Abstract
Objective
Examine the diurnal variation of salivary cortisol in adults with spinal cord injury (SCI) and the effect of stressors on cortisol and mood.
Method
Ecological momentary assessment (EMA) to capture cortisol, stress and mood from 25 persons with SCI and 26 without SCI. Data were analyzed using linear mixed models.
Results
There were no systematic differences between groups on missing data. Diurnal variation of cortisol of participants with SCI reflected an expected pattern. No significant group differences for cortisol diurnal pattern, stress or mood; when group interactions were significant, results indicated lower cortisol reactivity to stress in participants with SCI. Stress had a significant impact on positive, negative and agitated moods.
Conclusions
Stress in daily life and its association with cortisol and mood were largely similar between persons with and without SCI. A key methodological contribution is the demonstration of using EMA to collect biological and behavioral data in the field from participants with SCI. The use of EMA in rehabilitation psychology research has great potential to advance our understanding of the dynamics of daily life with disability.
Keywords: spinal cord injuries; stress, psychological; hydrocortisone; ecological momentary assessment
Introduction
A large body of literature has emerged over the last 50 years showing the inextricable link between psychological factors, such as stress, and the functioning of the hypo-pituitary-adrenal (HPA) axis. The HPA axis controls the release of cortisol, among other hormones, which is associated with psychological, physiological and physical health (Dickerson & Kemeny, 2004). Psychological stress is characterized by the perception of threat of a situation or event in the environment. Threats can include environmental stressors such as work, home or neighborhood, major life events, trauma or abuse (McEwen & Seeman, 1999). This perceived threat from the environment drives behavioral and physiological responses. Perception of threat is highly individualistic and influenced by the individual's experiences, genetics, prior stress exposure, social environment and individual characteristics such as social connection, social status, personality and coping style (Glei, Goldman, Chuang, & Weinstein, 2007; McEwen & Seeman, 1999; Olff, Langeland, & Gersons, 2005). Determinants of salivary cortisol to challenge are also highly variable and include gender and sex steroids, genetics, nicotine, coffee and alcohol, and pre- and post-natal stress (Kudielka, Hellhammer, & Wust, 2009) .
“Allostatsis” refers to maintaining stability or homeostasis through change and was first introduced to describe how the cardiovascular system adjusts to resting and active states of the body (Sterling & Eyer, 1988). McEwen and Seeman (1999) expanded this concept to capture physiological responses, such as the secretion of cortisol, to environmental and psychosocial situations and demands. The body's response to psychological stress involves the activation of two systems: the HPA axis and the sympathetic nervous system (SNS), with the former releasing cortisol and corticotrophin-releasing hormone (McEwen, 1998). When the HPA axis is functioning properly, elevated levels of these hormones are limited in magnitude and temporary in duration, and reduce to normal levels with the cessation of the stressor. However, under circumstances involving high intensity or chronic stress, hormonal dysregulation can lead to physical, psychosomatic, and psychological disorders (Ehlert & Straub, 1998). Long-term effects of critical or traumatic life events also appear to be associated with distinct dysregulation of the HPA axis (Ehlert & Straub, 1998) and, with this, vulnerability to disease and psychological dysfunction.
“Allostatic load” refers to the wear and tear of repeated neuroendocrine responses to chronic environmental challenges and is the “price” of allostasis, or adaptation (McEwen, 1998). Biological systems that provide protection (“fight or flight”) against acute stressors can eventually become damaging if that adaptive response (allostasis) stays “on.” Physiologic response to an acute psychological stress can begin within seconds and peaks 15 to 20 minutes after the onset of the stressor (Kudielka et al., 2009). However, over time, chronic stress (characterized by a prolonged state of or exposure to stress) leads to dysregulation of these protective systems. There is also debate over whether hyper- or hypo-responsivity to an acute stressor is influenced by the presence of chronic stress or exhaustion in an individual (Kudielka et al., 2009). Dysregulation resulting from prolonged activation of protective systems – allostatic load – is characterized by elevated (or in some cases diminished) levels of biomarkers reflecting SNS, HPA axis, immune system and cardiovascular activity (Glei et al., 2007). There is also evidence that acute and chronic stressors play an important role in the development of disorders having psychological features, such as depression, with the neuroendocrine system at the center of this causal pathway (Miller, Chen, & Zhou, 2007; vanEck, Berkhof, Nicolson, & Sulon, 1996).
Despite the importance of the adverse effects of stress on health, the study of HPA reactivity to stress in persons with various disabling conditions is very limited. In persons with spinal cord injury (SCI), the autonomic nervous systems involved in regulating the stress response is compromised by the direct effects of injury, such as sympathetic denervation and central neurotransmitter alteration (Palmer, 1985). A vulnerable health status is common among many persons with SCI due to direct and indirect effects of injury. Changes in the body as a result of dysregulated cortisol and autonomic activity can have long-term health effects that include bone mineral loss and abdominal fat deposits (Gold, 2005), increased cardiovascular risk (McEwen, 2003, 2005), and insulin resistance (Bauman, 1997), all of which are already compromised due to the effects of SCI. Stress-mediated immunity is also important in connection with infection, slow wound healing (Ebrecht et al., 2004), and pressure sores, the latter of which are among the leading causes of re-hospitalization and morbidity in persons with SCI (Cardenas, Hoffman, Kirshblum, & McKinley, 2004). The presence or absence of a concomitant brain injury may also affect cortisol response (Bay, Sikorskii, & Gao, 2009) although this has not been reported in the SCI literature.
The study of cortisol secretion in response to an acute stressor or multiple stressors has been primarily conducted in laboratory settings. While this allows for the standardization of stressors (e.g., mental arithmetic, public speaking), generalization to real-life stress conditions is limited (Biondi & Picardi, 1999). Field studies have focused on the occurrence of stressful events experienced in daily life, ranging from minor hassles to major traumatic events. Studies featuring momentary collection of cortisol in natural settings are predicated on the notion that more information is needed about psychoendocrinological responses to stressors encountered in daily life to better understand the mechanisms through which stress leads to disorders (Jacobs et al., 2007).
Ecological momentary assessment (EMA) refers to a variety of methods that involve repeated sampling (usually multiple times during a day across several or more days) of current behaviors and experience in real time and in natural environments. This approach was developed, in part, in response to the limitations of retrospective recall. EMA maximizes ecological validity, reduces recall bias, and allows for the study of “microprocesses” that influence behavior in real-world settings (Shiffman, Stone, & Hufford, 2008). Momentary assessment captures what the respondent is doing or feeling at the moment. This approach is also very useful when examining biological factors, such as salivary cortisol, that change in response to factors such as daily stressors and time of day. Capturing behavior in context is particularly important for understanding the association of daily stressors with cortisol and mood. Because EMA involves the collection of data in the respondent's natural environment, generalization to the real-world and real-life is enhanced, in contrast to laboratory studies.
