Abstract
Importance of the field
Asenapine is a new atypical antipsychotic medication with high affinity for D2 and 5HT2A receptors that has been approved by the FDA in adults for the acute treatment of schizophrenia in the United States. The purpose of this review is to describe the compound and examine whether it addresses some of the unmet clinical needs in treating schizophrenia.
Areas covered in this review
The development of asenapine is described with attention to its chemistry, pharmacodynamic and pharmacokinetic profile. Pre-clinical and clinical trials of safety and efficacy are reviewed. The advantages and disadvantages of asenapine relative to other antipsychotic medications are discussed.
What the reader will gain
Asenapine will be evaluated for whether it: a) causes a reduction in symptoms of schizophrenia; b) has a side-effect profile minimizing extrapyramidal symptoms, weight gain, and cardiac effects; and c) affects negative and/or cognitive symptoms.
Take home message
Asenapine is a recently approved agent with an acceptable cardiometabolic profile that exhibits similar efficacy as other antipsychotic medications, primarily on positive symptoms of schizophrenia. Relatively less weight gain compared to other agents may confer a notable advantage. Sublingual administration may have positive and negative effects on patient compliance. Potential “pro-cognitive” effects of asenapine are preliminary and require further investigation.
Keywords: antipsychotic, asenapine, bipolar disorder, dopamine, SAPHRIS, schizophrenia, serotonin
1. INTRODUCTION
Schizophrenia is a brain disease which affects approximately 1% of the population and is characterized by psychotic symptoms such as hallucinations and delusions, disorganized thought and behavior, and impairments in cognitive functions such as attention, learning, memory, and executive functioning. It presents a serious international health problem as it is associated with significant disability in social, occupational, and day-to-day functioning that can oftentimes be permanent and, in some cases, progressive. Suicide is prevalent in individuals with schizophrenia; anywhere from 9 to 13% sufferers eventually take their own life [1].
The serendipitous discovery of the antipsychotic properties of the phenothiazine chlorpromazine in the 1950’s initiated a revolution in the treatment of schizophrenia and psychotic conditions. Other compounds with dopamine D2 receptor antagonist properties soon followed, but these so-called “typical” antipsychotic medicines had high incidences of side effects, notably motor and extrapyramidal symptoms (EPS) as well as enduring and serious conditions such as tardive dyskinesia. Clozapine was the first of the wave of second-generation, “atypical” antipsychotic medications with a far lesser incidence of unwanted motor side effects. The primary disadvantage of clozapine, and reason for its infrequent use, is a risk of drug-induced agranulocytosis. The 1990’s saw the introduction of olanzapine, risperidone, and quetiapine, followed by ziprasidone, aripiprazole, and paliperidone among others. These medications have a lower propensity for causing EPS, however some of these agents cause weight gain, hyperglycemia, hyperlipidemia, and other metabolic problems which are not trivial and have been shown to shorten life expectancy in individuals treated with these compounds [2]. Furthermore, several antipsychotics, typical and atypical, have been associated with at least mild QTc prolongation [3]. Therefore one as-yet unmet clinical need in the treatment of schizophrenia is an effective agent which minimizes the motor and cardiac as well as the serious metabolic adverse events that characterize the side effect profiles of the existing medications.
Existing typical and atypical antipsychotic medications are relatively equally effective in treating what are known as the positive symptoms of schizophrenia, as evidenced by the interpretation of the findings of the government-funded Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study [4]. Thus, current treatment guidelines do not favor one class of antipsychotic over the other, rather suggest that “the choice of an antipsychotic medication and its dose, and subsequent decisions about changes in treatment, require careful initial consideration and ongoing, shared decision making between the patient and clinician” [5] (p. 934). What has been prominently lacking, however, is an agent that also treats the negative symptoms as well as the substantial cognitive impairment of schizophrenia. An effective antipsychotic medication with these “pro-cognitive” properties has as of yet remained largely elusive. This is particularly troubling given the strong correlation between cognitive performance in patients and functional outcome [6,7].
2. OVERVIEW OF THE MARKET
In addition to the atypical compounds mentioned above, the typical antipsychotic medication haloperidol remains a popular drug of choice for treating psychosis, especially acute episodes of psychosis and agitation as haloperidol can be administered emergently in intramuscular (IM) and even intravenous (IV) form with a rapid onset of effectiveness. The Food and Drug Administration does, however, warn that use of haloperidol, particularly off-label IV use, can result in serious cardiac events and sudden death. Risperidone had the unique advantage of being the first atypical agent on the market with a long-acting depot formulation. Paliperidone, which is the active metabolite of risperidone, is now available in an extended-release IM formulation. Aripiprazole, ziprasidone, and risepridone are also available in an IM formulation. Olanzapine, another atypical antipsychotic, is available in an oral formulation as well as sublingual and IM, and its depot formulation (olanzapine pamoate) is now also available. Depot formulations of fluphenazine and haloperidol have also been widely used. The remaining antipsychotic medications currently on the market are primarily administered in oral (non-dissolving) form.
