Abstract
Background and objectives: Urine IL-18 (uIL-18) has demonstrated moderate capacity to predict acute kidney injury (AKI) and adverse outcomes in defined settings. Its ability to predict AKI and provide prognostic information in broadly selected, critically ill adults remains unknown.
Design, setting, participants, & measurements: The study prospectively evaluated the capacity of uIL-18 measured within 24 hours of intensive care unit (ICU) admission to predict AKI, death, and receipt of acute dialysis in a large mixed-adult ICU population.
Results: Of 451 patients, 86 developed AKI within 48 hours of enrollment and had higher median uIL-18 levels [426 (interquartile range [IQR]: 152 to 1183) pg/mg creatinine] compared with those without AKI [248 (IQR: 120 to 559) pg/mg]. The area under the receiver operating characteristic curve for uIL-18 predicting subsequent AKI within 24 hours was 0.62 (95% CI: 0.54 to 0.69) and improved modestly to 0.67 (95% CI: 0.53 to 0.81) in patients whose enrollment eGFR was ≥75 ml/min per 1.73 m2. The highest median uIL-18 levels were observed in patients with sepsis at enrollment [508 (IQR: 230 to 1281) pg/mg], those receiving acute dialysis [571 (IQR: 161 to 1614) pg/mg] or dying [532 (IQR: 210 to 1614) pg/mg] within 28 days of ascertainment. After adjustment for a priori selected clinical predictors, uIL-18 remained independently predictive of composite outcome of death or acute dialysis within 28 days of ascertainment (odds ratio, 1.86 [95% CI: 1.31 to 2.64]).
Conclusions: uIL-18 did not reliably predict AKI development, but did predict poor clinical outcomes in a broadly selected, critically ill adult population.
The successful translation of promising preclinical treatments for acute kidney injury (AKI) has been hindered by a lack of early, accurate, and reliable indicators of injury. Furthermore, the discovery and validation of biologic markers able to differentiate between patients with mild or reversible forms of AKI and those that will progress to dialysis or not survive may also assist in risk-stratification for clinical trials. Recent efforts to identify biologic markers with early diagnostic and prognostic potential have yielded several candidates (1), including neutrophil gelatinase-associated lipocalin (NGAL) (2), liver fatty acid binding protein (L-FABP) (3), kidney injury molecule 1 (KIM-1) (4), cystatin C (5), and IL-18 (urine IL-18 [uIL-18]) (6,7).
IL-18, a proinflammatory cytokine of the IL-1 superfamily, is found in monocytes, fibroblasts, and proximal renal tubular epithelial cells (8). The ability of IL-18 to mediate ischemic proximal tubular injury in mice and proinflammatory responses via its actions on the Toll-like receptor 4 has provided a rationale for its use as a human AKI biomarker (9,10). Previous studies have explored the ability of uIL-18 levels to predict AKI among children undergoing cardiopulmonary bypass (11), adults receiving a kidney transplant (12), and in children requiring mechanical ventilation (13). More recently, early translational studies in humans with limited sample sizes have demonstrated that uIL-18 levels can provide important prognostic information for AKI patients after cardiac surgery (11), 3-month graft function in kidney transplant patients (11), and survival in acute respiratory distress syndrome (ARDS) and in critically ill children (6,13).
Using a large cohort of critically ill adults participating in the National Institutes of Health (NIH)-sponsored Validation of Biomarkers for Acute Lung Injury Diagnosis (VALID) study; we examined the ability of uIL-18 to predict both the development of AKI and clinically relevant outcomes including mortality and dialysis in a heterogeneous intensive care unit (ICU) population. Results were also compared independently and in combination with the previously reported performance of urine NGAL (uNGAL) (14).
Materials and Methods
Patients
The first 588 patients enrolled between February 2006 and January 2007 in the previously reported NIH-sponsored VALID study were studied. All adult (≥18 years of age) patients admitted to one of four ICUs (Medical, Cardiac, Surgical, Trauma) at Vanderbilt University Medical Center (VUMC) who remained in the ICU at ICU day 2 were eligible for enrollment (14). Patients were excluded if they experienced a cardiac arrest before enrollment, had transfer orders written or anticipated within 4 hours, died or were discharged within 48 hours of ICU admission, were admitted for uncomplicated overdose, had chronic lung disease requiring oxygen supplementation at baseline or pulmonary fibrosis, had a history of ESRD or renal transplant (49 subjects), received acute dialysis at or before enrollment (15 subjects), or received ICU care for greater than 3 days before assessment for eligibility. Of the 524 remaining patients, 490 had urine specimens available for analysis. Twenty-three patients had no serum creatinine (SCr) monitoring for all 3 follow-up days because the primary team deemed creatinine measurement unnecessary due to imminent death, discharge, or lack of a condition requiring intensive surveillance of kidney function (e.g., isolated trauma). The study protocol and consent forms were approved by the VUMC Human Subjects Institutional Review Board before study initiation and were in accordance with the Declaration of Helsinki.