Purpose
The study of HPA reactivity to stress has several important applications in the context of SCI. Although clinical experience suggests that persons with SCI experience elevated levels of stress related to the consequences and demands of injury, there are few studies that explicitly measure daily stressors, prospectively or otherwise; most studies typically measure perceived stress which is conceptualized as a global perception of burden (Cohen, Kamarck, & Mermelstein, 1983). Studies have shown that global perceived stress is not associated with injury characteristics or level of physical independence but, instead, to adjustment and coping (Gerhart, Weitzenkamp, Kennedy, Glass, & Charlifue, 1999). Global perceived stress has been related to depressive symptoms and anxiety in men with SCI, with low levels of social support also implicated in increased vulnerability to the negative impact of stress on psychological well-being (Rintala, Robinson-Whelen, & Matamoros, 2005). While global assessment of stress and its association with physiological parameters is valuable for understanding link between chronic stress and disease in the context of allostatic load and the consequence of chronic stress conditions, global assessments, in general, limit our understanding of dynamic changes in behavior across time and situations (Shiffman et al., 2008) and their effect on physiological outcomes such as cortisol responsivity.
One of the most important yet unanswered questions with respect to SCI and cortisol secretion is: to what degree do the direct effects of the injury on bodily systems involved in the stress response alter cortisol secretion in response to stress, particularly in the natural environment? Studies of cortisol amplitude in SCI have produced conflicting results of low, normal, and high circulating concentrations of cortisol, with most studies collecting only one or two time points to establish diurnal variation (Zeitzer, Ayas, Shea, Brown, & Czeisler, 2000). Differences in overall levels of cortisol between persons with and without SCI have not been consistently found (Campagnolo, Bartlett, Chatterton, & Keller, 1999; Huang, Wang, & Chen, 2000; Zeitzer et al., 2000) and very few studies have compared diurnal variation among persons with SCI with that of their peers without SCI (Zeitzer et al., 2000). In one of the few investigations of adrenal function and psychological outcomes in the context of SCI, in a small sample of persons with SCI meeting criteria for major depressive disorder (MDD), a dexamethasone suppression test, used to assess HPA function in psychiatric disorders, lacked adequate sensitivity and specificity for MDD in the sample (Frank, Kashani, Wonderlich, Lising, & Visot, 1985). Examination of associations of cortisol secretion and the corresponding experience of acute psychological stressors in either laboratory or natural settings or the effects of chronic psychological stress for persons with SCI has not been reported.
Given how little is known about HPA responsivity to stress in the context of SCI and implications for the impact of dysregulation on health, the primary aim of this pilot study was to examine cortisol secretion in response to daily stressors in a natural setting among persons with SCI using EMA. The following questions (Q) were addressed in this study: 1) does the diurnal pattern of cortisol secretion of persons with SCI correspond with expected elevation in the morning and decline toward evening (Q1); 2) is this pattern significantly different from persons without SCI (Q2); 3) is there a difference in the mean level of cortisol secretion between persons with SCI and without SCI (Q3); and 4) is there a difference in the experience of daily stressors and their effect on cortisol secretion and mood between persons with SCI and without SCI (Q4).
Methods
Rationale for momentary sampling design
The key to this study is examining the effect of daily stressors on cortisol and mood in a sample of individuals with SCI. Daily stressors were chosen over measuring global stress because we wanted to capture experience in daily life, which has not been addressed in the literature. The choice to use EMA in this study was based on two key considerations. The first was to reduce the influence of recall bias on the experience of events and associated stress and mood ratings. This is important because these factors can vary during the course of a single day and recall is heavily influenced by the individual's current state. The second was that sampling moments randomly throughout the day reduces the risk of over-sampling certain conditions or contexts (such as only work or only home environments) that may be systematically related to cortisol secretion, mood and/or the occurrence of daily stressors.
Participants
Fifty one adults with SCI (N = 25) and without SCI (N = 26) participated in this study. The goal for recruitment (N = 50) was met; a total of 35 persons with SCI and 38 persons without SCI were screened for this study. Two persons with SCI and one without dropped out after enrollment. Individuals with SCI sustained their injuries (at any level) after the age of 16 years, either traumatically (i.e., external forces) or non-traumatically (i.e., disease processes). They were primarily non-ambulatory and used either a power or manual wheelchair. Participants without SCI were matched on age (± 5 years) and gender. Participants with SCI were recruited from the University of Michigan SCI Research Registry; participants without SCI were recruited from the University of Michigan Engage Research Registry. All participants were paid $40 for their participation. Demographic and injury characteristics of the sample are given in table 1. The study was approved by the University of Michigan Institutional Review Board and conducted in 2008.
Table 1.
Demographic Characteristics of Sample
| |
With SCI (N = 25) |
Without SCI (N = 26) |
|---|---|---|
| Current age (mean, SD) |
45.96 (11.28) |
46.08 (11.49) |
| Gender (male; N, %) |
16 (64.0) |
15 (57.7) |
| Ethnicity (Caucasian; N, %) |
24 (96.0) |
24 (92.3) |
| Education (N, %) | ||
| High school or less | 2 (8.0) | 1 (3.8) |
| Some college or college degree | 18 (72.0) | 16 (61.5) |
| Graduate degree | 5 (20.0) | 9 (34.6) |
| Occupational status (Working, %) |
10 (40.0) |
19 (73.1) |
| Age at injury (mean, SD) |
29.88 (13.81) |
NA |
| Time since injury (years; mean, SD) |
16.0 (12.8) |
NA |
| Injury level (paraplegia vs. tetraplegia; N) | 12 vs. 13 | NA |
Enrollment and selection of study days
All participants were screened via a telephone interview prior to enrollment to assure that eligibility criteria were met. They were also screened for self-reported swallowing problems due to the saliva collection device. Data collection took place over two consecutive days, which were selected on the basis of participant choice. Participants were encouraged to select days that would represent their normal daily routines. Travel was not required to participate in this study which reduced participant burden and allowed for a broader geographic area from which to draw participants. Data collection materials and detailed instructions were mailed to participants; collected materials were returned to the study team by parcel pick up from the participants’ homes (or other location of their choosing).
Design of data collection materials
One of the most important considerations and aims of this study was the design of data collection materials for field study during daily routines. The study materials were designed in order to eliminate or minimize environmental barriers that may be encountered by participants with SCI. To accomplish this, a group of consultants consisting of two individuals with SCI (one with tetraplegia and one with paraplegia), two registered occupational therapists with expertise in SCI and rehabilitation, and study investigators worked together to design data collection kits that were easily accessible to SCI participants, particularly those with limited hand function.
