Abstract
Although the telencephalon of ray-finned fishes has garnered considerable attention from comparative neuroanatomists, detailed descriptions of telencephalic organization are available for only a few species. This necessarily limits our understanding of telencephalic evolution, particularly in light of the extraordinary diversity of ray-finned fishes. Thus, we have charted the cyctoarchitecture of the telencephalon of the African cichlid fish, Astatotilapia (Haplochromis) burtoni. We examined tissue sectioned in the transverse plane, and categorized cell groups based on size, shape, and staining intensity of cells, the density and distribution of cells, cell-poor zones, and relationship of cell groups to the anterior commissure and external sulci. In addition, to facilitate visualization of the transitions among cell groups, we aligned and animated a series of 100 sequential brain sections. We found that the A. burtoni telencephalon was similar to other percomorphs in being highly elaborated with many distinct cell groups. In the pallium, Dm, Dl, and Dc had a large number of cell groups, whereas Dd and Dp were more uniform. Although we recognized many similarities between the pallium of A. burtoni and other teleosts, we also recognized two cell groups (Dl-g and Dm-2) that might represent specializations of cichlids. We found that the subpallium had a similar organization to that of other ray-finned fishes.
Key Words: Telencephalon, Cichlid, Actinopterygian, Ray-finned fish, Neuroanatomy, Cytoarchitecture, Fish
Introduction
The brains of ray-finned fishes (actinopterygians) have diverged morphologically from those of tetrapods, and this has generated a great deal of interest from comparative neuroanatomists [reviewed in Nieuwenhuys and Meek, 1990]. These morphological differences apparently reflect the fact that actinopterygians have solved some basic organizational challenges differently from tetrapods, and they have done so under a different set of constraints. For example, actinopterygians relay ascending sensory information to the telencephalon primarily through the posterior tuberculum rather than the dorsal thalamus, which is the tetrapod pattern [reviewed in Butler and Hodos, 1996]. In addition, perhaps due to constraints on head size or shape [Striedter and Northcutt, 2006], the telencephalon of actinopterygians folds outward during development. The tetrapod telencephalon, in contrast, folds inward during development. This difference in initial developmental processes is coupled with subsequent cellular rearrangements [Braford, 1995; Yamamoto et al., 2007] that together set the stage for numerous differences among homologous brain structures, particularly in the pallium. Thus, understanding the structure and function of actinopterygian brains and how these differ from those of tetrapod brains may reveal some of the fundamental principles of brain evolution. The motivation to understand fundamentals of brain evolution has fueled a rich tradition of comparative neuroanatomy of ray-finned fish brains [Ariëns Kappers et al., 1960; Nieuwenhuys and Meek, 1990; Nieuwenhuys et al., 1998].
Neuroanatomical studies of ray-finned fish brains have been limited to a relatively small subset of extant species, with very few of the cichlid fishes. Cichlid fish brains might be particularly interesting from an evolutionary perspective because they are so highly derived [Salzburger et al., 2005]. We chose to describe the basic cytoarchitecture of the telencephalon of Astatotilapia burtoni, an African cichlid from Lake Tanganyika, because it is an important model species in molecular neuroethology [Hofmann, 2003; Fernald, 2006; Robinson et al., 2008], the cytoarchitecture of its diencephalon has been described [Fernald and Shelton, 1985], and its complex social behavior has been well documented [Fernald, 1977; Fernald and Hirata, 1977]. A better understanding of the basic morphology of the A. burtoni telencephalon, therefore, has the potential to open the door to further functional and hodological studies that can be directly related to its complex social behavior. We began with a description of the telencephalic cell groups as revealed by Nissl staining because this allows straightforward comparisons with prior studies.
We found that the telencephalon of A. burtoni share many features with telencephalons of other teleosts. In particular, we recognized all the major cell groups in the subpallium, although we note some disagreement about the pallial-subpallial boundary among authors. The pallium of A. burtoni was similar to other percomorphs in having a large number of distinct groups in Dm, Dl, and Dc. In addition, we report two unusual features (Dl-g and Dm-2) that are similar to cell groups recognized in tilapia [Pepels et al., 2002], suggesting that they might be specializations of cichlids. Because our descriptions are based on Nissl staining alone, however, they should be considered tentative until additional studies are comp-leted.
Materials and Methods
We used adult male and female A. burtoni from a laboratory stock originally derived from Lake Tanganyika, Africa. We sacrificed animals by rapid decapitation, exposed the dorsal surface of the brain within the braincase and fixed the head in Carnoy's fixative (30% chloroform, 60% ethanol, 10% acetic acid) overnight. Brains were rinsed in 70% ethanol, dehydrated, embedded in paraffin, sectioned at 15 μm on a microtome in the transverse plane, and stained with 1% cresyl violet. We also examined tissue prepared by Fernald and Shelton [1985] which was fixed in Bouin's fluid, embedded in paraffin, sectioned at 10 μm, and stained with Bodian and thionin [for methodological details, see Fernald and Shelton, 1985]. We distinguished cell groups using the following criteria: size, shape, and staining intensity of cells; density and distribution of cells; cell-poor zones; relationship of cell groups to the anterior commissure and external sulci. Where possible, we adopted the nomenclature of Northcutt [2006] which is a modification of the nomenclature used by Northcutt and Braford [1980].