Several compounds are in development as atypical antipsychotics. These include the major metabolite of clozapine, N-desmethylclozapine (norclozapine). Norclozapine has a similar but distinct receptor pharmacological profile to clozapine [8,9], with its muscarinic agonist properties providing hope that it may exhibit pro-cognitive efficacy. Clinical studies to date have been disappointing however, and further studies may be limited. Another metabolite of an already approved antipsychotic is paliperidone palmitate (9-hydroxy-risperidone). Paliperidone is a major plasma metabolite of risperidone and an ER IM formulation has been approved for acute treatment of schizophrenia. Consistent with risperidone, it has limited effects at muscarinic receptors thus may have limited cognitive deleterious effects [10]. In clinical trials paliperidone improved positive and negative symptoms compared to placebo, with higher completion rates in paliperidone groups. These findings require peer-reviewed publication however. Iloperidone has recently been approved by the FDA for the treatment of schizophrenia [11]. Consistent with atypical antipsychotics, iloperidone exhibits high affinity for 5-HT2 and D2 receptors [12]. Iloperidone exhibits antipsychotic efficacy; furthermore some preclinical evidence suggests that it may ameliorate negative symptoms (see review by [13]). Bifeprunox, similarly to aripiprazole, is a partial D2 receptor agonist while also exhibiting little efficacy at 5-HT2A, 5-HT2C, or noradrenergic receptors [14]. Dopamine partial agonists may prove to be a new class of antipsychotics [15], and a recent double-blind study suggested bifeprunox may be efficacious at treating symptoms in patients with schizophrenia [16].
3. INTRODUCTION TO ASENAPINE
Early preclinical studies suggested that asenapine (Box 1) may prove to be a novel antipsychotic with therapeutic potential for psychosis and a limited low propensity to induce EPS [17–19]. Moreover, preclinical evidence suggested that asenapine may not be cognitively deleterious at lower doses (<0.1 mg/kg), with sedation affecting performance at higher doses, while comparator atypical antipsychotics may result in bradyphrenic-like effects [20].
Box 1
Drug Summary
| Drug Name | Asenapine |
|---|---|
| Phase | FDA approved |
| Indication | Acute treatment of schizophrenia in adults Acute treatment of manic or mixed episodes associated with bipolar I disorder in adults |
| Pharmacological description/ Mechanism of Action |
5-Hydroxytryptamine 2A antagonist 5-Hydroxytryptamine 2C antagonist 5-Hydroxytryptamine 7 antagonist D2 antagonist |
| Route of administration | Sublingual |
| Chemical structure | 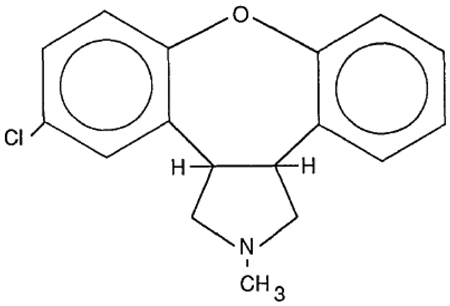 |
| Pivotal Trial(s) | Trial 041004: In this randomized, double-blind, Phase II trial in 174 schizophrenia patients (ITT population) over 6 weeks, asenapine 5 mg twice a day produced significant improvement on the primary endpoint of PANSS total scores, as well as on secondary endpoints of PANSS positive and negative symptom scores and CGI scores. Trial 041023: In this randomized, double-blind Phase III trial in 448 schizophrenia patients (ITT population) over 6 weeks, asenapine 5 mg twice a day produced significant improvement on the primary endpoint of PANSS total scores. There was a statistically significant difference between placebo and asenapine 10 mg twice a day in PANSS total scores using MMRM analysis, but not using ANCOVA with LOCF. Trial 041021: In this randomized, double-blind Phase III trial in 386 schizophrenia patients (ITT population), asenapine 5 mg or 10 mg twice a day or olanzapine 15 mg daily failed to result in significant decreases in PANSS total scores, but asenapine 5 mg decreased PANSS positive symptom scores. Olanzapine significantly reduced PANSS positive symptoms. |
ITT: intent-to-treat; PANSS: Positive and Negative Syndrome Scale; CGI: Clinical Global Impression; ANCOVA: Analysis of Covariance; LOCF: last observation carried forward; MMRM: mixed model for repeated measures
3.1 CHEMISTRY
Asenapine (trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenz[2,3:6,7]oxepino[4,5-c]pyrrolidine) maleate (Org 5222) was developed by altering the structure of mianserin by Organon laboratories. The molecular formula of asenapine maleate is C17H16CINO.C4H4O4 with a molecular weight of 401.84. Asenapine is quite stable in crystalline form although excessive light can induce degradation [21]. Clinical studies have used fast-dissolving (10 s) highly porous asenapine tablets (5 and 10 mg, with 1–4 mg tablets used during initial titration periods).
3.2 PHARMACODYNAMICS
3.2.1 In vitro pharmacology
Consistent with other atypical antipsychotics asenapine exhibits a higher binding affinity for the 5HT2A receptor compared to D2 receptors. Moreover, asenapine exhibits a broad range of effects on other neurotransmitter systems (Table 1) including 5-HT2C, 5-HT7, 5-HT2B, 5-HT6, α2B, D3, H1, D4, α1A, α2A, α2C, D2L, D1, D2S, 5-HT1A, 5-HT1B, and H2 receptors [22]–[23]. One major difference between asenapine and most other atypical antipsychotics (except risperidone, ziprasidone, and aripiprazole) is that it exhibits little muscarinic receptor antagonist effects [23–26], which may produce a less cognitively deleterious profile [27]. Given that D2 receptor occupancy has been deemed as vital for antipsychotic efficacy [28], it is important to note that 5 mg tablets result in ~75% D2 receptor occupancy, while occupancy was at 85% with 10 mg tablets [29].
