the literature, that is, a deeper understanding and characterization of the dynamic wounded tissue throughout the course of healing. These cellular markers are important if we are to evaluate the impact of novel treatment options. This is a fundamental baseline study of wound healing at the cellular level that future endeavors will evaluate at the molecular levels.
Figure 7.
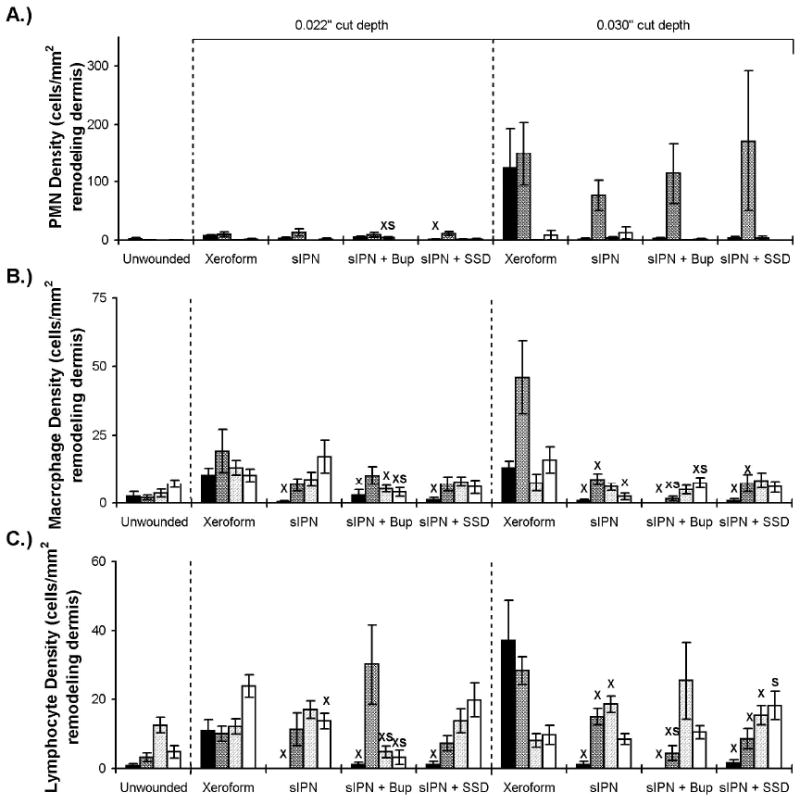
Dermal inflammation features of wounds dressed with Xeroform™, sIPN, sIPN with 0.5% bupivacaine (Bup), and sIPN with 1% silver sulfadiazine (SSD) examined histologically at 7 (■), 9 ( ), 14 (
), 14 ( ), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Polymorphonuclear leukocyte (PMN) density (including neutrophils, basophils, and eosinophils) (A), macrophage density(B), and lymphocyte density(C) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Polymorphonuclear leukocyte (PMN) density (including neutrophils, basophils, and eosinophils) (A), macrophage density(B), and lymphocyte density(C) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
Figure 8.
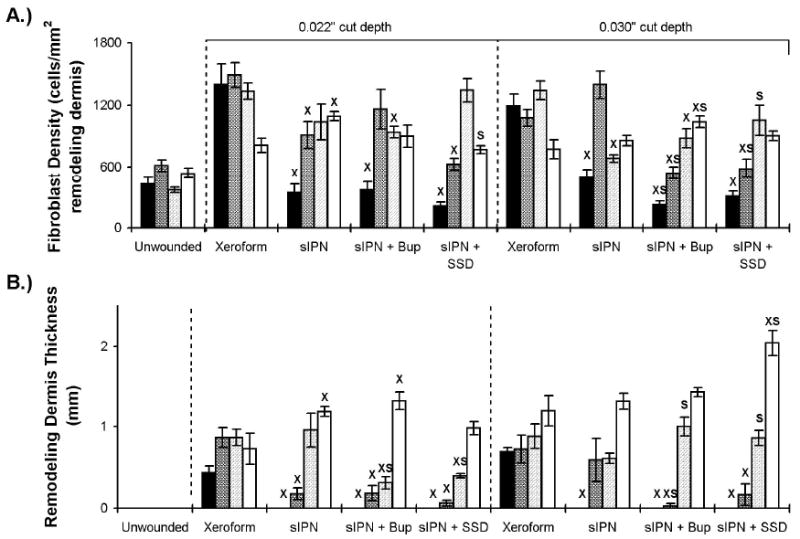
Dermal extracellular matrix remodeling features of wounds dressed with Xeroform™, sIPN, sIPN with 0.5% bupivacaine (Bup), and sIPN with 1% silver sulfadiazine (SSD) examined histologically at 7 (■), 9 ( ), 14 (
), 14 ( ), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Fibroblast density (A) and remodeling tissue thickness(B) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
), and 21 (□) days. The 0.016 in dermatome cut depth wounds are shown on the left, whereas 0.022 in wounds are on the right. Fibroblast density (A) and remodeling tissue thickness(B) are displayed. Data is reported as average of 10 total viewing regions from biopsies of two separate pigs ± standard error. “X” indicates significant difference from Xeroform™ treated tissue, and “S” indicates significant difference from sIPN without drug treatment (P<0.05).
