Abstract
Small molecule BCL-2 inhibitors are being examined as monotherapy in phase I/II clinical trials for several types of tumors. However, few data are available about the effect of BCL-2 inhibitors on immune function. The aims of this study were to investigate the effect of a small molecule BCL-2 inhibitor on immune function and determine the most effective way of combining this inhibitor with a recombinant vaccine to treat tumors. The in vitro effect of the pan-BCL-2 inhibitor GX15-070 was assessed in mouse CD8 T lymphocytes at two different stages of activation as well as regulatory T lymphocytes (Treg). The in vivo effect of GX15-070 after recombinant vaccinia/fowlpox CEA-TRICOM vaccination was analyzed in tumor-infiltrating lymphocytes, and in splenocytes of mice bearing subcutaneous tumors.
The therapeutic efficacy of such sequential therapy was measured as a reduction of pulmonary tumor nodules. Activated mature CD8 T lymphocytes were more resistant to GX15-070 as compared to early-activated cells. Treg function was significantly decreased after treatment with the BCL-2 inhibitor. In vivo, GX15-070 was given after vaccination so as to not negatively impact the induction of vaccine-mediated immunity, resulting in increased intratumoral activated CD8:Treg ratio, and significant reduction of pulmonary tumor nodules.
This study is the first to show the effect of a small molecule BCL-2 inhibitor on the immune system and following a vaccine. It is also the first to demonstrate the efficacy of this sequence in reducing tumors in mouse models, providing a rationale for the design of combinational clinical studies.
Keywords: vaccine, tumor immunotherapy, GX15-070, small molecule inhibitor, lung cancer
Introduction
The current goal of vaccine therapy is to generate antigen-specific immunity to cancer cells to enhance immune-mediated destruction of tumors. Preclinical and clinical studies have shown that cancer vaccines carrying tumor-associated antigens (TAAs) can induce and enhance immune response against those antigens 1, 2. The use of therapeutic cancer vaccines in combination with other cancer therapies offers a new paradigm that may yet lead to vaccines being used to treat several types of cancer 3. There has also been increasing use of targeted small molecule inhibitors in the treatment of many tumor types 2, 4–10.
Approaches that alter the balance between pro-survival and pro-death BCL-2 family members have shown potential benefit in preclinical cancer models 11, 12. Nonetheless, few data are available about the effect of small molecule BCL-2 inhibitors on immune function. We hypothesize that modulating the sensitivity of effector cells to the small molecule pan-BCL-2 inhibitor GX15-070 could change the balance between cancer cells and immune-effector cells, resulting in greater antitumor immune activity. GX15-070 is a synthetic derivative of bacterial prodiginines belonging to the polypirrole class of molecules 13. It is a BH3-only mimetic molecule classified as a pan-BCL-2 inhibitor because of its ability to bind all antiapoptotic BCL-2 family members, including BCL-2, BCLxL, BCL-w, MCL-1, and BAK 14. GX15-070 has been shown to induce apoptosis in hematologic and solid tumor cells in vitro and in vivo 15–19 and is being investigated in clinical trials 20–22.
With this study we investigated the effect of GX15-070 on both tumor and immune-effector cells, and then rationally designed a vaccine combination therapy regimen. The vaccine platform used was a recombinant poxviral vaccinia (rV) prime and one fowlpox (rF) boost with each vector containing transgenes for the carcinoembryonic antigen (CEA) and a triad of T-cell costimulatory molecules (B7-1, ICAM-1, and LFA-3; designated CEA/TRICOM) 1, 23. Here we show that GX15-070 toxicity on lymphocytes is dependent on their activation status, indicating that it would be beneficial to administer GX15-070 after vaccination. Furthermore, the BCL-2 small molecule inhibitor significantly decreased the function of Treg lymphocytes. Sequential therapy using a recombinant poxviral vaccinia (rV) prime and one fowlpox (rF) boost with each vector containing transgenes CEA/TRICOM 1, 23, followed by GX15-070, was shown to be effective in reducing orthotopic pulmonary tumors in immunocompetent mice, suggesting a rationale for the design of such combinational protocols for clinical studies.
Materials and Methods
Drug preparation
GX15-070 (obatoclax; Gemin X Pharmaceuticals, Malvern, PA) was dissolved in dimethyl sulfoxide (DMSO). For in vitro experiments, GX15-070 was dissolved in appropriate medium at concentrations of 0.1, 0.25, 0.5, and 1 µM. For in vivo experiments, GX15-070 was dissolved in PBS and used at 2 mg/kg based on a previous report in which 4 mg/kg of inhibitor were injected for 10 days over a 15-day period into nude mice in a plasmacytoma xenograft model 18.
Animals
Eight- to 12-week-old female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). A breeding pair of CEA-transgenic (CEA-Tg) mice homozygous for expression of human CEA was generously provided by Dr. John Shively (Beckman Research Institute, City of Hope National Medical Center, Duarte, CA) and used as a self-antigen model 24, 25. F5 mice (Taconic Farms, Hudson, NY) are transgenic for a T-cell receptor direct against the NP68 peptide, an epitope of nucleoprotein of influenza virus A/NT/60/68 (366ASNENMDAM374); NP68 flu peptide is presented by H-2Db 26, 27. Mice were housed and maintained in microisolator cages under specific pathogen-free conditions in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All experimental studies were approved by the National Cancer Institute’s Intramural Animal Care and Use Committee.
Tumor cell lines
LL/2 murine lung adenocarcinoma tumor cells were the gift of Dr. Chandan Guha (Albert Einstein College of Medicine, New York, NY). LL/2 tumor cells expressing human carcinoembryonic antigen (LL2-CEA) were generated by retroviral transduction with CEA cDNA, as previously described 28. Cells were maintained in complete medium (DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin).
CD8 T lymphocytes
Splenocytes were collected from TCR-Tg F5 mice. Cells were cultured for three days in complete CTL medium (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin) enriched with 10−4 µg/mL of F5 TCR 366ASNENMDAM374 ligand peptide 68 (NP68) (American Peptide Company Inc., Sunnyvale, CA). After three days, lymphocytes were used for the in vitro GX15-070 sensitivity assay as early-activated CD8 T cells. Early activation was defined as CD8a+/CD44+/CD69+ immunophenotype by flow cytometry. To obtain activated mature CD8 T lymphocytes, after early activation, viable lymphocytes were sorted by gradient centrifugation and cultured for an additional 7 days in complete CTL medium enriched with 140 ng/mL IL-15 (PeproTec, Rocky Hill, NJ). Mature activation was defined as CD8a+/CD44+/CD69− immunophenotype by flow cytometry.
In vitro sensitivity to GX15-070
GX15-070 was used in concentrations of 1, 0.5, 0.25, and 0.1 µM, with DMSO 0.0005% as a control. For the 24-h incubation, cells were cultured with different concentrations of GX15-070 before analysis. For the 72-h incubation, cells were cultured with different concentrations of GX15-070 for 24 h; GX15-070 was then eluted and cells were maintained in culture in complete medium for an additional 48 h before analysis. All experiments were performed at least three times.