Clinical Data Collection
The general study schematic has been described previously (14). Demographic data including age, gender, weight, ethnicity, and ICU admission diagnoses were collected at the time of enrollment. Acute Physiology and Chronic Health Evaluation (APACHE) II (15) and Simplified Acute Physiology Score (SAPS) II (16) were calculated at enrollment. The presence of the systemic inflammatory response syndrome (SIRS), sepsis, or severe sepsis was determined on a daily basis according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus definition (17). Vasopressor use, nephrotoxin exposure, blood product transfusion, 24-hour urine output, and fluid balance were collected during the ensuing 72-hour follow-up period. A history of chronic kidney disease (CKD) was considered present if recorded within the admission documentation or by a GFR of <60 ml/min per 1.73 m2 as estimated by the abbreviated modification of diet and renal disease (MDRD) equation [GFR (ml/min per 1.73 m2) = 186 × (Scr)−1.154 × (Age) −0.203 × (0.742 if female) × (1.210 if African American)] (18,19) using the lowest recorded SCr within 1 year of enrollment, if available. The presence of organ failure was determined using the Brussels Organ Failure Criteria for the following systems (circulatory, hepatic, coagulation, renal) (20).
Patients were followed prospectively until hospital discharge and ICU and hospital length of stay, and hospital mortality was recorded. The VALID database has been cross-referenced to the Social Security Death Index to allow for longitudinal determination of mortality. The Social Security Death Index is 88.2% sensitive for death for the general population (21). Patients without a recorded death in the death index were considered alive at 28 days after the study enrollment. Renal-specific outcomes were also collected including the need for nephrology consultation, indication and type of renal replacement therapy, and dialysis-dependence at discharge.
Laboratory Data Collection
The highest and lowest SCr values were recorded daily from ICU admission through 72 hours after enrollment. AKI was defined as a 0.3 mg/dl or 50% increase in SCr from the value closest to enrollment (generally the routine morning draw) to the maximum SCr within 48 hours as suggested by acute kidney injury network (AKIN) (22).
uIL-18 and creatinine samples were collected the morning of enrollment from the proximal meter reservoir of the Foley catheter, immediately placed on ice, pipetted into 400-μl aliquots, and frozen at −80°C within 1 hour of collection. IL-18 levels were measured in urine using the human IL-18 ELISA no. 036 (MBL International, Gentofte, Denmark). Since the Human IL-18 ELISA kit from MBL was only validated by the manufacturer for plasma and serum, we did an in-lab validation for measurement in human urine. Recombinant IL-18 protein was spiked into normal, control urine. After subtracting the concentration of the human IL-18 of the unspiked control from the recovered values in the spiked samples, we determined that there was good correlation between the spiked and recovery concentrations in the upper region of the standard curve (64 to 1000 pg/ml). However, there was poor sensitivity below 64 pg/ml. To improve sensitivity, the manufacturer's protocol was revised to incubate the urine samples with the primary monoclonal antibody for 24 hour instead of 1 hour, which resulted in excellent sensitivity below 64 pg/ml with a lower limit of detection of 2 pg/ml. Samples were run in duplicate, and lab personnel were blinded to the injury status of each patient. The mean intra-assay coefficient of variation in our laboratory was 3.1%. Urine creatinine was measured at the VUMC General Clinical Research Center core laboratory facility using a quantitative colorimetric assay (VITROS Chemistry Products; Ortho-Clinical Diagnostics Inc., Rochester, NY).
Statistical Analysis
Baseline characteristics of patients were described as medians with interquartile range (IQR) for continuous variables and compared using the Wilcoxon rank sum test. Categorical variables were expressed as proportions and compared using the Pearson χ2.
The ability of uIL-18 measured at enrollment to predict AKI development was analyzed separately at 24 and 48 hours using logistic regression. We selected patients who had at least one measured creatinine during the first day of enrollment for the 24-hour analysis, and for two consecutive days for the 48-hour analysis to avoid survivor or surveillance bias. Controls were selected with creatinine data at 24 hours and without evidence of AKI (0.3 or 50% increase in SCr from enrollment). For the 48-hour analysis, controls were also required to have a second creatinine measured and not meet criteria for AKI from enrollment.
The ability of uIL-18 to discriminate between AKI and non-AKI during the follow-up period was determined using receiver operating characteristic (ROC) curves providing sensitivity and specificity at different cutoff values of uIL-18 to detect AKI and the area under the curve (AUC). To reduce the contribution of pre-existing AKI, we performed sensitivity analyses restricting analysis to those patients with an enrollment eGFR of >75 ml/min per 1.73 m2. To assess the independent predictive ability of uIL-18 compared to a priori specified predictors of AKI such as age, modified APACHE II score, the presence of sepsis, organ failure, SCr closest to enrollment, ICU location, and previously measured uNGAL levels, a multivariable logistic regression modeling was used. A modified APACHE II score was calculated based on the total APACHE II score minus the points derived from the SCr value to avoid multicolinearity with SCr when these variables were simultaneously included in a multivariable regression.
Adjusted effects of uIL-18 were presented as odds ratios (OR) with 95% confidence intervals (CI). The effect modification of sepsis status on the association between uIL-18 and AKI was assessed by stratification analyses and by including a cross-product (IL-18 × sepsis) in multivariable logistic regression models. We also examined for multiplicative effects of uIL-18 and uNGAL on AKI by interaction analyses with a cross-product term in model (IL-18 × NGAL).
