Abstract
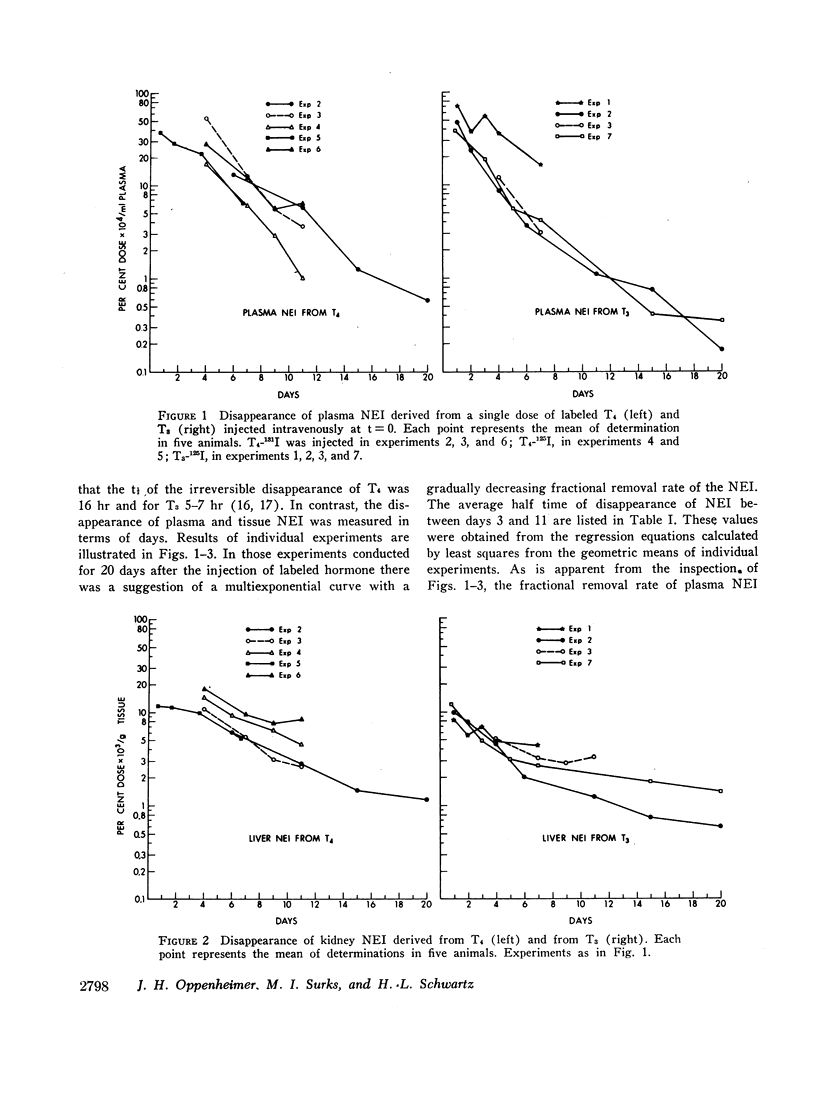
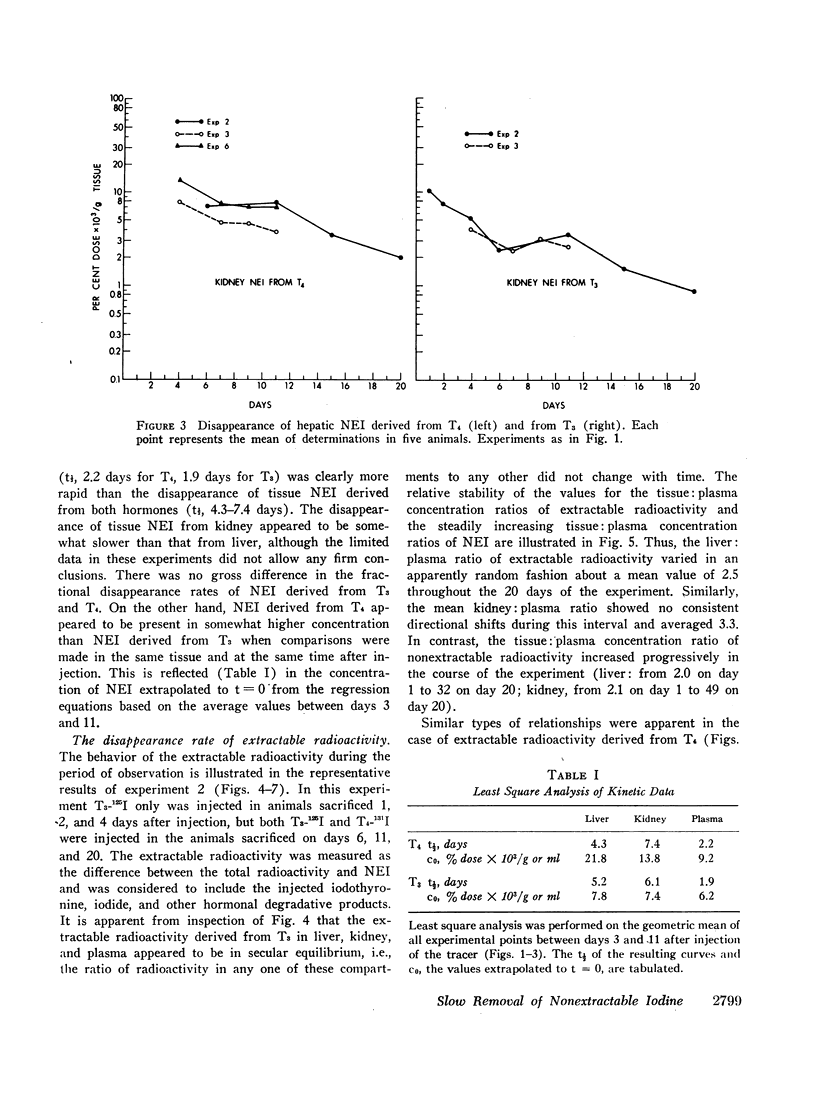
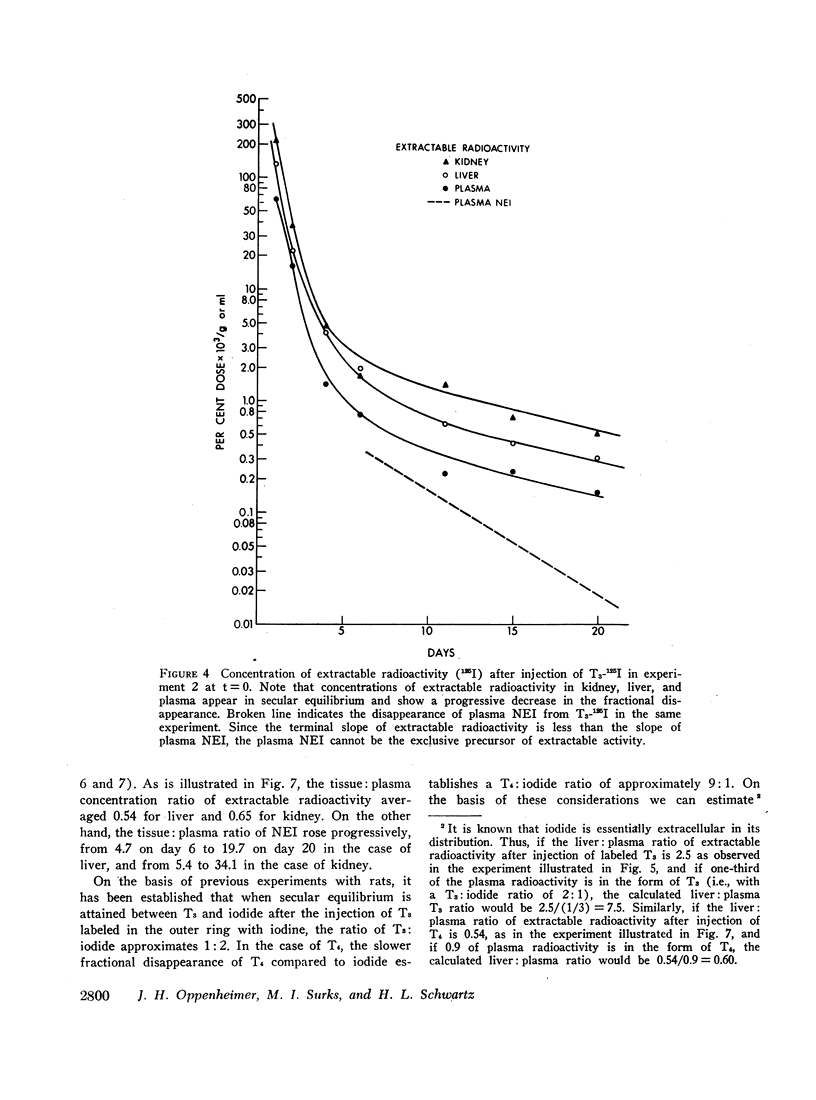
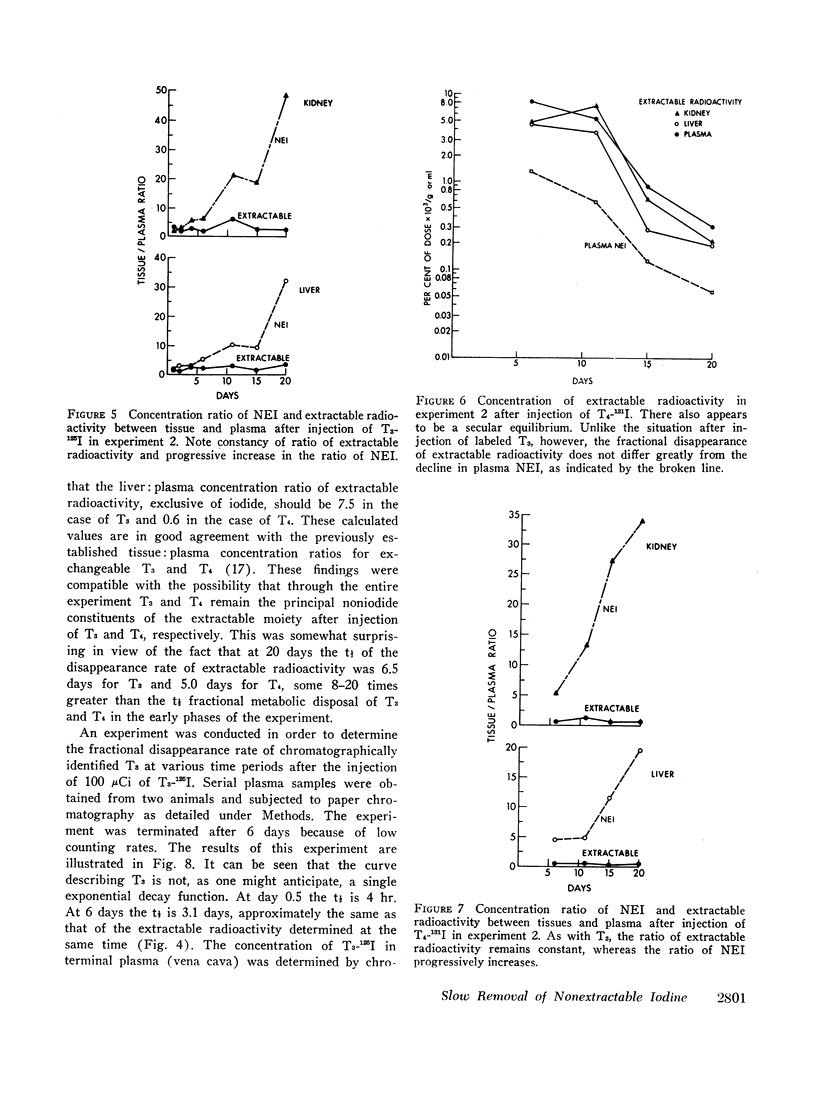
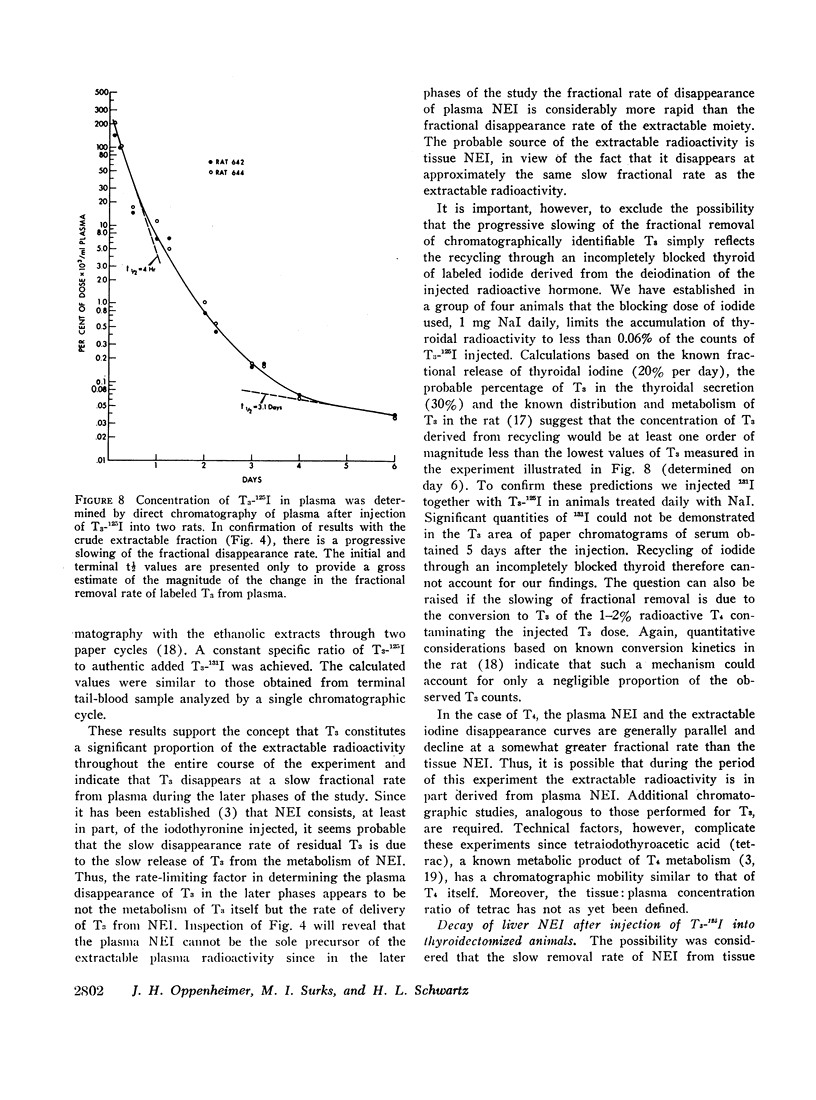
Previous studies have shown that a small but significant proportion of radioiodine from labeled L-thyroxine (T4) and 3,5,3′-triiodo-L-thyronine (T3) is incorporated into plasma and tissue proteins and is not, therefore, extractable with ethanol or other organic solvents. Other studies have shown that the complex consists, at least in part, of the iodothyronine in apparent covalent linkage with protein. In the present series of experiments the disappearance rate of nonextractable iodine (NEI) was determined in plasma, liver, and kidney after the injection of rats with a single dose of T4 and T3 labeled with radioiodine in the phenolic ring. The t½ of NEI decay was substantially longer than the t½ of the initial metabolic removal of T4 (16 hr) and T3 (4-6 hr). Thus, between days 3 and 11 the average t½ of plasma NEI derived from T4 was 2.2 days, from T3, 1.9 days; kidney NEI from T4, 7.4 days, from T3, 6.1 days; hepatic NEI from T4, 4.3 days, from T3, 5.2 days.