Table 1.
Equilibrium dissociation constants for various atypical antipsychotics including asenapine (Asen, aka Org 5222), clozapine (Cloz), olanzapine (Olanz), risperidone (Risp), and sertindole (Sert). Efficacy at dopamine D2 receptors is shaded given that clinical efficacy of antipsychotics require ~70% D2 occupancy. Muscarinic effects are boxed given that efficacy at muscarinic receptors have been linked to weight gain/cognitive dysfunction side effects. The typical antipsychotic haloperidol (Halo) is added for comparative purposes.
| Equilibrium dissociation constants for antipsychotics at human brain receptors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Asen | 5HT2C 0.27 |
5HT2A 0.77 |
α1 1.1 |
D2 2 |
H1 9.3 |
5HT1D 10.2 |
5HT1A 15 |
α2 16 |
M 7000 |
| Cloz | 5HT2A 2.59 |
H1 3.1 |
5HT2C 4.8 |
α1 6.8 |
M 9 |
α2 15 |
5HT1D 130 |
5HT1A 160 |
D2 210 |
| Olanz | H1 0.087 |
5HT2A 1.48 |
5HT2C 4.1 |
D2 20 |
M 36 |
α1 44 |
5HT1D 150 |
α2 280 |
5HT1A 610 |
| Risp | 5HT2A 0.15 |
α1 2.7 |
D2 3.77 |
5HT1D 3.9 |
H1 5.2 |
α2 8 |
5HT2C 32 |
5HT1A 190 |
M 34000 |
| Sert | 5HT2A 0.14 |
D2 2.7 |
α1 3.9 |
5HT2C 6 |
5HT1D 20 |
α2 190 |
H1 320 |
5HT1A 1050 |
M 5000 |
| Halo | D2 2.6 |
α1 17 |
5HT1D 40 |
5HT2A 61 |
H1 260 |
α2 600 |
5HT1A 1800 |
5HT2C 4700 |
M >10000 |
3.2.2 In vivo pharmacology
Initial studies demonstrated that intra-accumbel administration of asenapine could block the hyperactive effects of intra-accumbal dopamine in rats [18]. Thus asenapine reversed the dopaminergic-induced hyperactivity model of dopaminergic disruption in schizophrenia consistent with other antipsychotics [30–33]. The effects of asenapine alone were not presented however [18], thus it was unclear whether the asenapine reversal of dopamine-induced hyperactivity was simply due to asenapine-induced reduction in activity alone. Recently it was demonstrated that systemic administration of asenapine does reduce spontaneous activity alone – consistent with other antipsychotics [20]. Asenapine also reversed apomorphine-induced disruption of prepulse inhibition (PPI; [20]), and alters conditioned avoidance response, two paradigms which have been used as an animal models for antipsychotic activity [19,31]. This model has been given prominent validity for antipsychotic efficacy where antipsychotic-induced reversal of deficits correlated strongly with clinical potency [28,34].
While the evidence that asenapine acts as an atypical antipsychotic grows, there has been an increased drive toward developing pro-cognitive therapeutics to treat schizophrenia [35–39]. The functional outcome of patients with schizophrenia correlates with neurocognitive indices, thus the NIH and the Food and Drug Administration has funded and agreed upon a test-battery by which a drug can be approved as pro-cognitive [36]. A preliminary study with asenapine in patients with schizophrenia suggested that it may exert some pro-cognitive efficacy [40]. These data were only presented at a meeting however and data published to date in large clinical trials do not report cognitive effects of asenapine on patients with schizophrenia. Likewise in animal models of phencyclidine (PCP)-induced impairment in cognition, asenapine normalized cognitive performance [41–43], but these data have only been presented in abstract form and have yet to be published. To date, numerous studies have reported that antipsychotics, both typical and atypical, can reverse PCP- or other pharmacological-induced disruption of cognitive performance in rodents (see [39] for a review). It is generally accepted however, that these antipsychotics are insufficient to treat disrupted cognition in schizophrenia [4] and so the reliability of these models have been questioned. The cognitive effects of asenapine in normal rats in a short-term memory and sustained attention task have been presented however, and compared with olanzapine and risperidone [20]. These data suggest that asenapine may not be directly cognitively deleterious where effects on short-term memory and attention were only observed due to sedation. Thus pro-cognitive evidence for asenapine presented to date remains far from convincing.
3.3 PHARMACOKINETICS AND METABOLISM
Sublingual administration of asenapine results in a rapid absorption with peak plasma concentrations within 0.5–1.5 hours and moderate (35%) bioavailability. This is in the lower to mid range of other antipsychotics which exhibit 20–70% bioavailability at appropriate doses (see [44]). Oral dosing of asenapine results in low bioavailability (<2%) due to first pass metabolism in the gut and the liver. The primary metabolic pathways of asenapine are direct glucuronidation by glucuronidyl transferases and oxidative metabolism by cytochrome P450 isoenzymes. Thus coadministration of asenapine with known inhibitors, inducers or substrates of these metabolic pathways, including the CYP1A2 inhibitor fluvoxamine, can alter the metabolism of asenapine. Such interactions are not uncommon among atypical antipsychotics however [44,45]. Importantly given the high rate of smoking among schizophrenia patients [46], concomitant smoking during administration does not alter the pharmacokinetics of asenapine [47]. The reduced bioavailability via oral consumption means however, that eating or drinking within 10 minutes can alter the bioabilability of asenapine.
