Neointimal hyperplasia in response to arterial injury is a complex process, classically believed to be the consequence of vascular smooth muscle cell proliferation and migration, and the synthesis of extracellular matrix 1, 2. Recently, it has been proposed that the neointimal lesion also consists of progenitor cells attracted to the site of vascular injury. In this review, we will summarize the reports that demonstrate an important role for progenitor cells in the development of intimal hyperplasia. We will also examine the involved cell types as well as the mechanisms underlying progenitor cell recruitment to the injured arterial wall.
Bone marrow derived progenitor cells contribute to the neointimal lesion after arterial injury
The contribution of bone marrow derived cells to the neointimal lesion has been demonstrated repeatedly using bone marrow transplant in conjunction with mouse models of vascular injury 3–6. One of the earliest studies of this phenomenon was performed by Sata et al using bone marrow cells, which express β-galactosidase (a product of the LacZ gene), from ROSA26 mice. Cells from these mice are easily identified as blue when stained with X-gal 3. The techniques were as follows. Wild type mice were lethally irradiated, then injected via tail vein with bone marrow cells derived from ROSA26 mice. After confirmation that the ROSA26 bone marrow cells had reconstituted the bone marrow and blood cell lines of the wild type mice, a wire injury of the femoral artery was performed. Histological analysis of the subsequent neointimal lesion at specified time points revealed the presence of LacZ positive, or transplanted bone marrow derived cells. Furthermore, double staining revealed that the LacZ positive cells had differentiated into both smooth muscle (α-smooth muscle actin or α-SMA positive) as well as endothelial (CD31+) like cells. As shown in Table 1, numerous subsequent studies have confirmed the existence of bone marrow derived cells in the neointima. While in most of these studies the mouse bone marrow transplant model was employed, differing methods of labeling bone marrow cells as well as different types of injury have contributed to a wide range of values for the percentage of neointimal cells that are of bone marrow origin (ranging from 20 to 66%)4, 5. Furthermore, circulating white blood cells in an animal after bone marrow transplant are also bone marrow derived. As has been previously demonstrated, these inflammatory cells, specifically macrophages, can contribute to the neointimal lesion. Thus, it is important to evaluate the neointima in these models with specific staining to determine whether bone marrow derived cells have progenitor cell, smooth muscle cell, endothelial cell, or white blood cell markers.
Table 1.
Contribution of bone marrow derived cells to mouse arterial injury models. Since progenitor cells are believed to differentiate into smooth muscle cells or endothelial cells, some studies have also analyzed the percentage of bone marrow derived cells that also express smooth muscle cell markers (SMA+) or endothelial markers, thus signifying differentiation into different types of arterial wall cells.
| Experimental model | Determination of cell origin | Time | % BM derived neointimal (NI) or endothelial cells | % BM derived medial cells | publication |
|---|---|---|---|---|---|
| Mouse iliac artery wire injury | BMT Male→female Y chromosome ISH |
4 wks | 56% of total cells in NI, 44% of SMA+ cells | No medial SMA+ cells | Han C et al. 20016 |
| Mouse femoral artery wire injury | BMTROSA26→WT X-gal staining |
4 wks | 63.0±9.3% of total NI cells | 45.9±6.9% total cells | Sata M et al. 20023 |
| Mouse carotid wire injury model | Labeling with retrovirus expressing GFP | 2 wks | ~10% of total endothelial cells | Not reported | Werner et al. 2002.27 |
| Mouse femoral artery wire injury | BMTeYFP→WT IF |
4 wks | 66±12% of SMA+ NI cells | Not reported | Wang C-H et al. 2006.5 |
| Mouse femoral artery wire injury | BMTGFP→WT IF |
4 wks | 20.5±5.7% total NI cells | 39.3±3.1% total cells | Tanaka K et al. 2003.4 |
ISH= in-situ hybridization, BMT=bone marrow transplant, NI=neointima, SMA=smooth muscle actin, IF=immunofluoresence (to visualize GFP or YFP positive cells)
The progenitor cell contribution to the neointima appears to be determined by the type of injury
Whereas the mouse wire injury model was used in all of the studies cited in Table I, it has been shown that the contribution of bone marrow derived progenitor cells to the arterial wall may vary depending on the type of injury 4. Using a model that transplanted bone marrow cells from a transgenic mouse that expresses GFP (GFP+ mouse) to a wild type mouse, Tanaka et al showed that three distinct types of mechanical injury produced varying degrees of bone marrow derived cell contribution to the arterial wall. In the first injury model, a 0.38mm straight spring wire was inserted into the mouse femoral artery to denude and dilate the artery. This model best recapitulates angioplasty procedures in humans, since it involves both vessel wall dilatation and endothelial denudation. In the second model, a polyethylene tube was placed around the mouse femoral artery (perivascular cuff induced injury). In the last model, the mouse common carotid artery was ligated just proximal to the bifurcation. Wire injury led to large numbers of GFP+ cells in both the media and the neointima, whereas perivascular cuff placement and carotid artery ligation resulted in significantly fewer GFP+ medial and neointimal cells. (Table 2) The authors also studied the fate of the bone marrow derived cells by examining α-SMA expression. Whereas a significant number of GFP+ cells in the neointima and the media after wire injury were also α-SMA+, only a few of the GFP+ cells expressed α-SMA in the other two injury models 4. Finally, the authors showed that while each mode of injury induced differing degrees of inflammation, the degree of inflammation did not correlate with the contribution of bone marrow derived cells to the neointima. In all models, inflammatory cells were predominately macrophages, with the greatest infiltration of macrophages reported after perivascular cuff placement. At 4 weeks after injury, few macrophages were detected in the lesions produced by wire injury or carotid ligation, even though these modes of injury resulted in the greatest number of bone marrow derived cells in the arterial lesion.