To facilitate visualization of the transition among cell groups, we created BrainAlign, an ImageJ (NIH, Bethesda, Md., USA) macro, to align and animate a series of 100 sequential 15-μm sections stained with cresyl violet. BrainAlign uses existing ImageJ functions and plug-ins to create masks to extract each brain section from the background and align the images into a stack, as follows. Briefly, to extract the sections from the background, we first blurred them with a Gaussian function before using the threshold function to create a mask of each section, which we then used to extract each section from the background. To align the images, BrainAlign uses two ImageJ plug-ins that utilize rigid body transformation [Thevenaz et al., 1998]. To download the macro, see www.burmeisterlab.org. Once aligned, we animated the stack to better visualize the changes in organization of cell groups throughout the rostral-caudal extent of the brain.
All work was performed in accord with standards of humane animal care that were approved by the Stanford University Administrative Panel on Laboratory Animal Care committee.
Results
Ventral Telencephalon (V)
The ventral telencephalon is generally considered to be homologous to the subpallium of tetrapods [Nieuwenhuys and Meek, 1990]. In A. burtoni, the ventral telencephalon shares many similarities with other teleosts (table 1). The four main cell groups, the ventral (Vv), dorsal (Vd), supracommissural (Vs), and postcommissural (Vp) nuclei, which are found in all other actinopterygians, were clearly evident in A. burtoni. A. burtoni also has central/commissural (Vc), lateral (Vl), and intermediate (Vi) nuclei, which tend to be more variable in their presence among teleosts.
Table 1.
Proposed comparison among the descriptions of the telencephalons of some teleosts1
| A. bur- toni | Tilapia2 | Sea bass3 | Sun- fish4 | Rock- fish5 | Goldfish6 |
|---|---|---|---|---|---|
| Dc-1 | part of Da | Dc1 | Dc-1 | Dc | − |
| Dc-2 | part of Da | − | ? | − | − |
| Dc-3 | Dmdv | part of Dm3 | ? | − | Dc |
| Dc-4 | Dcl/Dcd? | − | Dc-3/Dc-4 | Dc | − |
| Dc-5 | Dcl/Dcd? | − | Dc-3/Dc-4 | Dc | − |
| Dd-d | Dd | Dd? | Dd? | Dd? | Dd |
| Dd-v | Dlp | Dlp | Dl-p | − | ? |
| Dl-d | part of Da | Dld | Dl-d | dDl? | Dld |
| Dl-g | Dld | ? | − | dDl? | − |
| Dl-v | Dla | Dlv | Dl-v | vDl | Dlv |
| NT | NT | NT | NT | − | Dlvv/NT? |
| Dm-1 | Dma | Dm1 | Dm-1 | vDm? | Dmr |
| Dm-2 | Dcm | Dm2 | − | − | Orc? |
| Dm-3 | Dmdd | Dm3 | Dm-3 | dDm | Dmc |
| Dx | ? | − | − | − | − |
| Dp | Dp | Dp | Dp | Dp | Dp |
| Vc | Vc | Vc | Vc | − | Vc |
| Vd | Vd+Vn | Vd | Vd | Vd | Vd |
| Vi | Vi | Vi | Vi | Vi | Vi |
| Vl | Vl | Vl | Vl | Vl | Vl |
| Vp | ? | ? | − | − | Vp |
| Vs-l | ? | Vs | ? | Vs+Vp | Cr |
| Vs-m | Dmv | Dm4 | Dm-4+ Vs+Vp | vDm | Vs |
| Vv | Vv | Vv | Vv | Vv | Vv |
When corresponding brain regions appeared to be lacking, we indicated this with a dash. When correspondences appear particularly uncertain, we indicated this with a question mark.
Pepels et al., 2002;
Cerdá-Reverter et al., 2001;
Northcutt and Braford, 1980;
Murakami et al., 1983;
Northcutt, 2006.
Rostral to the anterior commissure, the ventral telencephalon consists of Vd, Vv, Vl, and Vc (fig. 1; video sections 28–55). Ventral to Dm-1, the rostral part of Vd (Vd-r) appears just as the olfactory bulbs recede (fig. 1B; section 28). The cells of Vd-r are clearly distinguished from the Dm-1 cells, as they are larger, arranged in clusters, and are more lightly staining. The cells of Vv appear ventral to Vd-r (fig. 1B; section 28) and are smaller and more darkly staining than those of Vd-r. As Vv becomes prominent, Vd-r takes a progressively more dorsal position. Both Vd-r and Vv extend caudally to commissural levels. Just rostral to the anterior commissure, the caudal part of Vd (Vd-c) appears laterally and medially as two tight clusters of cells that subsequently merge (fig. 2B, 3A; sections 53–69). The precommissural subpallium is also characterized by a scattering of migrated cells that are interspersed with medial and lateral fiber tracts. Although we describe the more lateral cells as Vl and the more medial cells as Vc, they are nearly continuous at several points. In addition, there is some heterogeneity in Vc cells along the rostral-caudal axis; additional studies are necessary to determine whether Vc is one cell group or, alternatively, can be further subdivided.