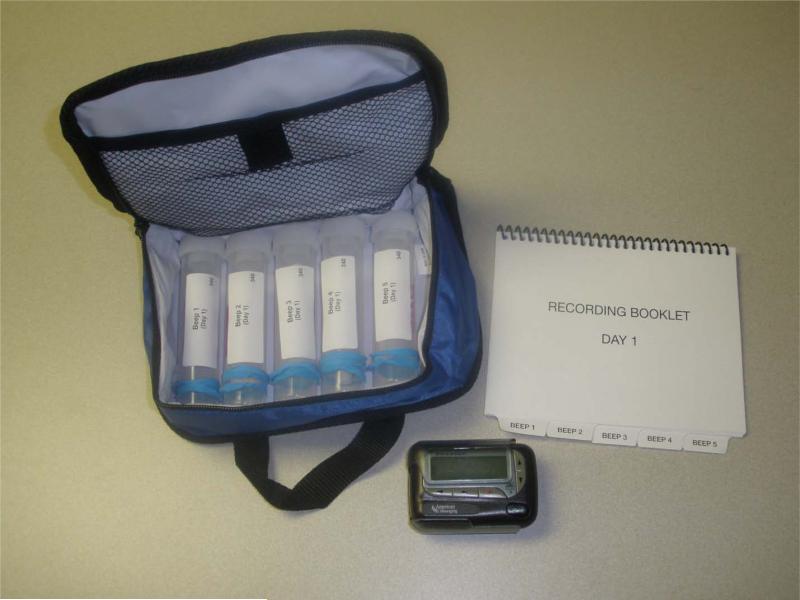
Kits were designed for ease in transporting them during each day of the data collection period. The quasi-random schedule of data collection required that kits would be carried at all times during data collection hours. Easy retrieval, particularly for wheelchair users, was carefully considered in kit design. Two small, zippered lunch bags, made out of soft water-resistant fabric were each equipped with five saliva collection tubes and a daily diary; see figure 1. Kit designs followed two patterns, one for the participants without SCI and those with paraplegia and a second one for those with tetraplegia and limited hand function. A set of instructions was developed for each style of kit and included photographs of the collection tubes to illustrate and clarify data collection steps. Kits assembled for those with tetraplegia included larger saliva collection tubes and twist caps, rings attached to zipper pulls on bags, and a large, ergonomically designed pen for recording in dairies. At the start of the study, participants were mailed a packet of materials that included the applicable set of instructions and two kits (one for each day), an electronic pager, and two bags to transport study materials throughout each data collection day.
Figure 1.
Data collection kit (rubber band at bottom of each saliva collection tube for participants with tetraplegia for ease of opening)
Daily diaries
The Experience Sampling Method is a structured diary technique that is used to assess individuals in the context of their daily living environments and has been shown to be a reliable way to study the immediate effects of stressors on mood and reduce recall bias (Csikszentmihalyi & Larson, 1987). Because this was a pilot study and, to our knowledge, the first investigation of its kind to involve persons with SCI, the design of measures was drawn directly from several published studies investigating the effect of daily stressors on cortisol and mood (Jacobs et al., 2007; Myin-Germeys et al., 2003; Myin-Germeys, van Os, Schwartz, Stone, & Delespaul, 2001) in favor of using untested measures. Each diary was divided into five tabbed sections corresponding to each beep time. Each section assessed the participant's current stress experience, social context, anticipation of stress and mood.
Stress was captured in four different ways using measures from Jacobs et al.'s (2007) study. Presence of stress was defined by a “yes” or “no” response to whether they were currently experience any stress at the time of the beep. Activity related stress captured stress related to activity performed at the time of the beep and was defined by the mean score of two items rated on 7-point Likert scales (strongly disagree to strongly agree): “I’d rather be doing something else” and “This activity requires effort.” Social stress captured the social context when others were present and was defined by the mean score of two items rated on 7-point Likert scales (strongly disagree to strongly agree): “I don’t like the present company” and “I would rather be alone.” Event related stress captured the most significant event to occur since the previous beep and was rated on 7-point Likert scales (very unpleasant to very pleasant).
Current mood was assessed by a 15 adjective mood checklist with three subscales (i.e., positive, negative and agitated) which were based on Jacobs et al.'s (2007) analysis of factor loadings of the 15 mood adjectives. Each were rated on 7-point Likert scales (from not at all to very). Cheerful, satisfied, energetic, and enthusiastic form the positive mood scale; insecure, lonely, anxious, blue, guilty, and suspicious form the negative mood scale; and relaxed (reverse coded), calm (reversed coded), and harried form the agitated mood scale.
Salivary cortisol collection
Salivary cortisol was collected at the time of each beep. The Salivette® device (Sarstedt Inc., Rommelsdorf, Germany) was used to collect saliva and consisted of a cotton swab chewed for 45-60 seconds and placed in a plastic collection tube. Bar coding and labeling were used on each Salivette® device to ensure accuracy. At the end of each collection day, participants were asked to store their saliva samples in the refrigerator until they could be returned to the study team. After they were received, they were transported to the laboratory and the samples were frozen at −20° C until assayed. Previous work suggests that cortisol concentrations from saliva are stable during extended periods without freezing (Clements & Parker, 1998).
Data collection schedules
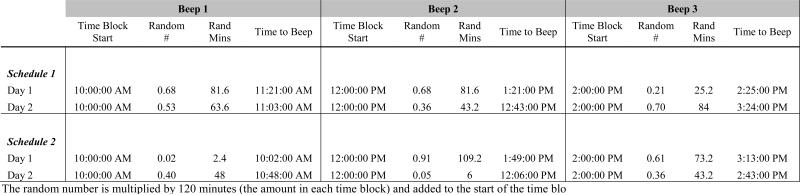
Data collection schedules were created using a stratified random sampling plan. Compliance with salivary cortisol sampling has been shown to be greater with intensive, random time sample vs. fixed (Jacobs et al., 2005). Data were collected between 10:00 AM and 8:00 PM; the later morning was chosen because of the extended amount of time many people with SCI require to complete morning self-care routines and a concern over additional participant burden. Five two-hour blocks beginning at 10:00 AM were created; the number of minutes from the start of each time block was randomly generated using a web-based, random number generator (http://www.random.org/decimal-fractions/). This was done by generating a random fraction and multiplying this by 120 minutes (the duration of each time block); the number of minutes was then added to the start of each time block to determine the beeping time for that block (see figure 2). In this manner, six beeping schedules (consisting of 10 beep times, five for each day) across the two days of data collection were generated. Minutes were rounded to the nearest 5-minute intervals due to limitations in software to send email alerts to pagers (Groupwise software). Assignment of beeping schedules to participants was done by randomly generating a list of numbers 1 through 6; participants were consecutively assigned one of the six random beeping schedules as they enrolled in the study.
Figure 2.