Table 2.
Percentage of GFP+ cells in the neointima and media of BMTGFP→WT mice 4 weeks after injury. (n=4)4
| Wire injury | Perivascular cuff | Carotid artery ligation | |
|---|---|---|---|
| Neointima | 38.9±5.8% | 7.0±2.1% | 24.1±5.3% |
| Media | 61.4±5.8 | 15.1±2.2% | 33.1±8.2% |
The findings of these studies clearly demonstrate that bone marrow cell contribution to arterial lesions can vary widely depending on the animal model of arterial injury. Similarly, previous studies of human vascular lesions suggest that the cellular constituents of vascular lesions also vary with the type of injury, for example, atherosclerosis vs. restenosis or restenosis after balloon angioplasty vs. vein graft intimal hyperplasia after vascular bypass 7, 8. In humans, the specific contribution of progenitor cells to vascular disease is less well defined. Stem cell therapy for vascular disease in humans is currently being tested in clinical trials, 9, 10 even though it has never been proven with the elegance of the animal models that progenitor cells actually contribute to the arterial wall response to injury. The animal models, however, remain crucial in achieving a better understanding of the mechanisms underlying progenitor cell recruitment. In this review, we focus specifically upon mouse models of vascular injury that result in intimal hyperplasia and restenosis. However, it is important to note that none of these models precisely recapitulate the human disease process.
Characterization of the progenitor cells that contribute to animal models of intimal hyperplasia
Bone marrow cells
In the foregoing mouse models of restenosis, the entire contents of the bone marrow were transplanted into the recipient mouse. Bone marrow is primarily composed of cells of the blood cell lineages (e.g. myelocytes, lymphocytes, monocytes, megakaryocytes, erythrocytes) at various stages of differentiation. Stem cells, defined as cells that are capable of self-renewal and differentiation, constitute in the human only approximately 0.01% of the total bone marrow. Together, stem cells and also progenitor cells (cells that are more differentiated) account for approximately 0.1% of total bone marrow cells. Thus, it is only a small percentage of bone marrow cells that are capable of directly repopulating the neointimal or endothelial layers after arterial injury.
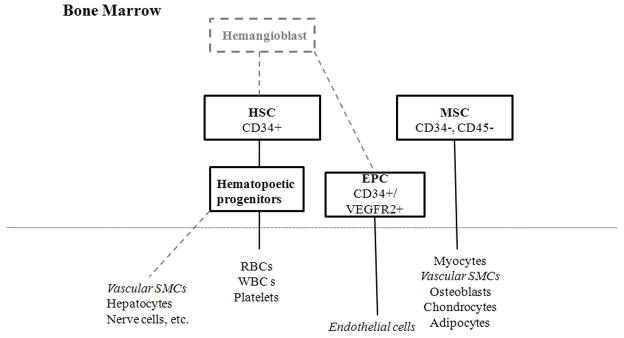
In humans, stem cells have been classified broadly into hematopoietic stem cells (HSC) (CD34+/CD38−) or non-hematopoietic or mesenchymal stem cells (MSC) (Figure 1) 11. HSCs, which differentiate into all the blood cell lines, are believed to be derived from a very early embryonic precursor – the hemangioblast. Of note, many believe that the hemangioblast is a common precursor for both HSCs and endothelial progenitor cells (EPC), although this point remains controversial.12–14 EPCs, however, are thought to be important in adult vasculogenesis and, relevant to this review, may also participate in the neointimal lesion. MSCs, on the other hand, have been described to differentiate into muscle cells, osteoblasts, chondrocytes, or adipocytes. Important cell surface markers that distinguish between the different types of progenitor cells are summarized in Table 3. In the following sections we will review how both HSCs and MSCs may contribute to neointimal hyperplasia.
Figure 1.
Schematic of stem cell and progenitor cell classification. The hemangioblast is believed to be an embryonic precursor for HSCs and possibly also EPCs. HSCs differentiate into hematopoietic progenitors and then into the various blood cell lineages, but there is also evidence that HSCs can transdifferentiate into non-hematopoietic cells. MSCs are believed to differentiate into multiple mesenchymal lineages. Dotted grey lines indicate points that are still controversial.
Table 3.