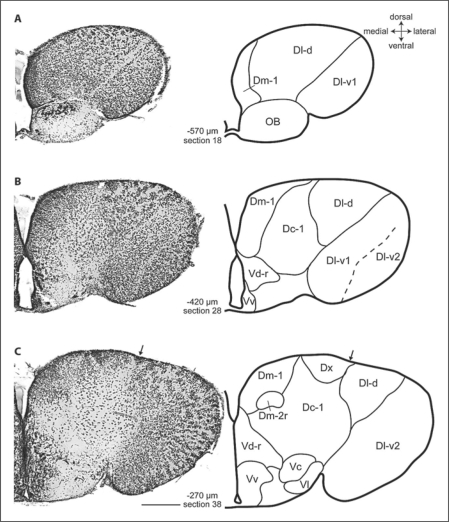
Fig. 1.
Photomicrographs and corresponding line drawings of the A. burtoni telencephalon stained with cresyl violet (A–C). Each panel shows a single hemisphere in the transverse plane. The arrow in panel C marks the slight indentation of the ventricular surface that demarcates the border between Dl and Dm. The rostral-caudal distance of each section from the rostral margin of the anterior commissure is indicated in micrometers. All panels are at the same magnification, the scale bar represents 200 μm, and the section number refers to the corresponding section in the supplementary video.
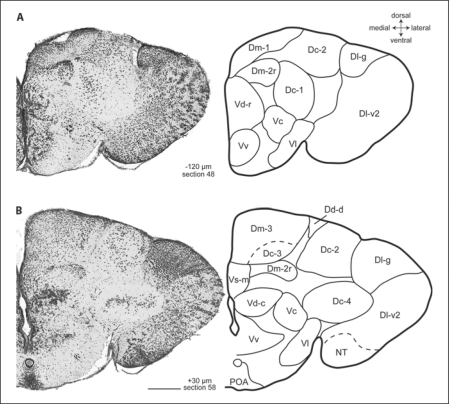
Fig. 2.
Photomicrographs and corresponding line drawings of the A. burtoni telencephalon stained with cresyl violet (A, B). Each panel shows a single hemisphere in the transverse plane. The rostral-caudal distance of each section from the rostral margin of the anterior commissure is indicated in micrometers. All panels are at the same magnification, the scale bar represents 200 μm, and the section number refers to the corresponding section in the supplementary video.
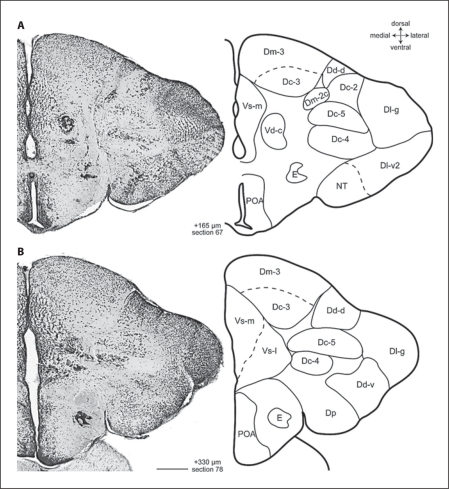
Fig. 3.
Photomicrographs and corresponding line drawings of the A. burtoni telencephalon stained with cresyl violet (A, B). Each panel shows a single hemisphere in the transverse plane. The rostral-caudal distance of each section from the rostral margin of the anterior commissure is indicated in micrometers. All panels are at the same magnification, the scale bar represents 200 μm, and the section number refers to the corresponding section in the supplementary video.
At commissural levels, the medial part of Vs (Vs-m) appears dorsomedial to Vd-c (fig. 2B; section 58), which it eventually replaces. At its rostral extent, the cells of Vs-m are small, lightly staining, and arranged in a laminar fashion (fig. 2B, 3A; sections 58–67). More caudally, this laminar arrangement is accompanied by the lateral part of Vs (Vs-l), which is characterized by clusters of cells ventrolaterally to Vs-m (fig. 3B; section 78). Postcommissurally, Vp appears dorsal to the preoptic area and ventral to Vs (fig. 4A; section 88). Vp cells are small and lightly staining. At its most caudal level, Vp cells appeared continuous with the migrated cells of Vi which extend to Dp. Postcommissurally, the small, tightly packed, and darkly staining cells of the entopeduncular nucleus (E) appear in a ventrolateral position in association with the lateral forebrain bundle (fig. 3A; section 67).
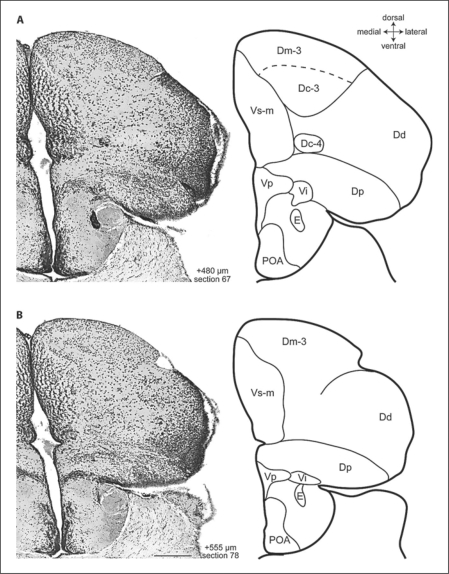
Fig. 4.