Example of Beeping Schedule Generation
Participant training and compliance
A detailed set of instructions including photographs were sent with data collection materials. Once materials were received, the study coordinator reviewed them over the phone with each participant before data collection commenced. Participants were instructed that once they received a beep, they were to collect the saliva sample within ten minutes following the beep. A greater than 15-minute lag between stressor and cortisol collection is considered less reliable and less valid (Jacobs et al., 2007); use of a ten-minute limit reduced the chance of exceeding this lag. If when receiving a beep, participants were unable to halt or interrupt their activities, they were instructed to indicate why they missed the beep in the corresponding diary section. Participants also were asked to record if they had eaten or drank within 30 minutes of the saliva collection beep. Because beeps were random throughout the day, participants were not asked to refrain from eating or drinking; rather we controlled for this in the analysis. The study coordinator was available by telephone during the work week and weekends to address any problems or questions participants had during data collection.
Salivary cortisol analysis
The salivary cortisol assay is based on a modification of the commercially available Bayer Cortisol Kit, (ACS:180 COR COMBO 300T 672303000). Standards and controls are diluted 1:10 with PBS to extend the lower range of the assay. Saliva samples are centrifuged prior to being assayed. This is a competitive chemiluminescent immunoassay. Cortisol in the saliva competes with acridinium ester-labeled cortisol (Lite Reagent) for binding to polyclonal rabbit anti-cortisol on the Solid Phase. The polyclonal rabbit anti-cortisol antibody is bound to monoclonal mouse anti-rabbit antibody, which is coupled to paramagnetic particles (Solid Phase). An inverse relationship exists between the amount of cortisol present in the saliva sample and the amount of relative light units (RLU) detected by the ACS:180 system. In the assay, 200 μl of Standard, QC or Sample is dispensed into a cuvette, followed by 50 μL of Lite Reagent and 250μl of Solid Phase. This mixture is incubated for 5 minutes at 37° C, separated, washed then treated with of 300 μL each of Trigger Reagents 1 and 2 to initiate the chemiluminescent reaction. The photomultiplyer tube then records the light output in RLU. The range of this assay is 0.012-9.29 μg/dL.
Statistical analysis
In this study, “beep” was the time variable, referring to the pager alert cueing data collection (beeps 1 through 5 are day 1 and beeps 6 through 10 are day 2). The beep variable was all ten beep times across both days (that is, beeps were not nested within days). Group membership refers to participants with SCI and without SCI. The primary outcome of interest in this study was salivary cortisol (μg/dL); in addition, three mood outcomes were tested: positive mood, negative mood and agitated mood. Stress predictors of these outcomes were: the presence of stress, activity-related stress, social stress and event-related stress.
Randomness of missing data (i.e., uncollected saliva samples and insufficient saliva samples) by group membership and beep time was examined using logistic regression. Confirmation that missing data were random guided the decision whether or not to employ imputation methods for missing data (Little & Rubin, 2002). The difference between beep time and actual time of data collection (recorded in daily diaries) was examined by group to ascertain if there was a greater lag for those with SCI. It was presumed that disparities between in personal clock settings and the pager setting would be random within and between groups, though this assumption could not be tested. Line graphs were used to visually examine diurnal cortisol level patterns across the two days of testing for participants with SCI (Q1) and cortisol level patterns between participants with and without SCI (Q2).
Linear mixed models (LMM) were used to analyze repeated measures data. LMM handle data where observations are not independent, correctly modeling errors where tests in the general linear model family (e.g., t-tests, ANOVA, correlation and regression) usually do not. Six covariance structures (Compound Symmetry [CS], Compound Symmetry Heterogeneous [CSH], Autoregressive, first order [AR(1)], Autoregressive, first order heterogeneous [AR(1)H], Toeplitz [TP] and Toeplitz Heterogeneous [TPH]) were considered for each outcome; the Akaike information criteria (AIC) value was used to compare models. Although no statistical test is available for differences in AIC values, models with the lowest AIC value are preferred (Tabachnick & Fidell, 2001). Because we were interested in the interaction of group and predictors, each model was tested in a hierarchical fashion. First, the model of main effects only was tested; then the interaction term was added to the model and tested again. If there was no statistically significant interaction effect, the main effects model was retained and estimates reported. If the interaction was statistically significant or marginally significant, post hoc testing was conducted and reported. Estimated marginal means with Bonferroni correction were used for post-hoc testing of significant interactions.
Group differences in cortisol levels (Q3), controlling for time, was modeled by beep, group, and group by beep interaction. Difference in the presence of stress (yes or no) was tested using logistic regression, controlling for time. Differences in the other three daily stressors (activity-related, social and event-related stress) was first examined to determine group differences (Q4), controlling for time, by modeling beep, group and beep by group. Then the effect of stress (Q4) on cortisol was modeled by beep, group, and group by stress (presence, activity-related, social and event-related). The same four stress models were then tested for their association with positive mood, negative mood and agitated mood; each model included beep, group and group by stress predictor. When cortisol was the outcome, consumption of food, alcohol or tobacco in the previous 30 minutes was included in models to control for their effects. Exploratory analysis also was preformed to compare participants with SCI by level of injury and time since injury on cortisol level across and within beep times, controlling for eating, drinking or smoking. SPSS® 16.0 was used to conduct all statistical analyses.
Results
Data Collection, Missing Data and Descriptives
Fifty one participants provided a total of 441 (86.4%) out of a possible 510 daily diary entries and saliva samples (with SCI = 213 and without SCI = 228). Nineteen of the collected saliva samples (4.3%) were insufficient for assaying (with SCI = 10 and without SCI = 9) and 50 saliva samples (10.2%) were not collected at all (with SCI = 27 and without SCI = 23). Some of the most common reasons for not collecting samples were not hearing the beep, not having the pager or being in the middle of self-care routines. There were no lag times between pager alert and collection time recorded in diaries that exceeded 15 minutes; thus all sample were retained for analysis. Uncollected saliva samples/dairy entries (X2 [2, N = 491] = 0.808, p = 0.668) and insufficient saliva samples (X2 [2, N = 460] = 3.43, p = 0.18) were not found to be systematic by group membership or beep time and therefore presumed to be random (thus no imputation methods were used for missing data). The lag time between pager beep time and time recorded on the diary was examined for group differences. Averaged across all beep times, participants with SCI had a significantly longer lag time (F = 5.091, p = 0.029); on average, those with SCI had a lag time of 0.89 minutes longer than those without SCI. Means and standard deviations of mood outcomes and stress predictors are given in table 2; means and standard deviations of cortisol values (grouped by morning, afternoon and evening and aggregated across both days of data collection) are given in table 3.
Table 2.
Descriptives of Stress and Mood Ratings and Cortisol values (aggregated across all beep times)
| With SCI M (SD) | Without SCI M (SD) | |
|---|---|---|
| Activity-related stress (range 1 through 7) | 3.64 (1.66) | 3.93 (1.47) |
| Social stress (range 1 through 7) | 1.67 (0.92) | 2.25 (1.30) |
| Event-related stress (range 1 through 7) | 3.43 (1.73) | 3.12 (1.76) |
| Positive mood (range 4 through 28) | 16.25 (4.87) | 18.00 (4.72) |
| Negative mood (range 6 through 42) | 11.99 (6.53) | 11.88 (6.93) |
| Agitated mood (range 3 through 21) | 9.08 (4.11) | 10.59 (4.43) |
Units for stress and mood are in their original units (1-point).