The role of uIL-18 in predicting clinical outcomes beyond AKI was evaluated using a multivariable logistic regression model for three different outcomes: (1) the need for inpatient acute dialysis within 28 days of measurement; (2) death within 28 days; and (3) combined endpoint of death or needing dialysis. Patients were monitored during hospitalization to record in-hospital survival or the need for acute dialysis. Death following discharge within 28 days was ascertained using either the electronic medical record or with the Social Security death index. Patients who were discharged alive without dialysis were considered as not having dialysis and being alive at 28 days because of the low likelihood of outpatient dialysis initiation within 28 days. Dialysis models were adjusted for APACHE score only to prevent overfitting, and all other models were adjusted for a priori specified-covariates as described above. The statistical software package R version 2.7.2 (www.r-project.org) and SAS version 9 were used for analyses, and two-sided P values of less than 0.05 were required to achieve statistical significance.
Results
Subject Characteristics
AKI was detected in 86 (19.1%) of 451 subjects with any available SCr values within 48 hours following enrollment. Approximately 15% of patients had acute lung injury (ALI)/ARDS upon study enrollment. Characteristics and uIL-18 levels of patients who developed AKI were compared with 305 subjects who had SCr data available within both 24 and 48 hours, and did not develop AKI (Table 1). Patients developing AKI were more likely to have CKD, diabetes mellitus, higher illness severity, sepsis, and require vasopressor support on enrollment than non-AKI patients (P < 0.05). AKI patients were also more likely to experience in-hospital death, require renal replacement therapy (RRT), and had fewer dialysis-free and ventilator-free days than non-AKI patients (P < 0.001). Supplemental Figure S1 shows the distribution of creatinine values between patients developing and not developing AKI at each time point.
Table 1.
Enrollment demographic and physiologic data of patients grouped according to AKI statusa
| No AKI (n = 305) | AKI (n = 86) | P | |
|---|---|---|---|
| Age | 52 (37 to 63) | 55 (43 to 68) | 0.064 |
| Male, n (%) | 170 (56%) | 56 (65%) | 0.12 |
| Caucasian, n (%) | 263 (86%) | 73 (85%) | 0.78 |
| DM, n (%) | 54 (18%) | 30 (35%) | <0.001 |
| CKD, n (%) | 27 (9%) | 23 (27%) | <0.001 |
| ICU type, n (%) | <0.001 | ||
| Medical | 151 (49%) | 47 (55%) | |
| Surgical | 54 (18%) | 29 (34%) | |
| Trauma | 92 (30%) | 7 (8%) | |
| Cardiac | 8 (3%) | 3 (3%) | |
| APACHE II | 22 (18 to 28) | 30 (23 to 34) | <0.001 |
| modAPACHE II | 21 (17 to 26) | 26 (20 to 31) | <0.001 |
| SAPS II | 44 (32 to 55) | 57 (41 to 69) | <0.001 |
| Sepsis, n (%) | 114 (37%) | 47 (55%) | 0.004 |
| Severe sepsis, n(%) | 109 (36%) | 46 (53%) | 0.003 |
| ALI/ARDS, n (%) | 42 (14%) | 16 (19%) | 0.27 |
| Contrast exposure Preceding 48 hours, n (%) | 41 (13%) | 9 (10%) | 0.47 |
| Vasopressor use, n (%) | 115 (38%) | 44 (51%) | 0.025 |
| Number of nephrotoxins at time of enrollment | 0 (0 to 1) | 0 (0 to 1) | 0.45 |
| Fluid balance from ICU admission to enrollment (extrapolated to 24 hours) | 2.0 (0.7 to 4.8) | 2.9 (0.7 to 7.8) | 0.036 |
| SCr closest to enrollment | 0.9 (0.7 to 1.2) | 1.5 (1.0 to 2.2) | <0.001 |
| Urine creatinine (mg/ml) | 0.88 (0.47 to 1.35) | 0.82 (0.52 to 1.37) | 0.9 |
| uIL-18 (pg/ml) | 215 (95 to 440) | 316 (144 to 716) | 0.002 |
| uIL-18/urine Cr (pg/mg) | 248 (120 to 559) | 426 (152 to 1183) | 0.004 |
| uNGAL (ng/ml) | 48.1 (12.7 to 179.3) | 126.6 (32.1 to 623.1) | <0.001 |
| uNGAL/urine Cr (ng/mg) | 57 (17 to 203) | 190 (32 to 995) | <0.001 |
DM, diabetes mellitus; APACHE II, Acute Physiology and Chronic Health Evaluation II score; modAPACHE II, modified APACHE II score; SAPS, Simplified Acute Physiology Score; MAP, mean arterial pressure. Potential nephrotoxins included radiocontrast within 48 hours prior to enrollment or aminoglycosides, angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, nonsteroidal anti-inflammatory agents, cyclooxygenase-2 inhibitors, trimethroprim-sulfamethoxazole, calcineurin inhibitors, or acyclovir at the time of enrollment. Values are presented as either proportions or median (IQR). Wilcoxon rank sum test was used to compare continuous variables and Chi-square test for categorical variables. P values <0.05 denote statistical significance.