Photomicrographs and corresponding line drawings of the A. burtoni telencephalon stained with cresyl violet (A, B). Each panel shows a single hemisphere in the transverse plane. The rostral-caudal distance of each section from the rostral margin of the anterior commissure is indicated in micrometers. All panels are at the same magnification, the scale bar represents 200 μm, and the section number refers to the corresponding section in the supplementary video.
Dorsal Telencephalon (D)
The dorsal telencephalon of actinopterygians is generally believed to correspond to the pallium [Nieuwenhuys and Meek, 1990]. The pallium of A. burtoni shares many features in common with other teleosts, and all major divisions are clearly recognizable. We found that the pallium of A. burtoni is characterized by extensive lateral (Dl), medial (Dm), and central (Dc) divisions with many distinct cell groups, and large, but more uniform dorsal (Dd) and posterior (Dp) divisions.
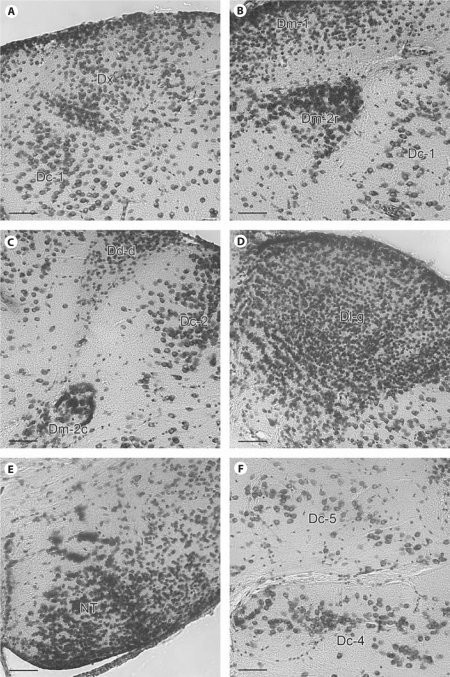
We recognized three subdivisions of Dm that we named sequentially in the order in which they appeared. Most anterior, Dm-1 is characterized by tightly packed, darkly staining, small cells at a position medial to Dl-d (fig. 1A, 5B; section 18). As the subpallium becomes more prominent, Dm-1 takes on a more dorsal position (fig 1; sections 18–54). A second subdivision, Dm-2, appears ventrally to Dm-1 as a cluster of cells (fig. 1C; section 38). Our Dm-2 does not appear to correspond to Dm-2 of other teleosts, although it shares some features with Orc in goldfish (see table 1). The cells of the rostral part of Dm-2 (Dm-2r) are slightly larger than Dm-1 cells, but smaller than the cells of Dc (fig. 5B). Dm-2r adopts a distinctive shape as it is displaced ventrally by Dm-3 at postcommissural levels (fig. 2B; section 58). Postcommissurally, the caudal part of Dm-2 (Dm-2c) appears within the lateral portion of Dm-2 (section 61). The cells of Dm-2c are slightly smaller than those of Dm-2r, and they are more tightly clustered (fig. 5C). Caudally, Dm-2c cells appear progressively laterally (fig. 3A; section 61–72) until Dd-d becomes prominent. This lateral transition of Dm-2c cells and their continuity with Dm-2r is particularly evident in the video (online suppl. fig. 1, sections 38–73, www.karger.com/doi/10.1159/000235613). At its most caudal extent, Dm-2c comes very close to Dd-d, but the cells of Dm-2c are easily distinguished from those of Dd-d (fig. 5C), which are very small and round. The close proximity between Dm-2r and Dm-1 and Dm-2c and Dd-d might suggest functional relationships in much the same way as Dc cell groups are thought to be related to more superficial cell groups.
Fig. 5.
Photomicrographs of transverse sections showing morphology and density of cell groups in the A. burtoni telencephalon. A Photomicrograph showing the clustering of medium-sized cells of Dx and the large cells of adjacent Dc-1. This image was taken from a section similar to that in figure 1C. B Photomicrograph showing the dense clustering of Dm-2r cells in contrast to the small round cells of Dm-1 and the larger cells of Dc-1. This image was taken from a section similar to that in figure 2A. C Photomicrograph showing the caudal part of Dm-2, the small round cells of Dd, and the large cells of Dc-2. This image was taken from a section similar to that in figure 3A. D Photomicrograph showing the small, densely packed cells of Dlg. This image was taken from a section similar to that in figure 2B. E Photomicrograph showing the clustering of cells in Dlvv. This image was taken from a section similar to that in figure 2B. F Photomicrograph showing the large loosely arranged cells of Dc-4 and Dc-5. This image was taken from a section similar to that in figure 3A. In all panels, the scale bars represent 40 μm.
At the point that Dm-2 first appears, we recognized another collection of medium-sized clustered cells along the ventricular surface between Dm-1 and Dl-d that we named Dx (fig. 1C, 5A; sections 34–45). More caudally, some clusters penetrate ventrally into the brain (sections 40–45). Rostrally, these cells are separated from Dm-1 by a wedge of Dc-1 cells, and caudally, they are separated from Dm-1 by a cell-poor zone. This cluster of cells is gradually replaced caudally by the larger, more loosely arranged cells of Dc-2. We found it difficult to clearly attribute these cells to either Dm or to Dl. On the one hand, the cells resemble those of Dl in size and shape, and they show a clustered pattern that is similar to the arrangement of cells in Dl; however, they do not appear to arise from Dl. Rather, they arise medial to a slight indentation of the ventricular surface that demarcates the border between Dl and Dm rostrally (fig. 1C; section 38). Likewise, this cluster of cells is clearly separated from those of Dm-1. Finally, it is also possible that these cells belong to Dc-2, which replaces them more caudally, although they are smaller than most cells we have attributed to Dc. Clearly, resolution of this issue requires additional data. Until such data are forthcoming, we concluded it was best to designate these cells as Dx.