Flow cytometric analysis and surface marker assays
Four-color cytometric analyses were done by a FACSCalibur cytometer (BD Biosciences, San Diego, CA) using 488 nm argon ion and 635 nm red diode lasers. For CD66 CEA staining, the anti-CEA monoclonal antibody (mAb) COL-1 (IgG2a) 29 and the negative-control murine myeloma mAb UPC-10 (IgG2a) (Cappel, Organon Teknika Corp., West Chester, PA) were used as primary antibodies. As a secondary antibody, fluorescein-labeled affinity purified antibody to mouse IgG (H+L) was used (KPL, Gaithersburg, MD). H-2Db MHC GIQNSVSA (CEA) and H-D2b MHC SQVTNPANI (HIV) tetramer peptides were purchased from Beckman Coulter Inc., Fullerton, CA. All other mAbs were purchased from BD Biosciences.
FoxP3 flow cytometric intracellular staining
Intracellular staining for APC-conjugated FoxP3 (eBioscience, San Diego, CA) was performed according to the manufacturer’s protocol.
Annexin V assays
Phosphatidylserine exposure was quantified by flow cytometry by surface Annexin V staining, according to the manufacturer’s protocol (BD Biosciences).
BrdU incorporation assay
For in vitro experiments, 8 h before the end of incubation, cells were labeled by adding 10 µM of BrdU to cultures. BrdU levels were detected with the BrdU Flow-Kit (BD Biosciences) according to the manufacturer’s instructions.
Flow cytometric analysis of cell-cycle phases
7AAD staining was performed to distinguish cell-cycle phases by BrdU incorporation and DNA content, according to the manufacturer’s instructions (BD Biosciences).
Flow cytometric analysis of cell-cycle
107/mL cells were labeled with 1 µM carboxyfluorescein-succinimidyl-ester (CFSE; Sigma-Aldrich, St. Louis, MO), incubated for 10 min in the dark at 37°C, then washed twice in PBS. 0.5×106 cells were extracted, fixed in 1% paraformaldehyde, and kept at 4°C to be used as the initial generation and as maximum level of fluorescence for FACS instrument setting. Cell cycle number was defined by proliferation of untreated LL2-CEA tumor cells over time.
Immunoprecipitation (IP) and immunoblotting (IB)
Cells were washed with 1X PBS and protein extracts were prepared by resuspending the cells in ice-cold IP buffer (50 mM Tris-HCl [pH 7.5], 250 mM NaCl, 1% NP-40, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF, 25 mM NaF, 1 mM orthovanadate, and protease inhibitors [p8340 SIGMA]) for 30 min. Insoluble debris was removed by centrifugation at 4°C for 10 min at 13,000 rpm. 500 µg of total protein were incubated with 1.5 µg of antibody overnight at 4°C with rotation, and then incubated with Protein G-sepharose beads (Invitrogen, Carlsbad, CA) for 1.5 h at 4°C with rotation. Beads were washed three times with IP buffer containing protein inhibitors, and IP proteins were eluted from the beads with 20 µL of 4X SDS-PAGE sample buffers at 95°C for 5 min. Proteins were separated on precast 4% to 20% SDS-PAGE gels (Invitrogen), and IB was performed on polyvinylidene fluoride membranes incubated overnight with primary antibody. Signals were detected using the corresponding secondary HRP-conjugated antibody, purchased from Amersham (GE Healthcare Bio-Sciences, Piscataway, NJ) and enhanced chemiluminescence purchased from Pierce (Thermo Fisher, Rockford, IL). Antibodies used for IB were rabbit anti-BAK from Upstate (Millipore, Billerica, MA), and rabbit anti-MCL-1 from Santa Cruz (Santa Cruz, CA). All densitometries were based on the amount of immunoprecipitated MCL-1 as a control.
Treg functional assay
Spleens of three TCR-Tg F5 mice and either three untreated C57BL/6 mice (control group) or three 2 mg/kg GX15-070-treated C57BL/6 mice (GX group) were removed and pressed through a 70-µm filter. CD8 T lymphocytes of TCR-Tg F5 mice were used as target cells. CD4+/CD25+ cells from both C57BL/6 groups were isolated using the CD4+ CD25+ Regulatory T Cell Isolation Kit from Miltenyi Biotec, according to the manufacturer’s instructions. CD4+/CD25+/FoxP3+ phenotype of Tregs was confirmed by flow cytometry. Proliferation assays were set up in 96-well plates in sextuplicate, with each well containing 3×104 CD8+ lymphocytes, or 3×104 CD8+ lymphocytes plus 3×104 Treg cells from untreated mice, or 3×104 CD8+ lymphocytes plus 3×104 Treg cells from GX-treated mice. CD8:Treg ratios examined were 1:1 and 1:2. NP68 peptide was added to each well in a final concentration of 10−4 µg/mL. In addition, a final concentration of 10−5 µg/mL of NP68 peptide was also studied. One µCi[3H]thymidine was added to each well after 48 h, and the plate was harvested and read 24 h later. Control wells containing CD8+ lymphocytes alone or Tregs alone were used to determine background levels of proliferation in culture. CD8+ cells and Tregs were incubated with NP68 plus concanavalin A as a positive control for proliferation. Lack of proliferation of selected CD4+CD25+ cells in control wells further confirmed their identification as Tregs.
Poxvirus constructs
Recombinant vaccinia (rV) and recombinant fowlpox (rF) viruses containing murine B7-1, ICAM-1, and LFA-3 transgenes in combination with human CEA transgene (rV/F-CEA-TRICOM) have been previously described 23. rF containing the gene for murine GM-CSF has also been described 30.
Subcutaneous LL2-CEA tumor model
5×105 LL2-CEA tumor cells were implanted subcutaneously in the right flank of C57BL/6 mice. On day 18 after tumor inoculation, tumors had grown from 0.5 to 0.8 cm3 and mice were divided into four groups with three mice/group: (a) untreated mice; (b) mice vaccinated on day 18 with 1×108 plaque-forming units (PFUs) rV-NP34-TRICOM plus 1×107 PFUs rF-GM-CSF; (c) mice vaccinated as in (b) and injected on day 23 with a single i.v. dose of 0.5 mg/kg GX15-070; (d) mice injected on day 23 with 2 mg/kg GX15-070. On day 24, mice were sacrificed and tumors were collected for flow cytometric analysis of tumor-infiltrating lymphocytes (TILs).
Orthotopic lung cancer treatment studies
CEA-Tg mice, where CEA is a self-antigen, were administered 3×105 LL2-CEA tumor cells i.v. Mice were then divided into four groups: (a) untreated mice; (b) mice given an i.v. dose of 2 mg/kg GX15-070 on days 19 and 23; (c) mice vaccinated on day 5 with 108 PFUs rV-CEA-TRICOM admixed with 107 PFUs rF-GM-CSF by s.c. injection, and boosted on day 12 with 108 PFUs rF-CEA-TRICOM admixed with 107 PFUs rF-GM-CSF; and (d) mice vaccinated as in (c), then given an i.v. dose of 2 mg/kg GX15-070 on days 19 and 23. Treatment response was assessed by counting the number of pulmonary tumor nodules.