Differential expression of cell surface markers between HSCs, EPCs, and MSCs. Markers may vary between species; mouse markers are shown because it is the most widely studied animal model. One of the most important distinctions between the groups is that while HSCs are CD34+ and CD45+, MSCs are CD34− and CD45−. CD117 is also known as c-kit.
| HSC | EPC | MSC | |||
|---|---|---|---|---|---|
| Human | Mouse | Human | Mouse | Human | Mouse |
| CD34+ | CD117+ | CD34+ | CD31+/dim | CD90+ | CD90+ |
| CD117+ | CD150+ | CD31+ | VE-cadherin+ | CD157+ | CD44+ |
| CD90+ | Sca-1+ | VE-cadherin+ | Stro-1+ | CD29+ | |
| CD243+ | VEGFR2+ | CD10+ | CD49e+ | ||
| CD133+ | E-selectin+ | CD105+ | |||
| CD45+ | CD202+ | CD71+ | |||
| ?AC133+ | |||||
| CD38− | CD48/CD41− | CD14− | CD45− | CD45− | |
| Lineage & minus; | Lineage & minus; | CD15− | CD11b− | CD34− | CD11b− |
Hematopoietic stem cells
HSCs are defined by the properties of self-renewal and the ability to differentiate into cells of all blood lineages. Although the accepted marker for human HSCs is CD34+, the gold standard for identifying these cells remains a reconstitution assay. Though impractical in humans, in the mouse a single HSC is capable of reconstituting the entire hematopoietic system for the life of the animal15, 16. In the mouse, HSCs are characterized by the combination of various markers, including c-kit+/sca-1+/lineage depleted, and are often referred to as the KSL cell population.
Some authors have proposed that HSCs can differentiate into muscle cells (including smooth and skeletal muscle as well as myocardial cells), neural cells, hepatocytes, as well as epithelial, kidney, intestinal and pancreatic cells 17–19. Moreover, early studies by Sata et al. have demonstrated that HSC’s are important in the bone marrow’s contribution to the neointima 3. HSCs, or KSL cells, were isolated from ROSA26 mice and just these cells, rather than the entire bone marrow, were transplanted into wild type mice. Mice then underwent femoral artery wire injury and the resulting neointimal lesion was shown to contain LacZ+ cells, some of which were also α-SMA positive. The authors therefore concluded that the HSC fraction of bone marrow could give rise to not only hematopoietic cells, but also vascular cells involved in intimal hyperplasia. Despite these findings, the ability of HSCs to differentiate into non-hematopoietic cells (i.e. SMCs) remains controversial 20. In studies where the mouse bone marrow has been reconstituted with a single HSC, transdifferentiation of HSCs into non-hematopoietic cells is extremely rare 21–23. In fact, when Sata’s group repeated their initial experiments using a single HSC to reconstitute the bone marrow rather than (c-kit+/sca-1+/lin−) cells, they found very few cells in the neointima that were the progeny of the single transplanted HSC, suggesting that the population of c-kit+/sca-1+/lin− cells used in their original experiments may have been contaminated with other progenitor cells 3, 20, 23.
Endothelial progenitor cells
EPCs were first isolated by Asahara et al. from human peripheral blood CD34+ cells 24. Asahara found that after 7 days of culture on fibronectin coated plates, not only were the cells morphologically different from freshly isolated CD34+ cells, but there was also a significantly higher percentage of cells that exhibited endothelial cell markers including CD31, Flk-1, Tie-2, or E-selectin. EPCs are currently defined as cells that express both progenitor and endothelial cell markers. In human cells, these markers would be CD34+ (progenitor) and VEGFR2+ (for endothelial cells) 25.
In the biology of intimal hyperplasia, it is believed that a faster rate of re-endothelialization after an arterial injury that results in intimal denudation, can reduce the formation of intimal hyperplasia 26. Werner et al. reported that bone marrow derived progenitor cells contributed to the endothelial layer after mouse carotid wire injury 27. Using retroviral infection to label bone marrow cells with a virus expressing GFP, these authors showed that up to 10% of ECs (vWF+ cells) were also GFP+, and therefore of bone marrow origin. Follow-up studies by this group reported that mouse spleen-derived mononuclear cells could differentiate into cells with characteristics of EPCs. Intravenous injection of these mouse-derived EPCs after wire carotid injury accelerated re-endothelialization and decreased neointimal hyperplasia, thus suggesting that EPCs may indeed play an important role in regulating neointimal formation 28.
Mesenchymal stem cells
In contrast to hematopoietic stem cells, there is also a population of non-hematopoietic stem cells or mesenchymal stem cells (MSC) that are believed to originate from bone marrow stromal cells and differentiate into myocytes, osteoblasts, chondrocytes, and adipocytes 11. Human MSCs typically express several cell surface markers such as CD105, CD44, CD90, CD71, and Stro-1, although none of these are specific to MSCs 11. MSCs are, however, distinct from HSCs and other hematopoietic cells in that they generally do not express CD34 and CD45. Since there are no reliable MSC markers, these cells are often isolated using specific cell culture conditions. Typically, a single-step purification method using adherence to plastic cell culture plates is employed. This results in a population of fibroblast-like cells, which are characterized as MSCs based on their ability to differentiate into multiple mesenchymal lineages (e.g. osteogenic, chondrogenic, myogenic, etc.) 29
Studies have shown that MSCs home to areas of injury after both site-directed and systemic administration 11. MSCs have been studied extensively in the context of cardiac tissue repair and are currently one the of the cell types being studied in clinical trials of cardiac regeneration following myocardial infarction 30, 31. The contribution of MSCs specifically to restenosis has only recently been explored 32. Irradiated wild type mice underwent bone marrow transplantation with MSCs derived from GFP+ mice. The mice were also transplanted via tail vein injection with whole bone marrow from GFP− mice since MSCs alone would not be expected to fully rescue these mice from myeloablative doses of radiation. Two months after transplant and successful engraftment, femoral artery wire injury was performed. After four weeks, the injured vessels developed a significant amount of intimal hyperplasia containing GFP+ cells (39±17%), indicating a robust contribution of bone marrow derived MSCs to this process.