Inflammation
Inflammation was examined as indicator of the relative material biocompatibility of each dressing type. Density of PMNs, macrophages, and lymphocytes were measured cells/mm2 within the remodeling dermis. (Figure 7) Within the first seven days after wounding and treatment, a thick mixture of fibrin clot, dead PMNs, and degraded extracellular matrix components were observed at the interface between all dressing treatments and the healthy underlying dermis. Cellular death was suggested by morphologic changes to the nuclei and cellular membrane structures consistent with apoptosis. This mixture at the dermis-clot interface sloughed off following epidermis formation. Viable inflammatory cells within the dermis indicate the early stages of remodeling and granulation. The greatest abundance of viable inflammatory cells within the dermis occurred subsequent to epidermis formation. In general, PMN density decreased with increasing treatment time for all test groups. PMN density showed a general trend with respect to time where densities peak at 10 days and decreased until 21 days (Figure 7A). There was a trend toward increased density of all inflammatory cell types in the viable dermis at the 0.030″ cut depth compared to the 0.022″ depth in all dressings. There remains statistically greater lymphocyte density at the 0.022″ cut depth compared to the 0.030″ depth. (Figure 7C) Recent literature shows this is not the only study counting cells by morphology based on hematoxylin and eosin (H&E) staining. Gross histology and cell counts are still valid and useful especially since this data was evaluated comparatively. Any significant differences in one cell type wound certainly justify a deeper look into other techniques. Morphological counting is no mean the best method, but is corroborated by other methods and is a bona fide stand alone evaluation method both clinically and in basic science research. Most histopathologists would agree melanocytes and neutrophils have unmistakable morphology with (H&E) stain. Leukocytes and neutrophils are currently counted using (H&E) and/or Giemsa stain11, 13-18. There are also others that continue to count lymphocytes11, macrophages16, and keratinocytes12. The keratinocyte and melanocyte normalization ratio has also been previously described12.
Dermal Extracellular Matrix Remodeling
The remodeling of the dermis was observed as a potential indicator of the magnitude of new extracellular matrix production and organization. Remodeling tissue thickness increased from seven days to a maximum 21 days in response to all dressing treatments (Figure 8B). A concomitant increase was observed in fibroblast density from seven days to a maximum at 7 or 14 days in response to all dressing treatments (Figure 8A). Remodeling tissue thickness and fibroblast density served as indicators of a permanent abnormality in the thickness, composition, and structure of the dermis that could form a hypertrophic scar19. This finding was shown in our previous work when the wounds were assessed at 42 days9.
Comparison of sIPN
Overall, there were no significant differences seen in epidermal healing, (Figure 6) inflammation, (Figure 7) or dermal extracellular matrix remodeling (Figure 8) when comparing the drug loaded sIPN to sIPN. There were no clinical differences seen during dressing removal or during tissue preparation other than what has been described above. We conclude that the addition of bupivacaine or silver sulfadiazine to sIPN does not affect healing of partial thickness wounds in our animal model compared to sIPN alone. We did not set out to identify whether the drugs would have their desired clinical or pharmacological effect.
Conclusion
There were overall no differences in the structures and cells associated with wound healing have been observed among treatment types. All treatments elicited healing without outstanding, untoward effects. We found that the different dressings elicited distinct histological features throughout the course of healing. Although the clinical outcome was comparable, the addition of silver sulfadiazine and bupivacaine did not dramatically alter these histological features. The addition of soluble bupivacaine and silver sulfadiazine to sIPN remains a feasible treatment option that will require further investigation to determine the drug effectiveness. As we expected, each of the treatments facilitated the removal of dead tissue from the dermis-clot interface and there were no clinical or histologic signs of infection.
Figure 3.

Representative histologic photomicrographs of wounded tissues 9 days after treatment. The 0.022 in dermatome cut depth wounds are shown on top, whereas 0.030 in wounds are on bottom. Tissue are aligned so that approximately the top 3rd of each photo contains dead or necrotic tissue such as stratum corneum, fibrin clot, dead cells, or degraded extracellular matrix. (H & E stain, objective magnification ×10).