Statistical analysis
Where not specified, results of tests of significance are reported as P values, derived from a 2-tailed Mann-Whitney test. All P values were calculated at 95% using GraphPad Prism 5® statistical software (GraphPad Software, La Jolla, CA).
Results
GX15-070 is cytostatic for LL2-CEA tumor cells
To investigate the in vitro antitumor effect of GX15-070 against LL2-CEA tumor cells, increasing concentrations of inhibitor were added to cultures. Viable cells were counted by Trypan Blue exclusion. As depicted in Fig. 1A, at 24 h, 1 µM GX15-070 induced a 41% reduction of viable LL2-CEA cells; at 72 h, there was a 64% decrease in viable cells with 1 µM GX15-070, and a 38% decrease with 0.5 µM GX15-070. The number of viable cells did not significantly decrease with concentrations of inhibitor lower than 0.5 µM. GX15-070 is known to inhibit the antiapoptotic machinery. After 24 h, phosphatidylserine externalization did not increase compared to DMSO until the concentration of GX15-070 reached 0.5 µM, rising with 1 µM of inhibitor (Fig. 1B). No appreciable increase in apoptosis was observed at 72 h. LL2-CEA tumor cells do not express BCL-2 but overexpress MCL-1, another member of the BCL-2 family (data not shown). We assessed the MCL-1/BAK binding as an indicator of GX15-070 effect on intrinsic apoptosis machinery. MCL-1/BAK immunoprecipitation at 6 h showed a 68% release of BAK from MCL-1 when cells were cultured with 1 µM GX15-070 (Fig. 1B), which was in agreement with the 24-h Annexin V increase. To assess the effect of GX15-070 on proliferation, cell division was investigated (Fig. 1C). CFSE flow cytometric assays revealed that GX-treatment resulted in fewer cells in more advanced generations. At 24 h, LL2-CEA control cells (DMSO) were in second generation, while after treatment with 1 µM GX15-070 they were in first generation. Furthermore, at 72 h, control cells were in fourth generation while GX-treated cells were divided between the second and third generation. We then studied the cell cycle phases at 72 h of incubation with 1 µM GX15-070. As shown by BrdU/7AAD staining (Fig. 1D), GX15-070 blocked cell cycle in S-G2 phase, as demonstrated by the decrease of cells in G1 and G2-M phases and the increase of cells in S-G2 phase. Collectively, these data demonstrate that, at the concentrations used, the in vitro GX15-070-dependent cytoreduction of LL2-CEA cells was caused by antiproliferative activity at low concentrations while at higher doses by both antiproliferative and proapoptotic effects.
Fig. 1. GX15-070 is cytostatic at low concentrations and cytotoxic at higher concentrations on LL2-CEA lung carcinoma cells.
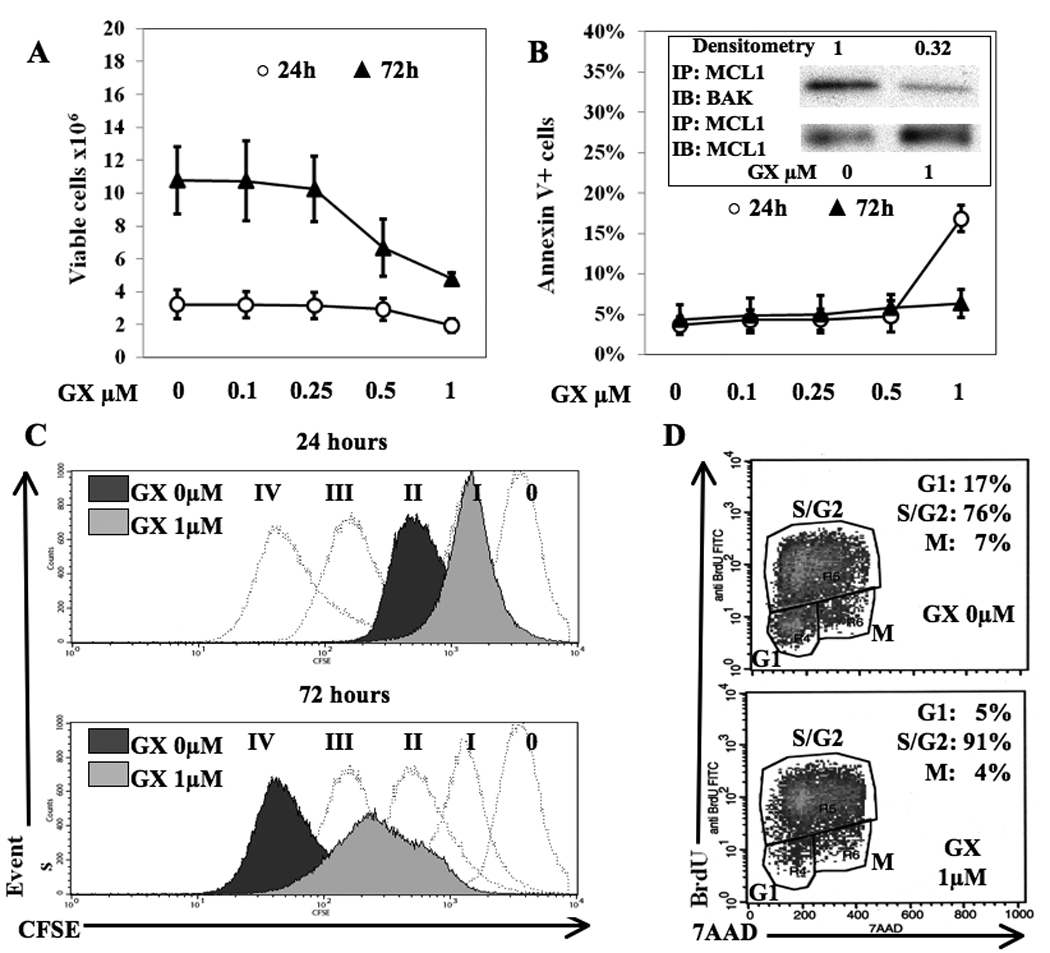
A, number of viable cells by Trypan Blue exclusion; B, apoptosis by Annexin V+ cells. Bars represent standard deviations of four independent experiments. INSERT, reduced MCL-1 binding to BAK after 6 h of treatment with GX15-070. MCL-1/BAK band intensity was calculated by densitometry compared to MCL-1/MCL-1 control and normalized with respect to DMSO control (GX 0 µM). Bands are representative of three independent experiments. C, cell division analysis. Dashed histograms: subsequent generations as defined by proliferation of untreated tumor cells over time; dark grey shaded histograms: DMSO control cells (GX 0 µM); light grey shaded histograms: cells treated with 1 µM GX15-070. Roman numerals indicate generations. Histograms are representative of three independent experiments. D, cell cycle analysis at 72 h of LL2-CEA treated with DMSO control (top panel) or 1 µM GX15-070 (bottom panel). Dot plots are representative of three independent experiments.