Circulating progenitor cells
In addition to progenitor cells residing in the bone marrow, a constant small population of peripheral circulating progenitor cells has also been described 24, 33. It is thought that this population of circulating progenitor cells can be isolated from peripheral mononuclear cells and have the ability to differentiate into other cell types, such as endothelial cells or smooth muscle cells34, 35. Simper et al. for example, demonstrated that smooth muscle cells could be derived from the peripheral blood of normal, healthy human subjects 34. Specifically, these authors isolated mononuclear cells and cultured these cells in media containing PDGF-BB which resulted in the induction of smooth muscle cell differentiation. Immunocytochemistry and western blot analysis of these cells revealed that they not only possessed typical SMC markers (α-SMA, smooth muscle myosin heavy chain, and calponin), but they also stained positive for the progenitor marker CD34 and the VEGF receptors (Flt1 and Flk1).
Similarly, Zhao et al. have shown that peripheral blood mononuclear cells can give rise not only to smooth muscle progenitor cells, but also pleuripotent stem cells 35. They identified a subset of peripheral blood monocytes that display a fibroblast-like morphology but exhibit both monocyte (CD14) and HSC (CD34 and CD45) markers. These cells could be induced to differentiate into macrophages, T lymphocytes, epithelial cells, endothelial cells, neuronal cells, and hepatocytes 35. Their findings suggest that progenitor cells that contribute to tissue repair after injury can be derived from the population of circulating cells and not necessarily directly from the bone marrow itself.
To further address whether circulating progenitor cells, as opposed to bone marrow derived progenitor cells, contribute to the arterial response after injury, Tanaka et al. developed a parabiotic model in which a GFP transgenic mouse was conjoined subcutaneously (no direct vascular anastamoses) with a wild type mouse 36. These authors found as early as 10 days and up to 20 weeks after surgery, that 35–40% of circulating leukocytes in a wild type mouse were GFP+. After femoral artery wire injury of the wild-type mouse, GFP+ cells were detected in both the neointima (14.8±4.5%) and media (31.1±8.8%), thereby suggesting that a portion of the cells in the injured arterial wall were derived from the pool of circulating peripheral cells. Furthermore, GFP+ cells found in the injured arterial wall were also shown to stain positively for CD31 and α-SMA, implying the presence of a cohort of circulating progenitor cells that has the potential to give rise to both endothelial and smooth muscle cells.
Mechanisms underlying progenitor cell recruitment to sites of arterial injury
Bone marrow derived progenitor cell recruitment to neointimal hyperplasia after vascular injury can be conceptualized in three stages: 1) mobilization of cells from the bone marrow, 2) migration and recruitment of bone marrow cells to the site of injury, and 3) differentiation of bone marrow cells into mature vascular cells, such as endothelial or smooth muscle cells. Cytokines and chemokines that have been shown to be important in these steps include but are not limited to Granulocyte Colony Stimulating Factor (G-CSF), Stromal derived factor-1α (SDF-1α), c-kit and c-kit ligand (KitL, also known as Stem Cell Factor or SCF), Matrix Metalloproteinase -9 (MMP-9), and Vascular endothelial growth factor (VEGF) as well as its receptor (VEGFR). In the following sections we will review each stage of progenitor cell recruitment and the current understanding of the important involved chemokines and cytokines.
Mobilization of progenitor cells from the bone marrow
It is believed that bone marrow progenitor cells (HSC or MSCs) are mobilized into the peripheral circulation in response to stress signals produced at the time of injury 37. In the bone marrow, progenitor cells exist in a complex environment consisting of bone marrow stromal cells and extracellular matrix (ECM) rich in fibronectin, collagens, and various proteoglycans 37. In order to exit the bone marrow, progenitor cells must migrate through a vascular barrier (bone marrow venous sinuses) that separates the hematopoietic compartment from the circulation. Quiescent progenitor cells are believed to be attached to bone marrow stromal cells or ECM through specific binding interactions, including VLA-4/VCAM-1, SDF-1α/CXCR4, CD44/HA (hyaluronic acid), and interactions between P-E- and L- selectin 37. For example, VCAM-1 is constitutively expressed by bone marrow endothelial and stromal cells, and disruption of the VCAM-1/VLA-4 interaction by antibodies to VCAM-1 or VLA-4 ultimately leads to progenitor cell mobilization.. 38, 39 Additionally, α4 and β2 integrins have also been shown to play a role in the interactions between progenitor cells and the bone marrow microenvironment, and defects in integrin expression have also lead to increased progenitor cell mobilization. 40, 41
G-CSF has been well established as a mobilizer of stems cells in both humans and mice 42, 43. Treatment with G-CSF leads to the accumulation of proteases, particularly neutrophil elastase and cathespin G, in the bone marrow and concurrent downregulation of their inhibitors. Neutrophil elastase and cathespin G, in turn, lead to cleavage of key adhesion molecules, including VCAM-1. Mobilization of progenitor cells by G-CSF has also been shown to be dependent on MMP-9, as Heissig et al have reported that G-CSF-induced progenitor cell mobilization was impaired in MMP-9−/− mice 44.