Figure 4.
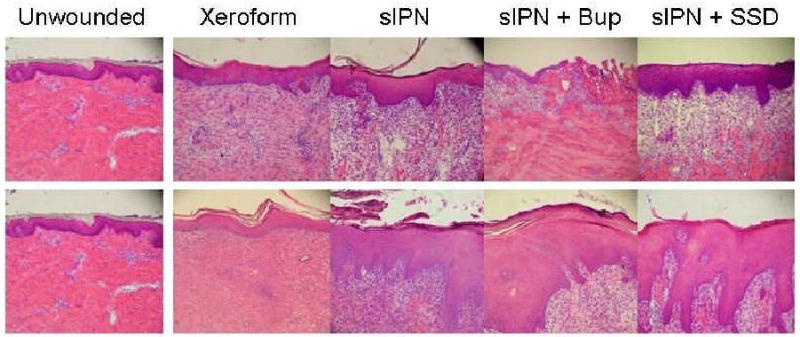
Representative histologic photomicrographs of wounded tissues 14 days after treatment. The 0.022 in dermatome cut depth wounds are shown on top, whereas 0.030 in wounds are on bottom. Tissue are aligned so that approximately the top 3rd of each photo contains dead or necrotic tissue such as stratum corneum, fibrin clot, dead cells, or degraded extracellular matrix. (H & E stain, objective magnification ×10).
Table 1.
Summary of small sampling of tissues processed for histology via cryosectioning (cryo) or formalin-fixed paraffin embedded (FFPE) sectioning. Values are shown as an average of five fields of view ± standard deviation. * indicates significant difference between cryosection and FFPE section (p < 0.05).
| Unwounded | 7d, 0.030″, sIPN + SSD | 9d, 0.022″, sIPN + SSD | 21d, 0.022″, sIPN | |||||
|---|---|---|---|---|---|---|---|---|
| Cell Type | Cryo | FFPE | Cryo | FFPE | Cryo | FFPE | Cryo | FFPE |
| Keratinocytes (cells/mm2 spinosum) |
4134.8 ± 427.4 | 3721.0 ± 470.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1729.6 ± 751.6 | 988.7 ± 220.7 |
| Melanocytes (cells/mm basale) |
9.5 ± 2.1 | *4.3 ± 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.7 0.9 |
0.0 |
| PMNs (cells/mm2 dermis) |
0.8 ± 1.8 | 2.4 ± 3.6 | 6.5 ± 10.5 | 33.9 ± 26.4 | 16.9 ± 7.8 | *40.3 ± 21.0 | 0.0 | *12.9 ± 10.0 |
| Macrophages (cells/mm2 dermis) |
7.3 ± 5.3 | 1.6 ± 3.6 | 1.6 ± 3.6 | 7.3 ± 6.6 | 12.9 ± 9.2 | 10.5 ± 2.2 | 23.4 ±23.6 | 8.1 ± 5.7 |
| Lymphocytes (cells/mm2 dermis) |
2.4 ± 3.6 | 4.8 ± 1.8 | 3.2 ± 3.4 | *11.3 ± 4.4 | 11.3 ± 7.8 | 19.4 ± 6.0 | 10.5 ±7.9 | 6.5 ± 6.1 |
| Fibroblasts (cells/mm2 dermis) |
425.0 ± 87.7 | *150.8 ± 48.6 | 442.7 ± 102.5 | *129.0 ± 79.4 | 716.9 ± 151.0 | *310.5 ± 60.0 | 1133.9 ±165.5 | *618.5 ± 40.6 |
Acknowledgments
Grant Information: Department of Surgery Research Grant, Surgical Science Foundation, MatriLab, and NIH R01EB6613
Bibliography
- 1.Brett DW. A review of moisture-control dressings in wound care. J Wound Ostomy Continence Nurs. 2006 Nov-Dec;33(6 Suppl):S3–8. doi: 10.1097/01.won.0000278581.53694.b6. [DOI] [PubMed] [Google Scholar]
- 2.Ayello EA. New evidence for an enduring wound-healing concept: moisture control. J Wound Ostomy Continence Nurs. 2006 Nov-Dec;33(6 Suppl):S1–2. doi: 10.1097/01.won.0000278580.46070.19. [DOI] [PubMed] [Google Scholar]
- 3.Waldeck H, Chung AS, Kao WJ. Interpenetrating polymer networks containing gelatin modified with PEGylated RGD and soluble KGF: synthesis, characterization, and application in in vivo critical dermal wound. J Biomed Mater Res A. 2007 Sep 15;82(4):861–871. doi: 10.1002/jbm.a.31054. [DOI] [PubMed] [Google Scholar]
- 4.Phillips JM, Kao WJ. Macrophage adhesion on gelatin-based interpenetrating networks grafted with PEGylated RGD. Tissue Eng. 2005 May-Jun;11(5-6):964–973. doi: 10.1089/ten.2005.11.964. [DOI] [PubMed] [Google Scholar]
- 5.Witte RP, Blake AJ, Palmer C, Kao WJ. Analysis of poly(ethylene glycol)-diacrylate macromer polymerization within a multicomponent semi-interpenetrating polymer network system. J Biomed Mater Res A. 2004 Dec 1;71(3):508–518. doi: 10.1002/jbm.a.30179. [DOI] [PubMed] [Google Scholar]