Mature CD8 T lymphocytes are more resistant to GX15-070 than early-activated CD8 T lymphocytes
We next investigated the in vitro effect of GX15-070 on murine CD8 T lymphocytes at two different activation stages: early-activated and mature. Compared to DMSO control, the number of early-activated lymphocytes treated with GX15-070 decreased in a dose-dependent manner at either 24 or 72 h (Fig. 2A), while mature lymphocytes were reduced to a lesser degree (Fig. 2C). Similarly, the proapoptotic effect of GX15-070 was distinct between the two stages of activated CD8 T lymphocytes, being higher in early-activated (Fig. 2B) than in mature cells (Fig. 2D). Further confirming the differences in sensitivity to GX15-070 between differently activated T cells was the immunoprecipitation from early-activated lymphocytes which at 6 h showed 67% release of BAK from MCL-1 with 1 µM of GX15-070 (Fig. 2B), while in mature lymphocytes it was 24% (Fig. 2D). Mature CD8 T lymphocytes were also more resistant to the antiproliferative effect of GX15-070 compared to early-activated cells. In the latter, treatment with 1 µM GX15-070 diminished BrdU incorporation compared to DMSO control by 29% at 72 h; in mature cells it decreased by 0.5%. These data, taken together, indicate that the sensitivity of activated CD8 T lymphocytes to GX15-070 depends on activation status, being higher in early-activated cells than in mature cells.
Fig. 2. Mature CD8 are more resistant to GX15-070 than early-activated lymphocytes.
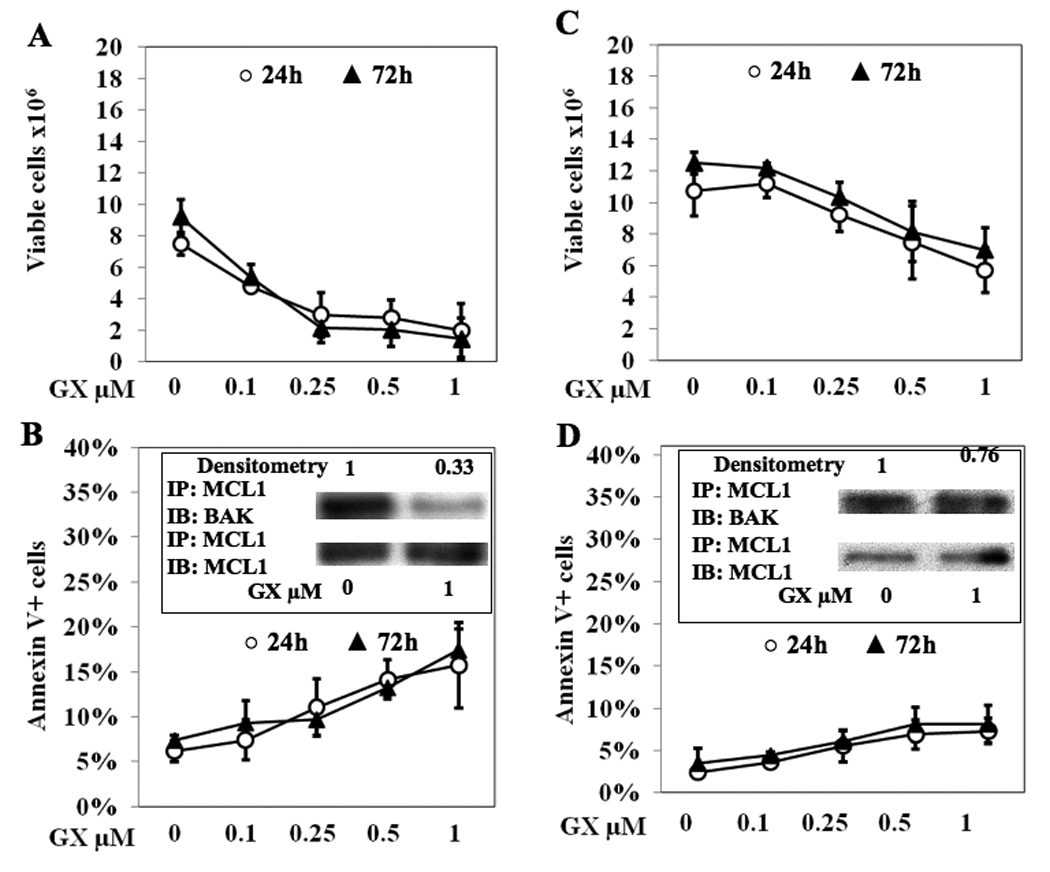
Two types of CD8 T cells were used: early-activated, defined as CD8+/CD44/ CD69+ (A–B) or mature, defined as CD8+/CD44+/CD69− (C–D). A and C, viable cell numbers as determined by Trypan Blue exclusion. B and D, apoptosis as determined by Annexin V+ cells. Bars represent standard deviations of three independent experiments. INSERTS, MCL-1 binding to BAK after 6 h of treatment with GX15-070. MCL-1/BAK band intensity was calculated by densitometry compared to MCL-1/MCL-1 control and normalized with respect to DMSO-treated cells (GX 0 µM). Bands are representative of three independent experiments.
GX15-070 inhibits Treg function
The administration of GX15-070 did not affect the overall number of Tregs (CD4+/CD25+/FoxP3+) in the spleen; there were 1.7% ± 0.17% SD in untreated versus 1.6% +/− 0.05% SD in GX-treated mice. In order to address whether GX15-070 affects Treg function, CD4+/CD25+/FoxP3+ cells from untreated and GX-treated mice were purified and used in an in vitro functional Treg assay. The proliferation of CD8+ cells in the presence of Tregs from untreated mice was significantly lower than in the presence of Tregs from GX-treated mice (Fig. 3), demonstrating that GX15-070 inhibits the suppressor function of Tregs. Similar results were obtained stimulating lymphocytes with two concentrations of NP68 peptide (10−4 or 10−5 µg/mL), as well as using two different CD8:Treg ratios (1:1 or 1:2). The Treg suppression was not accompanied by inhibition of proliferation, as BrdU incorporation was 0.76% ± 0.18% SD in untreated and 0.75% ± 0.11% SD in GX-treated mice.
Fig. 3. GX15-070 inhibits Treg function.