Near the level of the anterior commissure, Dm-3 appears dorsomedially to Dm-2, which it quickly replaces (fig. 2; section 49–59). Dm-3 is a prominent group of medium-sized, lightly staining, loosely arranged cells that persist to the caudal pole of the telencephalon (fig. 4B; section 49–100). The cells of Dm-3 that are near the ventricle are smaller in comparison to the large cells of the ventrolateral part of Dm-3. Following Northcutt [2006], we categorized the large cells of Dm-3 as Dc-3 (see below).
The dorsal part of the dorsal division of the pallium (Dd-d) appears dorsolaterally at about the level of the anterior commissure as a triangular wedge of small round cells (fig. 2B, 5C; section 58). As this cell group expands laterally, it replaces Dc-2 (fig. 3B; sections 58–78). At this point and more caudally, small recesses in the ventricular surface clearly demarcate Dd-d's borders with neigh boring Dm and Dl. Caudally, a ventrolateral arm of Dd (Dd-v) appears between Dlg and Dp (fig. 3B; section 72–78) which eventually merges with Dd-d at the caudal pole of the telencephalon (sections 72–100). If viewed in reverse (i.e., caudal to rostral), the continuity of Dd is readily apparent, and one sees that Dd-d and Dd-v are separated by Dl at rostral levels.
The Dl of the A. burtoni pallium is large and highly elaborated. As in many other teleosts [Northcutt and Davis, 1983; Cerdá-Reverter et al., 2001; Northcutt, 2006], the rostral pole of the telencephalon is dominated by Dl-d, dorsomedially, and Dl-v, laterally (fig. 1A; section 18). The cells of both Dl-d and Dl-v are medium-sized, and are characterized by a clustered pattern at the ventricular surface, giving the appearance of perpendicular lamina. A rostral location, medium-sized cells, and lamina are traits that A. burtoni Dl-d shares with Dl-d of other teleosts [Northcutt and Davis, 1983; Cerdá-Reverter et al., 2001]. Rostrally, the division between Dl-d and Dl-v is clearly demarcated by a cell-free zone (fig. 1A, B; sections 10–33), but more caudally, the division between them becomes obscured (fig. 1C; section 33–42).
Dl-v is a prominent feature of Dl that extends from the most rostral aspect of the telencephalon to postcommissural levels when it is replaced by Dd-v (fig. 3B; section 78). We recognized two divisions of Dl-v. Most rostrally, the cells of Dl-v1 are evenly spaced (sections 1–20). Mid-rostrally, Dl-v1 is replaced by Dl-v2, which is characterized by perpendicular lamina near the ventricle (fig. 1B, C, 2A; sections 28–48). The transition from Dl-v1 to Dl-v2 is indicated by a faint cell-sparse zone that first appears close to the ventrolateral edge of Dl-v and then moves progressively more dorsomedially (sections 21–35). A similar division of Dl-v was recognized in the sea bass [Cerdá-Reverter et al., 2001]. At caudal levels, there is a group of tightly packed cells within Dl-v at the most ventral pole that we recognized at the nucleus taenia (NT; fig. 2B, 3A; sections 54–68). As in sea bass [Cerdá-Reverter et al., 2001], these cells are slightly smaller than the cells of Dl-v, are darker staining and more tightly clustered (fig. 5E). The cells of NT persist until Dl is replaced by Dp (sections 49–72). Given the size and complexity of Dl-v in A. burtoni, we anticipate that hodological and histochemical studies will reveal subdivisions that are not apparent by cytoarchitecture alone [for example, see Yamamoto and Ito, 2008].
At about the level at which Dm-2 becomes distinct (fig. 2A; section 48), Dl-d is replaced by a group of very small, tightly packed, darkly staining cells that form lamina parallel to the ventricular surface (fig. 5D). The location, cell-size, cell-density, and staining pattern of this cell group are unlike other previously described subdivisions of Dl, leading us to believe that it is a previously undescribed feature of Dl. We named this cell group the granular zone of Dl (Dl-g) because of its characteristically small cells. Dl-g is separated from the more medial aspects of D by a deeply recessed sulcus ypsiloniformis (fig. 2A; section 48). Caudally, the lamina in the ventromedial portion of Dl-g are sparser than the cells of the dorsolateral portion (e.g., fig. 3A; section 67). At more caudal levels of the pallium, there is a complex transition from Dl-v2 and Dl-g to Dd and Dp (fig. 3, 4; sections 67–89). During the transition, the dorsal part of Dl-v2 is replaced by an expanding Dl-g, and the appearance of Dp replaces NT. At about this point, Dd-v emerges between Dl-g and Dp (fig. 3B; section 78). Eventually, the expanding Dd replaces Dl-g (fig. 4A; section 88). Dp appears postcommissurally in the ventrolateral pole of the pallium (fig. 3B; section 78). The cells of Dp are medium to small in size, very lightly staining, and more loosely arranged than those of Dd. The border between Dp and Dd is also indicated by a small indentation in the surface of the brain.