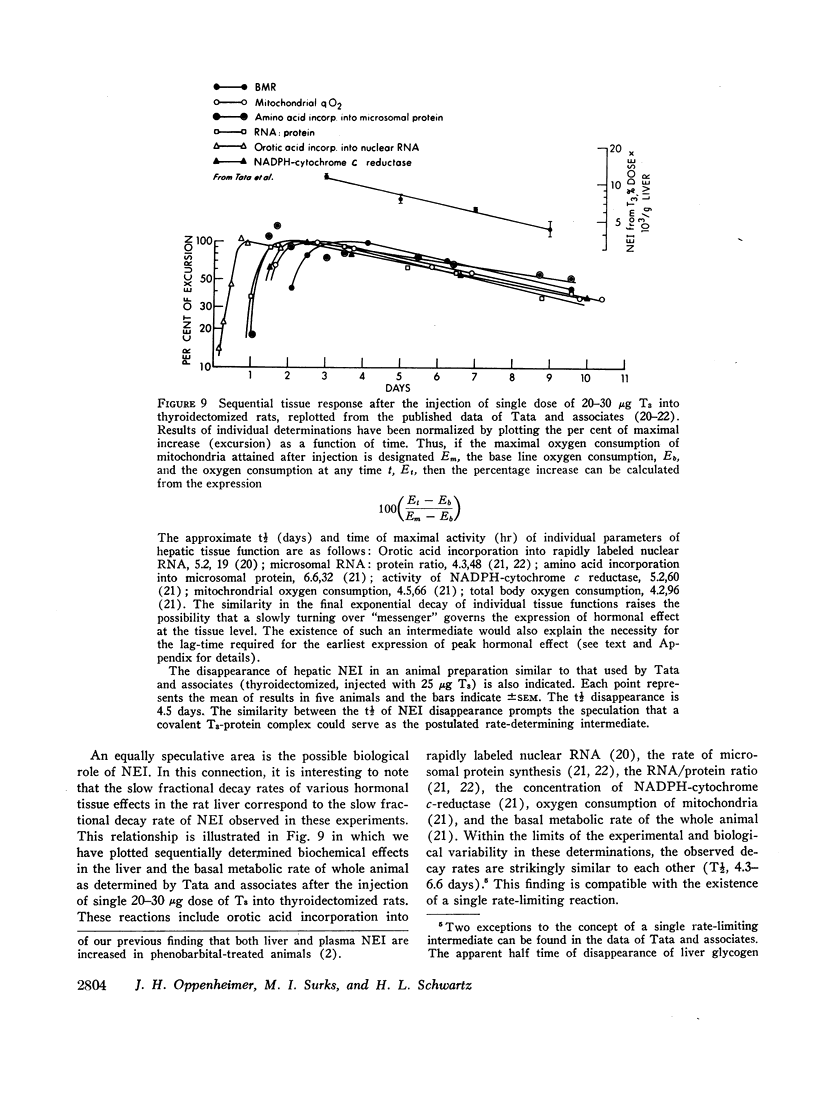
The slow disappearance of liver NEI was of special interest in connection with an analysis of previously published data by Tata and associates dealing with the sequential tissue effects after the injection of a single dose of T3 into thyroidectomized rats. The t½ of decay of the various biological effects measured, primarily in the liver, appeared similar to each other, averaging between 4 and 6 days. These findings are compatible with the existence of a single long-lived intermediate governing the tissue expression of thyroid hormone. The t½ of hepatic NEI in similarly prepared animals (thyroidectomized and injected with 25 μg of T3) was found to be 4.5 days. The coincidence in the slow fractional disappearance rates of hepatic NEI and the dissipation of hormonal tissue effects raises the distinct possibility that T3 interacts with specific cellular receptor sites to form covalent complexes which are slowly removed and serve both to initiate and to perpetuate hormonal action. A mathematical analysis of hormonal reaction mechanisms, based on the assumption of a linearly responsive system, a t½ of T3 of 4 hr, and a t½ of 4.5 days for the postulated long-lived “messenger” suggests that maximal expression of hormonal activity cannot be attained before 20 hr after the injection of a hormone pulse. This value is broadly consonant with the observed data accumulated by Tata and associates. The existence of a long-lived messenger, possibly a species of NEI, would therefore explain not only the slow dissipation of hormonal effects but also the well-recognized “lag-time” in the expression of hormonal action.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., KEATING F. R., Jr The role of the gastrointestinal tract, including the liver, in the metabolism of radiothyroxine. Endocrinology. 1952 Nov;51(5):427–443. doi: 10.1210/endo-51-5-427. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman M. B., Richter M. E., Lynch H. A. Incorporation of thyroxin carbon in protein fractions of Rana catesbiana tadpole nervous system, liver and tail. Endocrinology. 1970 Feb;86(2):217–224. doi: 10.1210/endo-86-2-217. [DOI] [PubMed] [Google Scholar]
- GALTON V. A., INGBAR S. H. The mechanism of protein iodination during the metabolism of thyroid hormones by peripheral tissues. Endocrinology. 1961 Jul;69:30–38. doi: 10.1210/endo-69-1-30. [DOI] [PubMed] [Google Scholar]
- JACQUEMIN C., NUNEZ J., ROCHE J. [On the intermediate products and mechanism of deiodination of thyroid hormones]. Gen Comp Endocrinol. 1963 Jun;3:226–238. doi: 10.1016/0016-6480(63)90017-0. [DOI] [PubMed] [Google Scholar]
- Kozyreff V., Surks M. I., Oppenheimer J. H. Formation of nondissociable hormone-protein complexes during the in vitro incubation of L-thyroxine and 3,5,3'-triiodo-L-thyronine with hepatic microsomes. Endocrinology. 1971 Sep;89(3):749–755. doi: 10.1210/endo-89-3-749. [DOI] [PubMed] [Google Scholar]