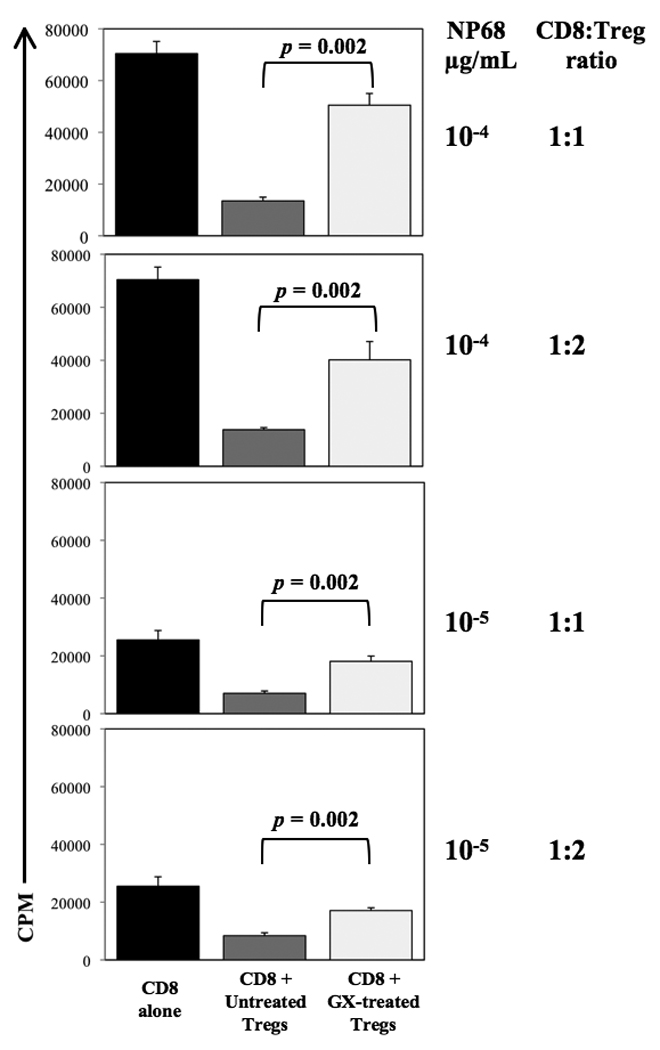
CD8 T lymphocytes from TCR-Tg F5 mice were stimulated with NP68 peptide in the absence or presence of Treg cells. Tregs were purified from spleens of untreated or GX15-070-treated C57BL/6 mice. Proliferation of CD8 cells was measured by [3H]thymidine incorporation. CD4+/CD25+/FoxP3+ phenotype of Tregs was confirmed by flow cytometry.
GX15-070 treatment following vaccination does not affect the ratio of intratumoral activated CD8 T:Treg lymphocytes
To analyze the effect of GX15-070 on intratumoral lymphocytes, we studied tumor-infiltrating lymphocytes in unvaccinated and vaccinated mice bearing advanced subcutaneous LL2-CEA tumors. As shown in Table 1, CD8 naïve lymphocytes decreased after GX15-070 treatment, while activated CD8 cells decreased to a lesser extent. This is consistent with the in vitro data described above (Fig. 2). Interestingly, although in mice receiving GX15-070 alone Treg lymphocytes were reduced 50% as compared to control mice, the activated CD8:Treg ratio did not change significantly because of the simultaneous decrease in activated CD8 T lymphocytes. Instead, the ratio between activated CD8 T lymphocytes and Tregs increased 3-fold after vaccination compared to control (untreated) mice as a consequence of the concomitant increase of activated CD8 T lymphocytes and decrease of Treg lymphocytes. Such a ratio did not change in vaccinated mice that received GX15-070. Taken together, these data indicate that (a) activated CD8 T lymphocytes are more resistant to GX15-070 than naïve cells and (b) after vaccination GX15-070 was not detrimental to activated CD8 T lymphocytes or to the activated CD8 T:Treg ratio.
Table 1.
Effect of GX15-070 on Tumor-Infiltrating Lymphocytes (TILS)
| Activated CD8 | CD8 naïve | Treg | Activated CD8 : Treg ratio |
|||
|---|---|---|---|---|---|---|
| Control | 15.4% | Control = 1X* |
1.3% | Control = 1X* |
2.0% | 7.7 |
| GX | 9.2% | 0.6 X | 0.3% | 0.2 X | 0.9% | 10.2 |
| Vaccine | 31.8% | 2.1 X | 9.9% | 7.6 X | 1.4% | 22.7 |
| Vaccine + GX | 25.2% | 1.6 X | 4.6% | 3.5 X | 1.2% | 21.0 |
C57BL/6 mice (n = 3/group) bearing s.c. LL2-CEA tumors were vaccinated on day 18 after tumor implant. GX15-070 was given on day 23. On day 24 mice were sacrificed and TILs were evaluated. TIL-gate was drawn in the lymphocytoid region. Activated CD8: CD8+/CD44+ cells. CD8 naïve: CD8+/CD44− cells. Treg: CD4+/CD25+/FoxP3+ cells. X*: treatment:control ratio.
Intratumoral CD8+/CD44int lymphocytes increased after vaccination are resistant to GX15-070
In order to investigate whether GX15-070 could affect antigen-reactive proliferation of CD8 lymphocytes, the differential intensity of expression of CD44 was analyzed on CD8+ tumor-infiltrating lymphocytes. As shown in Fig. 4, before vaccination the majority of intratumoral activated CD8 lymphocytes were CD44hi while after vaccination they were CD44int, indicating that vaccination triggered expansion of clones expressing only intermediate intensity of CD44. The administration of GX15-070 after vaccination did not alter the intensity of CD44 expression (Fig. 4A). Moreover, the increase of CD8+/CD44int lymphocytes was statistically significant in both groups that received vaccine when compared with either GX-treated or untreated non-vaccinated mice (Fig. 4B). The ratio between these and Treg was also significantly increased (Fig. 4D). BrdU incorporation studies showed that such an increase on CD8+/CD44int lymphocytes was accompanied by an accelerated proliferation rate. These data indicate that the subpopulation of CD8 lymphocytes that proliferated after vaccination was not affected by GX15-070.
Fig. 4. Intratumoral CD8+/CD44int lymphocytes increased after vaccination are resistant to GX15-070.
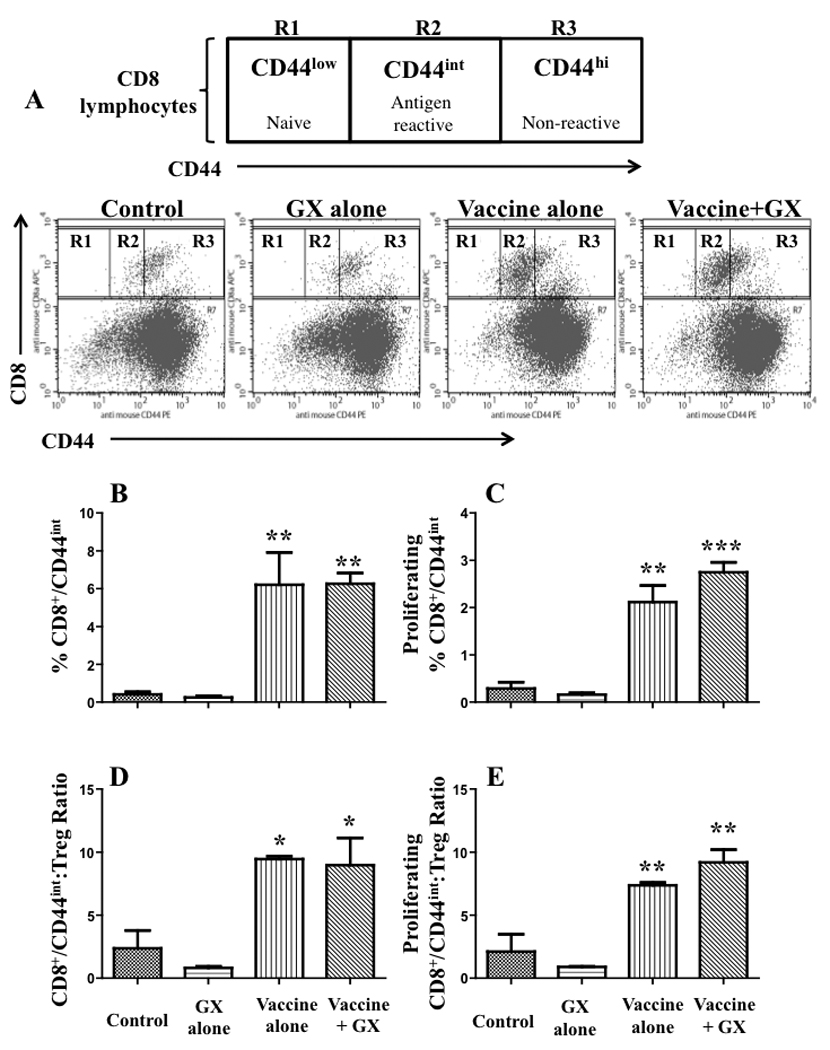
C57BL/6 mice (n = 3/group) bearing s.c. LL2-CEA tumors were vaccinated on day 18 after tumor implant. GX15-070 was given on day 23. On day 24 mice were sacrificed and TILs were evaluated. Statistical analysis based on one-way ANOVA test vs. control; *: P<0.05; **: P<0.005; ***: P<0.0005.