AKI was identified during the 48-hour period following enrollment.
Association between uIL-18 and AKI Development
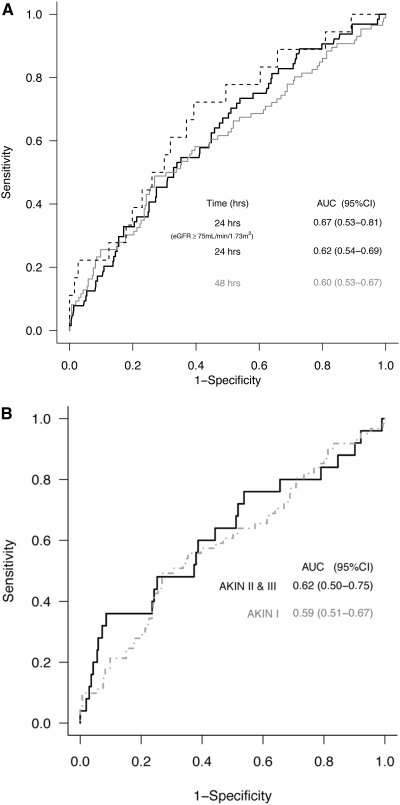
Table 1 shows creatinine-corrected biomarker concentrations measured at enrollment grouped by AKI status within 48 hours of enrollment. Median uIL-18 levels of the 64 patients who developed AKI within 24 hours [412 (IQR: 150 to 1477) pg/mg] were higher than in the 386 non-AKI subjects [230 (IQR: 109 to 534) pg/mg] (P = 0.003). By 48 hours, 86 subjects had developed AKI with a higher median uIL-18 level [426 (IQR: 152 to 1183) pg/mg] than the 305 subjects without AKI at any time during this interval [median 248 (IQR: 120 to 559) pg/mg] (P = 0.004). The AUC-ROCs for the utility of uIL-18 for prediction of AKI within 24 and 48 hours were 0.62 (95% CI: 0.54 to 0.69) and 0.60 (95% CI: 0.53 to 0.67), respectively (Figure 1A). By 72 hours after measurement, the AUC further decreased to 0.56 (95% CI: 0.49 to 0.63). A sensitivity analysis restricting the population to those with an eGFR of ≥75 ml ml/min per 1.73 m2 at enrollment (n = 275) was done to limit the contribution of preexisting AKI at enrollment. Eighteen of these patients developed AKI within 24 hours and were compared with the 257 patients without AKI. Despite similar enrollment median SCr values between the two groups [AKI 0.80 (IQR: 0.60 to 0.90) versus non-AKI 0.85 (IQR: 0.53 to 1.15), P = 0.22], median uIL-18 for patients developing AKI was 346 (IQR: 209 to 633) pg/mg and 189 (IQR: 98 to 390) pg/mg for non-AKI patients (P = 0.013). The AUC for AKI prediction in this subgroup of patients was 0.67 (95% CI: 0.53 to 0.81) (hashed line). We also explored the association between uIL-18 and AKIN staging (Figure 1B). Of subjects with AKI, 61 (70.9%) met AKIN stage I, 7 (8.2%) met AKIN stage II, and 18 (20.9%) met AKIN stage III criteria as assessed by change in SCr or by having received RRT within 48 hours of enrollment. Given the paucity of subjects that met stage II injury criteria, we combined them with individuals meeting stage III injury. The AUC of uIL-18 to predict the subsequent occurrence of AKIN stage 1 was 0.59 (95% CI: 0.51 to 0.67) and for the combined AKIN stages II and III, the AUC was 0.62 (95% CI: 0.50 to 0.75).
Figure 1.
(A) The AUC-ROCs for AKI development within 24 hours (black line) and 48 hours (gray line) of uIL-18 measurement. A sensitivity analysis restricted to patients in eGFR at enrollment of ≥75 ml/min/m2 was also performed (hashed line). The AUC values and 95% CI are listed within the figure. (B) The AUC-ROCs stratified by AKIN stage (I, II + III) over 48 hours. The AUC values and 95% CI are listed within the figure.
Previous studies have suggested that IL-18 levels may be influenced by the presence of sepsis (13). Median uIL-18 levels in the 184 patients with sepsis at enrollment were significantly higher [508 (IQR: 230 to 1281) pg/mg] than in the 306 patients without sepsis [170 (IQR: 88 to 322) pg/mg] (P < 0.001). Of the 184 patients with sepsis, 35 patients developed AKI within 24 hours and had median enrollment uIL-18 levels of 591 (IQR: 227 to 1825) pg/mg, which was not statistically different than uIL-18 levels in the 139 patients without AKI at 24 hours among those with SCr data available [465 (IQR: 246 to 1070) pg/mg] (P = 0.223). However, in the 306 patients without sepsis at enrollment, median uIL-18 levels at enrollment were higher in the 29 patients experiencing AKI by 24 hours [261 (IQR: 144 to 599) pg/mg] than in the 247 nonseptic subjects without AKI among those with SCr available [median uIL-18 163 (IQR: 86 to 305) pg/mg] (P = 0.041). We assessed for effect modification of sepsis on the association between uIL-18 levels and the outcome of AKI at 24 hours using logistic regression. Although the sepsis analysis suggested a potential differential association between uIL-18 and AKI in the absence of sepsis [OR 2.15 (95% CI: 1.21 to 3.81)] relative to those with sepsis [OR 1.39 (95% CI: 0.87 to 2.22)], the interaction term did not reach statistical significance (P = 0.25).