The possibility that treatment with G-CSF may increase intimal hyperplasia was suggested by the results of the MAGIC Cell trial, a randomized control clinical trial examining the effect of G-CSF mobilized peripheral blood stem cells in cardiac function after myocardial infarction and coronary stenting 45, 46. The study found a trend towards increased restenosis in the patient cohort treated with G-CSF. This finding prompted follow-up studies of how G-CSF might affect neointimal hyperplasia in animal models. In a model of rabbit iliac artery stenting, Cho et al reported that at 60 days after stenting, rabbits treated with G-CSF developed significantly more intimal hyperplasia when compared to rabbits treated with placebo (0.34±0.04 vs. 0.26±0.04, p=0.015) 47. Furthermore, the authors showed that at early time points after injury, treatment with G-CSF not only increased total peripheral white blood cell count, but specifically increased the number of putative EPCs (CD31+, VE-cadherin+, CD34+, KDR+) and smooth muscle progenitor cells (VE-cadherin+/a-SMA+ or CD31+/a-SMA+). Culture of these cells over 3 weeks with VEGF or PDGF resulted in endothelial (cobble stone shape and CD31+) and smooth muscle like cells (hill and valley morphology and a-SMA+), respectively. Therefore, increasing the number of mobilized progenitor cells may be beneficial to some types of injury (myocardial infarction), but may exacerbate others (arterial injury).
In addition to the effects of G-CSF, the interaction between SDF-1α and its receptor CXCR4 has also been shown to be important in regulating progenitor cell survival, cell cycle and mobilization 37. Intravenous administration of exogenous SDF-1α as well as treatment with a specific CXCR4 inhibitor (AMD-3100) rapidly induces progenitor cell mobilization in both humans and mice 48. Heissig et al. have shown that SDF-1α induces MMP-9 in the bone marrow, leading to cleavage of membrane bound KitL (mKitL) to soluble KitL (sKitL). This in turn results in increased progenitor cell cycling and enhanced cell motility, and ultimately leads to progenitor cell mobilization 5, 44, 48, 49. MMP-9-induced cleavage of mKitL to sKitL has also been demonstrated to be essential in the formation of intimal hyperplasia. While vascular injury in MMP-9−/− mice resulted in minimal intimal hyperplasia, treatment with exogenous sKitL was found to “rescue” these mice and increase intimal hyperplasia by 2.5 fold 5. Furthermore, mobilization of bone marrow derived cells, and consequently intimal hyperplasia, can be inhibited by administration of the drug Gleevec, a c-kit inhibitor 5.
Progenitor cell mobilization has been studied extensively in the context of wound healing and vasculogenesis. The data from these studies have shown that certain disease states may alter bone marrow cell mobilization in response to injury. Of note, these disease states are often the co-morbidities present in patients with peripheral vascular disease. Patients with diabetes have recently been found to have fewer circulating progenitor cells, as demonstrated by peripheral blood analyses. 50–52 Both diabetes and advanced age have been shown to impair progenitor cell mobilization in mice. 53, 54 In their studies of wound healing in diabetic mice, Gallagher et al. have shown that impaired nitric oxide synthase (NOS) activation results in decreased EPC mobilization and therefore impaired vasculogenesis and wound healing. Hyperbaric oxygen therapy, by activating NOS, increases nitric oxide (NO) production and therefore increases EPC mobilization from the bone marrow. 53 Furthermore, the group showed that injection of exogenous SDF-1α into the wound acted synergistically with hyperbaric oxygen treatment in mobilizing EPCs into the peripheral circulation. Also related to an impaired response to tissue hypoxia, work by Bosch-Marce et al demonstrated in mice that aging leads to a gradual loss-of-function in hypoxia-inducible-factor 1α (HIF-1α). This resulted in decreased progenitor cell mobilization, decreased expression of angiogenic cytokines, and ultimately poor recovery of limb perfusion following ischemic injury. 54 These effects could be reversed, however, by administration of an adenoviral vector that expressed a constitutively active form of HIF-1α. While the process of vasculogenesis is distinct from that of intimal hyperplasia and restenosis, the findings from the wound healing literature will likely be important in achieving a better understanding of progenitor cell mobilization after vascular injury. The forgoing studies reflecting the numerous cytokines, chemokines, and signaling cascades that are involved in progenitor cell mobilization will ultimately need to be considered in the context of pre-existing disease states such as diabetes or advanced age, which may have significant consequences for efficient progenitor cell mobilization and recruitment.