3.4 CLINICAL EFFICACY
Not all of the clinical trials testing asenapine for the treatment of schizophrenia have published (see [48] for a review) but their results are summarized in a recent FDA briefing document which concluded that asenapine twice daily showed efficacy in the acute treatment of schizophrenia in adults [29]. The seminal published study on short-term efficacy of asenapine was a pivotal Phase II trial comparing a 5 mg twice a day dose of asenapine to 3 mg twice daily risperidone and placebo in 174 schizophrenia patients (intent-to-treat or ITT population) over 6 weeks [49]. Asenapine was superior to placebo in reducing Positive and Negative Syndrome Scale (PANSS) total scores as well as scores on both the positive and negative subscales of this measure, whereas in this study risperidone was superior to placebo in only reducing positive symptoms and not negative symptoms (but see [49–52] for studies that have found risperidone to reduce negative symptoms).
A pivotal Phase III trial included 448 (ITT population) subjects at 43 sites, randomly assigned to placebo, asenapine 5 mg twice a day, asenapine 10 mg twice a day, or haloperidol 4 mg twice a day [53]. As above, change in PANSS total scores was the primary index of efficacy. This trial found definitive evidence for the efficacy of the asenapine 5 mg twice daily dose as well as haloperidol using the prespecified primary efficacy analysis, an Analysis of Covariance (ANCOVA) with Last Observation Carried Forward (LOCF), as well as a prespecified secondary efficacy analysis, a mixed model for repeated measures (MMRM) statistical approach. The efficacy of the asenapine 10 mg twice daily dose was supported using the MMRM approach but not the ANCOVA, however, this higher dose was shown to be superior to placebo in reducing PANSS positive symptom scores. In contrast, an analysis of six placebo-controlled clinical trials of asenapine concluded that the 5 and 10 mg twice a day doses showed similar efficacy on reducing total PANSS scores [54].
Another Phase III trial (Trial 041021) on 386 subjects (ITT population) randomly assigned to placebo, one of the two asenapine dosing regimens, or olanzapine 15 mg daily failed to find significant decreases in PANSS total scores when comparing asenapine and placebo at the study endpoint, but asenapine at the 5 mg dose decreased positive symptom scores from the PANSS. Treatment with olanzapine significantly reduced PANSS total scores as well as PANSS positive symptoms. Finally, in a fourth short-term trial (Trial 041022), neither asenapine nor olanzapine significantly separated from placebo in PANSS total score change after 6 weeks of treatment.
Theoretically, asenapine has promise as a pro-cognitive agent, given that it has a high affinity for 5HT2A antagonism [23], which has been suggested as a mechanism for decreasing negative symptoms and ameliorating cognitive deficits [55]. As described above, a 6-week study compared asenapine (5 mg twice daily) and risperidone (3 mg daily) to placebo on their impact on cognitive functions, which was a secondary endpoint measure, in acutely ill schizophrenia patients [40] and suggested that asenapine did improve processing speed, verbal learning, and memory compared to placebo. The authors report in this poster that the effect sizes for cognitive function improvement were greater with asenapine versus placebo than with risperidone versus placebo.
Post marketing surveillance of a drug is conducted by the FDA as not all possible side-effects can be anticipated during its review process. Any adverse events occurring are reported and catalogued so the product label can be updated. To date no post-marketing research has been conducted on asenapine though plans are in place.
3.5 SAFETY AND TOLERABILITY
As with many antipsychotic agents, the prescribing information for asenapine includes a box warning about increased mortality in elderly patients with dementia-related psychosis [47]. QT interval does appear to be mildly increased with asenapine compared to placebo [47];[29] prompting a warning against use in patients who are taking other drugs that increase QT interval or patients at risk for QT prolongation. However, an exposure-response analysis on 148 schizophrenia patients (treated population) measured with repeated electrocardiograms (ECG) over 16 days of asenapine treatment showed that QTc prolongation in asenapine was less than 5 milliseconds as compared to 7–8 milliseconds with quetiapine, calling into question whether there is indeed a relevant clinical effect on QT interval with asenapine [56].
The majority of efficacy studies suggest that asenapine is generally well-tolerated. Somnolence, usually transient, akathisia, and oral hypoesthesia are among the most common side effects, with an occurrence of at least 5% and at least twice that of placebo [29,48,49,53,57]. Some weight gain is seen with asenapine treatment compared to placebo, but less so than with risperidone or olanzapine [49,57,58]; for example over the 6-week published clinical trial, 4.3% of patients in the asenapine group showed a 7% or greater increase in their body weight as compared to 1.9% of patients in the placebo group, while the risperidone-treated group showed 17% incidence of significant weight gain [49]. Both asenapine and haloperidol resulted in minimal (less than 6%) weight gain over 6 weeks [53]. In a one-year safety study, asenapine caused less weight gain than olanzapine [57]. Incidence of other side effects common to many antipsychotics, such as hyperprolactinemia and alterations in glucose and lipid profiles, have generally been low [53,58].