Higher scores indicate higher stress or mood levels
Table 3.
Cortisol Values
| With SCI M (SD); # samples | Without SCI M (SD); # Samples | |
|---|---|---|
| Day 1 | ||
| Morning Cortisol (μg/dL) | 0.784 (0.36); 21 | 0.767 (0.22); 21 |
| Afternoon cortisol (μg/dL) | 0.738 (0.23); 42 | 0.664 (0.21); 49 |
| Evening Cortisol (μg/dL) | 0.664 (0.45); 44 | 0.575 (0.12); 46 |
| Day 2 | ||
| Morning Cortisol (μg/dL) | 0.763 (0.26); 22 | 0.743 (0.23); 23 |
| Afternoon Cortisol (μg/dL) | 0.655 (0.20); 44 | 0.666 (0.44); 44 |
| Evening Cortisol (μg/dL) | 0.574 (0.18); 40 | 0.559 (0.16); 44 |
Morning cortisol = 10:00 AM to 12:00 PM (beep 1 and 6)
Afternoon cortisol = 12:00 PM to 4:00 PM (beeps 2/3 and 7/8)
Evening cortisol = 4:00 PM to 8:00 PM (beeps 4/5 and 9/10)
Diurnal Pattern of Cortisol Secretion (Q1, Q2 and Q3)
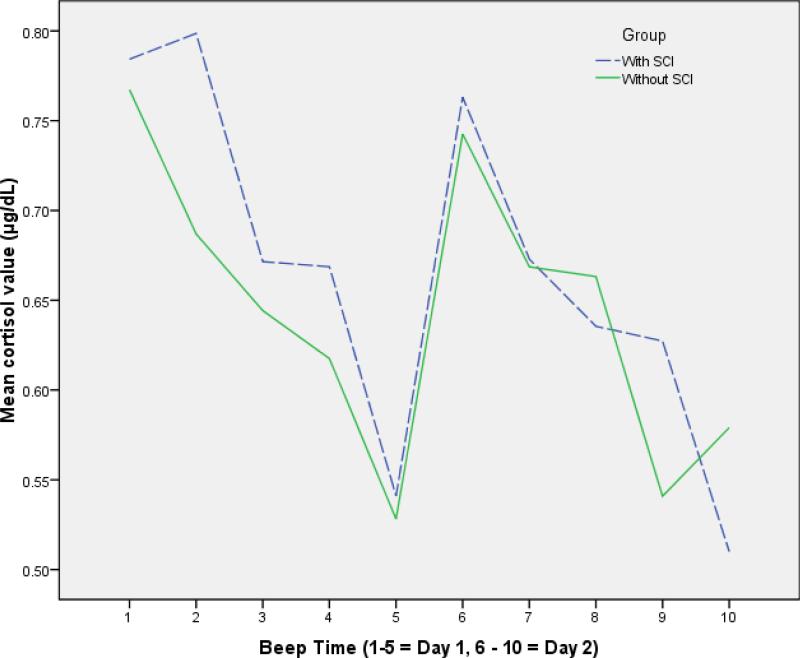
As shown in figure 3, the diurnal pattern of cortisol across each day for both groups showed a significant effect of time such that levels were higher early in the day with a gradual decline towards evening (F = 6.321, p ≤ 0.001). There was no statistically significant main effect for group or interaction with respect to cortisol level indicating that diurnal variation was similar between groups.
Figure 3.
Diurnal cortisol patterns for participants with SCI* and without SCI
*One outlier value removed for beep 5 (cortisol value = 3.01 μg/dL)
Experience of daily stressors (Q4)
Before examining the effects of stress on cortisol and mood outcomes, we examined group differences in the experience of the four kinds of stress. There was no difference in the presence of stress between SCI and controls. There also was no group main effects for activity related stress, social stress or event-related stress indicating that there were no differences in the experience of daily stressors between SCI and controls. However, there was a main effect of time of activity-related stress (F = 2.548, p = 0.007) such that activity-related stress generally declined throughout the day with peaks in the earlier part of the day and lowest in the evening. There was not a significant interaction of time by group indicating the effect of time on activity-related stress was similar between groups.
Effects of daily stressors on cortisol (Q4)
The presence of stress was first modeled on cortisol. There was a significant interaction by group such that for participants with SCI, when stress was present, cortisol values were significantly lower than when stress was not present (0.646 [0.032] μg/dL vs. 0.712 [0.029] μg/dL, p = 0.029; 95% CI: -0.011, 0.122); there was no within group difference for controls. There was a significant interaction of social stress such that for every unit of increased social stress, cortisol is lower for participants with SCI compared to those without. For activity-related or event-related stress, there were no main effects, group main effects or interaction effects on cortisol values.
Effects of daily stressors on mood (Q4)
For positive mood, there was no significant main effect of stress presence; however, there were significant main effects for activity-related, social and event-related stress, such higher stress was associated with lower the positive mood. There were no significant group main effects or interaction effects for any of the kinds of stress indicating the effect (or lack of effect) of these stressors on positive mood was similar between groups.
For negative mood, there was no main effect for the presence of stress or social stress, but there were significant main effects for activity-related and event-related stress. There were no significant group main effects or interaction effects for these four kinds of stress. For agitated mood, there was a significant interaction by group for the presence of stress such that for those without SCI, when stress was present, its effect on agitated mood was significantly higher than when stress was not present (z-scores; 0.448 vs. -0.025, S.E. = 0.127, p < 0.001; 95% CI: 0.224, 0.722); the same was not found for those with SCI. There was a significant main effect for activity-related stress and social stress and event-related stress such that greater stress was associated with higher agitated mood. There were no group main effects or interactions for any of these three models. Model estimates and covariance structures for cortisol and mood outcomes for each kind of stress are given in table 4.
Table 4.