We recognized five distinct groups of Dc. Our primary criterion for recognizing Dc was cell size and spacing, as Dc cells have typically been defined as the largest in the telencephalon. Most anterior is Dc-1, a group of loosely arranged cells that lie lateral to Dm-1, ventral to Dl-d, and medial to Dl-v (fig. 1B, C, 5A; sections 28, 38). At more caudal levels, Dc-1 coalesces into a more distinct group of cells that is clearly separated from other brain regions by cell-free zones (fig. 2A, 5B; section 48) and disappears at commissural levels. Dc-2 appears dorsally near the ventricular surface and between Dm and Dl (fig. 2A, B; sections 48, 58). These cells persist postcommissurally until they are replaced by an enlarged Dd-d (fig. 3; sections 62–71). The cells of Dc-2 are unusual for Dc in their ventricular position, but in other respects they are similar in size and staining to other Dc groups in A. burtoni and other species (fig. 5C). Dc-3 is a large-celled region within Dm-3 (fig. 2B, 3, 4A; sections 58–88). The border between Dc-3 and Dm-3 is indistinct. At commissural levels, Dc-4 appears ventrolaterally where it is clearly separated from other regions by large cell-free zones (fig. 2B; section 58) and persists until Dd becomes prominent (fig. 4A; section 88). Finally, the last group of Dc cells to appear is Dc-5, which appears dorsally to Dc-4 just as Dc-2 is receding.
Discussion
The brains of ray-finned fishes differ morphologically from tetrapods, and, among ray-finned fishes, there is significant diversity in basic cytoarchitecture, particularly within the telencephalon. We examined the cytoarchitecture of A. burtoni, a member of one of the most derived groups of ray-finned fishes, the cichlids. We examined cytoarchitecture as revealed by Nissl staining because this facilitates comparisons to prior studies, many of which also used Nissl-stained tissue. Overall, the telencephalon of A. burtoni shares many features with other teleosts. Broadly, the A. burtoni telencephalon was similar to other percomorphs [Murakami et al., 1983; Northcutt and Davis, 1983; Cerdá-Reverter et al., 2001; Pepels et al., 2002] in being highly elaborated with many distinct cell groups; in most cases, Dm, Dl, and Dc are the divisions with the greatest number of distinct cell groups. Not surprisingly, the telencephalon of A. burtoni is most similar to tilapia [Pepels et al., 2002]. In spite of this general similarity, there is a great deal of diversity evident among species (table 1). Some of these differences in cytoarchitecture might represent differences in opinion among authors, but some appear to represent actual species differences.
The Dl of A. burtoni is a dominant feature of the telencephalon. In many respects, it resembles Dl of other teleosts (table 1). For example, as in other teleosts, the rostral part of Dl is characterized by a dorsal and ventral part, and both are often characterized by perpendicular lamina [Northcutt and Braford, 1980; Murakami et al., 1983; Cerdá-Reverter et al., 2001]. More caudally, Dl is often subdivided further, and authors have often recognized an NT and a Dlp. We did not recognize a Dlp, but instead suggest that a caudal cell group similar to Dlp of many authors is part of Dd (table 1). One consequence of this designation is that Dd of A. burtoni is much larger than has been recognized in other teleosts [e.g., Cerdá-Reverter et al., 2001; Pepels et al., 2002; Northcutt, 2006]. In both A. burtoni and sea bass, Dl-v has a rostral part (Dl-v1) and caudal part (Dl-v2). In addition to these similarities, we observed one unusual feature in Dl of A. burtoni (Dl-g) that did not clearly correspond to previously described cell groups. The cells of Dl-g are distinctly smaller, more darkly staining, and more densely packed than the other cells that comprise Dl. The cells themselves most closely resemble those of Dd (fig. 5C, D), although they are most closely associated with Dl. Although these cells effectively replace Dl-d, it is not clear whether these cells are a specialization of Dl-d, Dl-v, or neither. Interestingly, there appears to be a comparable cell group in tilapia, another cichlid, that Pepels et al. [2002] assigned to Dl-d. Initially, we too suspected that A. burtoni Dl-g was comparable to Dl-d of other actinopterygians; however, Dl-d has previously been recognized as having medium-sized cells, perpendicular lamina, and a rostral location [Northcutt and Davis, 1983; Cerdá-Reverter et al., 2001; Northcutt, 2006], criteria that A. burtoni Dl-d clearly meets. Our Dl-g, in contrast, has small cells that are densely packed, parallel lamina, and a more caudal location. Given the position and shape of tilapia Dl-d, we believe it is comparable to A. burtoni Dl-g, but we cannot be confident in the similarity because Pepels et al. [2002] did not include descriptions of cell staining, size, or density. Nonetheless, if we are correct that both A. burtoni and tilapia share this cell group, it would suggest that it is a specialization of cichlids. Of course, this hypothesis needs testing in future studies that compare cytoarchitecture, hodology, and function of Dl-g in A. burtoni and other cichlids to findings in closely related non-cichlids.