Reduced weight gain with asenapine could be due to its lack of muscarinic M3 antagonism [59]. Asenapine has limited affinity for muscarinic receptors in comparison with clozapine and olanzapine [23,25]. Muscarinic antagonist effects could also deleteriously affect cognitive performance and may contribute to the deleterious effect on cognition observed with olanzapine and other atypical antipsychotics. Despite asenapine having no appreciable affinity for muscarinic receptors however, chronic asenapine administration (twice daily for 4 weeks) increased muscarinic receptor binding in the frontal cortex and hippocampal regions of rats [60]. These findings are consistent with the regionally specific asenapine-induced increases in AMPA and decreases in NMDA binding despite limited affinity for these receptors [61]. Thus asenapine administration produces some interaction with muscarinic receptors producing increased receptor expression comparable to the effects of olanzapine despite a lack of muscarinic receptor affinity in comparison to the latter [26]. Such effects may explain the limited weight-gain side effects of asenapine if indeed antipsychotic-induced weight gain occurs via a muscarinic M3 receptor antagonist mechanism [59]. The effects of asenapine treatment on muscarinic receptor binding could be as a result of indirect mechanisms mediated by one if its metabolites. These studies have yet to be conducted/published however.
Rates of EPS with asenapine treatment have been reported as lower than with haloperidol and lower or equivalent to risperidone [29,40], but a long-term safety study did find that asenapine was associated with more frequent EPS than olanzapine [57]. Increases in akathisia were observed with the 10 mg twice a day dose compared to the 5 mg twice daily regimen [29,53].
3.6 REGULATORY AFFAIRS
Asenapine is currently approved by the Food and Drug Administration for the acute treatment of schizophrenia as well as for the acute treatment of manic or mixed episodes associated with bipolar 1 disorder with or without psychotic features. Both these indications are for adults.
3.7 CONCLUSION
Asenapine 5 mg twice a day has shown clinical efficacy in reducing the symptoms of acute schizophrenia over 6-week trials and over one year-long trial, with the most robust effect on positive symptoms. Side effects and adverse reactions include somnolence, akathesia, and oral hypoesthesia, but the drug is generally well-tolerated and, importantly, seems to result in less clinically relevant weight gain than some other atypical antipsychotics.
4. EXPERT OPINION
One of the main difficulties in assessing the utility of asenapine over other antipsychotics across negative, and cognitive symptoms, is the general lack of published data on these two domains. Asenapine certainly appears to confirm to the standards of antipsychotics as it reduces positive symptomology in patients with schizophrenia. The effects of asenapine on negative and cognitive symptoms are however less clear. While there is some evidence of beneficial effects on negative and cognitive symptoms [40], these effects have primarily been reported in abstract format [40] and require further long-term evidence. It must be made clear, that although similar equivocal data are found for other antipsychotics being used today, those antipsychotics have at least more extensively published studies to draw conclusions from. The advantages, if any, that asenapine will have over its competitors will be in terms of weight gain and route of administration.
Asenapine is administered using sublingual tablets, which could be an advantage in a patient population as it is less likely to be ‘cheeked’. As it only takes 10 s for asenapine to dissolve, the tablet is unlikely to be ingested in a manner that would reduce its bioavailability. The disadvantage of this route of administration is, however, that patients cannot eat or drink for 10 min after ingestion. Given that asenapine is reported to have a bitter taste, strict compliance with the administration instructions may prove challenging for patients, especially as the drug has to be taken twice daily in comparison to once-a-day dosing for most other antipsychotic agents (ziprasidone is also dosed twice daily). The twice daily dosing requirement confers its own disadvantage independent of the sublingual administration, as increases in dosing frequencies appear to have a significant negative effect on schizophrenia patients’ adherence to antipsychotic medication regimens [62]. Thus compliance may prove to be one of the primary issues psychiatrists consider when choosing whether or not to prescribe asenapine.
Two major advantages asenapine may have are less EPS than typical antipsychotics and less weight gain than some other atypical antipsychotics, observed in both short- and long-term studies. Moreover, asenapine has no appreciable effect on glucose and lipids. Thus physicians may elect to switch patients who have gained substantial weight from other “tried and true” atypicals (e.g., olanzapine) to asenapine.
While extensive studies on pro-cognitive effects have yet to be published for asenapine, some data can be gleaned from animal studies. It is apparent that asenapine can improve executive functioning in rats, albeit in rats with medial prefrontal cortical lesions [63]. Although no patient with schizophrenia equates to frontal lobe lesioned patients, there are some similarities in executive dysfunction [64]. We are not suggesting here that prefrontal lesioned rats are a model for schizophrenia, nor did the authors [63], but the cognitive profile of asenapine may be further elucidated by assessing the effects of the drug in frontal lobe lesioned patients. Moreover, the doses used in this study were lower than those used to counter amphetamine-induced hyperactivity or apomorphine-induced disruption in PPI [20]. Given that the 5 mg tablet may produce D2 receptor occupancy at higher levels than is required for the demonstration of antipsychotic activity derived from other antipsychotics [28], perhaps a lower dose formulation could be assessed. Further support for such a formulation comes from the suggestion that asenapine may not impair cognitive functioning as measured by attention and short term memory in normal rats until sedative doses are reached, unlike olanzapine and risperidone [20].