Migration of progenitor cells to the site of injury
Once progenitor cells are in the peripheral circulation, they must be attracted to the site of tissue injury. In addition to playing an essential role in progenitor cell mobilization, the SDF-1α/CXCR4 axis has been reported to be involved in progenitor cell recruitment in numerous injury models including neointimal hyperplasia 55–57. In vitro studies have shown that SDF-1α induces EPC and CD34+ cell migration and CD34+ cell adhesion 58–60. In the wound healing literature, SDF-1α has been shown to play an important role in the recruitment of EPCs to diabetic wounds in mice. 53 In the mouse wire injury model, Zernecke et al demonstrated that blockade of SDF-1α signaling with either a SDF-1α blocking antibody, lentiviral-based local gene transfer of a mutant SDF-1α, or by transplantation of bone marrow cells deficient in CXCR4 resulted in decreased intimal hyperplasia which was associated with decreased bone marrow derived neointimal smooth muscle cells 57. The authors reported that SDF-1α induced platelet adhesion at the site of injury, and that subsequent release of platelet p-selectin led to progenitor cell adhesion and recruitment. These same mechanistic findings demonstrating an important role of SDF-1α and platelets in progenitor cell recruitment were confirmed by Massberg et al who studied this process in vivo using real-time video-fluorescence microscopy 61.
On a molecular level, integrins such as VLA-4, LFA-1 and α5β1 have been shown to play essential roles in progenitor cell adhesion 5, 60, 62. Notably, the α5β1 integrin has been shown to be upregulated by statin therapy 62. Consequently, administration of simvastatin to rats undergoing carotid balloon injury resulted in accelerated and more complete re-endothelialization due to increased EPC incorporation into the injured artery 62.
Other factors, such as VEGF and its receptor, have also been shown to stimulate progenitor cell migration and recruitment to sites of tissue injury 63–65. This complex process clearly involves numerous signaling pathways which are just beginning to be understood.
Differentiation of progenitor cells into mature vascular cells
The differentiation of bone marrow derived progenitor cells into vascular smooth muscle cells or smooth muscle-like cells or endothelial cells is the final step in the recruitment of bone marrow cells into the neointimal lesion. This phenomenon has been studied in vitro using several different stem and progenitor cell lines. TGF-β, through Smad3 signaling, has been shown to induce neural crest stem cells to differentiate into smooth muscle cells, 66 and PDGF-BB has been demonstrated to induce TR-BME2 cells, a mouse bone marrow derived EPC line, to differentiate into contractile and synthetic SMCs 66, 67. Both cytokines have been shown to be upregulated after arterial injury. In terms of endothelial cell differentiation, VEGF is the most studied chemokine. Of note, although EPCs are believed to be the major source of ECs, it has also been reported that MSCs cultured in the presence of VEGF can also differentiate into cells with phenotypic and functional features of endothelial cells 68.
It has been proposed that the direct cell/cell contact may be a stimulus of progenitor cell differentiation. Several studies have shown that direct cell-to-cell contact between MSCs and cardiomyocytes or smooth muscle cells results in MSC differentiation into these two cell types 69,70. Interestingly, it has also been reported that co-culture of MSCs with EPCs drives MSCs to differentiate into endothelial-like cells 32. Finally, many of the factors that have been found to be important in progenitor cell mobilization and/or migration may also play a role in differentiation. For example, co-culture of eYFP (yellow fluorescent protein) expressing progenitor cells with VSMCs expressing Kit Ligand stimulated differentiation of progenitor cells into smooth muscle cells, as reflected by cells that were double positive for eYFP and α-SMA. Consequently, addition of anti-KitL antibody to stimulated VSMCs expressing KitL resulted in less differentiation 5.
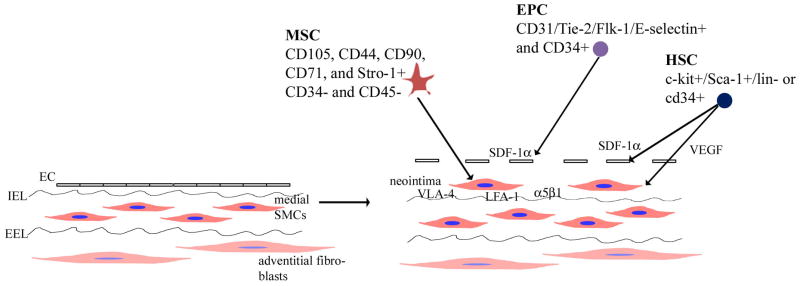
The contribution of different progenitor cells to the arterial response to injury is summarized in Figure 2. Together, these findings demonstrate that although the process of progenitor cell recruitment to sites of injury can be conceptualized in three steps, it is apparent that the steps are closely related in that many signaling molecules play important roles throughout this entire complex process.
Figure 2.