Association between uIL-18 and Clinical Outcomes
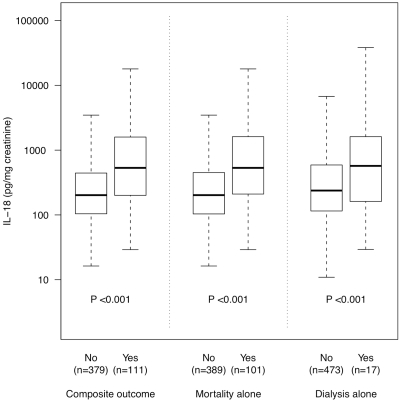
Figure 2 compares uIL-18 levels with an a priori specified composite endpoint of death or acute inpatient dialysis within 28 days in all 490 patients with urine samples available. Median enrollment uIL-18 levels were higher in the 111 patients experiencing the composite outcome [532 (IQR: 200 to 1593) pg/mg] than the 379 who did not [202 (IQR: 104 to 443) pg/mg] (P < 0.001), and in 101 who died [532 (IQR: 210 to 1614) pg/mg] versus the 389 alive at 28 days [202 (IQR: 104 to 451 pg/mg)] (P < 0.001). Median uIL-18 levels in the 17 patients receiving acute inpatient dialysis within 28 days [571 (IQR: 161 to 1614) pg/mg] were also higher than in the 473 patients who did not [238 (IQR: 115 to 591) pg/mg], although this did not reach statistical significance (P = 0.075). uIL-18 remained independently associated with the a priori selected outcome of death or need for dialysis [OR 1.86 (95% CI: 1.31 to 2.64)] in multivariable logistic regression model that adjusted for age, modified APACHE II score, SCr closest to enrollment, sepsis status at enrollment, organ failure, and ICU location (Table 2). While the major focus of this manuscript was to ascertain if uIL-18 provides early diagnostic and prognostic information, AKI is a known risk factor for poor clinical outcomes. As a result, a sensitivity analysis was performed assessing the ability of uIL-18 to predict our composite outcome after adjustment for age, sepsis, modified APACHE II, enrollment SCr, sepsis, organ failure, ICU location, and AKI within 24 hours of biomarker measurement. As expected, AKI event over 24 hours was predictive of death or the need for dialysis [OR 5.61 (95% CI: 2.80 to 11.26)]. However, uIL-18 measured before this detectable AKI event remained independently associated with our composite outcome [OR 1.76 (95% CI: 1.19 to 2.59)]. The details of this analysis can be found in Supplemental Table S1.
Figure 2.
Enrollment uIL-18 levels grouped according the composite outcome of 28-day mortality or acute dialysis during hospitalization within 28 days, 28-day mortality alone, or inpatient dialysis within 28 days. Values are displayed as Box-plot summaries with P values <0.05, denoting statistical significance.
Table 2.
Multivariable logistic regression model for the composite outcome (n = 111) of death (n = 101) or acute dialysis (n = 17)
| Variablea | Odds Ratio (95% CI) | P |
|---|---|---|
| Log10 IL-18 (IQR: 0.71) | 1.86 (1.31 to 2.64) | <0.001 |
| Age (IQR: 25 years) | 1.48 (1.03 to 2.14) | 0.036 |
| Modified APACHE II (IQR:11 units) | 2.36 (1.58 to 3.55) | <0.001 |
| Sepsis at enrollment | 1.89 (1.06 to 3.37) | 0.031 |
| Log10 SCr (IQR: 0.70) | 1.18 (0.91 to 1.52) | 0.221 |
| Location | 0.005 | |
| SICU:MICU | 0.29 (0.14 to 0.61) | |
| Trauma:MICU | 0.79 (0.37 to 1.69) | |
| Organ failure | 1.12 (0.61 to 2.05) | 0.71 |
| Overall model | <0.001 |
Continuous variables ORs are represented for IQR difference (SCr and IL-18 were log10 transformed in the model). ORs are adjusted for all variables shown.