Sequential therapy with rV/F-CEA-TRICOM and GX15-070 significantly reduces pulmonary tumor nodules
The demonstration that lymphocytes were more resistant in vitro to GX15-070 after activation, along with the observation that the BCL-2 inhibitor did not affect in vivo CD8 T lymphocytes when preceded by vaccination, suggested that temporally separated vaccine and GX15-070 could be beneficial. We thus investigated the effect of sequential therapy in CEA-Tg mice bearing LL2-CEA tumors with rV/F-CEA-TRICOM vaccine followed by GX15-070. Comparison of differences between variances of tumor nodules showed a significant reduction between sequential therapy vs. control and vs. GX15-070 alone treatment (Fig. 5). Moreover, the mean number of tumors on sequential therapy was almost half of vaccine alone group (Table 2). Overall, there were no differences noted in the sizes of individual pulmonary metastases regardless of treatment.
Fig. 5. Antitumor activity of vaccine followed by GX15-070.
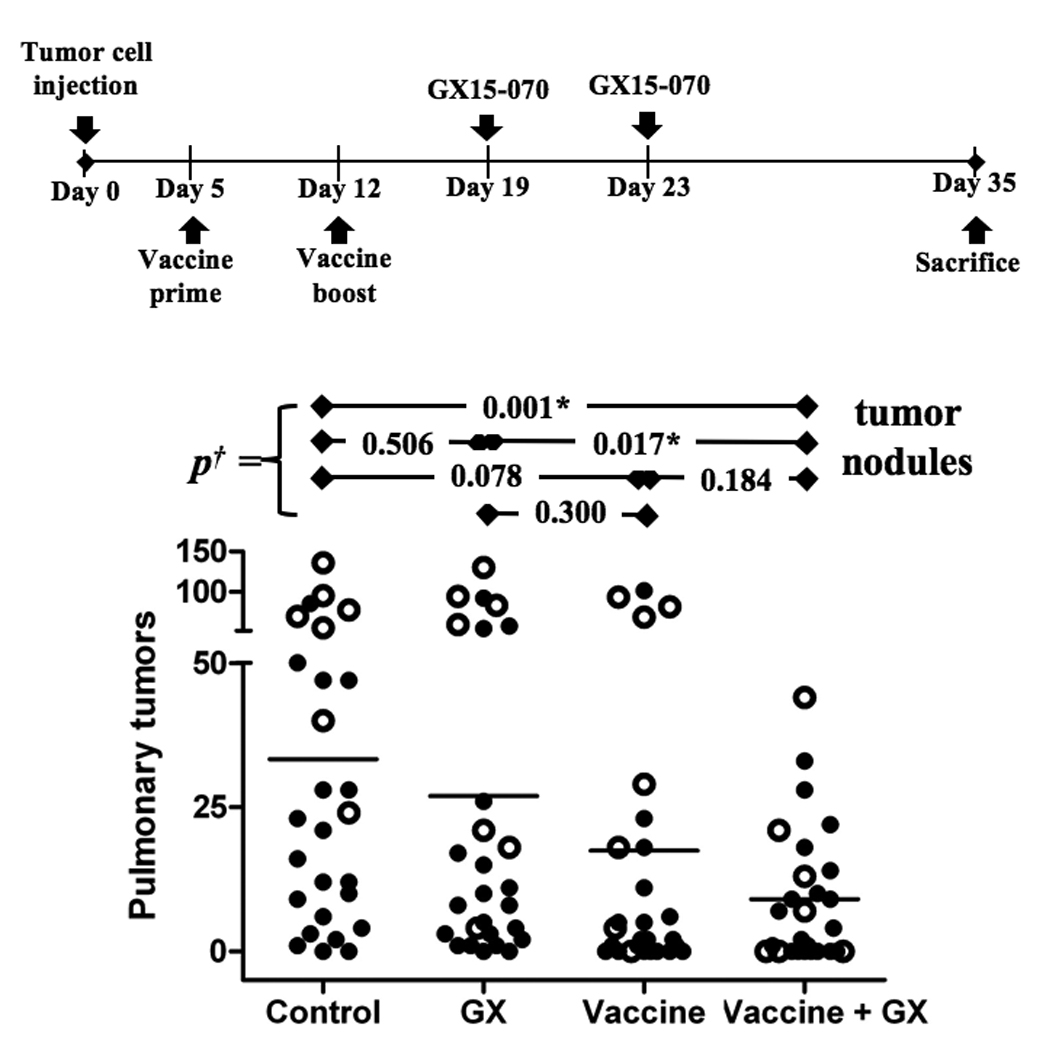
CEA-Tg mice, where CEA is a self-antigen, were administered 3×105 LL2-CEA tumor cells i.v. Mice were then divided into four groups: (a) untreated mice; (b) mice given an i.v. dose of 2 mg/kg GX15-070 on days 19 and 23; (c) mice vaccinated on day 5 with rV-CEA-TRICOM and boosted on day 12 with rF-CEA-TRICOM; (d) mice vaccinated as in (c), then given an i.v. dose of 2 mg/kg GX15-070 on days 19 and 23. Data from two independent experiments are represented, the first (open circles) with 7 mice per group and the second (closed circles) with 20 mice per group. †: Analysis of difference of variances of number of pulmonary tumors by two-tailed unpaired t-test. *: Statistical significance.
Table 2.
Sequential treatment of GX15-070 followed by vaccine decreases pulmonary tumors.
| Treatment | Tumor-free mice (%) |
Pulmonary tumors mean ±SE |
|---|---|---|
| Control | 2/27 (7%) | 33 ±7 |
| GX | 2/27 (7%) | 27 ±7 |
| Vaccine | 8/27 (30%) | 17 ±6 |
| Vaccine + GX | 10/27 (37%) | 9 ±2 |
CEA-Tg mice (n = 27/group) bearing LL2-CEA pulmonary tumors were vaccinated on days 4 (prime) and 12 (boost) after tumor implant, and then treated with GX15-070 on days 19 and 24. On day 35 mice were sacrificed and lungs inflated with India-ink for tumor nodules enumeration.