- 6.Einerson NJ, Stevens KR, Kao WJ. Synthesis and physicochemical analysis of gelatin-based hydrogels for drug carrier matrices. Biomaterials. 2003 Feb;24(3):509–523. doi: 10.1016/s0142-9612(02)00369-1. [DOI] [PubMed] [Google Scholar]
- 7.Stevens KR, Einerson NJ, Burmania JA, Kao WJ. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J Biomater Sci Polym Ed. 2002;13(12):1353–1366. doi: 10.1163/15685620260449741. [DOI] [PubMed] [Google Scholar]
- 8.Sawhney AS, Pathak CP, Hubbell JA. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials. 1993 Oct;14(13):1008–1016. doi: 10.1016/0142-9612(93)90194-7. [DOI] [PubMed] [Google Scholar]
- 9.Kleinbeck KR, Faucher L, Kao WJ. Multifunctional in situ photopolymerized semi-interpenetrating network system is an effective donor site dressing: a cross comparison study in a swine model. J Burn Care Res. 2009 Jan-Feb;30(1):37–45. doi: 10.1097/BCR.0b013e3181921f98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinbeck KR, Bader RA, Kao WJ. Concurrent in vitro release of silver sulfadiazine and bupivacaine from semi-interpenetrating networks for wound management. J Burn Care Res. 2009 Jan-Feb;30(1):98–104. doi: 10.1097/BCR.0b013e3181921ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahadeva S, Wyatt JI, Howdle PD. Is a raised intraepithelial lymphocyte count with normal duodenal villous architecture clinically relevant? J Clin Pathol. 2002 Jun;55(6):424–428. doi: 10.1136/jcp.55.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson JA, Grabowski R, Mu XC, Del Rosario A, Malfetano J, Slominski A. Possible mechanisms of hypopigmentation in lichen sclerosus. Am J Dermatopathol. 2002 Apr;24(2):97–107. doi: 10.1097/00000372-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Carmack S, Taddei T, Robert ME, Mistry P, Jain D. Increased T-cell sinusoidal lymphocytosis in liver biopsies in patients with chronic hepatitis C and mixed cryoglobulinemia. Am J Gastroenterol. 2008 Mar;103(3):705–711. doi: 10.1111/j.1572-0241.2007.01603.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng W, Zheng C, Tian J, Shi G. T helper cell population and eosinophilia in nasal polyps. J Investig Allergol Clin Immunol. 2007;17(5):297–301. [PubMed] [Google Scholar]
- 15.Diebold Y, Chen LL, Tepavcevic V, Ferdman D, Hodges RR, Dartt DA. Lymphocytic infiltration and goblet cell marker alteration in the conjunctiva of the MRL/MpJ-Fas(lpr) mouse model of Sjogren's syndrome. Exp Eye Res. 2007 Mar;84(3):500–512. doi: 10.1016/j.exer.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Park KJ, Oh YT, Kil WJ, Park W, Kang SH, Chun M. Bronchoalveolar lavage findings of radiation induced lung damage in rats. J Radiat Res (Tokyo) 2009 May;50(3):177–182. doi: 10.1269/jrr.08089. [DOI] [PubMed] [Google Scholar]
- 17.Verma MJ, Mukaida N, Vollmer-Conna U, Matsushima K, Lloyd A, Wakefield D. Endotoxin-induced uveitis is partially inhibited by anti-IL-8 antibody treatment. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2465–2470. [PubMed] [Google Scholar]
- 18.Wang CR, Chen SY, Wu CL, et al. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005 Apr;52(4):1319–1324. doi: 10.1002/art.20991. [DOI] [PubMed] [Google Scholar]
- 19.Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 2006 Sep;32(6):669–677. doi: 10.1016/j.burns.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