As with Dl, Dm of A. burtoni resembles Dm of other teleosts in many of its features (table 1). Notably, many authors recognize a rostral cell group (Dm-1) characterized by small, darkly staining cells and a more caudal cell group (Dm-3) characterized by more loosely arranged medium- to large-sized cells [e.g., Northcutt and Braford, 1980; Cerdá-Reverter et al., 2001]. The presence and characteristics of additional groups assigned to Dm are more variable. In A. burtoni, for example, we recognized a Dm-2 that does not have any obvious correspondence to other cell groups assigned to Dm. The most similar cell group appears to be one assigned to Dc in tilapia [Dcm; Pepels et al., 2002]. The central position of A. burtoni Dm-2 is generally similar to Dc, suggesting that it might, indeed, be more appropriately attributed to Dc. We chose, however, to assign this cell group to Dm and not to Dc, because, historically, Dc cells are described as among the largest within the pallium, and the cells of A. burtoni Dm-2 are noticeably smaller than other centrally migrated cell groups in A. burtoni (fig. 5). Finally, some authors have recognized a more caudal group within Dm, sometimes characterized by parallel lamina (e.g., see vDm in rockfish, Dm-4 of sunfish, and Dmdv of tilapia). We observed a similar cell group, but assigned it to the subpallium (see below).
In most species of ray-finned fishes, authors recognize one or more groups of large, widely spaced migrated cell groups, typically referred to as Dc. The number of recognized Dc groups varies considerably; for example, only one Dc group was recognized in rockfish [Murakami et al., 1983] and goldfish [Northcutt, 2006], but six were recognized in tilapia [Pepels et al., 2002]. In some cases, authors have speculated that each Dc group is closely associated with a particular subdivision of D. In A. burtoni, we recognized five distinct groups of Dc cells based primarily on cell size, spacing, and staining. For the most part, our Dc groups are similar to Dc groups recognized previously and, in some cases, it is possible to hypothesize about their relationship to other groups of D. For example, our Dc-3 is similar to goldfish Dc and appears to be closely associated with Dm-3. Caudally, we recognized two Dc groups (Dc-4 and Dc-5) that appear closely associated with Dl and are similar to cell groups in tilapia that are part of the indirect telencephalo-cerebellar pathway [Imura et al., 2003]. In other cases, the relationships are less clear. In addition, we recognized a group of Dc cells (Dc-2) that violates the general expectation that Dc groups should be centrally located within the telencephalon. Our Dc-2 is located near the ventricle between Dm-3 and Dl-g. We chose to assign this cell group to Dc, and not to Dm, Dd, or Dl, because the cells of Dc-2 are most similar to other groups of Dc. Future studies will be necessary, however, to determine whether this cell group is best assigned to Dc or some other division of the pallium.
In contrast to the differences we have noted within the pallium, the subpallium of A. burtoni does not appear to possess any derived cell groups (table 1), but our description of Vs appears to deviate from previous descriptions [e.g., Northcutt and Braford, 1980]. In A. burtoni, Vs-m first appears ventral to Dm and dorsal to Vd at about the level of the anterior commissure. As Vs-m becomes more prominent, it displaces Vd laterally (fig. 3A). At its caudal pole, the ventrolateral portion of Vs is characterized by the clustered cells of Vs-l. Several aspects of our Vs are similar to goldfish Vs; in particular, goldfish Vs appears between Dm and Vd and, more caudally, goldfish Vs has a distinct cluster ventrolaterally which was called the cell ring of Vs [Cr; Northcutt, 2006]. Our Vs, however, is most similar to the combined cells of sunfish Dm-4, Vs, and Vp. In particular, our Vs-m is similar to sunfish Dm-4 whereas our Vs-l is similar to sunfish Vs+Vp. In addition, our Vs-m might be similar to Dmv of tilapia [Pepels et al., 2002]. Thus, there appears to be some disagreement as to the location of the pallial-subpallial boundary that can only be resolved by additional studies. In any case, we attribute these differences to differences in opinion among authors and not to differences in the elaboration of the subpallium among species.
In summary, our primary objective for this paper has been to provide a basic description of the A. burtoni telencephalon that might serve as a basis for future hodological, histochemical, and functional studies. Because our descriptions are based on Nissl staining alone, they should, necessarily, be considered tentative until additional studies are forthcoming. In particular, our placement of the pallial-subpallial boundary requires confirmation, and our designation of some cell groups such as Dl-g, Dx, and Dm-2 would be strengthened by supporting data. In addition, future studies are likely to identify additional subdivisions that are not readily apparent by cytoarchitecture alone.
Acknowledgements
We thank Glenn Northcutt for several useful discussions and for preparation of some of the A. burtoni tissue that we used here. In addition, we thank Ping Fu and Jennifer Staab for their help in creating the animated stack of brain sections. Supported by NIH NS42984 and NSF IOB 0445682 to S.S.B., and NIH J. Javits Award NS34950 to R.D.F.