The possibility of using lower doses is perhaps emphasized from clinical data in patients with bipolar disorder, as asenapine (albeit at the 10 mg BID dose) has been indicated to treat acute mania also [65,66]. Given that the 10 mg twice daily dose may cause more adverse effects in the form of akathisia but hasn’t been shown to be substantially more effective in treating schizophrenia, lower doses of asenapine than 5 mg may also be worth testing. The research from asenapine effects on bipolar disorder also suggests that physicians who have patients with a prominent mood component (i.e., symptoms of mania) to their schizophrenia may find asenapine useful.
Ultimately, more long term studies are required for asenapine before definitive judgments on its utility in the treatment of schizophrenia can be made. Asenapine certainly proves efficacious in acute schizophrenia, but its putative less deleterious effects on cognition will only be disseminated following long-term studies. Given the data on cognition from animal work, which can inform research when assessed in the MATRICS test battery of cognition for schizophrenia [39], lower doses and tests selective for cognitive domains should be employed in these longer term studies.
Acknowledgments
This paper was funded by NIH grants: R01 MH071916 and R21 MH085221.
Footnotes
Declaration of interest: The authors declare no conflicts of interest.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Perenyi A, Forlano R. Suicide in schizophrenia. Neuropsychopharmacol Hung. 2005;7(3):107–117. [PubMed] [Google Scholar]
- 2.Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, et al. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. Journal Clin Psych. 2004;65 Suppl 7:4–18. [PubMed] [Google Scholar]
- 3.Taylor DM. Prolongation of QTc interval and antipsychotics. Amer J Psych. 2002;159(6):1062. doi: 10.1176/appi.ajp.159.6.1062. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter WT, Buchanan RW. Lessons to take home from CATIE. Psychiatr Serv. 2008;59(5):523–525. doi: 10.1176/ps.2008.59.5.523. [DOI] [PubMed] [Google Scholar]
- 5.Parks J, Radke A, Parker G, Foti ME, Eilers R, Diamond M, et al. Principles of antipsychotic prescribing for policy makers, circa 2008. Translating knowledge to promote individualized treatment. Schiz Bull. 2009;35(5):931–936. doi: 10.1093/schbul/sbn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Amer J Pscyh. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. * Highlights the need for the development of pro-cognitive therapeutics in the treatment of schizophrenia
- 7.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psych. 2006;67 Suppl 9:3–8. discussion 36–42. [PubMed] [Google Scholar]
- 8.Burstein ES, Ma J, Wong S, Gao Y, Pham E, Knapp AE, et al. Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J Pharmacol Exp Therap. 2005;315(3):1278–1287. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- 9.Kuoppamaki M, Syvalahti E, Hietala J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol. 1993;245(2):179–182. doi: 10.1016/0922-4106(93)90126-t. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DM, Fischetti C, Sparshatt A, Thomas A, Bishara D, Cornelius V. Risperidone long-acting injection: a prospective 3-year analysis of its use in clinical practice. J Clin Psych. 2009;70(2):196–200. [PubMed] [Google Scholar]
- 11.Citrome L. Iloperidone redux: a dissection of the Drug Approval Package for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762–1784. doi: 10.1111/j.1742-1241.2010.02344.x. [DOI] [PubMed] [Google Scholar]
- 12.Kalkman HO, Subramanian N, Hoyer D. Extended radioligand binding profile of iloperidone: a broad spectrum dopamine/serotonin/norepinephrine receptor antagonist for the management of psychotic disorders. Neuropsychopharmacology. 2001;25(6):904–914. doi: 10.1016/S0893-133X(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 13.Marino J, Caballero J. Iloperidone for the treatment of schizophrenia. Ann Pharmacother. 2010;44(5):863–870. doi: 10.1345/aph.1M603. [DOI] [PubMed] [Google Scholar]
- 14.Newman-Tancredi A, Cussac D, Depoortere R. Neuropharmacological profile of bifeprunox: merits and limitations in comparison with other third-generation antipsychotics. Curr Opin Investig Drugs. 2007;8(7):539–554. [PubMed] [Google Scholar]
- 15.Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS drugs. 2004;18(4):251–267. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- 16.Casey DE, Sands EE, Heisterberg J, Yang HM. Efficacy and safety of bifeprunox in patients with an acute exacerbation of schizophrenia: results from a randomized, double-blind, placebo-controlled, multicenter, dose-finding study. Psychopharmacology. 2008;200(3):317–331. doi: 10.1007/s00213-008-1207-7. [DOI] [PubMed] [Google Scholar]
- 17.Broekkamp CL, De Graaf JS, van Delft AM. Behavioural pharmacology of trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro- 1H-dibenz[2,3:6,7]oxepino-[4,5-c]pyrrolidine maleate, a compound interacting with dopaminergic and serotonergic receptors. Arzneimittelforschung. 1990;40(5):544–549. [PubMed] [Google Scholar]