Discussion
Targeted therapies that alter the balance between pro-survival and pro-death BCL-2 family members have shown potential benefit against cancer in preclinical models 12, 14–18, 31–34. The pan-BCL-2 inhibitor GX15-070, granted orphan drug status by the Food and Drug Administration for treatment of chronic lymphocytic leukemia, is one of the best candidates for targeted tumor therapy because it overcomes the MCL-1-mediated resistance, differentiating it from other BCL-2 antagonists such as ABT-737 and to the proteasome inhibitor bortezomib 14. BCL-2 pathway is broadly present in all eukaryotic cells as a modulator of survival. While in cancer cells aberrant BCL-2 activity can make them resistant to apoptotic signals, in immune-system BCL-2 molecules are indispensable for activation and maturation of T lymphocytes after antigen presentation 35.
In this study we evaluated the effect of combining GX15-070 and the TAA-specific vaccine rV/F-CEA-TRICOM in orthotopic pulmonary tumor models. The GX15-070-mediated reduction of viable tumor cells in vitro was caused mainly by antiproliferative activity and partially by a proapoptotic effect (Figs. 1–2). In particular, the cytostatic effects were noted at lower concentrations. Our findings confirm those reported by Konopleva et al., who described S-G2 block at low concentrations of GX15-070 on OCI-AML3 cells 33. Wang and co-authors 36 reported that the BCL-2 small molecule inhibitor TW-37 blocked cell growth of pancreatic cancer cells in S phase involving NOTCH-1 signaling pathway, confirming that the function of BCL-2 in cancer biology is beyond its classic role in cell survival.
We found that GX15-070 did not decrease vaccine-mediated immunity when administered after vaccination (Table 1). This is important because of the particular MCL-1/BAK binding inhibition exerted by GX15-070 that could affect the development and maintenance of T lymphocytes 35. Wang et al. recently reported a genomic/transcriptional analysis of apoptosis during activation of human T lymphocytes showing upregulation of the MCL-1 gene within 10 h after TCR ligation, indicating that MCL-1 is involved in early T cell activation 37. Our data corroborate and extend these findings. That study did not look at the effect of GX15-070 on T lymphocytes in different states of activation. Our in vitro results showed that CD8 T lymphocyte sensitivity to GX15-070 toxicity is dependent on their activation status, early-activated being more sensitive than activated mature lymphocytes (Fig. 2). Similarly, in vivo, intratumoral naïve CD8 cells decreased markedly more than activated lymphocytes after treatment with GX15-070 (Table 1), suggesting that vaccinating mice before GX-treatment could make T lymphocytes resistant to the inhibitor. It has been shown that CD8+/CD44int lymphocytes undergo a high proliferative response with IFN-γ production after antigen stimulation while CD8+/CD44hi do not 38. We found that the rate of CD8 proliferation after vaccination was restricted to cells expressing intermediate intensity of CD44, while CD44hi lymphocytes did not expand (Fig. 4) and that there was no effect of GX15-070 on this subpopulation after vaccination, confirming that vaccine-stimulated effector lymphocytes are resistant to GX15-070. It is important to note that intratumoral Tregs did not expand after vaccination, indicating that a differential response of immune-effector cells and Treg lymphocytes can be achieved by such a combination.
We concluded that if vaccination precedes GX15-070 treatment by an interval sufficient to overcome early activation, T lymphocytes would not be negatively affected by the inhibitor.
We also found that GX15-070 decreases Treg function. As shown in Fig. 3, the proliferation of CD8 T lymphocytes was significantly higher in co-culture with Tregs obtained from GX-treated mice than with those from untreated animals, indicating that GX15-070 inhibits Treg function. The inhibition of Treg suppression was not accompanied by inhibition of their proliferation, as BrdU incorporation was similar with or without GX15-070 treatment. This suggests that GX15-070 can mediate an increase in the immune anti-tumor activity by decreasing Treg-dependent immune suppression. This effect, along with the increased intratumoral activated CD8: Tregs ratio in mice that were first vaccinated and then treated with the inhibitor, suggests that a favorable milieu for immune activity against cancer cells can be achieved by such combination.
We found that LL2-CEA cells treated with GX15-070 were not more susceptible to CEA-specific CTL killing at 6, 24, or 72 h compared to cells cultured with DMSO (data not shown). Moreover, after treatment of LL2-CEA cells in vitro with GX15-070, supernatant concentrations of TNF, IFN-γ, TGF-β, MCP-1, IL-2, IL-4, IL-5, IL-6, IL-10, and IL12-p70, as well as surface expression of MHC-I, CEA, and FAS, did not change significantly at either 24 or 72 h (data not shown). This concurs with the study reported by Begley et al., which showed that the BCL-2-inhibitor ABT-737 increased the antitumor activity of specific peptide-pulsed DC vaccination on CT26 colon carcinoma in vivo though it failed to sensitize tumor cells to CTL-specific killing in vitro 39, suggesting that GX15-070 treatment does not make tumor cells intrinsically more sensitive to immune-mediated killing. In that study, vaccine was given both prior to and after tumor implantation. In the studies reported here, the vaccine was administered after tumor implantation.
An orthotopic pulmonary tumor model, where CEA is part of the self-antigen repertoire, showed that sequential administration of rV/F-CEA-TRICOM followed by GX15-070 significantly reduced the number of pulmonary tumor nodules, compared to untreated mice or mice receiving inhibitor or the vaccine alone (Fig. 5). Moreover, the mean number of tumors on sequential therapy was almost half of vaccine alone group (Table 2). There, sequential therapy did not decrease splenocytes’ IFN-γ production and CD8+ cell percentage (not shown). We hypothesize that the sequential therapy is effective by the suppression of function of Tregs coupled with a lack of toxicity to activated CD8 T cells when the small molecule inhibitor is administered after vaccination. The direct antitumor effect of GX15-070 increasing tumor cell apoptosis and slowing tumor proliferation may also have a role in the activity of sequential therapy. Future studies will examine this sequential therapy on overall survival for tumor bearing mice depleted of selected T-cell subsets.
It is important to emphasize that, when employing such a combination, the appropriate interval between administration of each agent is important. Vaccine-induced immunity may be reduced when the BCL-2 small molecule inhibitor is administered during or shortly after vaccine, since during initial activation lymphocytes are extremely sensitive to GX15-070. Thus it is important that vaccine be administered long enough before GX15-070 for lymphocytes to efficiently activate antigen-specific T cells. Given on an appropriate schedule, sequential therapy with a vaccine followed by GX15-070 appears to achieve therapeutic effects. Clinically, GX15-070 is usually administered once every three weeks. It is conceivable that patients could be vaccinated shortly after diagnosis, receive the GX15-070 two to three weeks later, and receive booster vaccines at intervals between GX15-070 infusions.