Comparative Analysis of uIL-18 to uNGAL
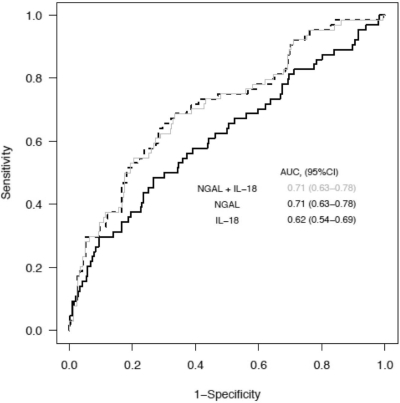
We previously studied the utility of uNGAL for prediction of AKI within the same cohort of patients and reported that it performed moderately with an AUC-ROC within 24 hours of AKI of 0.71 (95% CI: 0.63 to 0.78) relative to 0.62 (95% CI: 0.54 to 0.69) for uIL-18 (14). Supplemental Figure S2 illustrates the relative distribution of enrollment uIL-18 and uNGAL values between patients developing and not developing AKI patients over the first 24 hours. In this cohort, uIL-18 and uNGAL were significantly correlated with a Spearman coefficient of ρ = 0.55 (P < 0.001). Figure 3 shows the independent and combined AUC-ROCs for the prediction of AKI within 24 hours of measurement of uIL-18 [0.62 (95% CI: 0.54 to 0.69)] (black line), uNGAL [0.71 (95% CI: 0.63 to 0.78)] (gray line), and both biomarkers added [0.71 (95% CI: 0.63 to 0.78)] (hashed line). The addition of uIL-18 did not improve the ability of uNGAL to predict AKI as assessed by ROC curve. We also explored for multiplicative effects between uIL-18 and uNGAL for the prediction of AKI within 24 hours and did not find a statistically significant interaction.
Figure 3.
The AUC-ROCs for AKI development within 24 hours of measurement for uIL-18 (black line), uNGAL (hashed line), and in combination (gray line). AUC values and 95% CI are listed within the figure.
Separate logistic regression models for uIL-18 and uNGAL were adjusted for age, modified APACHE II, sepsis status at enrollment, and SCr closest to enrollment for AKI within 24 hours (Table 3). For the outcome of dialysis alone (n = 17), the model was adjusted for APACHE II score only. Only uNGAL was predictive of AKI [OR 1.71 (95% CI: 1.12 to 2.60)]. Both uNGAL [OR 1.53 (95% CI: 1.07 to 2.19) and uIL-18 [OR 1.86 (95% CI: 1.31 to 2.64)] were independently predictive of the composite outcome of death or acute dialysis within 28 days. Exploratory analysis revealed that uNGAL was predictive of acute dialysis [OR 2.76 (95% CI: 1.41 to 5.41)] when adjusted for APACHE II score alone and approached significance for 28-day mortality alone when adjusted for age, modified APACHE II, enrollment SCr, sepsis, organ failure, and ICU location [OR 1.44 (1.00 to 2.07)]. In comparison, after adjusting for APACHE II score alone, uIL-18 did not reliably predict patients requiring acute dialysis [OR 1.05 (95% CI: 0.56 to 1.98), however, was a better predictor of 28-day mortality than uNGAL after adjusting for age, modified APACHE II, enrollment SCr, sepsis, organ failure, and ICU location relative to uNGAL [OR 2.02 (95% CI: 1.41 to 2.89)].
Table 3.
The associations between uIL-18 and uNGAL with AKI within 24 hours, 28-day mortality, receipt of acute dialysis during hospitalization within 28 days, and the composite outcome
| IL-18a |
NGALa |
|||
|---|---|---|---|---|
| ORa (95% CI) | P | ORa (95% CI) | P | |
| Outcome variable: | ||||
| AKI 24 hours (n = 64)b | 1.13 (0.75 to 1.69) | 0.554 | 1.71 (1.12 to 2.60) | 0.013 |
| Dialysis following enrollment (n = 17)c | 1.05 (0.56 to 1.98) | 0.872 | 2.76 (1.41 to 5.41) | 0.003 |
| 28-day mortality (n = 101)d | 2.02 (1.41 to 2.89) | 0.001 | 1.44 (1.00 to 2.07) | 0.052 |
| 28-day mortality or dialysis (n = 111)d | 1.86 (1.31 to 2.64) | <0.001 | 1.53 (1.07 to 2.19) | 0.020 |
Biomarkers were log10 transformed. Odds ratios are represented for interquartile range difference of biomarkers concentrations.
Separate logistic regression models for IL-18 and NGAL were adjusted for:
Enrollment age, modified APACHE II, SCr, sepsis, and ICU location.
Dialysis model was adjusted for APACHE II score only to avoid overfitting.
Enrollment age, modified APACHE II, the presence of organ failure (circulatory, coagulation, hepatic, renal), SCr, sepsis, and ICU location.
Discussion
Although the critically ill are at high risk for developing AKI (23,24), the timing and origin of kidney injury in this setting is heterogeneous, which challenges biologic markers aiming to provide early diagnostic and prognostic information. uIL-18 measured within 48 h of AKI development did not alone reliably predict nor add to the ability of uNGAL to predict the subsequent development of AKI using changes in SCr. These results do not support the routine assessment of uIL-18 for this purpose in a mixed critically ill population. However, uIL-18 levels were independently associated with the risk of adverse clinical outcomes within 28 days and could provide important prognostic information.