- 18.Costall B, Domeney AM, Kelly ME, Naylor RJ, Tomkins DM. Actions of ORG 5222 as a novel psychotropic agent. Pharmacol, Biochem and Behav. 1990;35(3):607–615. doi: 10.1016/0091-3057(90)90298-v. [DOI] [PubMed] [Google Scholar]
- 19.Franberg O, Wiker C, Marcus MM, Konradsson A, Jardemark K, Schilstrom B, et al. Asenapine, a novel psychopharmacologic agent: preclinical evidence for clinical effects in schizophrenia. Psychopharmacology. 2008;196(3):417–429. doi: 10.1007/s00213-007-0973-y. [DOI] [PubMed] [Google Scholar]
- 20. Marston HM, Young JW, Martin FD, Serpa KA, Moore CL, Wong EH, et al. Asenapine effects in animal models of psychosis and cognitive function. Psychopharmacology. 2009;206(4):699–714. doi: 10.1007/s00213-009-1570-z. ** Useful presentation on the preclinical work that guides the clinical testing of putative antipsychotic drugs
- 21.Funke CW, Hindriks H, Sam AP. Physico-chemical properties and stability of trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H- dibenz[2,3:6,7]oxepino[4,5-c]pyrrolidine maleate. Arzneimittelforschung. 1990;40(5):536–539. [PubMed] [Google Scholar]
- 22.Cosi C, Koek W. Agonist, antagonist, and inverse agonist properties of antipsychotics at human recombinant 5-HT(1A) receptors expressed in HeLa cells. Eur J Pharmacol. 2001;433(1):55–62. doi: 10.1016/s0014-2999(01)01493-5. [DOI] [PubMed] [Google Scholar]
- 23.Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- 24.Bymaster FP, Hemrick-Luecke SK, Perry KW, Fuller RW. Neurochemical evidence for antagonism by olanzapine of dopamine, serotonin, alpha 1-adrenergic and muscarinic receptors in vivo in rats. Psychopharmacology. 1996;124(1–2):87–94. doi: 10.1007/BF02245608. [DOI] [PubMed] [Google Scholar]
- 25.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life sciences. 2000;24;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 26.Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharm. 2009;23(1):65–73. doi: 10.1177/0269881107082944. [DOI] [PubMed] [Google Scholar]
- 27.Bishara D, Taylor D. Upcoming agents for the treatment of schizophrenia: mechanism of action, efficacy and tolerability. Drugs. 2008;68(16):2269–2292. doi: 10.2165/0003495-200868160-00002. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psych. 1994;51(2):139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 29.Schering-Plough . Saphris (Asenapine) sublingual tablets. Breifing document (Background Package) Schering-Plough Research Institute; 2009. [Google Scholar]
- 30.Ellenbroek BA. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Therap. 1993;57(1):1–78. doi: 10.1016/0163-7258(93)90036-d. [DOI] [PubMed] [Google Scholar]
- 31.Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog in Neuropsychopharm and Biol Psych. 2003;27(7):1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Moore NA, Leander JD, Benvenga MJ, Gleason SD, Shannon H. Behavioral pharmacology of olanzapine: a novel antipsychotic drug. J Clin Psych. 1997;58 Suppl 10:37–44. [PubMed] [Google Scholar]
- 33.Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;14;602(2–3):334–342. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 34.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schiz Bull. 35(1):109–114. doi: 10.1093/schbul/sbn163. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schiz Bull. 2005;31(1):5–19. doi: 10.1093/schbul/sbi020. * Useful summary of the process the NIH-funded MATRICS initiative took to obtaining a road map by which the FDA could approve pro-cognitive therapeutics for schizophrenia
- 37.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Amer J Psych. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 38.Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schiz Bull. 2009;35(1):182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol & Therap. 2009;122(2):150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potkin S, Fleming K, Binnerman B, Keeller D, Alphs L, Panagides J. Effects of asenapine on cognitive function in acute schizophrenia: a placebo- and risperidone-controlled trial. Eur Neuropsychopharm. 2007;17:S466–S467. [Google Scholar]
- 41.Jentsch JD, Shahid M, Wong EHF, Roth RH. Asenapine improves cognitve function in monkeys repeatedly exposed to the psychotomimetic drug phencyclidine. Biol Psych. 2006;59 Suppl.:471. [Google Scholar]
- 42.Neill JC, Shahid M, Grayson B, Marston HM, Snigdha S. Asenapine improves a subchronic phencyclidine-induced deficit in object recognition memory in the rat. Biol Psych. 2008;63 Suppl.:75–76. [Google Scholar]
- 43.Neill JC, Shahid M, Wong EHF, Idris NF. Comparison of the efficacy of asenapine, risperidone, and olanzapine to improve a reversal learning deficit in the rat. Schiz Res. 2006;81 Suppl.:105–106. [Google Scholar]