- LISSITZKY S., ROQUES M., BENEVENT M. T. [Enzymatic deiodination of thyroxin and its derivatives. I. Purification and properties of thyroxin-deiodase from the rabbit muscle]. Bull Soc Chim Biol (Paris) 1961;43:727–742. [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Surks M. I. Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest. 1968 Jun;47(6):1399–1406. doi: 10.1172/JCI105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Shapiro H. C., Bernstein G., Surks M. I. Differences in primary cellular factors influencing the metabolism and distribution of 3,5,3'-L-triiodothyronine and L-thyroxine. J Clin Invest. 1970 May;49(5):1016–1024. doi: 10.1172/JCI106301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLASKETT L. G. Studies on the degradation of thyroid hormones in vitro with compounds labelled in either ring. Biochem J. 1961 Mar;78:652–657. doi: 10.1042/bj0780652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinwein D., Rall J. E. Nonenzymatic deiodination of thyroid hormones by flavin mononucleotide and light. J Biol Chem. 1966 Apr 10;241(7):1636–1643. [PubMed] [Google Scholar]
- Schwartz H. L., Surks M. I., Oppenheimer J. H. Quantitation of extrathyroidal conversion of L-thyroxine to 3,5,3'-triiodo-L-thyronine in the rat. J Clin Invest. 1971 May;50(5):1124–1130. doi: 10.1172/JCI106584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. C., Surks M. I., Oppenheimer J. H. Cellular and plasma protein determinants in the differential distribution and metabolism of D- and L-thyroxine in the rat. Endocrinology. 1971 Jan;88(1):93–101. doi: 10.1210/endo-88-1-93. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Incorporation of radioactivity from monoiodotyrosine by soluble systems. Biochim Biophys Acta. 1968 Feb 26;155(2):536–548. doi: 10.1016/0005-2787(68)90197-4. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Composition of nonextractable radioactivity formed after injection of labeled L-thyroxine and 3,5,3'-triiodo-L-thyronine in rats. Endocrinology. 1970 Sep;87(3):567–575. doi: 10.1210/endo-87-3-567. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Formation of iodoprotein during the peripheral metabolism of 3,5,3'-triiodo-L-thyronine-125I in the euthyroid man and rat. J Clin Invest. 1969 Apr;48(4):685–695. doi: 10.1172/JCI106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Metabolism of phenolic- and tyrosyl-ring labeled L-thyroxine in human beings and rats. J Clin Endocrinol Metab. 1971 Oct;33(4):612–618. doi: 10.1210/jcem-33-4-612. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schwartz H. L., Oppenheimer J. H. Tissue iodoprotein formation during the peripheral metabolism of the thyroid hormones. J Clin Invest. 1969 Nov;48(11):2168–2175. doi: 10.1172/JCI106183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R. Inhibition of the biological action of thyroid hormones by actinomycin D and puromycin. Nature. 1963 Mar 23;197:1167–1168. doi: 10.1038/1971167a0. [DOI] [PubMed] [Google Scholar]
- TATA J. R. Transiodination of proteins during enzymic de-iodination of thyroxine. Nature. 1960 Sep 17;187:1025–1026. doi: 10.1038/1871025a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYNN J., GIBBS R. Thyroxine degradation. II. Products of thyroxine degradation by rat liver microsomes. J Biol Chem. 1962 Nov;237:3499–3505. [PubMed] [Google Scholar]
- YAMADA T., IINO S., GREER M. A. Comparison of the effect of hypophysectomy and thyroxine administration on thyroid function in the rat. Endocrinology. 1961 Jul;69:1–12. doi: 10.1210/endo-69-1-1. [DOI] [PubMed] [Google Scholar]