uIL-18 has been studied in several settings to detect AKI and predict subsequent renal outcomes. While results have varied, early detection of AKI has proven more robust in settings of discrete ischemia-reperfusion injury such as cardiopulmonary bypass in children and after kidney transplantation (11,12,25). In contrast, prediction of AKI among co-morbid adults undergoing cardiopulmonary bypass (6,26), critically ill patients including children requiring mechanical ventilation (13), or patients with ARDS has been less robust. This observation may, in part, be explained by the association of the marker with inflammation (27). Kidney ischemia-reperfusion results in a localized inflammatory-mediated injury with important contributions from the pleiotropic effects of IL-18 on both innate and cognate immunity (28). However, in addition to renal tubular cells, expression has been observed within other cell lineages including macrophages, lymphocytes, intestinal epithelia, and fibroblasts raising the possibility of systemic production confounding the association between uIL-18 and AKI during acute illness or inflammatory conditions (27). Nevertheless, the amount of overlap observed in the distribution of uIL-18 levels between those who developed AKI and those who did not develop AKI may therefore limit its use for predicting subsequent rises in SCr in these settings.
Similarly, a relative lack of systemic production may also partially explain the apparent improved performance of this biomarker in the setting of relatively isolated ischemia-reperfusion injury in children (11), during transplantation of relatively “normal” kidneys (12), or in contrast nephropathy (29). Our examination of the interaction between uIL-18 and sepsis also suggested improved performance in nonseptic patients, though this did not reach statistical significance. As septic patients are at higher risk for developing AKI, it can be argued that a higher proportion were at risk for pre-existing AKI at the time of enrollment, which may have contributed to the observed lack of difference in uIL-18 levels within this group. However, our results are consistent with Washburn et al. (13), who recently demonstrated in a cohort of pediatric patients with established baseline kidney function requiring mechanical ventilation that a more robust association between uIL-18 and AKI development occurred only after the exclusion of septic patients. Whether using urine/plasma ratios of IL-18 in systemically ill patients would increase specificity for “kidney-specific” IL-18 production in AKI in such settings remains to be determined. Nevertheless, these observations highlight the challenges of validating candidate markers in settings such as critical illness where AKI phenotyping is more difficult to accomplish relative to those where the injury type is known or otherwise similar to the model in which the biomarker was initially characterized (e.g., ischemia-reperfusion).
Similar to the investigation of biomarkers for the diagnosis of other complex acute diseases such as ALI/ARDS (30), a single biomarker is unlikely to be sensitive to the multiple pathways (e.g., inflammatory, ischemic, nephrotoxic, coagulation cascade abnormalities, oxidative stress) involved in the generation of AKI in a broadly-selected patient population with acute illness. Consequently, we investigated the use of multiple markers to improve our ability to detect AKI early by examining the relative contribution of uIL-18 to uNGAL. When assessed by ROC curve, uIL-18 did not add substantially to the early discriminative or predictive power of uNGAL for the detection of AKI. In addition to the modest performance of uIL-18 alone, another reason may be the high degree of colinearity observed between these biomarkers. As a result, the incremental value of adding uIL-18 in AKI detection may be marginal because it is measuring information already captured by uNGAL. The failure of correlated biomarkers to provide substantial independent value has been illustrated in other disease states and underscores the importance of panels including “orthogonal” biomarkers that target distinct pathophysiologic mechanisms (31).
As SCr is commonly used but an imperfect gold standard to which novel candidate markers are being compared, extending analyses to the prediction of less ambiguous clinical endpoints remains an important area to be explored for all putative AKI biomarkers. Several mortality prediction models have been previously developed in AKI patients (32–35), although variations in case-mix, clinical data collection, and care processes between settings have often led to a lack of external validity (36). The use of more specific biologic indicators of renal injury may enhance the performance of these models in predicting renal-relevant outcomes between patient populations. Recent studies have suggested that uIL-18 may provide substantial prognostic information in different settings. Our results indicate that uIL-18 independently predicts the composite outcome of death or dialysis in critically ill patients even after adjustment for known clinical predictors. This finding is likely largely driven by death, which was the predominant event in this composite outcome, suggesting utility for uIL-18 as a marker of prognosis in critical illness. Sensitivity analyses in the small subgroup of patients requiring dialysis suggest that uNGAL may be a more specific predictor of AKI severity than uIL-18, a hypothesis further supported by its relatively superior performance for AKI prediction. Furthermore, we have previously demonstrated that uNGAL independently predicts the need for dialysis when death was treated as a competing risk (14). These findings need to be validated in larger cohorts and in those with AKI captured at the earliest stages of AKI diagnosis.
While a major strength of this study is the use of a large and diverse ICU-population, it remains a single-center study in need of external validation. Additional limitations include the use of a single time-point assessment of uIL-18 rather than serial measurements. Previous studies have demonstrated a transient peak in uIL-18 levels after a discrete ischemic event and thus, multiple urine samples may have increased the likelihood of observing a difference. However, it can be argued that for a biomarker to be useful in a setting where the type and timing of contributing insults is more variable, sustained expression following injury will be a requisite feature. Urine was also collected from the proximal urinary catheter reservoir and may have been subject to degradation after remaining at room temperature for up to an hour. However, data from our laboratory (CR Parikh, personal communication) suggest that urinary IL-18 is stable in a variety of storage and shipping conditions. Finally, while the higher enrollment SCr values in patients subsequently developing AKI versus controls were at least partially due to the higher underlying CKD rate in this group (27% versus 9%, P < 0.001), some subjects may have already developed AKI at the time of enrollment, thereby limiting the prediction of de novo injury. However, sensitivity analyses using more severe injury criteria (i.e., AKIN stages II and III) or restricting examination to those with preserved and similar eGFR between AKI and non-AKI groups at enrollment only modestly improved uIL-18 performance for early detection. Furthermore, these results are consistent with two recently published studies where baseline function was largely known (13,26) as well as a more selective case-control analysis by Parikh et al. in ARDS indicating only modest utility in this regard (6).