Summary
Bone marrow derived progenitor cells represent a new source of smooth muscle cells and endothelial cells that contribute to or modulate intimal hyperplasia after arterial injury. Numerous mouse models have been studied to gain insight into which progenitor cells are important, although the answers are still unclear. Whereas many studies have demonstrated the plasticity of HSCs, it is likely that MSCs also contribute to the smooth muscle cells and perhaps also the endothelial cells that repopulate the injured artery. Identification of the signaling mechanisms that underlie bone marrow progenitor cell recruitment to the neointima is only in its early stages. However several molecules, such as SDF-1α and KitL have emerged as potential targets for molecular therapy. Despite these advances in understanding progenitor cell recruitment, it is also imperative that these observations, made in mouse models of arterial injury, be validated in cases of human disease. If progenitor cells do indeed represent a significant fraction of neointimal cells, then an in-depth understanding of how the cells migrate from the bone marrow to the site of injury is essential for the development of targeted therapies for arterial restenosis.
Footnotes
No competing interests declared.
References
- 1.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. The Journal of pathology. 2000 Feb;190(3):300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arteriosclerosis, thrombosis, and vascular biology. 1997 Mar;17(3):417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 3.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nature medicine. 2002 Apr;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circulation research. 2003 Oct 17;93(8):783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, Fazel S, Li RK, Yau TM, Weisel RD, Stanford WL, Verma S. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circulation research. 2006 Sep 15;99(6):617–625. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 6.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. Journal of vascular research. 2001 Mar–Apr;38(2):113–119. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 7.Zalewski A, Shi Y, Johnson AG. Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circulation research. 2002 Oct 18;91(8):652–655. doi: 10.1161/01.res.0000038996.97287.9a. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Fan YS, Chow LH, Van Den Diepstraten C, van Der Veer E, Sims SM, Pickering JG. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circulation research. 2001 Sep 14;89(6):517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- 9.Miglionico M, Patti G, D’Ambrosio A, Di Sciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: a prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008 Apr 1;71(5):600–604. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 10.Adams B, Xiao Q, Xu Q. Stem cell therapy for vascular disease. Trends in cardiovascular medicine. 2007 Oct;17(7):246–251. doi: 10.1016/j.tcm.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells (Dayton, Ohio) 2007 Nov;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D. Hemangioblast does exist. Leukemia research. 2008 Jun;32(6):850–854. doi: 10.1016/j.leukres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Xiong JW, Yu Q, Zhang J, Mably JD. An Acyltransferase Controls the Generation of Hematopoietic and Endothelial Lineages in Zebrafish. Circulation research. 2008 Apr 3; doi: 10.1161/CIRCRESAHA.107.163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development (Cambridge, England) 1999 Nov;126(21):4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 15.Becker AJ, Mc CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963 Feb 2;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 16.Till JE, McCulloch EA, Siminovitch L. Isolation of Variant Cell Lines During Serial Transplantation of Hematopoietic Cells Derived from Fetal Liver. Journal of the National Cancer Institute. 1964 Oct;33:707–720. [PubMed] [Google Scholar]
- 17.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001 May 4;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 18.Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation. 2003 Oct 28;108(17):2070–2073. doi: 10.1161/01.CIR.0000099501.52718.70. [DOI] [PubMed] [Google Scholar]
- 19.Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nature medicine. 2003 Dec;9(12):1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- 20.Iwata H, Sata M. Potential contribution of bone marrow-derived precursors to vascular repair and lesion formation: lessons from animal models of vascular diseases. Front Biosci. 2007;12:4157–4167. doi: 10.2741/2377. [DOI] [PubMed] [Google Scholar]
- 21.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science (New York, NY. 2002 Sep 27;297(5590):2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 22.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004 Apr 8;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 23.Sahara M, Sata M, Matsuzaki Y, Tanaka K, Morita T, Hirata Y, Okano H, Nagai R. Comparison of various bone marrow fractions in the ability to participate in vascular remodeling after mechanical injury. Stem cells (Dayton, Ohio) 2005 Aug;23(7):874–878. doi: 10.1634/stemcells.2005-0012. [DOI] [PubMed] [Google Scholar]
- 24.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science (New York, NY. 1997 Feb 14;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 25.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circulation research. 2004 Aug 20;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi K, Nakamura S, Morishita R, Moriguchi A, Aoki M, Matsumoto K, Nakamura T, Kaneda Y, Sakai N, Ogihara T. In vivo transfer of human hepatocyte growth factor gene accelerates re-endothelialization and inhibits neointimal formation after balloon injury in rat model. Gene therapy. 2000 Oct;7(19):1664–1671. doi: 10.1038/sj.gt.3301284. [DOI] [PubMed] [Google Scholar]
- 27.Werner N, Priller J, Laufs U, Endres M, Bohm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arteriosclerosis, thrombosis, and vascular biology. 2002 Oct 1;22(10):1567–1572. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 28.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circulation research. 2003 Jul 25;93(2):e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Hisha H, Taketani S, Adachi Y, Li Q, Cui W, Cui Y, Wang J, Song C, Mizokami T, Okazaki S, Li Q, Fan T, Fan H, Lian Z, Gershwin ME, Ikehara S. Characterization of mesenchymal stem cells isolated from mouse fetal bone marrow. Stem cells (Dayton, Ohio) 2006 Mar;24(3):482–493. doi: 10.1634/stemcells.2005-0219. [DOI] [PubMed] [Google Scholar]