Effects of stressors on Cortisol and mood, as estimated in separate models for CORT, PM, NM and AM outcomes
| Models (independent variables)a | CORTb Est. (S.E.) | PMb Est. (S.E.) | NMb Est. (S.E.) | AMb Est. (S.E.) |
|---|---|---|---|---|
| Presence of stress (yes or no) | ||||
| Presence of stress | NA | -0.113 (0.116) | 0.160 (0.010) | NA |
| Group (Non-SCI vs. SCI [referent]) | NA | -0.407 (0.217) | 0.009 (0.176) | NA |
| Group by presence of stress | -0.103 (0.040)1 | NS | NS | -0.381 (0.181)1 |
| Covariance matrixc | AR(1)H | CS | CS | CS |
| Activity-related stress | ||||
| Activity-related stress | -0.005 (0.016) | -0.226 (0.042)3 | 0.938 (0.227)3 | 1.543 (0.191)3 |
| Group (Non-SCI vs. SCI [referent]) | 0.025 (0.032) | -0.381 (0.197) | 0.398 (1.560) | -1.173 (0.783) |
| Group by activity-related stress | NS | NS | NS | NS |
| Covariance matrixc | AR(1)H | CS | CS | CS |
| Social stress | ||||
| Social stress | NA | -0.186 (0.089)1 | -0.240 (0.404) | 1.067 (0.382)2 |
| Group (Non-SCI vs. SCI [referent]) | NA | -0.532 (0.308) | 1.556 (0.795) | -1.590 (1.097) |
| Group by social stress | -0.082 (0.034)1 | NS | NS | NS |
| Covariance matrixc | AR(1)H | CS | CS | CS |
| Event-related stress (Q4) | ||||
| Event-related stress | -0.009 (0.015) | -0.318 (0.308)3 | 1.036 (0.206)3 | 1.211 (0.191)3 |
| Group (Non-SCI vs. SCI [referent]) | 0.016 (0.034) | -0.280 (0.193) | -0.202 (1.682) | -1.816 (0.800)1 |
| Group by event-related stress | NS | NS | NS | NS |
| Covariance matrixc | AR(1)H | CS | CS | CS |
All models are corrected for beep time; CORT models also were corrected for consumption of food, alcohol or tobacco (not shown); continuous stress predictor variables are standardized (z-scores)
Dependent variables; estimates represent the change in outcome variable with one unit change in predictor; estimates are presented in the outcome units (salivary cortisol = μg/dL and mood = z-scores)
Covariance matrix (of the outcome variable) with the lowest AIC value are used for estimate values (AR(1)H = autoregressive heterogeneous and CS = compound symmetry)
CORT = cortisol value; PM = positive mood; NM = negative mood; AM = agitated mood
NA = main effects are not reported when there is a significant interaction; NS = non-significant interaction
Significance values for F tests; p ≤ 0.05
p ≤ 0.01
p ≤ 0.0001
Exploratory analysis by level of injury and time since injury
The association of cortisol and injury level was explored by modeling injury level (tetraplegia vs. paraplegia) on cortisol and the interaction of beep by injury level, controlling for eating, drinking or smoking. There was no statistically significant difference by level of injury on cortisol or interaction of beep by level injury indicating that mean cortisol across and within beep times was similar between persons with tetraplegia and paraplegia. There also was no effect of time since injury on cortisol, controlling for beep time and eating, drinking or smoking.
Discussion
This is the first study to examine diurnal variation of and effect of stressors encountered in daily life on salivary cortisol and mood in an SCI sample using EMA. A key methodological contribution of this study is the demonstration of the feasibility of utilizing EMA to collect biological and behavioral data from participants with significant mobility impairments, and the value of using prospective, momentary methods to capture the experience of daily life with disability. Ecological momentary assessment has rarely been used in rehabilitation research, but has promise for providing valuable insight into complex and dynamic processes of living with disability (Seekins, Ipsen, & Arnold, 2007). With careful planning, as we have shown, kits can be designed in an accessible way that facilitates the collection of biological and behavioral data in the field during everyday life. Although on average, the SCI participants took slightly longer to respond to beeps, this was statistically significant at only one time point and none exceeded the 15 minute maximum lag time. These findings support the success of the data collection kit design, which can be replicated in other studies and with other populations. Moreover, by capturing experience quasi-randomly throughout the day, we are able to describe a more complete picture of daily stressors than would have been be achieved by using a single retrospective survey.
By using repeated sampling, another key finding is that overall concentration and diurnal variation of salivary cortisol in participants with SCI was similar to those without SCI, with an expected pattern of a gradual decline from morning to evening. There were also no significant differences between groups for any of the types of stress reported or the presence of stress generally at any given point in daily life. Allowing for the fact that sampling was not done during the morning or later evening hours of self-care routines that may be more rigorous and demanding for persons with SCI, daytime experience of daily life stressors was generally similar between persons with and without SCI in this study.
While concentration and diurnal patterns of cortisol were similar between groups and by level of injury and time since injury, when there was an association between stress and cortisol, it was only for persons with SCI and in the reverse direction than expected. This is a provocative finding, albeit preliminary. In most studies, an increase in cortisol in response to stress is found, which exposes the body tissues to greater hormone concentrations resulting in damage and dysregulation. However, several recent studies have posited that, in some conditions, deficient cortisol signaling (i.e., blunted response or decline in output) also contributes to disease (Raison & Miller, 2003). Low levels of cortisol and responsiveness have been found in individuals exposed to trauma (Heim, Newport, Bonsall, Miller, & Nemeroff, 2001) and those with low levels of cortisol who have sustained a traumatic event have been shown to be more susceptible to develop post-traumatic stress disorder (PTSD) (Delahanty, Raimonde, & Spoonster, 2000). Low concentrations or secretions of cortisol have also been found in other chronic conditions, such as chronic fatigue syndrome (Van Houdenhove et al., 2001) and fibromyalgia (Vandenderen, Boersma, Zeinstra, Hollander, & Vanneerbos, 1992). In recent years, research has suggested that individuals exposed to severe stress or who have stress-related disorders have decreased cortisol secretion (Heim, Ehlert, & Hellhammer, 2000). All of these recent findings point to areas for further exploration in the context of SCI, particularly those injuries that are acquired traumatically thereby increasing a risk for developing PTSD.
Although the mechanisms involved in the development of diminished cortisol secretion are speculative (Heim et al., 2000), our preliminary findings of an inverse relationship of stress and cortisol secretion for participants with SCI may reflect similar dynamics. While we did not assess the presence of PTSD in our sample, 22 out of 25 individuals sustained their injuries in traumatic circumstances (e.g., car crashes, falls, and diving accidents). The direct and indirect effects of the injury itself may alter the responsiveness of the HPA axis to certain stressful stimuli. In our study, mean cortisol levels across time were similar between groups whereas in other studies of individuals exposed to trauma, baseline cortisol secretion has been significantly lower compared to depressed individuals (Yehuda, Teicher, Trestman, Levengood, & Siever, 1996). The mechanism for a lower cortisol response to stress in the context of SCI is not known and any relationship to the impact of relative insulin resistance or loss of spinal afferent feedback would be merely speculative without further investigation.