- 44.Spina E, de Leon J. Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007;100(1):4–22. doi: 10.1111/j.1742-7843.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 45.Prior TI, Chue PS, Tibbo P, Baker GB. Drug metabolism and atypical antipsychotics. Eur Neuropsychopharmacol. 1999;9(4):301–309. doi: 10.1016/s0924-977x(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 46.Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Aus and New Zeal J of Psych. 2009;43(3):277–282. doi: 10.1080/00048670802653372. [DOI] [PubMed] [Google Scholar]
- 47.Schering-Plough . Full Prescribing Information: Saphris (asenapine) 2009. [Google Scholar]
- 48.Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract. 2009;63(12):1762–1784. doi: 10.1111/j.1742-1241.2009.02228.x. [DOI] [PubMed] [Google Scholar]
- 49. Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psych. 2007;68(10):1492–1500. doi: 10.4088/jcp.v68n1004. ** This is the first clinical publication on the use of asenapine to treat schizophrenia
- 50.Curtis VA, Katsafouros K, Moller HJ, Medori R, Sacchetti E. Long-acting risperidone improves negative symptoms in stable psychotic patients. J Psychopharm. 2008;22(3):254–261. doi: 10.1177/0269881107082119. [DOI] [PubMed] [Google Scholar]
- 51.Lane HY, Liu CC, Chang WH. Risperidone for exclusively negative symptoms. Amer J Psych. 1999;156(2):335. doi: 10.1176/ajp.156.2.335. [DOI] [PubMed] [Google Scholar]
- 52.Riedel M, Spellmann I, Strassnig M, Douhet A, Dehning S, Opgen-Rhein M, et al. Effects of risperidone and quetiapine on cognition in patients with schizophrenia and predominantly negative symptoms. Eur Arch Psych Clin Neurosci. 2007;257(6):360–370. doi: 10.1007/s00406-007-0739-x. [DOI] [PubMed] [Google Scholar]
- 53. Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 30(2):106–115. doi: 10.1097/JCP.0b013e3181d35d6b. * Useful for corroborating the efficacy of Asenapine for treating the positive symptoms for schizophrenia in comparison to a typical antipsychotic
- 54.Friberg LE, de Greef R, Kerbusch T, Karlsson MO. Modeling and simulation of the time course of asenapine exposure response and dropout patterns in acute schizophrenia. Clin Pharmacol & Therap. 2009;86(1):84–91. doi: 10.1038/clpt.2009.44. [DOI] [PubMed] [Google Scholar]
- 55.Roth BL, Hanizavareh SM, Blum AE. Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacology. 2004;174(1):17–24. doi: 10.1007/s00213-003-1683-8. [DOI] [PubMed] [Google Scholar]
- 56.Chapel S, Hutmacher MM, Haig G, Bockbrader H, de Greef R, Preskorn SH, et al. Exposure-response analysis in patients with schizophrenia to assess the effect of asenapine on QTc prolongation. J Clin Pharmacol. 2009;49(11):1297–1308. doi: 10.1177/0091270009344855. [DOI] [PubMed] [Google Scholar]
- 57. Schoemaker J, Naber D, Vrijland P, Panagides J, Emsley R. Long-Term Assessment of Asenapine vs. Olanzapine in Patients with Schizophrenia or Schizoaffective Disorder. Pharmacopsychiatry. 2010 doi: 10.1055/s-0030-1248313. * Useful for corroborating the efficacy of Asenapine for treating the positive symptoms for schizophrenia
- 58.Weber J, McCormack PL. Asenapine. CNS drugs. 2009;23(9):781–792. doi: 10.2165/11200860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 59.Silvestre JS, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find Exp Clin Pharmacol. 2005;27(5):289–304. doi: 10.1358/mf.2005.27.5.908643. [DOI] [PubMed] [Google Scholar]
- 60.Choi YK, Wong EH, Henry B, Shahid M, Tarazi FI. Repeated effects of asenapine on adrenergic and cholinergic muscarinic receptors. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2010;13(3):405–410. doi: 10.1017/S1461145709990824. [DOI] [PubMed] [Google Scholar]
- 61.Tarazi FI, Choi YK, Gardner M, Wong EH, Henry B, Shahid M. Asenapine exerts distinctive regional effects on ionotropic glutamate receptor subtypes in rat brain. Synapse (New York, NY. 2009;63(5):413–420. doi: 10.1002/syn.20618. [DOI] [PubMed] [Google Scholar]
- 62.Pfeiffer PN, Ganoczy D, Valenstein M. Dosing frequency and adherence to antipsychotic medications. Psychiatr Serv. 2008;59(10):1207–1210. doi: 10.1176/ps.2008.59.10.1207. [DOI] [PubMed] [Google Scholar]
- 63.Tait DS, Marston HM, Shahid M, Brown VJ. Asenapine restores cognitive flexibility in rats with medial prefrontal cortex lesions. Psychopharmacology. 2009;202(1–3):295–306. doi: 10.1007/s00213-008-1364-8. [DOI] [PubMed] [Google Scholar]
- 64.Ornstein TJ, Sahakian BJ, McKenna PJ. Memory and executive impairment in schizophrenia: comparison with frontal and temporal brain damage. Psych Medicine. 2008;38(6):833–842. doi: 10.1017/S0033291707001468. [DOI] [PubMed] [Google Scholar]
- 65.McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. Bipolar disorders. 2009;11(7):673–686. doi: 10.1111/j.1399-5618.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre RS, Cohen M, Zhao J, Alphs L, Macek TA, Panagides J. Asenapine versus olanzapine in acute mania: a double-blind extension study. Bipolar disorders. 2009;11(8):815–826. doi: 10.1111/j.1399-5618.2009.00749.x. [DOI] [PubMed] [Google Scholar]