- 30.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American journal of cardiology. 2004 Jul 1;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature medicine. 2005 Apr;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 32.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, Lan YJ, Yeh CH, Stanford WL. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arteriosclerosis, thrombosis, and vascular biology. 2008 Jan;28(1):54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 33.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962 Jun;19:702–714. [PubMed] [Google Scholar]
- 34.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002 Sep 3;106(10):1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2003 Mar 4;100(5):2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka K, Sata M, Natori T, Kim-Kaneyama JR, Nose K, Shibanuma M, Hirata Y, Nagai R. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. Faseb J. 2008 Feb;22(2):428–436. doi: 10.1096/fj.06-6884com. [DOI] [PubMed] [Google Scholar]
- 37.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. Journal of cellular biochemistry. 2006 Oct 15;99(3):690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 38.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proceedings of the National Academy of Sciences of the United States of America. 1993 Oct 15;90(20):9374–9378. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proceedings of the National Academy of Sciences of the United States of America. 1995 Oct 10;92(21):9647–9651. doi: 10.1073/pnas.92.21.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001 Oct 15;98(8):2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 41.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Molecular and cellular biology. 2003 Dec;23(24):9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proceedings of the National Academy of Sciences of the United States of America. 2001 Aug 28;98(18):10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavallaro AM, Lilleby K, Majolino I, Storb R, Appelbaum FR, Rowley SD, Bensinger WI. Three to six year follow-up of normal donors who received recombinant human granulocyte colony-stimulating factor. Bone marrow transplantation. 2000 Jan;25(1):85–89. doi: 10.1038/sj.bmt.1702072. [DOI] [PubMed] [Google Scholar]
- 44.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002 May 31;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004 Mar 6;363(9411):751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 46.Kang HJ, Kim HS, Koo BK, Kim YJ, Lee D, Sohn DW, Oh BH, Park YB. Intracoronary infusion of the mobilized peripheral blood stem cell by G-CSF is better than mobilization alone by G-CSF for improvement of cardiac function and remodeling: 2-year follow-up results of the Myocardial Regeneration and Angiogenesis in Myocardial Infarction with G-CSF and Intra-Coronary Stem Cell Infusion (MAGIC Cell) 1 trial. American heart journal. 2007 Feb;153(2):237, e231–238. doi: 10.1016/j.ahj.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Cho HJ, Kim TY, Cho HJ, Park KW, Zhang SY, Kim JH, Kim SH, Hahn JY, Kang HJ, Park YB, Kim HS. The effect of stem cell mobilization by granulocyte-colony stimulating factor on neointimal hyperplasia and endothelial healing after vascular injury with bare-metal versus paclitaxel-eluting stents. Journal of the American College of Cardiology. 2006 Jul 18;48(2):366–374. doi: 10.1016/j.jacc.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 48.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S, Moore MA. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001 Jun 1;97(11):3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 49.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leukemia & lymphoma. 2003 Apr;44(4):575–582. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- 50.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008 Jul;51(7):1296–1305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 51.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arteriosclerosis, thrombosis, and vascular biology. 2006 Sep;26(9):2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 52.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006 Dec;49(12):3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. The Journal of clinical investigation. 2007 May;117(5):1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circulation research. 2007 Dec 7;101(12):1310–1318. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 55.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. The Journal of clinical investigation. 2003 Jul;112(2):160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney international. 2005 May;67(5):1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 57.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circulation research. 2005 Apr 15;96(7):784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003 Mar 11;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 59.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. The Journal of experimental medicine. 1997 Jan 6;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, Ben-Hur H, Lapidot T, Alon R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34(+) cells on vascular endothelium under shear flow. The Journal of clinical investigation. 1999 Nov;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. The Journal of experimental medicine. 2006 May 15;203(5):1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002 Jun 25;105(25):3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 63.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inoue S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004 Oct 19;110(16):2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 64.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. Journal of cellular and molecular medicine. 2007 Sep–Oct;11(5):1012–1030. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiedler J, Etzel N, Brenner RE. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. Journal of cellular biochemistry. 2004 Nov 15;93(5):990–998. doi: 10.1002/jcb.20219. [DOI] [PubMed] [Google Scholar]
- 66.Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. The Journal of biological chemistry. 2006 Jan 20;281(3):1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muto A, Fitzgerald TN, Pimiento JM, Maloney SP, Teso D, Paszkowiak JJ, Westvik TS, Kudo FA, Nishibe T, Dardik A. Smooth muscle cell signal transduction: implications of vascular biology for vascular surgeons. J Vasc Surg. 2007 Jun;45( Suppl A):A15–24. doi: 10.1016/j.jvs.2007.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem cells (Dayton, Ohio) 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 69.Wang T, Xu Z, Jiang W, Ma A. Cell-to-cell contact induces mesenchymal stem cell to differentiate into cardiomyocyte and smooth muscle cell. International journal of cardiology. 2006 Apr 28;109(1):74–81. doi: 10.1016/j.ijcard.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 70.Ball SG, Shuttleworth AC, Kielty CM. Direct cell contact influences bone marrow mesenchymal stem cell fate. The international journal of biochemistry & cell biology. 2004 Apr;36(4):714–727. doi: 10.1016/j.biocel.2003.10.015. [DOI] [PubMed] [Google Scholar]