Emotional reactivity to daily stressors in this study was similar to the findings of other investigations (Jacobs et al., 2007; Smyth et al., 1998). While there were no group differences for mood, persons without SCI had significantly greater agitated mood when stress was present than when it was not, which was not the case for persons with SCI. While preliminary, these results may hint at a certain emotional resistance to stress that living with SCI may confer while, physiologically, there may be greater sensitivity to the presence of stress, irrespective of type of stress as classified in this study. Although some differences were found with respect to the association of stress with cortisol and mood, group and interaction effects were largely non-significant in this study. These findings may suggest that the experience of stress in daily life and its association with cortisol and mood are, for the most part, similar between people living with SCI and their non-SCI peers. Presumptions that life with SCI is necessarily more stressful on any given day, at any given moment, may not be supported when using a prospective, repeated measures designs that are more sensitive to dynamics experienced in daily life. In fact, we advocate that such presumptions should be tested using methods that more fully capture the details of daily life with disability to pinpoint where disability and daily life interface to cause stress and threats to well being.
Limitations
There are several limitations of this pilot study. The small sample size limits generalization to the larger SCI population. Moreover, the SCI participants were drawn from a research registry and are likely to represent fairly high functioning and healthy participants, which may not be representative of other segments of the SCI population. The SCI participants in this study have been living with SCI for 16 years on average and this may account for much of the lack of group differences; a different picture may emerge with respect to daily stressors and their impact on outcomes in persons who are transitioning through early adjustment after injury, often a most difficult passage.
Other covariates known to effect stress, cortisol, and mood, such as sleep quality were not included in this study and may have had an effect on outcomes. We did not directly assess the presence of MDD, PTSD, use of psychoactive medication or history of concomitant brain injury at the time of SCI, all of which may also affect cortisol responsivity. Cortisol samples were not collected upon awakening and thus diurnal variation for the morning may not be fully representative had we collected samples earlier than 10 AM. Initially, we were concerned that collecting awakening cortisol would be additionally burdensome given an already demanding collection schedule for participants with SCI. However, given few problems with this protocol, future studies in this population can include collection of awakening cortisol. Compliance with sampling with respect to the time recorded in the daily diaries may be underestimated given previous research suggesting that self-reports tend to overestimate compliance (Broderick, Arnold, Kudielka, & Kirschbaum, 2004; Jacobs et al., 2005).
Directions for future research
The preliminary results from this pilot study point to an array of directions for future inquiry. One of the next steps with this study's data is to examine the qualitative aspects of what participants were doing when alerted to examine whether marked differences exist with respect to the kinds of activities comprising a typical day for each of the groups. Examining the adjustment process early after injury using this richer methodology can provide a level of insight into complex dynamics yet to be fully explored. By demonstrating the ability to capture salivary cortisol in the field in this population, further exploration of the association of biomarkers such as cortisol and factors relevant to the long-term health of persons with SCI can be pursued. Awakening cortisol response (ACR), which was not collected in this study, can provide further insight into the association of health and stress. For example, ACR has been associated with health factors particularly relevant for persons with SCI, such as bone density (Brooke-Wavell, Clow, Ghazi-Noori, Evans, & Hucklebridge, 2002), health status (Kudielka & Kirschbaum, 2003), and wound healing (Ebrecht et al., 2004).
A sex and gender difference in the impact of stress on health and well being, which also was not examined in this study, is another fruitful line of future inquiry. Because of the predominance of men in the SCI population, sex and gender differences are infrequently explored. Nevertheless, there is research to suggest that psychological stress can differentially impact health and well-being for women and men (Kunz-Ebrecht, Kirschbaum, Marmot, & Steptoe, 2004; Sandanger, Nygard, Sorensen, & Moum, 2004). Awakening cortisol response has also been associated with greater anticipatory stress on weekdays for women (Kunz-Ebrecht et al., 2004), a differential effect of psychosocial factors at work and home (Eller, Netterstrom, & Hansen, 2006), and the progression of artery wall thickening in women (Eller, Netterstrom, & Allerup, 2005).
The use of EMA in rehabilitation research has great potential to advance what we understand about daily life with disability and complex, interpersonal and broader social aspects of the adjustment processes. Factors such as uncontrollability and threats to the social self have been associated with HPA activation (Dickerson & Kemeny, 2004) and are particularly intriguing areas for further exploration as they manifest in the context of SCI and other disabilities. Moreover, the integration of biological data with behavioral data is an important advancement in rehabilitation psychology research. For example, physiological correlates of MDD or PTSD in this population are important targets of future investigation given the risk for developing these psychological disorders after injury. Moreover, very little is known about the physiological effects of HPA dysregulation associated with these disorders on long term health and risk of secondary condition exacerbation in persons with SCI.
As we have shown, the ability to capture biological and behavioral data in the field and during daily life in persons with significant mobility impairment is possible with careful planning. Advancements in technologies that allow for remote capture of these data make the conduct of such studies more and more feasible for clinical researchers. The integration of ambulatory measurement of biomarkers, such as cortisol, and the use of “biosensors” (i.e., instruments that record physiological or motor activity) provide useful data on onset, intensity, duration, and time course of physiological facets of behavior and causal and non-causal relationships between the environment, behavior, cognition, and physiology in natural settings (Haynes & Yoshioka, 2007).
Conclusions
The results of this pilot study highlight the importance of using more sophisticated methods than retrospective surveys to capture information about the interplay between psychological and physiological systems in the context of SCI, or any disabling condition, and in the context of daily life. The design and execution of this study demonstrate that EMA methods, such as those used here, can be feasibly and reliably used to capture psychological and biological data in the field from persons with significant mobility impairments. Using such methods to examine complex dynamics, such as stress reactivity in natural environments or during adjustment processes immediately after new injury, can help to refine biopsychosocial models in rehabilitation – bringing greater emphasis to the “bio” in these models and clarifying their links to psychological processes. Life stressors are complex, making it difficult to identify the unique contribution of each element of stress (Michaud, Matheson, Kelly, & Anisman, 2008). However, expansion and integration of our knowledge about stress and its effects across levels of organization require expansion and integration of methods across levels of analysis reflecting realities of life with SCI. Examining daily life stressors in tandem with their physiological concomitants allows a more full perspective for evaluating how specific stressors may impact physiological and behavioral outcomes for persons living with SCI and if, and where, meaningful differences truly exist in comparison with their non-SCI peers.
ACKNOWLEDGEMENTS
The authors thank the participants in this study for the time and effort they generously dedicated to this project and study coordinator Mary J. Burton, M.S. The authors also thank Kathleen Welch, Ph.D., University of Michigan Center for Statistical Consulting and Research, for her assistance with data analysis. This research was supported by a grant from the University of Michigan Clinical and Translation Science Award Pilot Grant Program (NIH, #UL1RR024986). The Rehabilitation Research Training program at the University of Michigan, Department of Physical Medicine and Rehabilitation, National Institute on Disability and Rehabilitation Research (#H133P030004), Office of Special Education and Rehabilitative Services, U.S. Department of Education provided support to Dr. Farrell.
Contributor Information
Claire Z. Kalpakjian, Department of Physical Medicine and Rehabilitation.
Debra J. Farrell, Department of Physical Medicine and Rehabilitation.
Kathie J. Albright, Department of Physical Medicine and Rehabilitation.