Abbreviations used in this paper
- D
Dorsal (pallial) division of the telencephalon
- Dc
Central division of D
- Dc-1,2,3,4,5
Subdivisions of Dc
- Dd
Dorsal division of D
- Dd-d
Dorsal part of Dd
- Dd-v
Ventral part of Dd
- Dl
Lateral division of D
- Dl-d
Dorsal subdivision of Dl
- Dl-g
Granular zone of Dl
- Dl-v
Lateral subdivision of Dl
- Dl-v1,2
Parts of Dl-v
- Dm
Medial division of D
- Dm-1,2,3
Subdivisions of Dm
- Dm-2c
Caudal part of Dm-2
- Dm-2r
Rostral part of Dm-2
- Dp
Posterior division of D
- Dx
Unassigned subdivision of D
- E
Entopeduncular nucleus
- NT
Nucleus taenia
- OB
Olfactory bulb
- POA
Preoptic area
- V
Ventral (subpallial) division of the telencephalon
- Vc
Central nucleus of V
- Vd
Dorsal nucleus of V
- Vd-c
Caudal part of Vd
- Vd-r
Rostral part of Vd
- Vi
Intermediate nucleus of V
- Vl
Lateral nucleus of V
- Vp
Postcommissural nucleus of V
- Vs
Supracommissural nucleus of V
- Vs-l
Lateral part of Vs
- Vs-m
Medial part of Vs
- Vv
Ventral nucleus of V
References
- Ariëns Kappers CU, Huber GC, Crosby EC. The Comparative Anatomy of the Nervous System of Vertebrates, Including Man. New York: Hafner Publishing Company; 1960. [Google Scholar]
- Braford MR., Jr Comparative aspects of forebrain organization in the ray-finned fishes: touchstones or not? Brain Behav Evol. 1995;46:259–274. doi: 10.1159/000113278. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. New York: Wiley-Liss; 1996. [Google Scholar]
- Cerdá-Reverter JM, Zanuy S, Muñoz-Cueto JA. Cytoarchitectonic study of the brain of a perciform species, the sea bass (Dicentrarchus labrax). I. The telencephalon. J Morphol. 2001;247:217–228. doi: 10.1002/1097-4687(200103)247:3<217::AID-JMOR1013>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Fernald RD. Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Anim Behav. 1977;25:643–653. [Google Scholar]
- Fernald RD. The social control of reproduction: Physiological, cellular, and molecular consequences of social status. In: Platek SM, Keenan JP, Shackleford TK, editors. Evolutionary Cognitive Neuroscience. Cambridge, MA: MIT Press; 2006. pp. 197–220. [Google Scholar]
- Fernald RD, Hirata N. Field study of Haplochromis burtoni: Quantitative behavioral observations. Anim Behav. 1977;25:964–975. [Google Scholar]
- Fernald RD, Shelton LC. The organization of the diencephalon and the pretectum in the cichlid fish, Haplochromis burtoni. J Comp Neurol. 1985;238:202–217. doi: 10.1002/cne.902380207. [DOI] [PubMed] [Google Scholar]
- Hofmann HA. Functional genomics of neural and behavioral plasticity. J Neurobiol. 2003;54:272–282. doi: 10.1002/neu.10172. [DOI] [PubMed] [Google Scholar]
- Imura K, Yamamoto N, Sawai N, Yoshimoto M, Yang CY, Xue HG, Ito H. Topographical organization of an indirect telencephalo-cerebellar pathway through the nucleus paracommissuralis in a teleost, Oreochromis niloticus. Brain Behav Evol. 2003;61:70–90. doi: 10.1159/000069353. [DOI] [PubMed] [Google Scholar]
- Murakami T, Morita Y, Ito H. Extrinsic and intrinsic fiber connections of the telencephalon in a teleost, Sebastiscus marmoratus. J Comp Neurol. 1983;216:115–131. doi: 10.1002/cne.902160202. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Meek J. The telencephalon of actinopterygian fishes. In: Jones EG, Peters A, editors. Cerebral Cortex. New York: Plenum Press; 1990. pp. 31–73. [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. The central nervous system of vertebrates. New York: Springer; 1998. [Google Scholar]
- Northcutt RG. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J Comp Neurol. 2006;494:903–943. doi: 10.1002/cne.20853. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Braford MR., Jr . New observations on the organization and evolution of the telencephalon of actinopterygian fishes. In: Ebbesson SOE, editor. Comparative Neurology of the Telencephalon. New York: Plenum Press; 1980. pp. 41–98. [Google Scholar]
- Northcutt RG, Davis RE. Telencephalic organization in ray-finned fishes. In: Davis RE, Northcutt RG, editors. Fish Neurobiology. Vol 2. Ann Arbor, MI: University of Michigan Press; 1983. pp. 117–163. [Google Scholar]
- Pepels PP, Meek J, Wendelaar Bonga SE, Balm PH. Distribution and quantification of corticotropin-releasing hormone (CRH) in the brain of the teleost fish Oreochromis mossambicus (tilapia) J Comp Neurol. 2002;453:247–268. doi: 10.1002/cne.10377. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5:17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF, Northcutt RG. Head size constrains forebrain development and evolution in ray-finned fishes. Evol Dev. 2006;8:215–222. doi: 10.1111/j.1525-142X.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ito H. Visual, lateral line, and auditory ascending pathways to the dorsal telencephalic area through the rostrolateral region of the lateral preglomerular nucleus in cyprinids. J Comp Neurol. 2008;508:615–647. doi: 10.1002/cne.21717. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ishikawa Y, Yoshimoto M, Xue HG, Bahaxar N, Sawai N, Yang CY, Ozawa H, Ito H. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav Evol. 2007;69:96–104. doi: 10.1159/000095198. [DOI] [PubMed] [Google Scholar]