To our knowledge, this is the first study to demonstrate the feasibility and efficacy of combining a vaccine and the small molecule BCL-2 inhibitor GX15-070 in the treatment of established tumors. We believe these results constitute a rationale for potential clinical trials employing sequential use of vaccine with BCL-2 inhibitors.
Acknowledgements
Grant Support: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH.
The authors acknowledge the excellent editorial assistance of Bonnie L. Casey and Debra Weingarten in the preparation of this manuscript.
References
- 1.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–1849. [PubMed] [Google Scholar]
- 2.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, Quick D, Franzusoff A, Greiner JW, Schlom J, Hodge JW. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14:4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25 Suppl 2:B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, Bastian A, Marte J, Tsang KY, Beetham P, Grosenbach DW, Schlom J, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 6.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo-Saito C, Schlom J, Camphausen K, Coleman CN, Hodge JW. The requirement of multimodal therapy (vaccine, local tumor radiation, and reduction of suppressor cells) to eliminate established tumors. Clin Cancer Res. 2005;11:4533–4544. doi: 10.1158/1078-0432.CCR-04-2237. [DOI] [PubMed] [Google Scholar]
- 8.Tsang KY, Palena C, Yokokawa J, Arlen PM, Gulley JL, Mazzara GP, Gritz L, Yafal AG, Ogueta S, Greenhalgh P, Manson K, Panicali D, et al. Analyses of recombinant vaccinia and fowlpox vaccine vectors expressing transgenes for two human tumor antigens and three human costimulatory molecules. Clin Cancer Res. 2005;11:1597–1607. doi: 10.1158/1078-0432.CCR-04-1609. [DOI] [PubMed] [Google Scholar]
- 9.Theoret MR, Arlen PM, Pazdur M, Dahut WL, Schlom J, Gulley JL. Phase I trial of an enhanced prostate-specific antigen-based vaccine and anti-CTLA-4 antibody in patients with metastatic androgen-independent prostate cancer. Clin Genitourin Cancer. 2007;5:347–350. doi: 10.3816/CGC.2007.n.017. [DOI] [PubMed] [Google Scholar]
- 10.Hodge J, Schlom J, Abrams S. Vaccines and immunostimulants. In: Kufe D, editor. Holland-Frei Cancer Medicine. 7th ed. Hamilton, ONT: BC Decker; 2006. pp. 786–801. [Google Scholar]
- 11.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haura EB, Cress WD, Chellappan S, Zheng Z, Bepler G. Antiapoptotic signaling pathways in non-small-cell lung cancer: biology and therapeutic strategies. Clin Lung Cancer. 2004;6:113–122. doi: 10.3816/CLC.2004.n.025. [DOI] [PubMed] [Google Scholar]
- 13.Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007;2:605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Belec L, Billot X, Acoca S, Purisima E, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–159. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mott JL, Bronk SF, Mesa RA, Kaufmann SH, Gores GJ. BH3-only protein mimetic obatoclax sensitizes cholangiocarcinoma cells to Apo2L/TRAIL-induced apoptosis. Mol Cancer Ther. 2008;7:2339–2347. doi: 10.1158/1535-7163.MCT-08-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, Shore GC, Campo E, Colomer D. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008 doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- 20.Haura E, Williams C, Chiappori A, Adams J, Northfelt D, Malik S, Van Echo D. A phase I trial of the small molecule pan-bcl-2 inhibitor obatoclax (GX15-070) in combination with docetaxel in patients with relapsed non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2008;26:19035. [Google Scholar]
- 21.Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, Yee K, Ravandi F, Giles F, Schuh A, Gupta V, Andreeff M, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, Viallet J, Cheson BD. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 24.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1469–1477. [PubMed] [Google Scholar]
- 25.Schmitz J, Reali E, Hodge JW, Patel A, Davis G, Schlom J, Greiner JW. Identification of an interferon-gamma-inducible carcinoembryonic antigen (CEA) CD8(+) T-cell epitope, which mediates tumor killing in CEA transgenic mice. Cancer Res. 2002;62:5058–5064. [PubMed] [Google Scholar]
- 26.Townsend AR, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 27.Mamalaki C, Elliott J, Norton T, Yannoutsos N, Townsend AR, Chandler P, Simpson E, Kioussis D. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev Immunol. 1993;3:159–174. doi: 10.1155/1993/98015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991;51:3657–3662. [PubMed] [Google Scholar]
- 29.Muraro R, Wunderlich D, Thor A, Lundy J, Noguchi P, Cunningham R, Schlom J. Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res. 1985;45:5769–5780. [PubMed] [Google Scholar]
- 30.Kass E, Parker J, Schlom J, Greiner JW. Comparative studies of the effects of recombinant GM-CSF and GM-CSF administered via a poxvirus to enhance the concentration of antigen- presenting cells in regional lymph nodes. Cytokine. 2000;12:960–971. doi: 10.1006/cyto.2000.0684. [DOI] [PubMed] [Google Scholar]
- 31.Lin TS. Novel agents in chronic lymphocytic leukemia: efficacy and tolerability of new therapies. Clin Lymphoma Myeloma. 2008;8 Suppl 4:S137–S143. doi: 10.3816/CLM.2008.s.009. [DOI] [PubMed] [Google Scholar]
- 32.Campas C, Cosialls AM, Barragan M, Iglesias-Serret D, Santidrian AF, Coll-Mulet L, de Frias M, Domingo A, Pons G, Gil J. Bcl-2 inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Exp Hematol. 2006;34:1663–1669. doi: 10.1016/j.exphem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, Bornmann W, Kantarjian H, Viallet J, Samudio I, Andreeff M. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez-Galan P, Roue G, Lopez-Guerra M, Nguyen M, Villamor N, Montserrat E, Shore GC, Campo E, Colomer D. BCL-2 phosphorylation modulates sensitivity to the BH3 mimetic GX15-070 (Obatoclax) and reduces its synergistic interaction with bortezomib in chronic lymphocytic leukemia cells. Leukemia. 2008;22:1712–1720. doi: 10.1038/leu.2008.175. [DOI] [PubMed] [Google Scholar]
- 35.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang S, Sarkar FH, Mohammad RM. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and induces apoptosis in pancreatic cancer: involvement of Notch-1 signaling pathway. Cancer Res. 2009;69:2757–2765. doi: 10.1158/0008-5472.CAN-08-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Windgassen D, Papoutsakis ET. A global transcriptional view of apoptosis in human T-cell activation. BMC Med Genomics. 2008;1:53. doi: 10.1186/1755-8794-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pihlgren M, Arpin C, Walzer T, Tomkowiak M, Thomas A, Marvel J, Dubois PM. Memory CD44(int) CD8 T cells show increased proliferative responses and IFN-gamma production following antigenic challenge in vitro. Int Immunol. 1999;11:699–706. doi: 10.1093/intimm/11.5.699. [DOI] [PubMed] [Google Scholar]
- 39.Begley J, Vo DD, Morris LF, Bruhn KW, Prins RM, Mok S, Koya RC, Garban HJ, Comin-Anduix B, Craft N, Ribas A. Immunosensitization with a Bcl-2 small molecule inhibitor. Cancer Immunol Immunother. 2009;58:699–708. doi: 10.1007/s00262-008-0592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