Anthony Chiodo, Department of Physical Medicine and Rehabilitation.
Elizabeth A. Young, Department of Psychiatry University of Michigan.
REFERENCES
- Bauman WA. Carbohydrate and lipid metabolism after spinal cord injury. Topics in Spinal Cord Injury Rehabilitation. 1997;2:1–22. [Google Scholar]
- Bay E, Sikorskii A, Gao FL. Functional status, chronic stress, and cortisol response after mild-to-moderate traumatic brain injury. Biological Research for Nursing. 2009;10:213–225. doi: 10.1177/1099800408326453. [DOI] [PubMed] [Google Scholar]
- Biondi M, Picardi A. Psychological stress and neuroendocrine function in humans: The last two decades of research. Psychotherapy and Psychosomatics. 1999;68:114–150. doi: 10.1159/000012323. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Brooke-Wavell K, Clow A, Ghazi-Noori S, Evans P, Hucklebridge F. Ultrasound measures of bone and the diurnal free cortisol cycle: A positive association with the awakening cortisol response in healthy premenopausal women. Calcified Tissue International. 2002;70:463–468. doi: 10.1007/s00223-001-2085-8. [DOI] [PubMed] [Google Scholar]
- Campagnolo DI, Bartlett JA, Chatterton R, Keller SE. Adrenal and pituitary hormone patterns after spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 1999;78:361–366. doi: 10.1097/00002060-199907000-00013. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: A multicenter analysis. Archives of Physical Medicine and Rehabilitation. 2004;85:1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. Journal of Nervous and Mental Disease. 1987;175:526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Ebrecht M, Hextall J, Kirtley LG, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology. 2004;29:798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Straub R. Physiological and emotional response to psychological stressors in psychiatric and psychosomatic disorders. Annals of the New York Academy of Sciences. 1998;851:477–486. doi: 10.1111/j.1749-6632.1998.tb09026.x. [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Allerup P. Progression in intima media thickness-the significance of hormonal biomarkers of chronic stress. Psychoneuroendocrinology. 2005;30:715–723. doi: 10.1016/j.psyneuen.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Hansen AM. Psychosocial factors at home and at work and levels of salivary cortisol. Biological Psychology. 2006;73:280–287. doi: 10.1016/j.biopsycho.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Frank R, Kashani J, Wonderlich S, Lising A, Visot L. Depression and adrenal function in spinal cord injury. American Journal of Psychiatry Research. 1985;142:252–253. doi: 10.1176/ajp.142.2.252. [DOI] [PubMed] [Google Scholar]
- Gerhart KA, Weitzenkamp DA, Kennedy P, Glass CA, Charlifue SW. Correlates of stress in long-term spinal cord injury. Spinal Cord. 1999;37:183–190. doi: 10.1038/sj.sc.3100804. [DOI] [PubMed] [Google Scholar]
- Glei DA, Goldman N, Chuang YL, Weinstein M. Do chronic stressors lead to physiological dysregulation? Testing the theory of allostatic load. Psychosomatic Medicine. 2007;69:769–776. doi: 10.1097/PSY.0b013e318157cba6. [DOI] [PubMed] [Google Scholar]
- Gold PW. The neurobiology of stress and its relevance to psychotherapy. Clinical Neuroscience Research. 2005;4:315–324. [Google Scholar]
- Haynes SN, Yoshioka DT. Clinical assessment applications of ambulatory biosensors. Psychological Assessment. 2007;19:44–57. doi: 10.1037/1040-3590.19.1.44. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Huang T, Wang Y, Chen S. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. American Journal of Physical Medicine & Rehabilitation. 2000;81:1582–1586. doi: 10.1053/apmr.2000.9173. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Nicolson NA, Derom C, Delespaul P, van Os J, Myin-Germeys I. Electronic monitoring of salivary cortisol sampling compliance in daily life. Life Sciences. 2005;76:2431–2443. doi: 10.1016/j.lfs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Little R, Rubin D. Statistical analysis with missing data. Wiley; New York, NY: 2002. [Google Scholar]
- McEwen BS. Stress, adaptation, and disease - Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? Journal of Psychiatry & Neuroscience. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress. 2008;11:177–197. doi: 10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Peeters F, Havermans R, Nicolson NA, deVries MW, Delespaul P, et al. Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatrica Scandinavica. 2003;107:124–131. doi: 10.1034/j.1600-0447.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Archives of General Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BPR. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neuroscience and Biobehavioral Reviews. 2005;29:457–467. doi: 10.1016/j.neubiorev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Palmer JB. Depression and adrenocortical function in spinal cord injury patients: a review. Archives of Physical Medicine and Rehabilitation. 1985;66:253–256. doi: 10.1016/0003-9993(85)90164-9. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Rintala DH, Robinson-Whelen S, Matamoros R. Subjective stress in male veterans with spinal cord injury. Journal of Rehabilitation Research and Development. 2005;42:291–304. doi: 10.1682/jrrd.2005.10.0155. [DOI] [PubMed] [Google Scholar]
- Sandanger I, Nygard JF, Sorensen T, Moum T. Is women's mental health more susceptible than men's to the influence of surrounding stress? Social Psychiatry and Psychiatric Epidemiology. 2004;39:177–184. doi: 10.1007/s00127-004-0728-6. [DOI] [PubMed] [Google Scholar]
- Seekins T, Ipsen C, Arnold NL. Using ecological momentary assessment to measure participation: A preliminary study. Rehabilitation Psychology. 2007;52:319–330. [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Smyth J, Ockenfels MC, Porter L, Kirschbaum C, Hellhammer DH, Stone AA. Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology. 1998;23:353–370. doi: 10.1016/s0306-4530(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostatis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. John Wiley and Sons; New York: 1988. pp. 629–649. [Google Scholar]
- Tabachnick B, Fidell L. Using Multivariate Statistics. 4th ed. Allyn & Bacon; Needham Heights, MA: 2001. [Google Scholar]
- Van Houdenhove B, Neerinckx E, Lysens R, Vertommen H, Van Houdenhove L, Onghena P, et al. Victimization in chronic fatigue syndrome and fibromyalgia in tertiary care - A controlled study on prevalence and characteristics. Psychosomatics. 2001;42:21–28. doi: 10.1176/appi.psy.42.1.21. [DOI] [PubMed] [Google Scholar]
- Vandenderen JC, Boersma JW, Zeinstra P, Hollander AP, Vanneerbos BR. Physiological effects of exhausting physical exercise in primary fibromyalgia syndrome (PFS): Is PFS a disorder of disorder of neuroendocrine reactivity. Scandinavian Journal of Rheumatology. 1992;21:35–37. doi: 10.3109/03009749209095060. [DOI] [PubMed] [Google Scholar]
- vanEck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. Journal of Clinical Endocrinology and Metabolism. 2000;85:2189–2196. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]