In summary, we found that uIL-18 measured within 24 hours of ICU admission modestly predicted subsequent AKI development in a heterogeneous critically ill adult population, although not independently of previously measured uNGAL levels. uIL-18 was independently associated with adverse clinical outcomes and may have a role in defining further risk-stratification in this patient population. As AKI remains a complex disease unlikely to be described completely by any single marker, the utility of uIL-18 in determining the prognosis of patients early in course of AKI and in combination with other biomarkers reflecting separate biologic pathways remains an important area for further study.
Disclosures
Chirag R. Parikh is a co-inventor on the IL-18 patent licensed by the University of Colorado and is a Consultant to Abbot Diagnostics.
Supplementary Material
Acknowledgments
The VALID study is supported by NIH Grant UO1 HL081332 from the National Heart, Lung and Blood Institute, K24 DK62849 from the National Institute of Diabetes, Digestive, and Kidney Diseases, and the Clinical Translational Science Award 1UL-1RR024975 from the National Center for Research Resources. E. Siew is supported by the National Kidney Foundation Research Fellowship Award and the Vanderbilt Mentored Clinical Research Scholar Program 5KL2 RR024977-02.
We express our appreciation and are indebted to the VALID Study Group (Caroline Gilmore, RN, Kathryn Carlson, RN, Emilia Nannie, RN, Nadine Rihani, Donna Staed, and Jeremy Stephens), who assembled the database and specimen repository for this study, and to the patients who took part in the study. We also would like to thank Andrew J. Vincz for his technical expertise.
Preliminary data from this manuscript was submitted in abstract form at the 2009 American Society of Nephrology Annual meeting; October 27 through November 1, 2009; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Coca SG, Yalavarthy R, Concato J, Parikh CR: Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 73: 1008–1016, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P: Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int 73: 465–472, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Herget-Rosenthal S, Marggraf G, Husing J, Goring F, Pietruck F, Janssen O, Philipp T, Kribben A: Early detection of acute renal failure by serum cystatin C. Kidney Int 66: 1115–1122, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL: Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol 16: 3046–3052, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gracie JA: Interleukin-18 as a potential target in inflammatory arthritis. Clin Exp Immunol 136: 402–404, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL: Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Washburn KK, Zappitelli M, Arikan AA, Loftis L, Yalavarthy R, Parikh CR, Edelstein CL, Goldstein SL: Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol Dial Transplant 23: 566–572, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Siew ED, Ware LB, Gebretsadik T, Shintani A, Moons KG, Wickersham N, Bossert F, Ikizler TA: Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol 20: 1823–1832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II: A severity of disease classification system. Crit Care Med 13: 818–829, 1985 [PubMed] [Google Scholar]
- 16.Le Gall JR, Lemeshow S, Saulnier F: A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957–2963, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Bone RC, Sibbald WJ, Sprung CL: The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101: 1481–1483, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Levey A, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Bernard G: The Brussels Score. Sepsis 1: 43–44, 1997 [Google Scholar]
- 21.Schisterman EF, Whitcomb BW: Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr 2: 2, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W: Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30: 2051–2058, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A: Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: A prospective observational cohort study. Crit Care 12: R96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracie JA, Robertson SE, McInnes IB: Interleukin-18. J Leukoc Biol 73: 213–224, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B, Chadban SJ: IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol 19: 2331–2341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q: Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract 108: c176–c181, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Levitt JE, Gould MK, Ware LB, Matthay MA: The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med 24: 151–167, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Gerszten RE, Accurso F, Bernard GR, Caprioli RM, Klee EW, Klee GG, Kullo I, Laguna TA, Roth FP, Sabatine M, Srinivas P, Wang TJ, Ware LB: Challenges in translating plasma proteomics from bench to bedside: Update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol 295: L16–L22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM: Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Liano F, Gallego A, Pascual J, Garcia-Martin F, Teruel JL, Marcen R, Orofino L, Orte L, Rivera M, Gallego N, et al. : Prognosis of acute tubular necrosis: An extended prospectively contrasted study. Nephron 63: 21–31, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH: Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. J Am Soc Nephrol 9: 692–698, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Chertow GM, Soroko SH, Paganini EP, Cho KC, Himmelfarb J, Ikizler TA, Mehta RL: Mortality after acute renal failure: Models for prognostic stratification and risk adjustment. Kidney Int 70: 1120–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Doig GS, Oudemans van Straaten H, Ronco C, Kellum JA: External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med 33: 1961–1967, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.